Abstract
Background
This study was designed to test the hypothesis that antioxidant Vitamin C prevents the impairment of endothelial function during prolonged sitting.
Material/Methods
Eleven men (24.2±4.4 yrs) participated in 2 randomized 3-h sitting trials. In the sitting without vitamin C (SIT) and the sitting with vitamin C (VIT) trial, participants were seated for 3 h without moving their legs. Additionally, in the VIT trial, participants ingested 2 vitamin C tablets (1 g and 500 mg) at 30 min and 1 h 30 min, respectively. Superficial femoral artery (SFA) flow-mediated dilation (FMD) was measured hourly for 3 h.
Results
By a 1-way ANOVA, there was a significant decline in FMD during 3 h of SIT (p<0.001). Simultaneously, there was a significant decline in antegrade (p=0.04) and mean (0.037) shear rates. For the SIT and VIT trials by a 2-way (trial × time) repeated measures ANOVA, there was a significant interaction (p=0.001). Pairwise testing revealed significant between-SFA FMD in the SIT and VIT trial at each hour after baseline, showing that VIT prevented the decline in FMD 1 h (p=0.009), 2 h (p=0.016), and 3 h (p=0.004). There was no difference in the shear rates between SIT and VIT trials (p>0.05).
Conclusions
Three hours of sitting resulted in impaired SFA FMD. Antioxidant Vitamin C prevented the decline in SFA FMD, suggesting that oxidative stress may contribute to the impairment in endothelial function during sitting.
Keywords: Antioxidants, Oxidative Stress, Sedentary Lifestyle
Background
Increased sitting time has been associated with increased risk of cardiovascular disease and mortality [1]. Prolonged sitting can create a unique physiological milieu in the lower extremity vasculature, leading to conditions conducive to the development of atherosclerosis [2]. For instance, 5 h of sitting has been shown to increase calf blood pooling and decrease thigh blood flow [3,4]. Greater than 3 h of sitting has also been shown to increase diastolic blood pressure (BP), mean arterial pressure (in the arm and leg), and total peripheral resistance [4,5]. Furthermore, only 2 h of sitting significantly increased whole-blood viscosity in the leg [6]. In the first experiment investigating the effects of sitting on vascular shear stress and endothelial function, Padilla et al. [5] discovered that 3 h of sitting significantly attenuated vascular shear in the popliteal artery; however, this was not associated with a concomitant decline in endothelial function measured by FMD. Contrary to this study, we recently discovered that 3 h of sitting results in a decline in mean shear rate in the SFA in addition to a significant decline in endothelial function when all measurements are made in the seated position [7]. Interestingly, in the vascular system, areas of low vascular shear stress have been associated with increased oxidative stress [8] and impaired endothelial function [8]. Additionally, low shear stress may be an etiological mechanism for atherosclerosis [9].
The human vasculature is internally coated with a single layer of cells called the endothelium, which becomes functionally impaired in the process of atherosclerosis [10]. Nitric oxide (NO), which is a critical molecule involved in maintaining the antiatherogenic properties of the endothelium [11], is synthesized from L-arginine by endothelial NO synthase (eNOS) in the presence of laminar shear stress and co-factors such as nicotinamide adenine dinucleotide phosphate (NADPH) and tetrahydrobiopterin (BH4) [12]. Shear stress associated with physical movement augments NO bioavailability [13] and thereby improves endothelial function [12]. Conversely, sitting decreases lower extremity shear rates [5,7], and hence it is possible that sitting may reduce NO bioavailability in the lower extremities. Indeed, low shear stress leads to decreased expression of NOS [8]. Our group has recently proposed that prolonged sitting may lead to low vascular shear-related impairment in endothelial function involving mechanisms related to oxidative stress [2]. We have recently address the first part of this hypothesis and shown that prolonged sitting decreases mean shear rate and impairs endothelial function in SFA [7].
The purpose of the current study was to determine if antioxidant Vitamin C prevents the decline in SFA endothelial function during prolonged sitting. We hypothesized that there would be a significant decline in SFA FMD from baseline during 3 h of sitting. Previous studies from our lab and others have demonstrated vitamin C to be effective in preventing the oxidative stress-mediated decline in endothelial function in a variety of physiological stressors, including, but not limited to, hypertension [14] and increased retrograde shear [15]. Therefore, we hypothesized that vitamin C would prevent the decline in SFA endothelial function during 3 h of sitting.
Material and Methods
Study design (Figure 1)
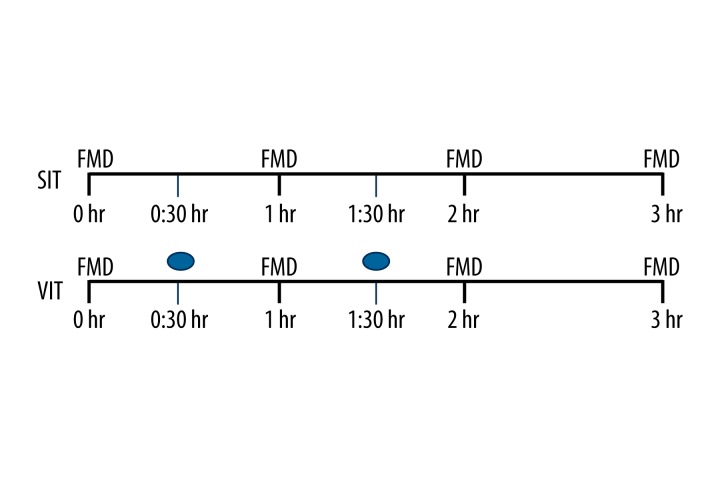
Figure 1.
Study design. SIT represents prolonged 3 h of sitting. VIT represents prolonged sitting with intermittent vitamin C. FMD was measured at 0, 1, 2, and 3 h. ● Represents vitamin C. Participants ingested 1 g vitamin C at 30 min and 500 mg at 1 h 30 min.
This study consisted of 2 screening visits and 2 sitting trials conducted in random sequence, the first with 3 h of uninterrupted sitting (SIT) and the second with oral Vitamin C during uninterrupted sitting (VIT). FMD was measured at baseline, 1 h, 2 h, and 3 h in both the trials. All procedures for the study were approved by the Indiana University Institutional Review Board, and participants gave written informed consent for their participation.
Participants
To be included, participants (all men) had to self-report that they were nonsmokers, and not taking any anti-hypertensive, lipid lowering, or anti-diabetic medications. Participants needed to have total cholesterol ≤240 mg·dl−1 and triglycerides ≤200 mg·dl−1 and fasting blood glucose <120 mg·dl−1. We recruited individuals who performed <150 min/week−1 of moderate-intensity physical activity or <75 min/week−1 of vigorous-intensity physical activity [16]. Participants were asked to maintain their regular diet patterns throughout the study duration and to discontinue any over the counter antioxidant supplement at least 7 days prior to the first session of testing. Based on our pilot data, for the primary dependent variable SFA FMD, we estimated that 12 participants would be needed to find a significant difference between trials with statistical power of >0.80 at α≤0.05.
Screening visits
At the first visit, all experimental procedures were explained to the participants and they were familiarized with the lab setting. If the participants volunteered to participate in the study, a written informed consent was obtained. Height, weight, and BP (in triplicate in a seated posture) were measured using standard procedures and a medical health history and habits questionnaire [7] was completed to screen for any preexisting cardiovascular or metabolic condition and physical activity levels. BP (in triplicate) was measured during an additional screening visit at the same time of day, to confirm previous measurements.
Testing trials
Participants arrived at the laboratory after an overnight fast of at least 6 h between 07:00 and 09:00 h. The arrival time was matched for both trials within each subject. Participants were asked to refrain from any caffeinated drink for at least 8 h prior to reporting to the lab. Upon their arrival in the lab, all participants self-reported adhering to this dietary schedule. During both trials, the participants remained seated for 3 h without moving their legs (perpendicular to the floor) and feet (flat on the ground). Participants were allowed to move their arms, although not vigorously; for example, to use a computer or do light reading during the non-testing periods of the trials. Arm movement was not quantified. The FMD and other vascular function parameters were measured at baseline, 1 h, 2 h, and 3 h. Baseline measurements were made after ~20 min of seated rest. The 2 trials were performed in random order, separated by a minimum of 2 and a maximum of 7 days. The SIT trial was uninterrupted sitting for 3 h, whereas in the VIT trial participants ingested 2 Vitamin C tablets (with water); 1 g at 30 min and 500 mg at 1h 30 min during the sitting interval. Doses were determined based on pilot data and were not tailored to body weight.
SFA FMD
SFA endothelial function was measured by FMD in accordance with current guidelines [17]. We chose SFA for 2 reasons: (1) It is a readily accessible artery to use for measuring FMD in a sitting position; and (2) SFA FMD has been shown to be largely NO-dependent [18]. Each measurement was performed in a dark, quiet, and climate-controlled room (22–25oC) room. A 5×84 cm automatic BP cuff (E-20 rapid cuff inflator; D.E. Hokanson, Bellevue, WA., USA) was placed on their right thigh about 7 cm above the knee joint, distal to the SFA recording location. Images of the SFA were obtained longitudinally 7–10 cm below the inguinal line with a 2-D high-resolution ultrasound system (Terason t3000, Teratech Corp., Burlington, MA, USA), using a 5–12-MHz multifrequency linear-array transducer. Once satisfactory images of near and far arterial walls were obtained, the transducer was secured and stabilized in a stereotactic clamp, and landmarks were made on the subject’s skin to ensure similar placement of the transducer for subsequent FMD procedures and shear rate assessments within and between trials. Participants were encouraged to maintain the landmarks between the 2 trials. In addition to imaging the arterial dimensions, Doppler ultrasound was used to concurrently measure SFA blood velocity. Doppler flow signals were corrected at an insonation angle of 60°, and the sample volume was placed in the middle of the artery.
Diameter images and Doppler measurements of blood velocity were continuously recorded for 45 s at baseline prior to cuff inflation. The automatic BP cuff was then rapidly inflated to 250 mmHg and maintained for 5 min until cuff deflation. Diameter and blood velocity recordings resumed prior to cuff deflation and continued for 5 min after deflation. Ultrasound images were continuously recorded at 5 frames/s−1 with Camtasia (TechSmith, Okemos, MI, USA), and stored as .avi files [7]. This procedure was repeated hourly across the sitting intervals. All measurements were made in the seated position.
Arterial diameters and blood velocities
Off-line analysis of diameters were performed using automated edge-detection software (Brachial Analyzer, Medical Imaging Applications LLC, Coralville, IA, USA) as previously described [7]. This software allows the technician to determine a region of interest where the near and far vessel walls are most clear. The vessel wall borders are then detected using an optimal graph search-based segmentation that uses a combination of pixel density and image gradient as an objective function. All analyzed images were reviewed by the technician and edited when needed to ensure that diameter measures were always determined from the intima-lumen interface at the near and far vessel wall. Blood velocities were determined using internally validated custom-made software by selecting a region of interest that surrounded the Doppler wave. The velocity-time integral was used to calculate the mean blood velocity. The peak dilation after cuff deflation was determined using the highest 3-s moving average and FMD was presented as a percentage change from baseline diameter (FMD%). SFA shear rate used as an estimate of arterial shear stress and was calculated for each FMD% at baseline and during the post-occlusion period using the following formula: Vm·D−1, where V m is mean blood velocity (cm·s−1) and D is mean arterial diameter (cm). All measurements and analysis were performed by a single researcher (ST) who was blinded to the participant identity and treatment condition for each image file.
Statistical analysis
Descriptive analysis was performed to summarize subject characteristics (Table 1). Within both trials, 1-way ANOVA was conducted on the baseline diameter as the dependent variable. Within the SIT trial, a 1-way ANOVA was conducted on the dependent variables baseline diameter, FMD%, and shear rates (antegrade shear rate, mean shear rate, retrograde shear rate, peak shear rate, and shear rate [area under the curve] SRauc (area from deflation up until peak dilation)). When an effect was found, pairwise comparisons were used to locate significant differences across time from baseline. Observed effect size was reported for ANOVA interactions as partial eta squared (η2). Comparisons across the 2 treatment conditions for FMD and shear rates (antegrade shear rate, mean shear rate, retrograde shear rate, peak shear rate, and shear rate [area under the curve] were performed using 2-way repeated measures ANOVA, evaluating the effect of sitting on the vasculature differed between conditions (with and without Vitamin C). We were only interested in the differences between SIT and VIT at each time-point; hence, pairwise comparisons were used to identify significant differences at each time-point if the main effects were positive. Alpha level for statistical significance was set a priori at 0.05. All statistical calculations were performed using IBM SPSS Statistics 22.0 software (IBM SPSS, Inc.).
Table 1.
Subject demographics.
| Variable | Value |
|---|---|
| N | 11 |
| Age (yrs) | 24.2±4.4 |
| BMI (kg·m2) | 23.6±3.4 |
| Systolic blood pressure (mmHg) | 116±10 |
| Diastolic blood pressure (mmHg) | 77.3±6 |
| Total cholesterol (mg/dl) | 171.5±32.7 |
| LDL cholesterol (mg/dl) | 98±29.6 |
| HDL cholesterol (mg/dl) | 57.8±13.6 |
| VLDL cholesterol (mg/dl) | 15.7±9.8 |
| Triglycerides (mg/dl) | 78±49 |
| Glucose (mg/dl) | 91±6.5 |
Data represented as mean ±SD.
Results
We recruited and tested 12 healthy, inactive participants (See Table 1 for subject characteristics). We had an incomplete ultrasound image at 1 time point on 1 subject and this subject was omitted from all analysis. As a result, data on 11 participants is presented.
Tests to identify the effects of SIT
SFA FMD% (Figure 2)
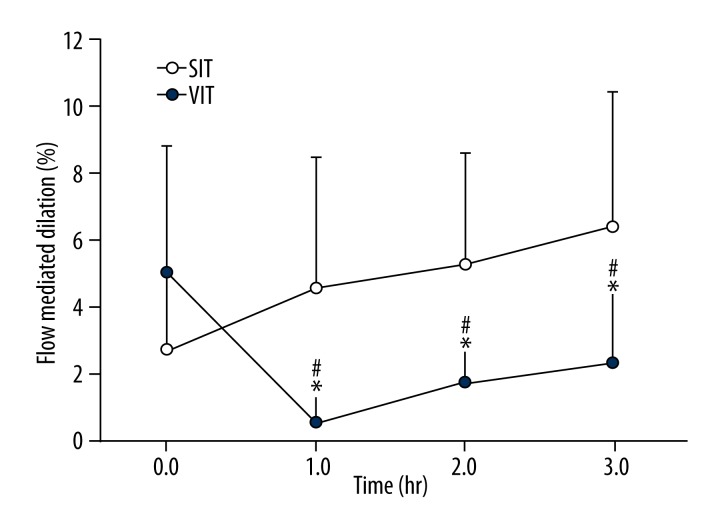
Figure 2.
FMD in the superficial femoral artery during 3 h of sitting during the sitting (SIT) and Vitamin C (VIT). Error bars represent standard deviations. * Indicates significant difference between trials at p≤0.05. # Indicates significant difference from baseline. Error bars represent standard deviation.
By one way ANOVA there was a significant decline in FMD% from baseline during 3 hr of sitting (p<0.001, η2=0.506). Pairwise comparisons indicated the difference to be between baseline and 1hr (p=0.003), 2 hr (p=0.014) and 3 hr (p=0.013).
SFA shear rates
There was a significant decline in antegrade shear rate (p=0.04, η2=0.238) and mean shear rate from baseline to 3 h (p= 0.037, η2=0.506). There were no significant differences in retrograde shear rate, peak shear rate, and shear rate area under the curve (SRauc) from baseline to 3 h (P>0.05).
Baseline diameters between SIT and VIT
Using pairwise comparisons, there was a significant difference between baseline diameters in the SIT and VIT trial (p=0.019). There was, however, no significant difference between the baseline diameters at 1 h, 2 h, or 3 h (p>0.05).
Tests to identify differences between SIT and VIT
SFA FMD% between SIT and VIT (Figure 2)
There was a significant trial × time interaction for SFA FMD (p=0.001, η2=0.406). The variable of interest was FMD% between each time-point. We conducted pairwise comparisons to investigate the differences between SIT and VIT FMD% at each hour. Baseline SFA FMD% did not differ between the 2 trials (p=0.093). However, FMD% was significantly different between trials at 1 h (p=0.009), 2 h (p=0.016), and 3 h (p=0.004) with VIT preventing the decline of FMD.
Using 2-way repeated measures ANOVA, there was a significant time × trial SRauc interaction. Pairwise comparisons indicated a significant difference in SRauc at baseline (p=0.017) and at 2 h (p=0.024) between the SIT and VIT trials. It is important to note that the SRauc analysis was for n=5 because there were multiple participants who did not have an arterial dilation in response to hyperemia. There were no significant differences between antegrade (p=0.193), mean (p=0.605), peak (p=0.187), or retrograde shear (0.723) rates between SIT and VIT trials (p>0.05). Baseline diameters and all flow data are presented in Table 2
Table 2.
Baseline diameters (mm), Shear rates (s−1), SRauc and normalized FMD in the SFA during the SIT and VIT trial at baseline, 1 hr, 2 hr and 3 hr.
| SIT | VIT | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 hr | 1 hr | 2 hr | 3 hr | 0 hr | 1 hr | 2 hr | 3 hr | |
| Baseline diameters | 6.32±0.66* | 6.36±0.74 | 6.50±0.70 | 6.33±0.77 | 6.61±0.55* | 6.42±0.76 | 6.29±0.74 | 6.38±0.76 |
| Antegrade | 7.7±1.36 | 5.77±2.39^ | 5.87±2.17^ | 6.48±1.79 | 7.03±3.5 | 8.58±6.91 | 6.56±3.08 | 6.22±2.39 |
| Mean | 5.03±1.58 | 2.9±2.94^ | 3.14±1.23^ | 3.69±1.28 | 5.17±7.54 | 4.44±4.23 | 2.99±2.6 | 3.17±2.14 |
| Retrograde | 2.65±1.16 | 2.87±1.79 | 2.73±1.55 | 2.80±1.33 | 3.31±3.06 | 3.87±3.39 | 3.30±1.84 | 2.83±1.32 |
| Peak | 54.21±69.10 | 21.01±13.50 | 28.51±13.50 | 23.10±10.30 | 24.56±16.28 | 24.74±16.10 | 23.30±17.76* | 17.78±10.94* |
| SRauc (a.u) | 831.9±228.1# | 439.5±294.2 | 359.6±220.7$ | 489.7±265.7 | 247.9±195.9# | 488.8±255.3 | 622.6±291.7$ | 351.7±228.7 |
| FMD: SRauc | 0.005±0.002 | 0.24±0.05 | 0.002±0.002 | 0.002±0.002 | 0.05±0.05 | 0.007±0.007 | 0.03±0.04 | 0.18±0.36 |
SFA shear rates (s−1) and baseline diameters (mm) during SIT and VIT trials. Data represented as Mean ±SD.
Indicates baseline diameters significantly different from each other at 0 hr only.
Indicates antegrade and mean shear rates significantly different from 0hr in SIT trial only.
indicate significant difference between SRauc in the SIT and VIT trial at baseline and at 2 hrs respectively. Data for SRauc and FMD: SRauc are from 5 participants. Please see limitations for details.
Discussion
The purpose of this study was to determine if antioxidant vitamin C prevents impairment of SFA endothelial function during prolonged sitting. This is the first study to our knowledge to experimentally investigate the role of oxidative stress during prolonged sitting. We hypothesized that there would be a significant decline in SFA FMD from baseline during 3 h of sitting and that antioxidant vitamin C would prevent the decline in SFA FMD during 3 h of sitting. Indeed, in support of our hypothesis, we found a significant decline in SFA FMD during 3 h of sitting (SIT trial), which was counteracted by Vitamin C (Figure 2). Our results suggest that endothelial function in the SFA is significantly impaired from baseline during a 3-h session of uninterrupted sitting, beginning sometime before the first hour. We also found that there was an associated decline in antegrade and mean shear rates from baseline to 3 h of sitting in the SIT trial. Based on the results from the VIT trial, the mechanism responsible for the decline in FMD during SIT is suggestive of an oxidative stress mechanism. There is only 1 other study to our knowledge that has directly investigated oxidative stress as a potential mechanism for endothelial dysfunction in a model of physical inactivity. In an animal model for chronic inactivity, Laufs et al. [19] found that 6 weeks of inactivity significantly increased NADPH oxidase, superoxide, and reactive oxygen species (ROS), and resulted in endothelial dysfunction and increased atherosclerosis in sedentary mice as compared to mice who voluntary trained on running wheels. It is well known that exercise-induced shear stress facilitates beneficial endothelial adaptations [20]; hence, it is tempting to speculate that the endothelial dysfunction from oxidative stress in Laufs’ [19] study may be a result of low shear stress in the sedentary mice as compared to the running mice. It is important to note that this study induced chronic inactivity, whereas we were looking at acute inactivity, but there is no other study to our knowledge which has looked at oxidative stress during inactivity. We have recently shown (and data presented in this paper) that there is a significant decline in mean shear rate during 3 h of siting. Low mean shear rate has been implicated in reduced NO bioavailability and in decreased activity of eNOS [21]. Hence, in the absence of biological markers (see limitations) to test if the impairment in endothelial function is caused by mechanisms related to oxidative stress, we used vitamin C in our research design.
Vitamin C and endothelial function
Use of vitamin C is a novel and critical aspect of this project because it provides suggestive evidence on the mechanisms for endothelial dysfunction during sitting. Vitamin C is an antioxidant and has been previously used in endothelial function to counteract oxidative stress (and to discover this mechanism) from smoking [22], high-fat meals [23], exercise [24], and increased oscillatory shear [15]. Our results show that vitamin C prevents the decline in SFA FMD during 3 h of sitting, suggesting a possible role of mechanisms involving oxidative stress causing the decline of FMD during sitting. The dose of vitamin C ingested by our participants was higher than the recommended daily allowance and was expected to saturate participants’ plasma [25]. There are several potential pathways by which vitamin C may have prevented the decline in SFA FMD during sitting. Vitamin C has been shown to restore NO activity [14] and decrease the activation of vascular NADPH oxidase (a pro-oxidant) [26]. Vitamin C supplementation has been found to protect BH4 and thus the activity of eNOS [27,28]. Vitamin C also allows the recycling of BH3 radicals to a stable BH4 in the presence of ROS [29], thereby maintaining NO production [30]. Indeed, vitamin C may have directly counteracted oxidative stress by scavenging elevated superoxide radicals [31]. Vitamin C, when ingested at 30 min and 1 h 30 min, prevented the decline in endothelial function during 3 h of sitting. This may suggest that mechanisms involving oxidative stress may be present during prolonged sitting, which are counteracted by vitamin C. Using a placebo in addition to vitamin C was also considered. Placebos are used in trials to conceal whether a treatment is being given or not and hence to control for the psychosomatic effects. It is highly unlikely that the vascular responses including blood flow patterns and diameters can be modulated by psychosomatic effects in a period of 3 h. Vitamin C also did not change blood flow patterns during the trial. Furthermore, use of vitamin C was based on pilot data and was meant only to uncover the oxidative stress mechanism.
Alternative mechanisms
There are alternative mechanisms which may attenuate endothelial function during prolonged sitting. For example, viscosity has been shown to be higher in the legs after a session of 2 h of sitting [6], and in the presence of low mean shear rates [32]. Viscosity has been positively associated with increased coagulation and inflammatory markers [33], which in turn have been strongly associated with impaired endothelial function [34]. It is possible that vitamin C counteracted the viscosity and inflammation in our experiment. Indeed, plasma vitamin C has anti-inflammatory effects and is inversely associated with viscosity [35]. Additionally, skeletal muscle inactivity results in an increase in intracellular calcium concentration, which may result in increased NAD(P)H oxidase activity elevating superoxide production [36].
Limitations
Our study is novel and the results have public health significance. However, it is not devoid of limitations. For example, not being able to obtain blood samples from the lower extremities is a clear limitation. Blood samples from the lower extremities would have increased our understanding about the physiological milieu in the legs during sitting and would have allowed us to measure viscosity and oxidative stress markers. Viscosity measurement would have allowed us to explore alternative mechanisms for FMD attenuation during sitting, and calculate shear stress rather than using shear rate as a surrogate measure for shear stress. Oxidative stress measurements from the legs would have conclusively supported the oxidative stress mechanism. It has to be understood, however, that obtaining continuous blood samples from a lower extremity vein in this design is difficult, and these investigators tried this technique in 5 people multiple times before it was deemed logistically impossible (technical difficulty in addition to subject discomfort) even with the use of a topical anesthetic. We did collect venous blood samples from the antecubital vein and analyzed the plasma for oxidative stress biomarkers. However, these biomarkers did not change during 3 h of sitting as compared to baseline, similar to the brachial artery FMD, implying that the local lower extremity effect could not be detected using the upper extremity measurements [37]. We did not measure BP during SIT. BP is known to increase during sitting [4,5] and may lead to endothelial dysfunction. Increased BP is also known to impair endothelial function via the oxidative stress mechanism [14]. Some of our participants had no FMD at some time-points, which did not allow us to calculate SRauc until the point of maximum dilation. Since SRauc is the stimulus that causes the dilation, we cannot speculate if it was the stimulus that was attenuated. Our study was conducted in young and inactive men and more research needs to be done to replicate these findings in women and subjects of different age groups. Despite these minor limitations, our study is the first to our knowledge to discover the mechanism behind vascular impairment during prolonged sitting.
Conclusions
Three hours of sitting leads to a significant decline in endothelial function, most likely through an oxidative stress mechanism. Sitting is a common activity of modern human beings. Repeated sitting sessions may lead to chronically altered shear patterns, oxidative stress, endothelial dysfunction, and a predisposition to atherosclerosis. With the prevalence of sedentary behavior increasing, vitamin C as a regular supplement taken during the seated sessions may play a role in maintaining vascular health in the inactive population.
Acknowledgements
The authors thank Samantha Mayhew for her assistance during data collection.
Footnotes
Source of support: This project was funded in part through American College of Sports Medicine Foundation’s Doctoral Student Research Grant, and IU School of Public Health and IU University Graduate School’s grant in aid awarded to SST
Competing interests
The authors report no competing interests.
All research was carried out at the Clinical Exercise Physiology laboratory at Indiana University, Bloomington.
References
- 1.Bauman AE, Chau JY, Ding D, Bennie J. Too much sitting and cardio-metabolic risk: an update of epidemiological evidence. Current Cardiovascular Risk Reports. 2013;7(4):293–98. [Google Scholar]
- 2.Thosar S, Johnson BD, Johnston JD, Wallace JP. Sitting and endothelial function: the role of shear stress. Med Sci Monit. 2012;18(12):RA173–80. doi: 10.12659/MSM.883589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shvartz E, Gaume J, White R, Reibold R. Hemodynamic responses during prolonged sitting. J Appl Physiol Respir Environ Exerc Physiol. 1983;54(6):1673–80. doi: 10.1152/jappl.1983.54.6.1673. [DOI] [PubMed] [Google Scholar]
- 4.Shvartz E, Reibold R, White R, Gaume J. Hemodynamic responses in orthostasis following 5 hours of sitting. Aviat Space Environ Med. 1982;53(3):226–31. [PubMed] [Google Scholar]
- 5.Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol. 2009;297(3):H1103–8. doi: 10.1152/ajpheart.00167.2009. [DOI] [PubMed] [Google Scholar]
- 6.Hitosugi M, Niwa M, Takatsu A. Rheologic changes in venous blood during prolonged sitting. Thromb Res. 2000;100(5):409–12. doi: 10.1016/s0049-3848(00)00348-0. [DOI] [PubMed] [Google Scholar]
- 7.Thosar SS, Bielko SL, Mather KJ, et al. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc. 2014 doi: 10.1249/MSS.0000000000000479. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282(21):2035–42. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 9.Phinikaridou A, Hua N, Pham T, Hamilton JA. Regions of low endothelial shear stress colocalize with positive vascular remodeling and atherosclerotic plaque disruption an in vivo magnetic resonance imaging study. Circ Cardiovasc Imaging. 2013;6(2):302–10. doi: 10.1161/CIRCIMAGING.112.000176. [DOI] [PubMed] [Google Scholar]
- 10.Giannotti G, Landmesser U. Endothelial dysfunction as an early sign of atherosclerosis. Herz. 2007;32(7):568–72. doi: 10.1007/s00059-007-3073-1. [DOI] [PubMed] [Google Scholar]
- 11.Palmer RM, Ferrige A, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–26. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 12.Vallance P, Chan N. Endothelial function and Nitric Oxide: clinical relevance. Heart. 2001;85(3):342–50. doi: 10.1136/heart.85.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium derived NO function in humans. J Physiol. 2004;561(1):1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taddei S, Virdis A, Ghiadoni L, et al. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97(22):2222–29. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BD, Mather KJ, Newcomer SC, et al. Vitamin C prevents the acute decline of flow-mediated dilation after altered shear rate patterns. Appl Physiol Nutr Metab. 2012;38(3):268–74. doi: 10.1139/apnm-2012-0169. [DOI] [PubMed] [Google Scholar]
- 16.CDC. How much physical activity do adults need? Downloaded from http://www.cdc.gov/physicalactivity/everyone/guidelines/adults.html.
- 17.Thijssen DHJ, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kooijman M, Thijssen D, De Groot P, et al. Flow-mediated dilatation in the superficial femoral artery is NO mediated in humans. J Physiol. 2008;586(4):1137–45. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laufs U, Wassmann S, Czech T, et al. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25(4):809–14. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 20.Tinken TM, Thijssen DHJ, Hopkins N, et al. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55(2):312–18. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 21.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282(21):2035–42. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 22.Heitzer T, Mu T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94(1):6–9. doi: 10.1161/01.cir.94.1.6. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Zhao SP, Gao M, et al. Vitamin C preserves endothelial function in patients with coronary heart disease after a high-fat meal. Clin Cardiol. 2002;25(5):219–24. doi: 10.1002/clc.4950250505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alessio HM, Goldfarb AH, Cao G. Exercise-induced oxidative stress before and after vitamin C supplementation. Int J Sport Nutr. 1997;7(1):1–9. doi: 10.1123/ijsn.7.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93(8):3704–9. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001;38(3):606–11. doi: 10.1161/hy09t1.094005. [DOI] [PubMed] [Google Scholar]
- 27.d’Uscio LV, Milstien S, Richardson D, et al. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and NO synthase activity. Circ Res. 2003;92(1):88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- 28.Huang A, Vita JA, Venema RC, Keaney JF. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000;275(23):17399–406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 29.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric oxide synthase. J Biol Chem. 2003;278(25):22546–54. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 30.Laursen JB, Somers M, Kurz S, et al. Endothelial regulation of vasomotion in apoE-deficient mice implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103(9):1282–88. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 31.Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun. 1975;63(2):463–68. doi: 10.1016/0006-291x(75)90710-x. [DOI] [PubMed] [Google Scholar]
- 32.Ku DN. Blood flow in arteries. Annual Review of Fluid Mechanics. 1997;29(1):399–434. [Google Scholar]
- 33.Kwaan HC. Role of plasma proteins in whole blood viscosity: a brief clinical review. Clin Hemorheol Microcirc. 2010;44(3):167–76. doi: 10.3233/CH-2010-1271. [DOI] [PubMed] [Google Scholar]
- 34.Weiner SD, Jin Z, Cushman M, et al. Brachial artery endothelial function and coagulation factors in the multi-ethnic study of atherosclerosis (MESA) J Am Coll Cardiol. 2011;57(14):E1414. [Google Scholar]
- 35.Son E-W, Mo S-J, Rhee D-K, Pyo S. Vitamin C blocks TNF-α-induced NF-κB activation and ICAM-1 expression in human neuroblastoma cells. Arch Pharm Res. 2004;27(10):1073–79. doi: 10.1007/BF02975434. [DOI] [PubMed] [Google Scholar]
- 36.Carr AC, Zhu B-Z, Frei B. Potential antiatherogenic mechanisms of ascorbate (vitamin C) and α-tocopherol (vitamin E) Circ Res. 2000;87(5):349–54. doi: 10.1161/01.res.87.5.349. [DOI] [PubMed] [Google Scholar]
- 37.Wallace J. Does brachial artery endothelial function represent the endothelial function during prolonged sitting? Medicine & Science in Sports & Exercise. 2013;46(5):13. [Google Scholar]