Abstract
Purpose of the review
Cardiomyocyte necrosis activates an inflammatory response that serves to clear the injured myocardium from dead cells, and stimulates repair, but may also extend injury.
Recent findings
Recently published studies have identified Interleukin (IL)-1α and RNA released by necrotic cardiomyocytes as key danger signals that trigger the inflammatory response following infarction. IL-1 promotes activation of a pro-inflammatory phenotype in leukocytes and fibroblasts, and delays myofibroblast transdifferentiation. Inhibitory lymphocytes play a crucial role in negative regulation of the post-infarction inflammatory response by modulating macrophage and fibroblast phenotype. Cardiac macrophages exhibit significant heterogeneity and phenotypic plasticity and may orchestrate the reparative response following infarction. In neonatal mice, resident embryonic macrophage subpopulations may promote a regenerative response. In contrast, in adult animals replacement of resident macrophage populations with monocyte-derived macrophages may induce inflammation while inhibiting cardiac regeneration. These exciting observations highlight the crucial role of macrophages in cardiac injury and repair, but should be interpreted with caution considering the limitations of murine models of neonatal myocardial injury.
Summary
Design of novel strategies to reduce cardiac injury, improve repair and promote regeneration is dependent on understanding of the cell biology of the inflammatory response.
Keywords: inflammation, myocardial infarction, cytokine, macrophage, lymphocyte
Introduction
Cardiomyocyte necrosis triggers an intense inflammatory cascade that serves to clear the injured myocardium from dead cells and sets the stage for activation of the reparative process. In myocardial infarction, necrotic cardiomyocytes release alarmins, activating innate immune signals, and inducing recruitment of pro-inflammatory leukocytes. Although inflammation is required for phagocytotic removal of dead cells and for activation of reparative mesenchymal cells, timely suppression and spatial containment of the inflammatory reaction is necessary to prevent extension of injury. Overactive, dysregulated, temporally prolonged, or spatially expanded inflammatory responses may cause death of viable cardiomyocytes, enhance matrix degradation (thus promoting dilative remodeling), and extend fibrosis[1]. The inflammatory cascade in myocardial infarction and heart failure is an attractive therapeutic target; however, implementation of anti-inflammatory strategies is challenging due to the pleiotropic and multifunctional effects of inflammatory mediators that may activate both injurious and reparative processes. Thus, dissection of the inflammatory signals implicated in cardiac injury and repair and identification of the effector cells involved in regulation of inflammation is of outstanding significance. This manuscript will present recent advances that significantly contributed to our understanding of myocardial inflammation.
Activation of pro-inflammatory cascades following cardiac injury. The “danger signals”
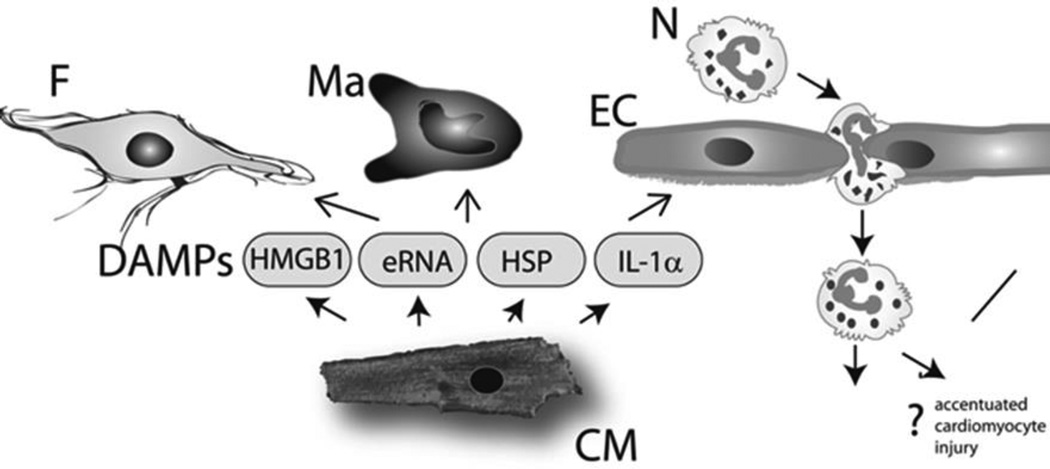
Necrotic cardiomyocytes are capable or releasing a wide range of damage-associated molecular patterns (DAMPs) that activate innate immune pathways triggering the inflammatory response (Figure 1)[2]. Although high mobility group box-1 (HMGB1) has been suggested as important cardiomyocyte-derived alarmin[3], [4]; the identity of the key primary stimulus that may trigger the inflammatory reaction following infarction remains unknown. Using a combination of in vivo and in vitro approaches, Lugrin and co-workers[5] demonstrated that necrotic cardiomyocytes release Interleukin (IL)-1α, a key early damage signal that activates the abundant fibroblasts in the myocardium, promoting their pro-inflammatory activation through MyD88-dependent, toll like receptor (TLR)-independent signaling (Figure 1). IL-1 signaling is critically implicated in activation of the post-infarction inflammatory response: in addition to IL-1 α released by necrotic cells, de novo synthesis and activation of IL-1β stimulates type 1 IL-1 receptor (IL-1R1) signaling in pro-inflammatory leukocytes and in fibroblasts[6]. In both leukocytes and fibroblasts, activation of IL-1 signaling induces cytokine expression and promotes matrix-degrading properties[7]. Moreover, IL-1 suppresses fibroblast proliferation[8] and inhibits transdifferentiation of fibroblasts into myofibroblasts[7], [9], delaying activation of a reparative response until the infarct is cleared from dead cells and matrix debris. IL-1α is not the only endogenous danger signal released by cardiomyocytes in the infarcted region. Chen et al identified release of RNA (including several microRNAs) by necrotic cardiomyocytes as an important injury-activated pro-inflammatory stimulus in the infarcted heart[10]. Extracellular RNA contributed to myocardial inflammation by activating a TLR-3/Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (Trif) cascade. In the pressure overloaded heart, mitochondrial DNA that escapes from autophagy has been reported to trigger TLR-9-dependent inflammation extending myocardial injury[11]. Whether a similar pro-inflammatory pathway is activated in the infarcted heart remains unknown. The cellular targets of these danger signals include cardiomyocytes, vascular cells, fibroblasts and infiltrating leukocytes[12]. Whether specific alarmins activate distinct cell types driving injurious or reparative functions remains unknown. A growing body of evidence suggests that activation of innate immune signaling in hematopoietic cells may exert deleterious actions, extending injury. Bone marrow transplantation experiments in mice suggested that activation of TLR2, or stimulation of complement-activated responses in leukocytes extend ischemic injury[13, 14].
Figure 1.
Release of damage-associated molecular patterns (DAMPs) in the infarcted myocardium initiates the inflammatory response and may extend injury. High mobility group box-1 (HMGB-1), extracellular RNA (eRNA), heat shock proteins (HSP) and Interleukin (IL)-1α are released by necrotic cardiomyocytes (CM) and activate innate immune signaling pathways in leukocytes (N, neutrophil; Ma, macrophage), endothelial cells (EC) and fibroblasts (F).
Removal of apoptotic cells as a mechanism for suppression of post-infarction inflammation
Repair of injured tissues is dependent on timely suppression and resolution of pro-inflammatory signaling. In the healing infarct, negative regulation of the inflammatory response is critical for protection of the infarcted heart from adverse remodeling. Clearance of dead cells by phagocytes activates an inhibitory program, serving as a key mechanism for termination of the pro-inflammatory cascade. Wan and co-workers provided the first demonstration of a critical role for cardiomyocyte efferocytosis (the process by which dying cells are removed by phagocytes) in suppression of acute inflammation following myocardial infarction[15]. Induction of macrophage myeloid-epithelial-reproductive tyrosine kinase (MERTK) in myocardial phagocytes was necessary for effective engulfment and removal of apoptotic cardiomyocytes from the infarct. Disruption of cardiomyocyte efferocytosis resulted in prolonged inflammation and accentuated adverse remodeling. Whether clearance of other apoptotic cell types involves distinct pathways remains unknown. In the healing infarct, the majority of apoptotic cells are non-cardiomyocytes; infiltrating neutrophils represent a large pool of cells programmed to undergo apoptosis. Their clearance from the infarcted heart may also activate an inhibitory program leading to resolution of inflammation.
Lymphocyte subpopulations: key effector cells implicated in negative regulation of inflammation
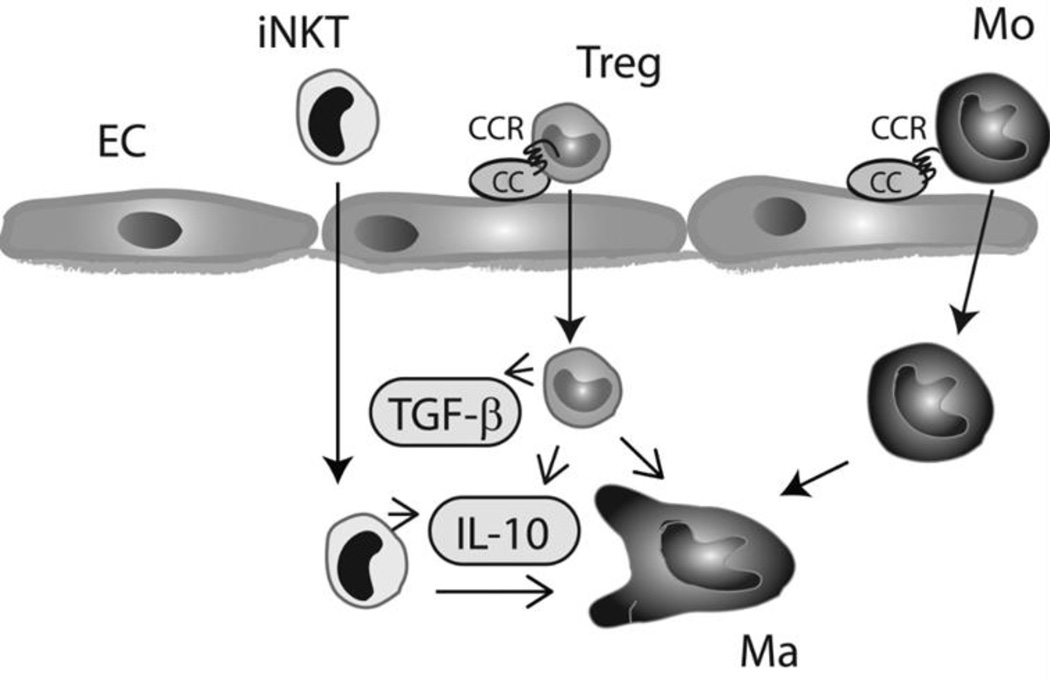
Although descriptive studies in large animal models have demonstrated that the infarcted hearts recruits lymphocytes expressing anti-inflammatory cytokines (such as IL-10)[16], the critical role of lymphocyte subpopulations in regulation of the inflammatory and reparative response has only recently been appreciated. Zouggari and co-workers demonstrated that B cells produce the CC chemokine CCL7 and mobilize pro-inflammatory monocytes in mouse infarcts increasing cardiomyocyte injury[17]. During the reparative phase, lymphocyte subsets with inhibitory properties, such as regulatory T cells (Tregs) are recruited in the infarct through pathways that may involve distinct chemokine/chemokine receptor pairs[18]. Recent studies using both genetic and antibody-mediated depletion strategies have suggested a critical role for Tregs in suppression of the inflammatory reaction following myocardial infarction[19]. Tregs may act by promoting differentiation of macrophages to an anti-inflammatory phenotype[19] and by modulating protease synthesis by cardiac fibroblasts[20]. The protective actions of Tregs may involve both secreted mediators and contact-dependent cell-cell interactions[21]. Selective activation of invariant Natural killer T cells (iNKT cells) through administration of α -galactosylceramide also suppressed inflammation and attenuated injury in experimental models of myocardial infarction[22], [23]. These studies suggest that, despite their small numbers[20], [24], infiltrating T cell subpopulations are capable of exerting profound effects on many other cell types involved in cardiac inflammation, repair and remodeling. (Figure 2)
Figure 2.
T cell subsets, such as regulatory T cells (Tregs) invariant Natural killer T cells (iNKT) are recruited in the infarct through CC chemokine-dependent pathways and may be important cellular effectors in negative regulation of the post-infarction inflammatory response. Lymphocyte subpopulations may modulate macrophage (Ma), endothelial cell (EC), and fibroblast phenotype by secreting soluble mediators (such as IL-10 and TGF-β), or through contact-dependent interactions (Mo, monocyte).
Are surviving cardiomyocytes a source of protective cytokines in the healing infarct?
In the early hours after infarction, necrotic cardiomyocytes release danger signals that initiate inflammation and may extend injury. However, spatial containment of the inflammatory response into the area of infarction is critical for optimal repair, and may be dependent on activation of inhibitory pathways in the border zone[25], [26]. Do viable cardiomyocytes in the infarct border zone secrete cytoprotective and anti-inflammatory mediators that may serve as a protective “barrier” preventing expansion of the inflammatory response? A recent study by Rainer and co-workers suggests that cardiomyocytes may secrete a wide range of cytoprotective mediators; expression of these signals may be regulated by the local cytokine environment[27]. Mice with cardiomyocyte-specific loss of Transforming Growth Factor (TGF)-β signaling were protected against rupture through an increase in levels of protective mediators, such as IL-33, Thrombospondin-4 and growth differentiation factor-15. The findings highlight the pleiotropic actions of TGF-β, suggesting that in addition to its effects on leukocytes, fibroblasts and vascular cells[28], it may also inhibit activation of a protective program in cardiomyocytes of the infarct border zone.
The cardiac macrophages
Despite their abundance in the infarcted heart, macrophages have remained understudied, in part due to their functional heterogeneity[29]. Over the last 2 years, several high-impact investigations have shed new light into the role of macrophages in cardiac homeostasis, injury and repair. The adult mouse heart contains a heterogeneous population of macrophages that can be subclassified into several subsets with distinct ontological origins [30], [31]. The cardiac macrophage population is derived from yolk-sac macrophages and from fetal monocyte progenitors and is maintained through local proliferation. As mice age, these resident macrophages are progressively replaced by monocyte-derived cells, even in the absence of inflammatory stimulation[32]. Following infarction, the heart is infiltrated by abundant macrophages; the majority of these newly recruited cells are derived from circulating Ly-6C(high) monocytes attracted in the healing infarct through interactions involving the CC chemokine monocyte chemoattractant protein-1/CCL2[33], [34], [35]. Infiltrating monocytes may be derived not only from the bone marrow, but also from the spleen[36], [37]. Expression of the transcription factor interferon regulatory factor 5 (IRF5) is increased in macrophages during the inflammatory phase of cardiac repair and drives a pro-inflammatory program[38]. As the infarct heals, reparative Ly-6C(low) F4/80 (high) macrophages, derived from the Ly-6C(high) monocyte subpopulation, proliferate and express inhibitory mediators, promoting repair[34]. If the majority of infarct macrophages are derived from monocytes, what is the fate of the resident cardiac macrophage subpopulations? Much like for other populations of interstitial cells, the susceptibility of macrophages to ischemic injury remains unknown. Evidence suggests that some resident macrophages may undergo apoptosis following permanent coronary occlusion[35]. Whether there is complete removal and replacement of the original resident macrophage population remains unknown.
Do macrophages hold the key to cardiac regeneration?
Recent experimental evidence suggests that macrophages may be critically involved in cardiac repair and regeneration. In an apical resection model, neonatal mice exhibited myocardial regeneration; this capacity was lost at 7 days of age[39]. Depletion of macrophages abrogated regeneration of the neonatal heart, suggesting that neonatal cardiac macrophages exhibit a unique regenerative phenotype and secrete soluble mediators that contribute to formation of new myocardium[40]. Similar observations were noted in a model of genetic cardiomyocyte ablation driven by a diphtheria toxin receptor-based system. In neonatal mice, cardiomyocyte ablation resulted in expansion of embryonic-derived resident macrophages that promoted cardiomyocyte proliferation. In contrast, in adult hearts, recruitment of pro-inflammatory monocytes inhibited repair. These findings suggested that embryonic resident macrophage populations may be essential cellular effectors in cardiac regeneration[41].
Although the emerging body of evidence suggesting a potential role of macrophage subsets in cardiac regeneration is of outstanding interest, caution is advised regarding interpretation of the findings. First, the observations on regeneration of the neonatal heart are predominantly derived from experimental models with a pathology that is quite different from infarction of ischemic origin. In the apical resection model, removal of cardiac tissue eliminates the main source of “danger signals”, responsible for activation of inflammation in infarctive cardiac injury. Myocardial regeneration may be much more complicated in an environment that contains abundant dying cells and fragmented matrix proteins. On the other hand, in the cardiomyocyte ablation model, preservation of vascular and interstitial cells, and possible differences in the mechanism of cardiomyocyte death, may result in distinct reparative responses that do not recapitulate repair of ischemic infarction. Second, to what extent the observations made in neonatal mice can be generalized in higher mammals, and in particular in humans, is unknown. The profile and properties of macrophages in human hearts has not been systematically investigated. Moreover, whether human neonatal macrophages exhibit reparative and regenerative properties has not been tested.
Clinical implications
The negative experience with implementation of early anti-inflammatory strategies in myocardial infarction[42] and the complex effects of inflammatory pathways in injury and repair have discouraged attempts to target inflammation in heart disease. In patients with overactive, or dysregulated, post-infarction inflammatory responses, inhibition of inflammation may be a promising strategy to attenuate adverse remodeling. Anti-IL-1 approaches are particularly attractive and have already been tested in pilot studies with promising results. Treatment of patients with ST elevation myocardial infarction with recombinant IL-1 receptor antagonist attenuated adverse remodeling [43] and reduced the incidence of heart failure [44]. Approaches targeting the MCP-1/CCR2 system may selectively inhibit pro-inflammatory monocytes preventing adverse remodeling[45]. Identification of patients with excessive post-infarction inflammation using suitable biomarkers, or imaging approaches, may be needed for optimal selection of patients likely to benefit from anti-inflammatory strategies[46]. Expansion of our understanding on the immune cell subsets that promote cardiac regeneration may lead to identification of new cell therapy approaches to generate new myocardium.
Conclusions
Cardiomyocyte death activates resident myocardial populations that serve to sense injury and to repair the damaged heart. These non-cardiomyocyte populations exhibit remarkable plasticity and undergo dynamic changes in the environment of the infarct. Moreover, danger signals released by dying cells recruit leukocyte subsets that play a critical role in clearance of the wound and in activation of a reparative program. Studies published over the last 12 months suggested that unique subpopulations of macrophages may be capable of inducing myocardial regeneration, implying that the inflammatory reaction to myocardial injury can be modulated towards a regenerative path. A lot remains to be learned regarding these leukocyte subsets, the basis for their reparative properties, and the pathways driving their differentiation. However, these observations stress the importance of the non-cardiomyocyte compartment in determining the fate of cardiomyocytes, and highlight the potential therapeutic benefits that could be gained by unraveling the secrets of the inflammatory response.
Key points.
Necrotic cardiomyocytes release danger signals that activate innate immune pathways and may extend injury, but also serve a crucial role in clearance of dead cells and in cardiac repair
Lymphocyte subpopulations play an important role in negative regulation of the post-infarction inflammatory response.
Macrophage subsets orchestrate reparative responses following cardiac injury.
Neonatal resident cardiac macrophage may promote cardiac regeneration.
Acknowledgments
Financial support: Dr Frangogiannis’ laboratory is supported by NIH grants R01 HL76246 and R01 HL85440.
Abbreviations
- DAMPs
Damage-associated molecular patterns
- HMGB1
high mobility group box-1
- IL
Interleukin
- MyD88
myeloid differentiation primary response gene 88
- TLR
toll-like receptor
- Trif
IL-1 receptor domain-containing adaptor inducing IFN-β
- Mertk
macrophage myeloid-epithelial-reproductive tyrosine kinase
- Tregs
regulatory T cells
- iNKT cells
invariant Natural killer T cells
- (TGF)-β
Transforming Growth Factor
- (IRF5)
interferon regulatory factor 5
Footnotes
Conflicts of interest: None
References and recommended reading
Papers of particular interest, published within the annual period of review, (18 months/ 2013–2014) have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011;8:292–300. doi: 10.1038/nrcardio.2011.38. [DOI] [PubMed] [Google Scholar]
- 3.Herzog C, Lorenz A, Gillmann HJ, et al. Thrombomodulin's lectin-like domain reduces myocardial damage by interfering with HMGB1-mediated TLR2 signalling. Cardiovasc Res. 2014;101:400–410. doi: 10.1093/cvr/cvt275. [DOI] [PubMed] [Google Scholar]
- 4.Andrassy M, Volz HC, Igwe JC, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 5. Lugrin J, Parapanov R, Rosenblatt-Velin N, et al. Cutting Edge: IL-1alpha Is a Crucial Danger Signal Triggering Acute Myocardial Inflammation during Myocardial Infarction. J Immunol. 2015;194:499–503. doi: 10.4049/jimmunol.1401948. *This interesting study demonstrates for the first time that IL-1a released by necrotic cardiomyocytes may be a critical danger signal that triggers the post-infarction inflammatory response.
- 6.Bujak M, Dobaczewski M, Chatila K, et al. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saxena A, Chen W, Su Y, et al. IL-1 Induces Proinflammatory Leukocyte Infiltration and Regulates Fibroblast Phenotype in the Infarcted Myocardium. J Immunol. 2013;191:4838–4848. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer JN, Hartogensis WE, Patten M, et al. Interleukin-1 beta induces cardiac myocyte growth but inhibits cardiac fibroblast proliferation in culture. J Clin Invest. 1995;95:2555–2564. doi: 10.1172/JCI117956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronnum H, Eskildsen T, Andersen DC, et al. IL-1beta suppresses TGF-beta-mediated myofibroblast differentiation in cardiac fibroblasts. Growth Factors. 2013;31:81–89. doi: 10.3109/08977194.2013.787994. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Feng Y, Zou L, et al. Role of extracellular RNA and TLR3-Trif signaling in myocardial ischemia-reperfusion injury. J Am Heart Assoc. 2014;3:e000683. doi: 10.1161/JAHA.113.000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oka T, Hikoso S, Yamaguchi O, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Hoog VC, Timmers L, Van Duijvenvoorde A, et al. Leucocyte expression of complement C5a receptors exacerbates infarct size after myocardial reperfusion injury. Cardiovasc Res. 2014;103:521–529. doi: 10.1093/cvr/cvu153. [DOI] [PubMed] [Google Scholar]
- 14.Arslan F, Smeets MB, O'Neill LA, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 15. Wan E, Yeap XY, Dehn S, et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113:1004–1012. doi: 10.1161/CIRCRESAHA.113.301198. ** The first direct demonstration of the critical role that clearance of apoptotic cardiomyocytes plays in repression and resolution of the post-infarction inflammatory reaction and in protection from adverse remodeling.
- 16.Frangogiannis NG, Mendoza LH, Lindsey ML, et al. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165:2798–2808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 17. Zouggari Y, Ait-Oufella H, Bonnin P, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. *This study demonstrates for the first time that B cells regulate infiltration of the infarct with pro-inflammatory monocytes.
- 18.Dobaczewski M, Xia Y, Bujak M, et al. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176:2177–2187. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weirather J, Hofmann UD, Beyersdorf N, et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 20.Saxena A, Dobaczewski M, Rai V, et al. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol. 2014 doi: 10.1152/ajpheart.00328.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang TT, Yuan J, Zhu ZF, et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012;107:232. doi: 10.1007/s00395-011-0232-6. [DOI] [PubMed] [Google Scholar]
- 22.Sobirin MA, Kinugawa S, Takahashi M, et al. Activation of natural killer T cells ameliorates postinfarct cardiac remodeling and failure in mice. Circ Res. 2012;111:1037–1047. doi: 10.1161/CIRCRESAHA.112.270132. [DOI] [PubMed] [Google Scholar]
- 23.Homma T, Kinugawa S, Takahashi M, et al. Activation of invariant natural killer T cells by alpha-galactosylceramide ameliorates myocardial ischemia/reperfusion injury in mice. J Mol Cell Cardiol. 2013;62:179–188. doi: 10.1016/j.yjmcc.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Yan X, Anzai A, Katsumata Y, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Frangogiannis NG, Ren G, Dewald O, et al. The critical role of endogenous Thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 26.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–1939. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 27. Rainer PP, Hao S, Vanhoutte D, et al. Cardiomyocyte-specific transforming growth factor beta suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ Res. 2014;114:1246–1257. doi: 10.1161/CIRCRESAHA.114.302653. *The first demonstration of an important role for cardiomyocyte-derived protective mediators in prevention of cardiac rupture. Cardiomyocyte-specific TGF-β signaling suppresses these protective signals.
- 28.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. *A highly informative review manuscript on the biology of tissue macrophages.
- 30.Pinto AR, Paolicelli R, Salimova E, et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One. 2012;7:e36814. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. **A detailed characterization of the origin and heterogeneity of cardiac macrophage populations in normal and infarcted hearts.
- 32.Molawi K, Wolf Y, Kandalla PK, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med. 2014;211:2151–2158. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dewald O, Zymek P, Winkelmann K, et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 34.Hilgendorf I, Gerhardt LM, Tan TC, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heidt T, Courties G, Dutta P, et al. Differential Contribution of Monocytes to Heart Macrophages in Steady-State and After Myocardial Infarction. Circ Res. 2014;115:284–295. doi: 10.1161/CIRCRESAHA.115.303567. *An interesting study on the fate of resident macrophages following cardiac injury and on the origin of infarct macrophages.
- 36.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ismahil MA, Hamid T, Bansal SS, et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–282. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courties G, Heidt T, Sebas M, et al. In Vivo Silencing of the Transcription Factor IRF5 Reprograms the Macrophage Phenotype and Improves Infarct Healing. J Am Coll Cardiol. 2014;63:1556–1566. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aurora AB, Porrello ER, Tan W, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. **A highly provocative report suggesting that neonatal macrophages may drive cardiomyocyte regeneration in a model of myocardial infarction induced through coronary ligation.
- 41. Lavine KJ, Epelman S, Uchida K, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. 2014;111:16029–16034. doi: 10.1073/pnas.1406508111. *This interesting study highlights the distinct role of macrophage subsets in repair and regeneration of the infarcted heart.
- 42.Christia P, Frangogiannis NG. Targeting inflammatory pathways in myocardial infarction. Eur J Clin Invest. 2013;43:986–995. doi: 10.1111/eci.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbate A, Kontos MC, Grizzard JD, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study) Am J Cardiol. 2010;105:1371–1377. doi: 10.1016/j.amjcard.2009.12.059. e1371. [DOI] [PubMed] [Google Scholar]
- 44.Abbate A, Van Tassell BW, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study] Am J Cardiol. 2013;111:1394–1400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majmudar MD, Keliher EJ, Heidt T, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127:2038–2046. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]