Abstract
A multifunctional FePt nanoparticle was developed that targets tumor microvasculature via “radiation-guided” peptides, and is detected by both near-infrared (NIR) fluorescence imaging and analytical mass spectrometry methods. Tumor specific binding was first measured by biotinylated peptide linked to fluorophore-conjugated streptavidin. This showed tumor selective binding to tumors using the HVGGSSV peptide. FePt nanoparticles were synthesized sequentially by surface modification with poly(l)lysine, poly(ethylene) glycol conjugation, and functionalized with HVGGSSV peptide and fluorescent probe Alexa fluor 750. NIR fluorescence imaging and ICP-MS analysis showed significant HVGGSSV-FePt nanoparticle binding to irradiated tumors as compared to unirradiated tumors and controls. Results indicate that multifunctional FePt nanoparticles have potential application for radiation-guided targeting and imaging of cancer.
Keywords: Radiation, Tumor targeting, Nanotechnology, Magnetic nanoparticles
INTRODUCTION
Targeted molecular imaging has become a rapidly growing area in medical research and drug development. Noninvasive molecular imaging modalities currently used include optical bioluminescence and fluorescence, ultrasound, magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), and positron emission tomography (PET).3 In addition, many hybrid systems combining two or more of these imaging modalities have recently emerged in order to utilize specific advantages of each imaging modality. Multifunctional nanoparticles have also been developed that combine targeting ligands, imaging agents, and therapeutic drugs within a single-nanoscale system.3,7 These multifunctional nanoparticles are of increasing importance in cancer imaging and treatment, due to their ability to selectively target, image, monitor, and treat cancer simultaneously.
Thus far, many nanoparticles have been developed for diverse applications including quantum dots, nanoshells, paramagnetic nanoparticles (MNPs), and liposomes.6 MNPs are of particular interest, as they can function both as contrast agents for MRI as well as therapeutic agents for hyperthermia-based treatment of cancer.3 Targeting of these MNPs to tumors can provide a valuable tool for real-time cancer imaging and therapy. MNPs including iron oxide (FeOx), iron cobalt (FeCo), and iron platinum (FePt) have been shown to be promising MRI contrast agents in previous studies.3,6,10 Here, we present the development of a novel, multifunctional FePt nanoparticle that targets tumor microvasculature via “radiation-guided” peptides, and can be detected by near-infrared (NIR) fluorescence imaging.
Site specific targeting of FePt MNPs requires surface modification and the addition of functional groups for attachment of ligands including targeting peptides and antibodies.7 These functionalized MNPs can be directed specifically to cancer by targeting receptors on the surface of tumor microvasculature. This targeting capability enables more contrast agent or drug to reach tumors and reduces nonspecific uptake in other tissues. In addition, since the development of new blood vessels (angiogenesis) is vital for tumor growth, targeting of tumor microvasculature provides an excellent means for monitoring the growth and size of tumors, as well as their response to therapy.4,13 The TIP-1 binding ligand, HVGGSSV, targets components of the vascular endothelium that are significantly expressed after treatment with ionizing radiation, but are not expressed in untreated vascular endothelium and normal tissues.4,5,11 This phenomenon can be exploited to “guide” the HVGGSSV peptide to specific tumor sites by pre-treating them with a low does of ionizing radiation to induce the expression of receptors within tumor vascular endothelium that bind the HVGGSSV ligand. This radiation-guided peptide is able to bind with high affinity to radiation-inducible receptors expressed in many tumor types including lung, brain, prostate, and pancreatic carcinomas.4 Binding of the HVGGSSV peptide to this receptor can enable targeting of FePt MNPs to cancer.
MATERIALS AND METHODS
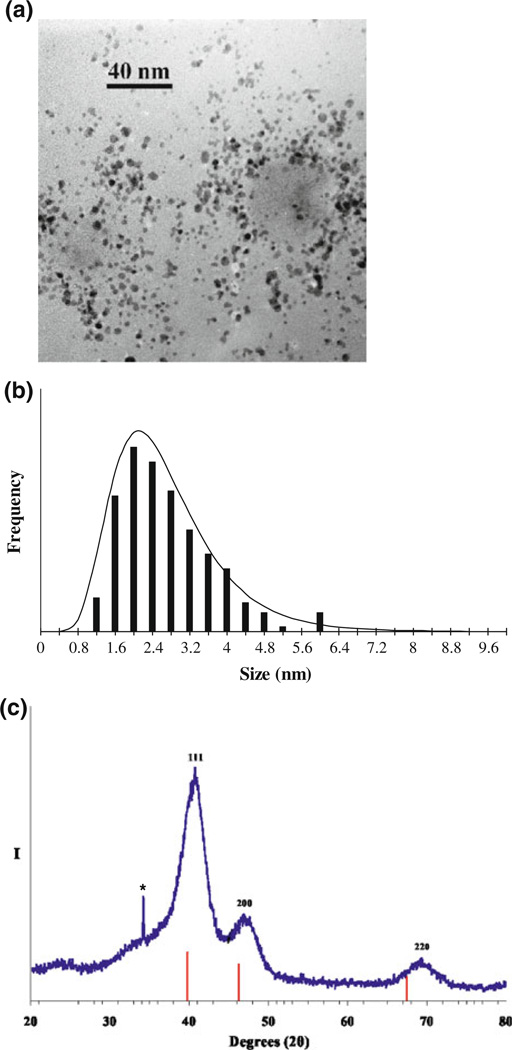
FePt MNPs (face centered cubic, fcc) were prepared in an adaptation of previously published work on ferromagnetic L10 nanoparticles by Rutledge, Wellons and colleagues.8,12 Organometallic reagents were purchased from Strem Chemicals (Newburyport, MA) and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO). Thermal treatments were conducted inside a quartz tube under continuous gas flow using a one-foot Linberg/Blue tube furnace. Sonication was performed in glass vials immersed in a Bransonic 2510R sonicator. Morphology and size distribution of FePt MNPs was determined by transmission electron microscopy (TEM) (Phillips CM 20T) at an accelerating voltage of 200 kV. A representative bright-field micrograph of the prepared FePt MNPs is shown in Fig. 1a. TEM micrographs were used to determine the monodispersity of FePt MNPs, and an average particle size of 2.7 ± 1.0 nm was calculated (Fig. 1b). Powder X-ray diffraction (XRD) spectrum of the FePt MNPs is shown in Fig. 1c. Scans were obtained on a Scintag X1 θ/θ automated powder X-ray diffractometer with Cu Kα radiation as target, a Peltier-cooled solid-state detector, and a zero-background Si(510) sample support. Peak indices in XRD diffraction pattern for FePt MNPs are assigned relative to those known for fcc FePt. Scherrer’s analysis of XRD peak widths gives an average volume-weighted fcc FePt particle size of 2.3 nm, which corresponds to average particle size calculated using TEM micrographs.
FIGURE 1.
(a) TEM micrograph (250 kx) and particle-size histogram (b) of prepared fcc FePt nanoparticles (calculated average particle size of 2.7 ± 1.0 nm). (c) XRD scan (Cu Kα radiation) of prepared fcc FePt nanoparticles along with the XRD line pattern of Pt metal, PDF (#4-802). Peak indices are assigned relative to those known for fcc FePt. Scherrer’s analysis of XRD peak widths gives an average volume-weighted fcc FePt particle size of 2.3 nm. Asterisk denotes diffraction intensity assigned to a trace amount of Na2CO3.
Multifunctional FePt nanoparticles were synthesized as shown in Scheme 1. FePt MNPs were first modified with folic acid (Sigma, St. Louis, MO) in an aqueous solution under continuous sonication. Excess folic acid was removed by washing with ethanol and centrifuging twice. MNPs were further treated with an aqueous poly(l)lysine solution (Sigma, St. Louis, MO) by dropwise addition under continuous stirring, followed by sonication, to provide an amine-functionalized surface (Scheme 1a).1 Amine-modified FePt was then conjugated to a heterobifunctional cross-linker, succinimidyl-[(N-maleimidopropionamido)-tetraethyleneglycol] ester (Pierce Biotechnology, Rockford, IL), producing a maleimide functionalized nanoparticle surface (Scheme 1b). The cysteine thiol group of CGGGKKKGGGNHVGGSSV was reacted with the maleimide functionalized nanoparticle surface (Scheme 1c) and the intermediate conjugate was purified using G-25 gel desalting columns (Pierce Biotechnology, Rockford, IL). A scrambled version of HVGGSSV peptide (CGGGKKKGGGSGVSGHVN) was used as a control and conjugated to FePt nanoparticles in a separate group. Monofunctional N-hydroxysuccinamide (NHS) esters of Alexa fluor 750 were then used to conjugate the fluorescent probe to the lysine ε-amino groups on the peptide for NIR fluorescence imaging (Scheme 1d). For conjugation, 1 mg Alexafluor 750 was dissolved in 100 µL of dimethyl sulfoxide (Sigma, St. Louis, MO) and added to HVGGSSV modified FePt nanoparticles for 1 h in a sodium bicarbonate buffer at a pH of 8. After the reaction was complete, the excess dye was removed from the solution by G-25 gel desalting columns (Pierce Biotechnology, Rockford, IL).
SCHEME 1.
Multifunctional FePt nanoparticle synthesis. Schematic diagram for synthesis of multifunctional FePt nanoparticles. (a) Surface modification of FePt-folate with poly(l)lysine, (b) addition of polyethylene glycol crosslinker, (c) conjugation of HVGGSSV peptide, and (d) conjugation of Alexafluor 750 fluorescent probe.
To evaluate tumor targeting of these multifunctional FePt nanoparticles, an in vivo xenograft model of lung cancer was used to study biodistribution. Athymic nude mice were injected with approximately 1 × 106 H460 human lung carcinoma cells (American Type Tissue Culture Collection, Manassas, VA) on the left and right hind limbs (Fig. 2). Tumors were allowed to reach 0.5–0.8 cm3 in size before beginning treatments. All mice were anesthetized using ketamine and xylazine solution prior to irradiation to inhibit mobility during treatment. Tumors were irradiated with 300 kV X-rays using a Pantak Therapax 3 linear accelerator system (Pantak, East Haven, CT) with an adjustable collimator set to limit dosage to the tumor region only. The tumor on the left hind limb of each mouse received a radiation dose of 3 Gy, and the tumor on the right hind limb served as an internal negative control receiving a sham radiation dose of 0 Gy. During irradiation procedures, 1-cm thick lead blocks were arranged above the rest of the body, leaving only the desired area on the hind limb exposed for treatment. Four hours following radiation, mice were administered either HVGGSSV-FePt nanoparticles or the scrambled peptide SGVSGHVN-FePt nanoparticles (50 µg of peptide per mouse) through tail vein injection.
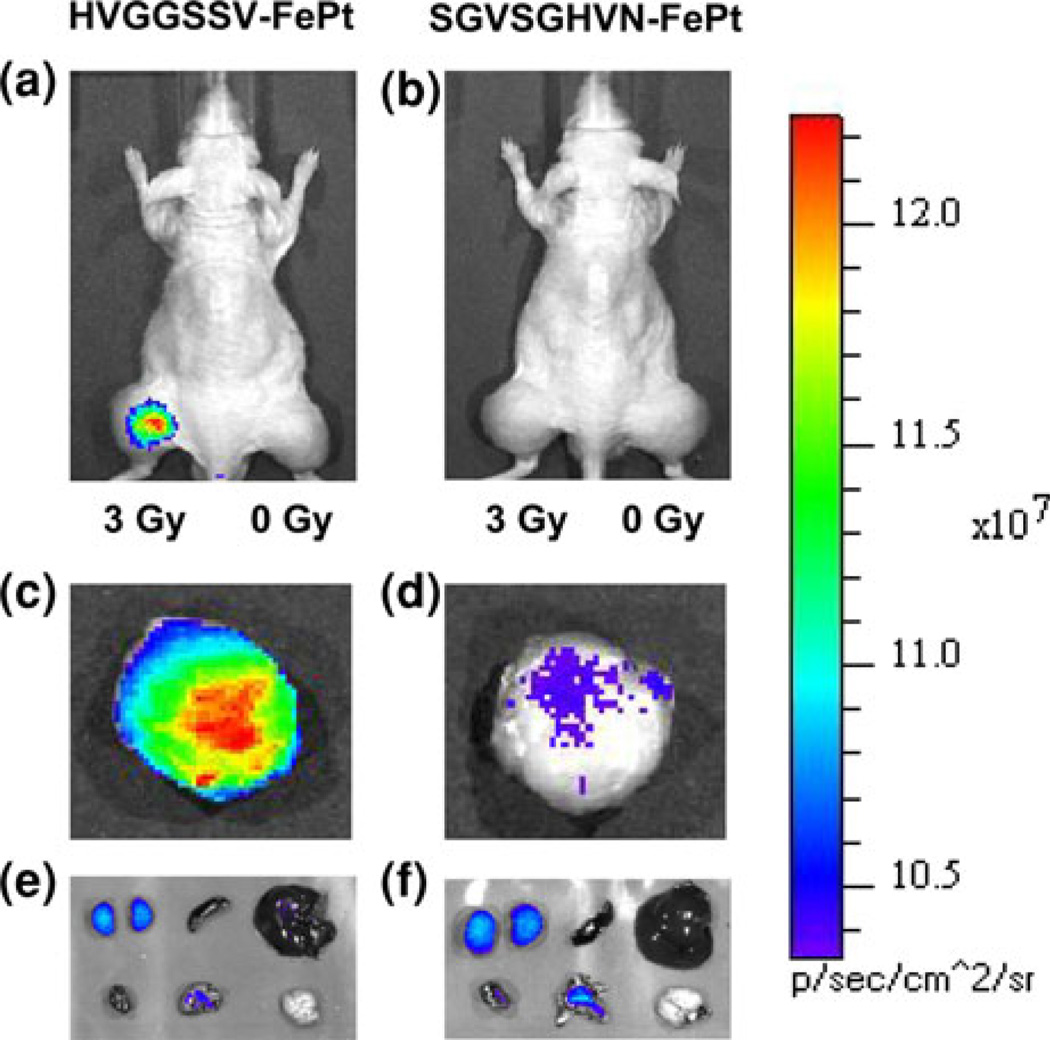
FIGURE 2.
In vivo NIR fluorescence imaging of subcutaneous H460 lung tumor-bearing nude mice (hind limbs) after intravenous tail vein injection of 50 µg of (a) targeted HVGGSSV-FePt nanoparticles labeled with Alexa fluor 750 and (b) scrambled SGVSGHVN-FePt nanoparticles labeled with Alexafluor 750. In both groups, the left hind limb tumor was treated with 3 Gy radiation to induce receptor expression, while the right hind limb tumor served as a control and received a sham radiation dose of 0 Gy. Images were acquired at 48 h following injection. Strong tumor binding is observed in the irradiated (left tumor) for the HVGGSSV targeted FePt nanoparticle. Ex vivo NIR fluorescence imaging of representative excised irradiated tumors at 48 h: (c) Targeted HVGGSSV-FePt nanoparticles labeled with Alexa fluor 750; (d) scrambled SGVSGHVN-FePt nanoparticles labeled with Alexa fluor 750; and internal organs (top row from left to right are kidneys, spleen, and liver; bottom row from left to right are heart, lungs, and brain). (e) Targeted HVGGSSV-FePt nanoparticles labeled with Alexa fluor 750 and (f) scrambled SGVSGHVN-FePt nanoparticles labeled with Alexa fluor 750. All NIR fluorescence images were acquired at 48 h and are normalized.
NIR fluorescence imaging studies were performed to confirm binding of HVGGSSV-FePt nanoparticles to irradiated tumors in vivo. Images were taken 48 h post-injection (p.i.), using a Xenogen IVIS 200 small animal imaging system (Xenogen Inc., Alameda, CA) with a Cy7 filter set with all mice under isoflurane anesthesia. Fluorescence images were acquired with 1 s exposure time using an f/stop of 2. Following imaging, mice were immediately euthanized by carbon dioxide inhalation, and their tumors and major organs were excised for ex vivo imaging. The total fluorescence intensity (photons/s/area/steradians) for each tissue was measured. For quantitative comparison, regions of interest (ROI) were drawn over tumors and normal tissues for a group of three animals, and the data was analyzed using Living Image software (Hopkinton, MA).
Inductively coupled plasma mass spectrometry (ICP-MS) studies were also performed to confirm binding of HVGGSSV-FePt nanoparticles to irradiated tumors in vivo. Tissue samples (tumor, kidney, and liver) were collected at 48 h p.i., homogenized, centrifuged at 3000 rpm and an aliquot of 0.5 g was digested in an aqueous 2% solution of nitric acid. Samples were vortexed and analyzed for Pt content by ICP-MS.
RESULTS
Specific targeting was observed with HVGGSSV-FePt nanoparticles to irradiated tumor (left leg) as compared to unirradiated tumor (right leg) and the rest of the body (Figs. 2a, 2b). No significant fluorescence intensity in either tumor was observed in mice treated with scrambled SGVSGHVN-FePt nanoparticles. Mice were killed and tumors and organs were excised and imaged ex vivo (Figs. 2c–2f). The ratios of irradiated tumor, unirradiated tumor and the kidneys, spleen, liver, heart, lungs, and brain are shown (Fig. 2). As shown in Figs. 2a and 2b, significantly greater fluorescence intensity is observed in the irradiated tumor of the mouse injected with HVGGSSV-FePt nanoparticles compared to SGVSGHVN-FePt nanoparticles, with approximately 3.35 times greater fluorescence. Figures 2e and 2f show imaging of internal organs for both treatment groups. Fluorescence intensity was highest in the kidneys for both groups, followed by liver, lungs, heart, brain, and spleen (Fig. 3). No significant difference was observed for organ biodistribution in either HVGGSSV-FePt or SGVSGHVN-FePt nanoparticle treated mice.
FIGURE 3.
Bar graph of tumor tissue treated with 3 Gy radiation vs. normal tissues in targeted HVGGSSV-FePt nanoparticles and scrambled SGVSGHVN-FePt nanoparticles both labeled with Alexa fluor 750 (control) (n = 3–5 mice). Fluorescence intensity is expressed as units of photons/s/area/steradians. *p<0.05.
To confirm specific targeting and delivery of FePt nanoparticles to irradiated tumor tissue, ICP-MS analysis of Pt content was performed on tumor, kidney, and liver samples from both treatment groups. Figure 4 shows that administration of HVGGSSV-FePt nanoparticles generates tumor Pt levels of 0.38 µg/mL at 48 h p.i., whereas administration of the SGVSGHVN-FePt nanoparticles only generates 0.05 µg/mL. It is apparent that the total level of FePt nanoparticles delivered to irradiated tumors via the HVGGSSV targeting peptide is approximately 7.3 times greater than that observed in the SGVSGHVN control peptide treated mice. The kidney and liver data for HVGGSSV-FePt nanoparticles also show Pt levels in kidneys of 0.08 and 0.09 µg/mL tissue, compared to 0.03 and 0.04 µg/mL when SGVSGHVN-FePt nanoparticles were given. The tumor/kidney and tumor/liver ratios of Pt content for HVGGSSV-FePt nanoparticles are approximately 4.7 and 4.3, compared to 1.8 and 1.5 for SGVSGHVN-FePt, respectively.
FIGURE 4.
Bar graph of total platinum levels in H460 lung tumor-bearing nude mice after intravenous injection of targeted HVGGSSV-FePt nanoparticles and scrambled SGVSGHVN-FePt nanoparticles (control) via tail vein (n = 3–6 mice) *p <0.05.
DISCUSSION
In summary, we report the use of multifunctional FePt nanoparticles for in vivo targeting and imaging of tumors by radiation-guided peptide ligands. The use of tumor microvascular targeting peptides such as these have become increasingly important for targeting, imaging, and treatment of cancer. The use of radiation-guided peptides is significant in that tumor microvasculature can be selectively targeted by the expression of radiation-inducible receptors.4,5,11 Unlike other targeting ligands, the HVGGSSV peptide binds specifically to irradiated tumor microvascular endothelial cells, and thus has the potential to serve as an effective tool for imaging irradiated tumors and delivering therapeutics.4,5,11 This radiation-guided peptide can be used to target drug delivery specifically to irradiated tumors, resulting in greater bioavailability of chemotherapeutics and higher therapeutic efficacy.5
Peptides have become increasingly more attractive as targeting ligands because they eliminate many of the limitations commonly associated with other larger ligands like antibodies. Despite the shorter half-lives of peptides in circulation, the smaller size of peptides allows for greater tissue penetration and cellular uptake in vivo.2,3 Due to their smaller size and lower steric hindrance, more peptides can be conjugated to the surface of one nanoparticle than antibodies, and this greater polyvalency may allow for stronger binding affinity and better targeting efficacy compared to antibodies.2,3 Using a nanoparticle scaffold for the conjugation of such peptides allows the addition of various other functionalities for imaging and drug attachment.
Use of the FePt MNPs as the foundation of these nanoparticles enables additional imaging possibilities with MRI for further preclinical and clinical studies.6 In order to validate peptide binding and tumor targeting in this study, we utilized NIR fluorescence imaging instead of MRI because it provides a faster and more efficient method for studying multiple animals.2 The amine-modified FePt MNPs were covalently attached to a 500 MW polyethylene glycol crosslinker in order to attach targeting units, improve circulation time, and enhance the biodistribution profile. Optical NIR fluorescence imaging takes advantage of recent developments in fluorescent probes in the NIR region of the spectrum. Fluorescent probes such as Alexa fluor 750 excite and emit in the NIR range and allow for improved visualization of anatomical, functional, and molecular events in mice with less interference from autofluorescence. These attributes make it ideal for studying tumor targeting, biodistribution and kinetics of radiation-guided peptides and nanoparticles such as HVGGSSV-FePt nanoparticles. Since NIR fluorescence intensity is a function of optical path length between excitation light and the subject, subcutaneous tumor models in nude mice were chosen for this study in order to minimize noise from light scattering. Imaging was done both in vivo and ex vivo with excised tumors in order to validate signal detected in in vivo images. As expected, fluorescence intensity was lesser in vivo as compared to direct imaging of dissected tissues. This is most likely caused by the loss of excitation and emission light by penetration of the skin, in addition to scattering caused by the skin.
In vivo NIR fluorescence imaging of mice with irradiated and unirradiated tumors given Alexa fluor 750 labeled HVGGSSV-FePt nanoparticles showed significant targeting to irradiated tumors at 48 h post-treatment. Binding of HVGGSSV-FePt nanoparticles in irradiated tumors was 3.35 times greater than scrambled peptide SGVSGHVN-FePt nanoparticle controls. Direct imaging of excised tumors confirmed these results with significant binding in irradiated tumors as compared to unirradiated tumors. Tumor binding was not observed in animals injected with SGVSGHVN-FePt nanoparticles.
NIR imaging is a non-invasive tool for visualizing tumor targeting in vivo, and a complement to more direct chemical quantification such as mass spectrometry.8 Measurement of radiance underestimates nanoparticle binding within the tumor because NIR light scatters throughout the mouse. Therefore, protons emitted from the tumor will light up the entire mouse analogous to a light bulb within a lantern. In contrast, ICP-MS analysis of Pt content measurements reflect concentration values determined from tissue homogenates. Since the fluorescence intensity values were determined from intact tissue and not homogenized tissue, it is possible that the fluorescence intensity is not homogeneously distributed in the tissue and thus the values may vary in different segments of the same tissue. ICP-MS analysis circumvents this limitation by analyzing tissue homogenates, giving a more homogenous distribution within tissue samples and more accurate results. The use of ICP-MS directly quantifies the content of metals such as Fe or Pt in tumors and other tissues.9 Like other mass spectrometry methods, it is highly sensitive tool for validating tumor targeting data provided by NIR optical imaging methods. Combining both optical imaging and mass spectrometry techniques enabled the confirmation of results using both imaging and chemical analysis of FePt nanoparticle targeting to tumors.
In this study, we have shown that targeting and imaging of cancer is possible with multifunctional FePt nanoparticles using radiation-guided peptides. We have demonstrated that FePt nanoparticles functionalized with the HVGGSSV peptide can specifically target irradiated tumor microvasculature via radiation-inducible receptors. Use of FePt MNPs allowed for detection of Pt in tumor and tissues by analytical mass spectrometry methods. The incorporation of a NIR fluorescent probe allowed for molecular imaging capability by fluorescence imaging. This multifunctional approach enabled us to study biodistribution of these targeted FePt nanoparticles by both noninvasive molecular imaging techniques as well as direct chemical analysis of ex vivo tissues in a murine lung cancer model. Future studies to further improve the in vivo targeting and imaging of HVGGSSV-FePt nanoparticles, including MRI studies to validate tumor targeting are under way.
ACKNOWLEDGMENTS
This work was supported by Vanderbilt-Ingram Cancer Center, and NIH grants P50CA128323, R01-CA112385, R21-CA128456, and R01-CA125757. We thank Rossane Delapp for technical assistance.
REFERENCES
- 1.Babic M, Horak D, Trchova M, Jendelova P, Glogarova K, Lesny P, Herynek V, Hajek M, Sykova E. Poly(l-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjugate Chem. 2008;19:740–750. doi: 10.1021/bc700410z. [DOI] [PubMed] [Google Scholar]
- 2.Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 3.Cai W, Shin D, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 4.Han Z, Fu A, Wang H, Diaz R, Geng L, Onishko H, Hallahan DE. Noninvasive assessment of cancer response to therapy. Nat. Med. 2008;14:343–349. doi: 10.1038/nm1691. [DOI] [PubMed] [Google Scholar]
- 5.Hariri G, Yan H, Wang H, Han Z, Hallahan DE. Radiation-guided drug delivery to cancer. Clin. Cancer Res. 2010;16:4968–4977. doi: 10.1158/1078-0432.CCR-10-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maenosono S, Suzuki T, Saita S. Superparamagnetic FePt nanoparticles as excellent MRI contrast agents. J. Magn. Magn. Mater. 2008;320:L79–L83. [Google Scholar]
- 7.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 8.Rutledge RD, Morris WH, III, Wellons MS, Gai Z, Shen J, Bentley J, Wittig JE, Lukehart CM. Formation of FePt nanoparticles having high coercivity. J. Am. Chem. Soc. 2006;128:14210–14211. doi: 10.1021/ja0633868. [DOI] [PubMed] [Google Scholar]
- 9.Smit T, Snyman JR, Neuse EW, Bohm L, van Rensburg CEJ. Evaluation of cisplatin and a novel platinum polymer conjugate for drug toxicity and drug distribution in mice. Anticancer Drugs. 2005;16:501–506. doi: 10.1097/00001813-200506000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, Olson J, Zhang M. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005;5:1003–1008. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Yan H, Fu A, Han M, Hallahan D, Han Z. TIP-1 translocation onto the cell plasma membrane is a molecular biomarker of tumor response to ionizing radiation. PLoS One. 2010;5:e12051. doi: 10.1371/journal.pone.0012051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wellons MS, Morris WH, Gai Z, Shen J, Bentley J, Wittig JE, Lukehart CM. Direct synthesis and size selection of ferromagnetic FePt nanoparticles. Chem. Mater. 2007;19:2483–2488. [Google Scholar]
- 13.Zhang C, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, Zentgraf H, Bock M, Eisenhut M, Semmler W, Kiessling F. Specific targeting of tumor angiogenesis by RGD-conjugated ultra-small superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007;67:1555–1562. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]