Abstract
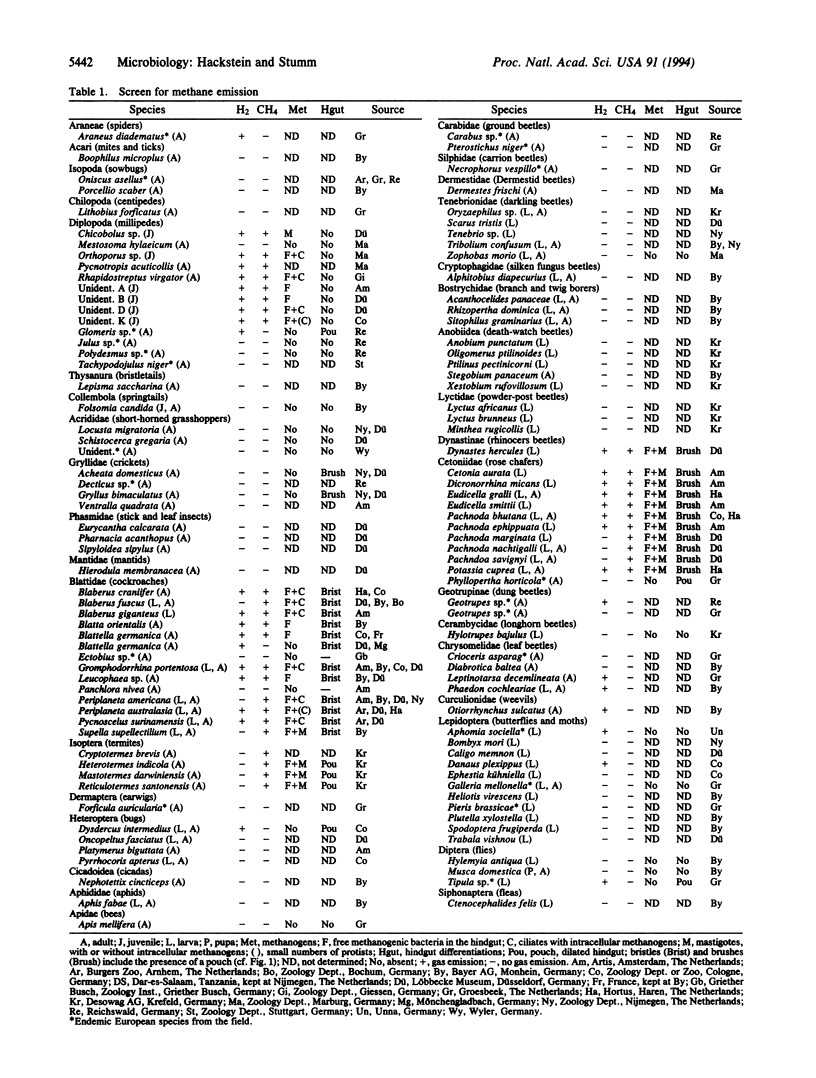
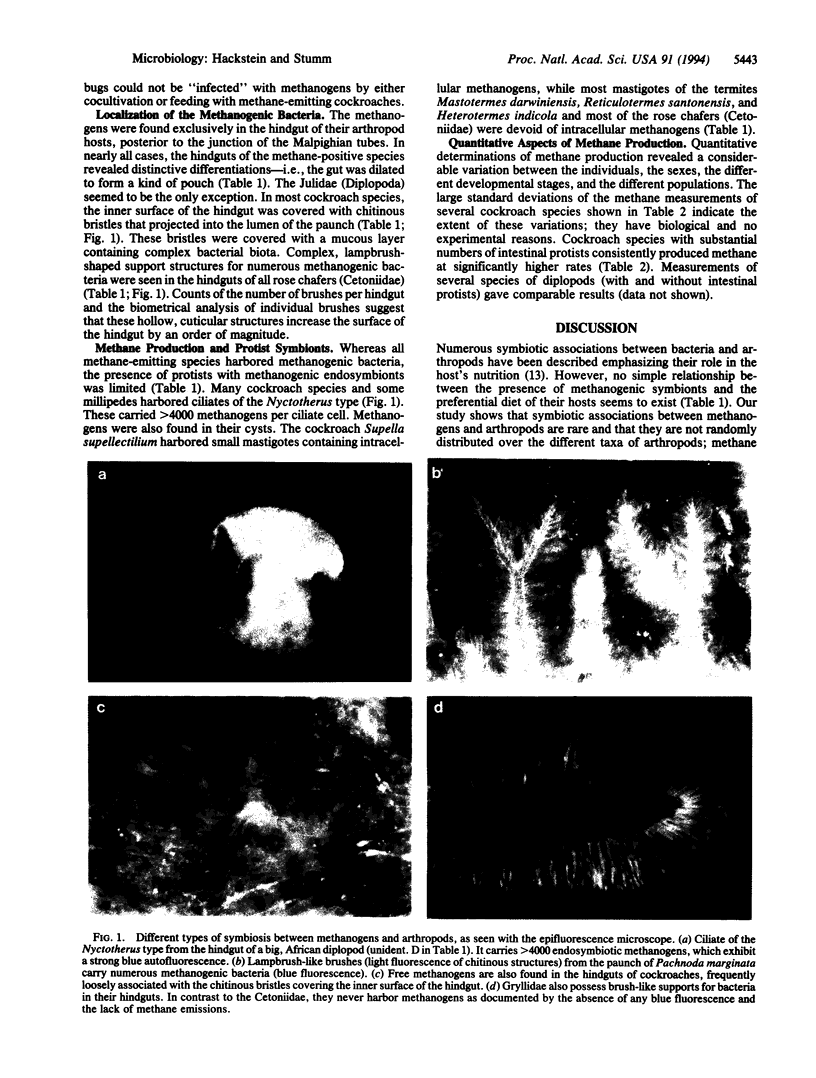
We have screened more than 110 representatives of the different taxa of terrestrial arthropods for methane production in order to obtain additional information about the origins of biogenic methane. Methanogenic bacteria occur in the hindguts of nearly all tropical representatives of millipedes (Diplopoda), cockroaches (Blattaria), termites (Isoptera), and scarab beetles (Scarabaeidae), while such methanogens are absent from 66 other arthropod species investigated. Three types of symbiosis were found: in the first type, the arthropod's hindgut is colonized by free methanogenic bacteria; in the second type, methanogens are closely associated with chitinous structures formed by the host's hindgut; the third type is mediated by intestinal anaerobic protists with intracellular methanogens. Such symbiotic associations are likely to be a characteristic property of the particular taxon. Since these taxa represent many families with thousands of species, the world populations of methane-producing arthropods constitute an enormous biomass. We show that arthropod symbionts can contribute substantially to atmospheric methane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brauman A., Kane M. D., Labat M., Breznak J. A. Genesis of acetate and methane by gut bacteria of nutritionally diverse termites. Science. 1992 Sep 4;257(5075):1384–1387. doi: 10.1126/science.257.5075.1384. [DOI] [PubMed] [Google Scholar]
- Breznak J. A. Intestinal microbiota of termites and other xylophagous insects. Annu Rev Microbiol. 1982;36:323–343. doi: 10.1146/annurev.mi.36.100182.001543. [DOI] [PubMed] [Google Scholar]
- Cruden D. L., Markovetz A. J. Microbial ecology of the cockroach gut. Annu Rev Microbiol. 1987;41:617–643. doi: 10.1146/annurev.mi.41.100187.003153. [DOI] [PubMed] [Google Scholar]
- Doddema H. J., Vogels G. D. Improved identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1978 Nov;36(5):752–754. doi: 10.1128/aem.36.5.752-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijzen H. J., Barugahare M. Contribution of anaerobic protozoa and methanogens to hindgut metabolic activities of the American cockroach, Periplaneta americana. Appl Environ Microbiol. 1992 Aug;58(8):2565–2570. doi: 10.1128/aem.58.8.2565-2570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijzen H. J., Broers C. A., Barughare M., Stumm C. K. Methanogenic bacteria as endosymbionts of the ciliate Nyctotherus ovalis in the cockroach hindgut. Appl Environ Microbiol. 1991 Jun;57(6):1630–1634. doi: 10.1128/aem.57.6.1630-1634.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M. D., Breznak J. A. Effect of host diet on production of organic acids and methane by cockroach gut bacteria. Appl Environ Microbiol. 1991 Sep;57(9):2628–2634. doi: 10.1128/aem.57.9.2628-2634.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman P. R., Greenberg J. P., Wandiga S. O., Crutzen P. J. Termites: a potentially large source of atmospheric methane, carbon dioxide, and molecular hydrogen. Science. 1982 Nov 5;218(4572):563–565. doi: 10.1126/science.218.4572.563. [DOI] [PubMed] [Google Scholar]




