Abstract
Due to the clinical importance of hearing and balance disorders in man, model organisms such as the zebrafish have been used to study lateral line development and regeneration. The zebrafish is particularly attractive for such studies because of its rapid development time and its high regenerative capacity. To date, zebrafish studies of lateral line regeneration have mainly utilized fish of the embryonic and larval stages because of the lower number of neuromasts at these stages. This has made quantitative analysis of lateral line regeneration/and or development easier in the earlier developmental stages. Because many zebrafish models of neurological and non-neurological diseases are studied in the adult fish and not in the embryo/larvae, we focused on developing a quantitative lateral line regenerative assay in adult zebrafish so that an assay was available that could be applied to current adult zebrafish disease models. Building on previous studies by Van Trump et al.17 that described procedures for ablation of hair cells in adult Mexican blind cave fish and zebrafish (Danio rerio), our assay was designed to allow quantitative comparison between control and experimental groups. This was accomplished by developing a regenerative neuromast standard curve based on the percent of neuromast reappearance over a 24 hr time period following gentamicin-induced necrosis of hair cells in a defined region of the lateral line. The assay was also designed to allow extension of the analysis to the individual hair cell level when a higher level of resolution is required.
Keywords: Developmental Biology, Issue 86, Zebrafish, lateral line regeneration, lateral line development, neuromasts, hair cell regeneration, disease models
Introduction
The lateral line (LL) system is a mechanosensory organ found in both fish and amphibians that is responsible for hearing, balance, rheotaxis and mediating behaviors such as schooling and predator avoidance1-5. It is composed of clusters of hair cells surrounded by supporting cells, both of which are positioned in structures called neuromasts6. These neuromasts are typically organized into vertical lines (called stitches) along the longitudinal axis of the body and tail with some horizontal stitches observed in the head of the fish. In the adult, neuromasts are significantly greater in number within the stitches as compared to embryonic or larval fish6. Biomedical studies in zebrafish have focused on the effect of antibiotic treatment, noise-induced trauma, chronic infection, etc. on hair cells7,8 in an attempt to better understand their effects in humans.
Unlike most vertebrates, teleosts, such as the zebrafish (Danio rerio), have the ability to regenerate lost hair cells. Zebrafish are particularly useful because of their rapid development time and high regenerative capacity. To date, however; zebrafish studies on lateral line development and/or regeneration have mainly utilized the embryonic and larval stage fish due to the reduced number of lateral line neuromasts which allows for easier counting and analysis6,9,10.
However, as many zebrafish models of neurological and non-neurological diseases11-16 are studied in the adult fish and not the larvae, we focused on developing a lateral line regenerative assay in adult zebrafish using gentamicin (an aminoglycoside previously used in zebrafish larvae and more recently used with adult fish17) so that an assay was available that could be applied to current adult zebrafish disease models. While previously published procedures by Van Trump et al.17 established the conditions for hair cell ablation in the adult fish, they did not establish a standard curve for neuromast regeneration which is required for quantitative comparison between control and experimental groups such as when using transgenic zebrafish lines or pharmacologically-induced disease states in zebrafish18. We therefore followed the procedures of Van Trump et al.17 for hair cell ablation, but built on their work to establish a standard curve of neuromast regeneration to enable investigators to use our data when comparing control and experimental groups such as with adult zebrafish disease models. The assay was also designed to allow extension of the analysis to the individual hair cell when a higher level of resolution is required.
Protocol
All procedures are performed following the guidelines described in "Principles of Laboratory Animal Care" (National Institutes of Health publication no. 85-23, revised 1985) and the approved Rosalind Franklin University Institutional Animal Care and Use Committee animal protocol 08-19.
1. Gentamicin-induction of Hair Cell Necrosis
Prepare gentamicin sulfate in normal saline at a final concentration of 0.004% (4.32 mM).
Place adult fish (D. rerio, 4-6 months of age) in a container containing the 0.004% (4.32 mM) gentamicin solution. Any container can be used, but we use a fish container from a Pharmacal Aquatic System which is 7 in wide, 6 in high, and 7 in long. Place the container with fish in an incubator set at 28 °C for 24 hr. Set the total volume of fluid in the tank at a sufficient level to maintain fish in a viable state for the 24 hr period. Note: Aeration of the gentamicin fluid is not necessary if sufficient volume is used for the number of fish being treated.
2. Vital Staining of Hair Cells
Prepare a 0.08% concentration (in normal saline) of the fluorescent vital dye [4-4-diethylaminostyryl)-N-methylpyridinium iodide (485 nm excitation λ and 603 nm emission λ in methanol) from a working stock solution of 15 mg/ml in ethanol.
To determine if gentamicin treatment was effective a subset of control and gentamicin treated fish are stained immediately by placing fish in the well of a 6 well culture plate containing the vital dye. Use a sufficient number of fish (and culture plates as required for statistical significance to be achieved. Based on the examiner's speed of neuromast counting, place the fish in the plates in a staggered manner over time so that fish are not stained for over 75 min as described in step 2.3.
Place the plates from step 2.2 in a bench drawer by the fluorescent microscope to be used for examination of stained neuromasts. Turn off the room lights to prevent quenching of the vital dye over the 1 hr staining period at room temperature.
Prepare both dye wash-out and anesthetic water tanks. Dye wash-out water is normal fish water and for anesthetic water, add sufficient 2-phenoxyethanol so that a 1:1,000 dilution in normal fish water is achieved.
Place fish in excess normal fish water to rinse excess vital dye and proceed to step 3.1 for observation of vital dye stained fish.
To examine regeneration of neuromasts, transfer gentamicin-treated fish that were washed in normal fish water to an incubator for between 8-16 hr at 28 °C.
At various times between 8-16 hr, fish are removed from the incubator, washed and stained as indicated in steps 2.1-2.4. Proceed to step 3.1 for observation of vital dye stained fish.
3. Anesthetizing Fish and Fluorescent Counting of Neuromasts
Blot each fish on a paper towel to remove excess fluid and then place it on a dampened piece of filter paper that is centered on the lid of a plastic Petri dish.
Place the lid on the stage of a fluorescent stereo microscope to obtain a digital image of the vital dye stained neuromasts of the mid body stitches.
Use a digital camera placed on the fluorescent stereo microscope set a magnification of 2X to capture images for subsequent quantitative analysis. Note: The magnification setting of the stereo microscope may depend on the brand of microscope used, but the setting should allow easy viewing and counting of individual neuromasts within the mid body stitches.
Determine the amount of regeneration by counting the number of visible neuromasts within the four designated stitches on the bottom-most ventral side of the fish just proximal to the right pectoral fin (see Figure 1). For statistical analysis use an appropriate test such as ANOVA or the Student's T-test. Experiments should utilize a minimum of 5 fish per time point and all experiments should be repeated a minimum of 3x.
Based on the neuromast regeneration time curve (see Figure 3), count neuromasts between 8-16 hr post gentamicin wash-out to be within the linear phase of the regeneration curve. Note: Use of the linear time phase allows for proper quantitative analysis between the control and experimental groups.
4. Fluorescent Counting of Individual Hair Cells for Obtaining Higher Resolution of the Quantitative Analysis if the Neuromast Analysis is Not Statistically Significant
If the quantitative analysis at the level of neuromasts is not significant, analysis at the level of the individual hair cell can also be utilized to obtain a higher degree of resolution. Select fish at a particular time point post gentamicin wash-out (time point based on the earlier neuromast studies), vital dye stain the fish as described in Protocol 2, and then euthanize the fish using 2-phenoxyethanol at a 1:500 dilution for 1-5 min.
In subdued light to prevent quenching, make four incisions so that a square skin flap preparation is made as follows. Make an incision along the upper ribs of the fish until it is aligned with the anal fins, then make an incision across the belly, and finally, make two vertical incisions on each side of these incisions so the square skin flap is created. Note: This skin preparation will incorporate the mid body stitches used in the neuromast experiments.
Place the skin specimen on a glass slide and then place a circular glass cover slip over the excised skin specimen to help anchor and flatten the tissue for subsequent digital imaging.
Using the skin specimens from step 4.3, obtain digital images of the hair cells within each neuromast of the mid body stitches. Take images at a magnification of at least 60X and then count the hair cells within individual neuromasts for comparative quantitative analysis of the control and experimental groups (see Figure 4).
Representative Results
Optimization of the procedures for quantifying neuromast regeneration of the lateral line in adult zebrafish.
The neuromasts of larval zebrafish are readily quantifiable; however, the lateral line of the adult zebrafish has a much greater number of neuromasts per stitch making quantitative analyses more difficult6,17,19,20. As seen in Figure 1A, the head has a significantly higher number of neuromasts compared to either the mid-section or tail; with the tail region having the least number of neuromasts as shown in Figure 1D. Because the pattern of stitches in the head is complicated and significantly greater in the number of neuromasts, it did not lend itself as a region for quantitative analysis. In addition, regardless of the gentamicin concentration we tested, complete ablation of neuromasts throughout the head was rarely attainable; leaving spots of neuromasts observed after gentamicin treatment as previously reported by Van Trump et al.17 In contrast, the tail has too few neuromasts, and as such, we selected the mid-body region (Figure 1B) to quantitatively analyze neuromast regeneration in the adult. In this region, we identified four stitches just posterior to the lateral pectoral fin that were consistent in neuromast number among all adults [61.45 (n = 95)] (Figures 1B and 1E). Importantly, we were able to consistently and completely ablate the neuromasts of this region by a 24 hr 0.004% gentamicin treatment (as previously reported in Van Trump et al.17) allowing for a subsequent accurate determination of neuromast regeneration (compare Figures 1B and 1E and inset with Figure 2, 0 hr).
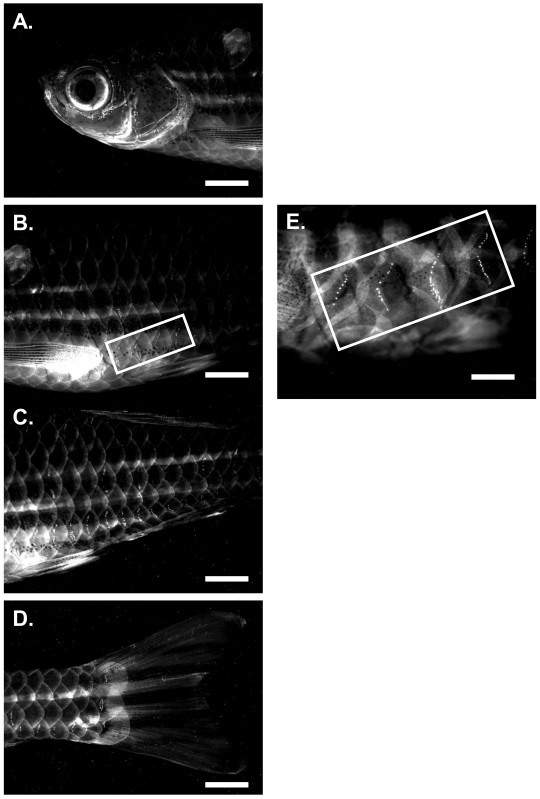 Figure 1.The fluorescent pattern of neuromast within stitches of the adult zebrafish is shown along the longitudinal axis.Panel A is the head region, Panel B is the Mid-body region with the four stitches used for quantitative analysis outlined with a box. Panel C is the Posterior-body region. Panel D is the caudal fin region. A higher magnification of the 4 stitches of the Mid-body region used for quantitative analysis is shown in Panel E. Magnification Power of 1X and 2X.
Figure 1.The fluorescent pattern of neuromast within stitches of the adult zebrafish is shown along the longitudinal axis.Panel A is the head region, Panel B is the Mid-body region with the four stitches used for quantitative analysis outlined with a box. Panel C is the Posterior-body region. Panel D is the caudal fin region. A higher magnification of the 4 stitches of the Mid-body region used for quantitative analysis is shown in Panel E. Magnification Power of 1X and 2X.
It has been reported that copper sulfate treatment is an effective chemical method to induce rapid necrosis of hair cells in embryos and larvae21. Here we tested copper sulfate treatment with the hope that it might shorten the time to induce neuromast ablation. Copper sulfate concentrations ranging from 5-50 mM for various exposure times up to 48 hr were utilized as was previously reported by Liang et al.21 It was found that copper sulfate was lethal at the higher concentrations and not effective at lower concentrations in adult fish (data not shown). Please click here to view a larger version of this figure.
Parameters used for fluorescent analysis of lateral line regeneration in adult zebrafish.
Regeneration was monitored after all neuromasts within the four mid-body stitches were ablated following 24 hr gentamicin-treatment (Figure 2, compare 0 hr with control) and positive regeneration was determined by the appearance of a minimum of three neuromasts within a stitch. (Figure 2, 0 hr). By 8 hpg, approximately one third of the fish had some sign of recovery (n = 34); although the intensity of neuromasts was faint in the regenerating stitches (Figure 2, 8 hr, faint stitches outlined by boxes). The number of neuromasts and their intensity continued to increase in a linear fashion until regeneration reached a plateau at 16 hpg (compare Figure 2, 16 hr with Figure 2, 8 hr). It was not until at least 24 hpg that all fish treated with gentamicin had fully recovered with both equal numbers and intensities of the neuromasts within the lateral line stitches as compared to controls (Figure 2, 24 hr). A time-line for neuromast regeneration following gentamicin withdrawal is shown in Figure 3 which shows the linear and plateau phases of the recovery curve. We note that in less than 5% of cases, the regenerating stitches did not appear as individual entities but instead appeared as a smear of fluorescence.
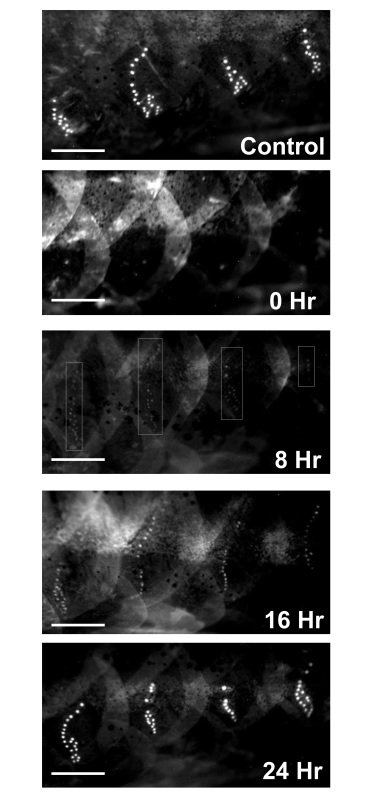 Figure 2. Fluorescent images of neuromasts. Control fish, 0 Hr fish (immediately following 24 hr of 0.004% gentamicin treatment), 8 Hr fish (8 hpg) with some faint staining of neuromasts within the 4 stitches used for quantitative analysis (only 30% of all fish showed this pattern of staining at 8 hpg; 70% showed no neuromast staining at this time point; the white boxes outline the faintly stained neuromasts that were seen in the 30% of fish that showed some degree of regeneration at 8 hpg), 16 Hr fish, and 24 Hr fish. Complete regeneration of neuromasts within the 4 stitches in regard to 1) the number of neuromasts and 2) intensity of staining of neuromasts was observed by 24 hpg. Please click here to view a larger version of this figure.
Figure 2. Fluorescent images of neuromasts. Control fish, 0 Hr fish (immediately following 24 hr of 0.004% gentamicin treatment), 8 Hr fish (8 hpg) with some faint staining of neuromasts within the 4 stitches used for quantitative analysis (only 30% of all fish showed this pattern of staining at 8 hpg; 70% showed no neuromast staining at this time point; the white boxes outline the faintly stained neuromasts that were seen in the 30% of fish that showed some degree of regeneration at 8 hpg), 16 Hr fish, and 24 Hr fish. Complete regeneration of neuromasts within the 4 stitches in regard to 1) the number of neuromasts and 2) intensity of staining of neuromasts was observed by 24 hpg. Please click here to view a larger version of this figure.
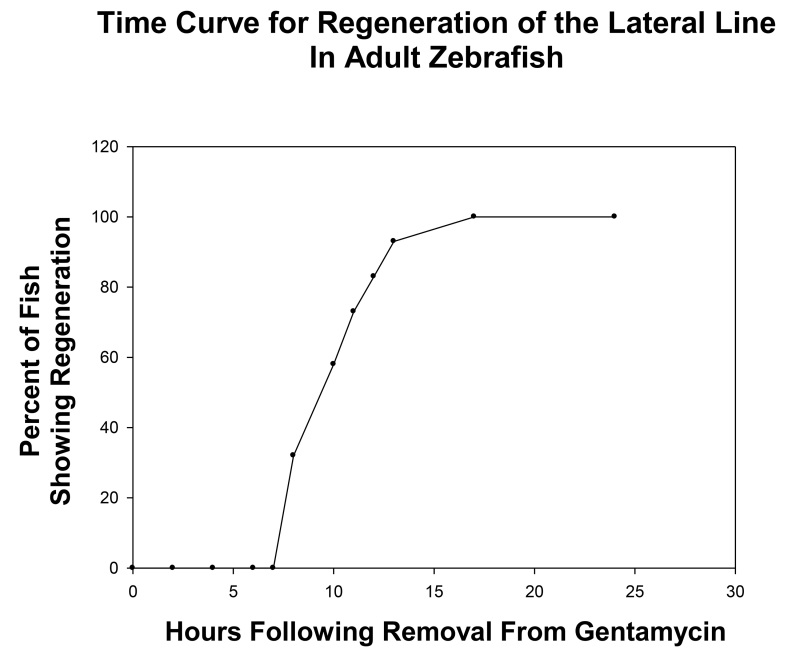 Figure 3. Graph showing the time course of neuromasts regeneration following withdrawal from 24 hr treatment of adult zebrafish with 0.004% gentamicin. As shown, recovery begins at the 8 hpg time point and reached a plateau at the 16 hpg time point. This defined the liner phase of neuromast regeneration between 8-16 hr and time points within this linear phase should be used to quantitatively compare control and experiment groups.
Figure 3. Graph showing the time course of neuromasts regeneration following withdrawal from 24 hr treatment of adult zebrafish with 0.004% gentamicin. As shown, recovery begins at the 8 hpg time point and reached a plateau at the 16 hpg time point. This defined the liner phase of neuromast regeneration between 8-16 hr and time points within this linear phase should be used to quantitatively compare control and experiment groups.
Neuromast toxicity is induced by prolonged exposure to fluorescent staining dyes.
In our estimation an ideal way to perform these experiments would be to stain the fish with the fluorescent dye prior to treatment, stain again at 0 hr then stain again at regeneration time points. However, we encountered a complication to these studies which is the fact that hair cell fluorescent staining dyes such as 4-Di-2-Asp, as can also be the case with other mitochondrial stains22 can have a toxic affect on hair cells23. This fact required us to use separate groups of fish since repeated staining of the same fish could not be employed. In all cases the experimental fish including controls were treated in parallel to eliminate experimental variability.
Confocal analysis at the level of individual hair cells.
If the results obtained from the neuromast analysis are not statistically significant between the control and experimental groups, one may extend these studies to the level of the individual hail cells to obtain a higher degree of resolution for quantitative comparisons. As indicated in Figure 4, neuromasts from a control group (a neuromast at 12 hr of regeneration is shown in this figure) can be viewed by confocal microscopy of skin preparations from the mid body region. At 8 hr, 10 hr, and 12 hr of regeneration time, we found that control groups (7 animals/group) had a range of 0-4 hair cells/neuromast. As expected for control groups, when quantitatively analyzed, no statistical difference between neuromasts was detected in terms of the number of hair cells per neuromast at the time points indicated above (P values ranged from 0.230-0.472). Such an approach may be taken between any control and experiment group when needed to extend or confirm the data obtained from the first phase of neuromast studies.
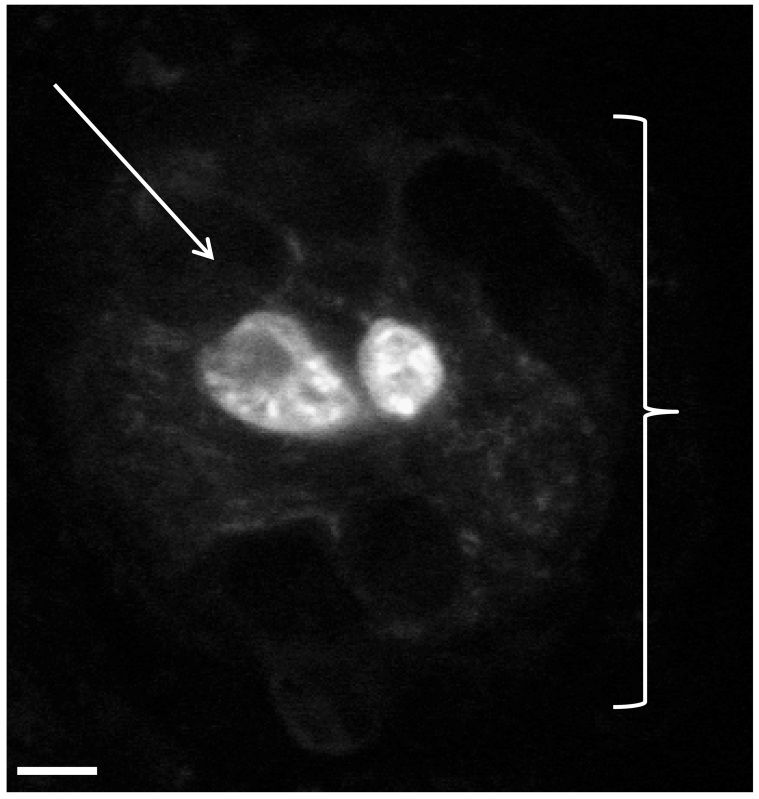 Figure 4. Analysis of hair cell/neuromast regeneration using skin preparations of the defined lateral line region described in protocol step 3.3. Fluorescent confocal image of hair cells within a neuromast obtained from a zebrafish skin preparation. Two vital dye stained hair cells are shown within a neuromast of a control fish (Figure 4). This image was obtained 12 hr post-removal of gentamicin (linear regeneration phase). The white bracket symbol ( ) indicates an individual neuromast while the white arrow indicates a supporting cell surrounding the hair cells. Supporting cells do not stain under these conditions and appear as black spaces. Magnification, 60X.
Figure 4. Analysis of hair cell/neuromast regeneration using skin preparations of the defined lateral line region described in protocol step 3.3. Fluorescent confocal image of hair cells within a neuromast obtained from a zebrafish skin preparation. Two vital dye stained hair cells are shown within a neuromast of a control fish (Figure 4). This image was obtained 12 hr post-removal of gentamicin (linear regeneration phase). The white bracket symbol ( ) indicates an individual neuromast while the white arrow indicates a supporting cell surrounding the hair cells. Supporting cells do not stain under these conditions and appear as black spaces. Magnification, 60X.
| 1 | 1. Gentamicin treatment [0.004% (4.32 mM)] of control and experimental fish for 24 hr at 28 °C using an incubator. |
| 2 | 2. Wash out of gentamicin to initiate regeneration of hair cells. Return fish to the 28 °C incubator for investigator selected time periods between 8-16 hr. |
| 2 | 3. Vital dye stain [0.08% 4-4-diethylaminostyryl-N-methylpyridinium iodide (4-Di-2-Asp)] control and experimental fish for 1 hr at room temperature and then wash out stain with fish water for fluorescent imaging. |
| 1 or 2 | 4. If necessary due to nonsignificant results from analysis of neuromasts, repeat Protocols 1-3 with a separate group of control and experimental fish, but then obtain a skin preparation for confocal analysis of individual hair cells. |
Table 1. A summary of the protocol outlined above.
Discussion
Based on the extensive body of literature that has been established for analysis of lateral line (LL) regeneration in embryonic and larval zebrafish8,24,25, the goal of our study was to develop a quantitative assay for lateral line regeneration in zebrafish that could be applied to disease models that are best studied in the adult fish. We found that certain critical points are important when applying procedures developed for embryonic/larval fish to the adult fish. The most important of these points regarded: 1) the number of lateral line neuromasts along the longitudinal axis of the fish, 2) the duration of staining of neuromast hair cells, 3) the concentration and duration of treatment of the aminoglycoside, and 4) the timing of lateral line regeneration following aminoglycoside treatment. These points will be addressed in the following discussion.
In regard to the number of neuromasts along the lateral line of zebrafish, embryonic and larval zebrafish have the distinct advantage that the neuromasts have a simple pattern because of their lower number within a given stitch as compared to adult fish. This low number has allowed investigators to clearly identify each neuromast and assign a name to it6. In this way, regeneration studies can quantify the reappearance of a particular neuromast by name at any time following withdrawal from an aminoglycoside agent. The greater number of neuromasts per stitch in the adult introduces significant difficulty in accurate counting and quantification during the reappearance of regenerating neuromasts when compared to that of embryos or larvae. As shown, we assessed the pattern of lateral line neuromasts within stitches of the adult and determined that the mid-body (Figures. 1 and 2) provided the optimal region of stitches for analysis.
Staining of neuromasts with hair cells dyes such as 2-[4-(dimethylamino)styryl]-N-ethylpyridinium iodide (DASPEI) or 4-Di-2-Asp allows one to visualize neuromasts of the lateral line using fluorescent stereomicroscopy26,27 in larval and juvenile fish. These same stains are also effective in the adult fish and our quantitative analysis indicated that no significant difference in the number of neuromasts within the mid-body region was observed among normal control zebrafish (with an average of 61.45 neuromasts in normal control adult fish).
It has been reported that hair cell dyes such as 2-[4-(dimethylamino) styryl]-N-ethylpyridinium iodide (DASPEI) or 4-Di-2-Asp can themselves be toxic to neuromast cells24, and fish cannot be repeatedly stained with these fluorescent agents if one is to monitor aminoglycoside-induced regeneration. Repeated staining of the same fish introduces multiple toxicity events (by both the stain and the aminoglycoside) that makes the experiment un-interpretable23. Accordingly, all experiments in our study required parallel 4-Di-2-Asp staining of multiple sets of fish in order to show that neuromasts were 1) present in the nontreated gentamicin control condition, 2) fully ablated immediately following gentamicin exposure, and 3) regenerating at some hour post gentamicin-treatment following withdrawal and washing out of this aminoglycoside. In this way, all fish was stained only once with the neuromast dye.
It should be pointed out that while the procedures of Van Trump et al.17 establish the conditions for hair cell ablation in the adult fish, they do not establish a standard curve for neuromast regeneration which is required for quantitative comparison between control and experimental groups. We therefore followed the procedures of Van Trump et al.17 for hair cell ablation (gentamicin concentration of 0.004% using a 24 hr exposure time, see Figure 2 for hair cell ablation results at 24 hr) but extended their work to establish a standard curve of neuromast regeneration. This allows for comparative analysis of LL regeneration in the adult zebrafish using the four stitches of the mid body region that we established for our assay conditions (see Figures 1 and 2). In order to determine if a shorter period for hair cell ablation could be obtained, we also tested the effect of copper sulfate which has been effectively used in larval fish for periods as short as 2 hr. Our studies indicated that copper sulfate (5-50 mM for various exposure times up to 48 hr as was previously reported by Liang et al.21 for larvae) was not found to be effective in adult fish as an agent for hair cell ablation. This highlights the fact that conditions used for ablation of hair cells in embryos and larvae cannot always be directly transferred for use with the adult fish.
As pertaining to the lateral line regeneration in the adult, we found similar time frames for regeneration of neuromasts between that of embryo/larval and adult fish. As reported previously by others, zebrafish embryos and larvae show neuromast regeneration at 12-24 hr post aminoglycoside wash-out8. We observed the linear phase of neuromast regeneration appearing in the 8-12 time-frame (not seen as full stitches for the 8 hr time point as shown in Figure 2) with a plateau reached at 16 hpg. Complete control-like appearance of neuromasts within stitches was not observed until 24 hpg as reported for embryos and larvae. Complete control-like appearance denotes both the number and intensity of neuromasts within all four stitches of the mid-body region in adult zebrafish. Additionally, if the quantitative results obtained at the level of the neuromasts are not statistically significant, the investigator can extend their studies to the level of the individual hair cells within the neuromasts using confocal microscopy as described by our procedures.
The neuromasts/hair cell regeneration assay described in this article can be applied to disease states that are best manifested in the adult zebrafish rather than in the early larval/embryonic stages. A limitation of the assay concerns the affect of the experimental condition whether it be 1) the transgenic strain that mimics a particular disease state or 2) a pharmacologically-induced disease state] on stem cells within the lateral line system of adult zebrafish. In this regard, a particular disease state of the adult zebrafish may or may not affect stem cells of the hair cell lineage, and it is important to note that neuromast regeneration is completely dependent on these stem cell proliferation/differentiation processes8,24,25.
As an example of this limitation, we will describe experiments performed on an adult zebrafish model of Type I diabetes. This particular disease model was developed in adult zebrafish in order to study the long term secondary complication induced by hyperglycemia28. For a number of reasons described previously28,29, these studies can only be performed using adult zebrafish. Because peripheral nerves along with the specialized cellular structures they innervate are adversely affected in patients with diabetes, we wanted to determine if lateral line regeneration of neuromasts/hair cells was also impaired in diabetic zebrafish. Using the lateral line regeneration assay no statistically significant delay was detected in neuromast regeneration. To confirm this negative result, the experiments were repeated at the more refined level of the individual hair cell. Again, no statistically significant difference was observed in hair cell regeneration between control and diabetic groups. Therefore, the data were inconsistent with the supposition that hyperglycemia impedes neuromast/hair cell regeneration; possibly due to the resistance of stem cells of the hair cell lineage to hyperglycemic conditions. With this limitation in mind, the neuromasts/hair cell regeneration assay described in this article does provide a means to test whether any particular adult zebrafish disease model involves dysfunction in the hair cell regenerative process as monitored using the lateral line system. Positive results would imply stem cell involvement and further studies would therefore be warranted.
Disclosures
This work was supported by a research grant from the Iacocca Family Foundation, National Institutes of Health Grant DK092721 (to R.V.I.), and Rosalind Franklin University start-up funds. No potential conflicts of interest relevant to this article were reported. G.C.P., S.M.M, and N.D. researched data. M.P.S. Jr. and RI oversaw the project, contributed to the discussion of the data, and oversaw the writing and editing of the manuscript.
Acknowledgments
The authors have nothing to disclose.
References
- Dambly-Chaudire C, Sapde D, Soubiran F, Decorde K, Gompel N, Ghysen A. The Lateral Line of Zebrafish: a Model System for the Analysis of Morphogenesis and Neural Development in Vertebrates. Biol. Cell. 2003;95(9):579–587. doi: 10.1016/j.biolcel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Montgomery J, Carton G, Voigt R, Baker C, Diebel C. Sensory Processing of Water Currents by Fishes. Phil. Trans. Royal Soc. London B Biol. Sci. 2000;355(1401):1325–1327. doi: 10.1098/rstb.2000.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck LM, Winter MJ, Redfern W, Whitfield TT. Ototoxin-Induced Cellular Damage in Neuromasts Disrupts Lateral Line Function in Larval Zebrafish. Hearing Res. 2012;284(1-2):1–2. doi: 10.1016/j.heares.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Engelmann J, Hanke W, Mogdans J, Bleckmann H. Hydrodynamic Stimuli and the Fish Lateral Line. Nature. 2000;408(6808):51–52. doi: 10.1038/35040706. [DOI] [PubMed] [Google Scholar]
- Olszewski J, Haehnel M, Taguch M, Liao JC. Zebrafish Larvae Exhibit Rheotaxis and Can Escape a Continuous Suction Source Using Their Lateral Line. PloS One. 2012;7(5):e36661. doi: 10.1371/journal.pone.0036661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible DW, Kruse GJ. Organization of the Lateral Line System in Embryonic Zebrafish. J. Comp. Neurol. 2000;421(2):189–198. [PubMed] [Google Scholar]
- Coffin AB, Reinhart KE, Owens KN, Raible DW, Rubel EW. Extracellular Divalent Cations Modulate Aminoglycoside-Induced Hair Cell Death in the Zebrafish Lateral. 2009;253(1-2):1–2. doi: 10.1016/j.heares.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-Induced Hair Cell Death and Rapid Regeneration in the Lateral Line of Zebrafish (Danio. 2003;4(2):219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW. Notch Signaling Regulates the Extent of Hair Cell Regeneration in the Zebrafish Lateral Line) J. Neurosci. 2008;28(9):2261–2273. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Raible DW, Stone JS. Feathers and Fins: Non-Mammalian Models for Hair Cell Regeneration. Brain Res. 2009;1277:12–23. doi: 10.1016/j.brainres.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibliowicz J, Tittle RK, Gross JM. Toward a Better Understanding of Human Eye Disease Insights From the Zebrafish, Danio Rerio. Prog. Mol. Biol. Transl. Sci. 2011;100:287–330. doi: 10.1016/B978-0-12-384878-9.00007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mione MC, Trede NS. The Zebrafish As a Model for Cancer. Dis. Model. Mech. 2010;3(9-10):9–10. doi: 10.1242/dmm.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W, Bally-Cuif L. Adult Zebrafish As a Model Organism for Behavioural Genetics. BMC. Neurosci. 2010;11 doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Guo S. Use of Zebrafish As a Model to Understand Mechanisms of Addiction and. Complex Neurobehavioral Phenotypes. Neurobiol. Dis. 2010;40(1):66–72. doi: 10.1016/j.nbd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius MS, Langenau DM. Zebrafish As a Model for Cancer Self-Renewal. Zebrafish. 2009;6(4):377–387. doi: 10.1089/zeb.2009.0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan DJ, MacRae CA. Zebrafish Genetic Models for Arrhythmia. Prog. Biophys. Mol. Biol. 2008;98(2-3):2–3. doi: 10.1016/j.pbiomolbio.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Trump WJ, Coombs S, Duncan K, McHenry MJ. Gentamicin Is Ototoxic to All Hair Cells in the Fish Lateral Line System. Hear. Res. 2010;261(1-2):1–2. doi: 10.1016/j.heares.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Littleton RM, Hove JR. Zebrafish: a Nontraditional Model of Traditional Medicine. J. Ethnopharmacol. 2013;145(3):677–685. doi: 10.1016/j.jep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-Induced Hair Cell Death and Rapid Regeneration in the Lateral Line of Zebrafish (Danio. 2003;4(2):219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski J, Haehnel M, Taguchi M, Liao JC. Zebrafish Larvae Exhibit Rheotaxis and Can Escape a Continuous Suction Source Using Their Lateral Line) PLoS One. 2012;7(5):36661–36. doi: 10.1371/journal.pone.0036661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Wang D, Renaud G, Wolfsberg TG, Wilson AF, Burgess SM. The Stat3/Socs3a Pathway Is a Key Regulator of Hair Cell Regeneration in Zebrafish [Corrected. J. Neurosci. 2012;32(31):10662–10673. doi: 10.1523/JNEUROSCI.5785-10.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae M, Asaoka R, Wada H, Sasaki K. Fluorescent Dye Staining of Neuromasts in Live Fishes: An Aid to Systematic Studies. Ichthyol Res. 2012. pp. 286–290.
- Magrassi L, Purves D, Lichtman JW. Fluorescent Probes That Stain Living Nerve Terminals. The J. Neurosci. 1987;7(4):1207–1214. doi: 10.1523/JNEUROSCI.07-04-01207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Coffin AB, Hong LS, Bennett KO, Rubel EW, Raible DW. Response of Mechanosensory Hair Cells of the Zebrafish Lateral Line to Aminoglycosides Reveals Distinct Cell Death Pathways. Hear. Res. 2009;253(1-2):1–2. doi: 10.1016/j.heares.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namdaran P, Reinhart KE, Owens KN, Raible DW, Rubel EW. Identification of Modulators of Hair Cell Regeneration in the Zebrafish Lateral. 2012;32(10):3516–3528. doi: 10.1523/JNEUROSCI.3905-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera AA, Banner LR. The Use and Effects of Vital Fluorescent Dyes: Observation of Motor Nerve Terminals and Satellite Cells in Living Frog Muscles. J. Neurocytol. 1990;19(1):67–83. doi: 10.1007/BF01188440. [DOI] [PubMed] [Google Scholar]
- Hickey PC, Jacobson D, Read ND, Louise Glass , L N. Live-Cell Imaging of Vegetative Hyphal Fusion in Neurospora Crassa. Fungal. Genet. Biol. 2002;37(1):109–119. doi: 10.1016/s1087-1845(02)00035-x. [DOI] [PubMed] [Google Scholar]
- Olsen AS, Sarras MP, Intine RV. Limb Regeneration Is Impaired in an Adult Zebrafish Model of Diabetes Mellitus. Wound Repair Regen. 2010;18(5):532–542. doi: 10.1111/j.1524-475X.2010.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen AS, Sarras MP, Leontovich A, Intine RV. Heritable Transmission of Diabetic Metabolic Memory in Zebrafish Correlates With DNA Hypomethylation and Aberrant Gene Expression. Diabetes. 2012;61(2):485–491. doi: 10.2337/db11-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]


