Abstract
Stem cells hold promise to treat diseases currently unapproachable, including Parkinson's disease, liver disease and diabetes. Seminal research has demonstrated the ability of embryonic and adult stem cells to differentiate into clinically useful cell types in vitro and in vivo. More recently, the potential of fetal stem cells derived from extra-embryonic tissues has been investigated. Fetal stem cells are particularly appealing for clinical applications. The cells are readily isolated from tissues normally discarded at birth, avoiding ethical concerns that plague the isolation embryonic stem cells. Extra-embryonic tissues are large, potentially increasing the number of stem cells that can be extracted. Lastly, the generation and sequestration of cells that form extra-embryonic tissues occurs early in development and may endow resident stem cell populations with enhanced potency. In this review we summarize recent work examining the plasticity and clinical potential of fetal stem cells isolated from extra-embryonic tissues.
Keywords: wharton's jelly, amnion, amniotic fluid, multipotent, cell replacement
Stem cells – an abridged history
Stem cells can be loosely classified into three broad categories based on their time of isolation during ontogenesis: embryonic, fetal and adult. In this section they will be discussed not in order of their appearance in the organism, but arranged chronologically by date of initial isolation and characterization.
The first stem cell population was identified in adult mouse bone marrow by McCulloch and Till in the early 1960s. Pioneering work on these colony forming unit-spleen cells, later termed hematopoietic stem cells (HSCs), established the two functional properties of a stem cell population: self-renewal and multipotency. HSCs divide readily in culture and give rise to progeny that retain the colony-forming properties of the parental cells, evidence of self-renewal. Progeny of HSCs differentiate into multiple specialized cell types, including erythrocytes granulocytes and megakaryocytes, fulfilling the criteria of multipotency [1–3]. These landmark studies have formed the foundation of stem cell biology – revolutionizing the manner in which physicians and scientists study and treat disease.
Recent years have witnessed an explosion in the number of adult stem cell populations isolated and characterized. Every tissue or organ, from brain to fat, apparently contains a stem cell population. While still multipotent, adult stem cells have long been considered restricted, giving rise only to progeny of their resident tissues. Recent, and currently controversial studies have challenged this dogma, suggesting that adult stem cells may be far more plastic than previously appreciated [4, 5]. If confirmed, these findings would have far reaching implications. Stem cells from easily harvested tissues (skin, bone marrow, fat) might be used clinically to treat disorders of more vulnerable and less accessible internal organs. Current research is investigating the utility of adult stem cells in the treatment of many disorders, including Parkinson's disease, cardiovascular disease and diabetes.
The roots of embryonic stem cell (ESC) research can be traced back more than half a century. Examination of mouse teratomas, complex tumors containing a mix of differentiated adult tissues, hinted at the existence of ESCs [6–8]. However, the generation and characterization of ESC lines from the blastocysts of mice and humans did not occur until the early 1980s and late 1990s, respectively [9,10]. Subsequent studies have shown ESCs to fulfill the criteria of a stem cell population as first proposed by McCulloch and Till. ESCs divide indefinitely in culture (self-renewal) while maintaining their capacity for extensive in vitro and in vivo differentiation (pluripotency). Isolation from the earliest stages of development has endowed ESCs with the plasticity to differentiate to derivatives of all germ layers, including clinically important cell types such as dopaminergic neurons and pancreatic beta-cells [11, 12]. ESCs reintroduced into blastocysts can participate in the development of all organs and tissues in the adult animal, confirming their intrinsic potency [13]. This demonstrated plasticity has made ESCs the benchmark against which stem cell potency is measured. Despite ethical and political concerns, ESCs remain a leading candidate for future regenerative medicine applications.
Fetal stem cells, comprising the final broad stem cell class, have a comparatively recent history. They can be isolated from two distinct sources, the fetus proper and the supportive extra-embryonic structures. Stem cells derived from the extra-embryonic sources are particularly interesting due to their potential clinical utility. Throughout this review, the term ‘fetal stem cells’refers exclusively to those populations isolated from extra-embryonic tissues.
Similar to their adult counterparts, the first isolated fetal stem cells were hematopoietic, derived from human umbilical cord blood. The isolated cells were capable of long-term self-renewal and differentiation to multiple hematopoietic lineages [14, 15]. Clinically, cord blood stem cells were successfully employed in a bone marrow transplant in 1988 [16]. In some countries parents routinely bank the cord blood of their newborns against the advent of childhood hematological maladies.
Over the past decades fetal stem cells have been isolated from multiple extra-embryonic tissues, reminiscent of gradual broadening of stem cell sources seen in the adult. Amniotic fluid (AF), Wharton's jelly, placenta and amnion have all generated putative stem cells (Fig. 1). The relative potency of these stem cell populations needs to be fully determined, and further investigation is ongoing.
1.
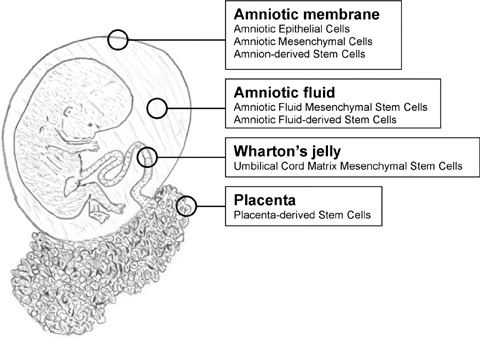
Extra-embryonic stem cell sources. Stem cells have been isolated from all extra-embryonic tissues, including the amniotic membrane, amniotic fluid, Wharton's jelly and placenta.
Fetal stem cells
Extra-embryonic tissues as stem cell reservoirs offer many advantages over both embryonic and adult stem cell sources. Extra-embryonic tissues, collectively known as the afterbirth, are routinely discarded at parturition, so little ethical controversy attends the harvest of the resident stem cell populations. The extracorporeal nature of fetal stem cell sources facilitates isolation, eliminating patient risk that attends adult stem cell isolation. Most significantly, the comparatively large volume of extra-embryonic tissues and ease of physical manipulation theoretically increases the number of stem cells that can be isolated.
Cord blood represents the prototypical fetal stem cell source. HSCs isolated from cord blood have been extensively studied and have demonstrated clinical utility. Excellent reviews of cord blood stem cells have been recently published, and they will not be discussed in detail in this review [17, 18]. Study of other fetal stem cells lags behind, and much work will be required to see if these stem cell populations measure up to the high standard set by cord blood HSCs. What we do know about fetal stem cells from other extra-embryonic sources is summarized below.
Amniotic fluid (AF)
For more than 70 years AF has been used as a safe and reliable tool for prenatal diagnosis of genetic disease. Recent evidence has suggested that AF may have utility beyond diagnostics, serving as an accessible reservoir of multipotent fetal cells. Of all the extra-ESC sources discussed in this review, AF is the only one where harvest typically occurs prior to parturition. This unique trait may become increasingly important as in utero cell-based therapies progress [19].
AF contains a heterogeneous population of cells displaying a range of morphologies. Most of these cells are epithelial in nature and have a limited capacity to proliferate in culture. Cells originating from the fetal skin, urogenital, respiratory and digestive tracts can be found within the AF. Additional cells from the inner surface of the amniotic membrane add to the mix. The cellular composition of AF changes with gestation, coinciding with the maturation of the fetus [20]. Inappropriate cell types, such as neural cells, can sometimes populate AF in cases of fetal anomalies [21]. The multitude of cell types existing within the AF lead to the hypothesis that stem cells might also be present. In fact, stem cells within AF were first isolated and described in 1993 by Toricelli et al. Cells with the characteristics of HSCs were isolated from human AF at 7–12 weeks of gestation [20]. This finding inspired further efforts to isolate additional stem cell populations from the AF.
The presence of mesenchymal cells in AF had been suggested for a number of decades, but the existence of a mesenchymal, non-hematopoietic, stem cell population has only recently been reported [22]. Amniotic fluid mesenchymal stem cells (AF-MSCs) were first isolated and characterized in 2003. AF-MSCs were initially isolated from human amniocentesis samples based on their preferential adherence to tissue culture plastic. These cells expressed a number of mesenchymal cell surface markers, including CD90 and CD105. Following in vitro expansion, the isolated cells were capable of differentiating in vitro into fibroblasts, adipocytes and osteoblasts. AF-MSCs can be isolated from as little as 2 ml of extracted AF from second trimester pregnancies, and expanded to >180 million cells within 4 weeks. This proliferative capacity meets or exceeds that described for adult human MSCs, suggesting that AF-MSCs may be particularly well suited for procedures requiring large numbers of donor cells [23].
Subsequent works have further characterized putative stem cell populations isolated from AF. Prusa et al. demonstrated the expression of Oct4 within a subset of AF cells. This is important, as Oct4 expression is associated with pluripotent cells such as embryonic germ cells and ES cells [24]. Demonstration of proliferation within this population further suggests that pluripotent stem cells can be both isolated and propagated from the AF of humans. Other groups have provided evidence that AF-MSCs express both mesodermal and ectodermal gene products [25, 26]. This is consistent with the emerging concept that stem cell populations exist in a multidifferentiated state. In the most comprehensive study to date, De Coppi et al. have examined the potential of amniotic fluid-derived stem cells (AFS cells) isolated from rodents and humans. Employing immunoselection, AF cells expressing the cell surface antigen c-Kit were purified from primary amniocentesis cultures. Isolated cells grew rapidly in culture and were capable of more than 250 population doublings. This demonstrated proliferative capacity far exceeds the Hayflick limit of 50 doublings established for most cultured somatic cells. Importantly, AFS cells display a normal karyotype and maintain telomere length during long-term culture. This latter attribute facilitated the establishment of clonal lines from AFS cells, necessary to establish ‘stemness’of a population. Clonal AFS lines differentiated in vitro to putative adipocytes, endothelial cells, hepatocytes, osteocytes, myocytes and neurons, derivatives of all germ layers (Fig. 2). This broad plasticity appeared to be a general attribute of the selected cells: nineteen different amniocentesis cultures yielded multipotent AFS cell clonal lines [27]. A more recent report has provided evidence of in vitro chondrogenic differentiation of AFS cells, providing further evidence of the plasticity and clinical potential of cells isolated from the AF [28].
2.
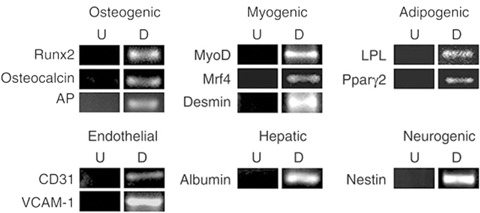
Amniotic fluid-derived stem cells are multipotent in vitro. RT-PCR analysis of mRNAs for lineages indicated using a retrovirally marked second round subclone of AFS cells. U: Control undifferentiated cells. D: cells maintained under conditions to promote osteogenic (8 days), myogenic (8 days), adipogenic (16 days), endothelial (8 days), hepatic (45 days), neurogenic (8 days) differentiation. Reprinted with permission Nature Biotechnology 25, 100–6 (2007).
Most clinical scenarios dictate that stem cells isolated from AF will need to be stored unaltered until they are needed. A recent study has demonstrated that AF cells cryopreseved for decades remain viable and proliferate following revival. Proliferating cells express the stem cell markers Oct4 and Rex1, but did not appear to express telomerase. In vitro differentiation of the revived AF cells to osteoblast- and neuron-like cells provides evidence that potency is maintained despite long-term storage [29].
Most relevant to ultimate clinical application, AF cells have been transplanted into animal models of disease. AF cells injected into the ischemic myocardium did not differentiate to cardiomyocytes, but did participate in neovascularization [30]. However, a more recent study documented rapid and complete rejection of AFS cells transplanted to a similar ischemic model [31]. More work is needed to resolve these apparently contradictory outcomes.
Survival of transplanted AF cells appears more robust in the brain. AF cells transplanted to the normal or ischemic brain survive and differentiate to neural lineages, predominantly astrocytes [32]. Clonal lines of human AFS cells pre-differentiated into neural cells in vitro engraft into a number of brain areas following transplantation to the mouse brain. Interestingly, the same clonal line was capable of bone formation under a different in vivo paradigm, further evidence of the plasticity and therapeutic potential of AFS cells. In all cases, the transplanted cells survived, appeared well behaved and did not show any evidence of tumor formation [27].
Wharton's jelly
The umbilical cord contains two arteries and one vein, protected by a proteoglycan rich connective tissue called Wharton's jelly. Within the abundant extracellular matrix of Wharton's jelly resides a recently described stem cell population called Umbilical Cord Matrix Stem Cells (UCMSCs). The cells are present in relatively high numbers, with an average of 400,000 cells isolated per umbilical cord [33]. This is significantly greater than the number of MSCs that can be routinely isolated from adult bone marrow, emphasizing a primary advantage of stem cell harvest from extra-embryonic sources. The isolated cells expressed CD29 and CD54, consistent with a mesenchymal cell type, and could be propagated in culture for more than 80 population doublings. UCMSCs expressed several stem cell markers, including c-Kit, Nanog, Oct4 and Sox2 [34, 35]. In vitro, the cells were capable of differentiation to multiple mesodermal cell types, including fat, bone and skeletal muscle [36, 37]. More surprisingly, defined in vitro treatments favored differentiation to putative cardiomyocytes and neurons [34, 37]. Generation of clinically important dopaminergic neurons has also been reported [38].
In a more recent study, Karahuseyinoglu et al. have demonstrated the existence of at least two apparently distinct progenitor cell populations within the umbilical cord matrix. Initially distinguished based on morphology, these type 1 and type 2 cells can be further identified by their differential expression of vimentin and cytokeratins [33]. Both populations of cells are multipotent, capable of differentiation to fat, bone and cartilage. Comparison to prototypical adult bone marrow stromal cells (MSCs) has revealed interesting differences. Adult MSCs appear to be more adept at in vitro differentiation to adipocytes, demonstrating more rapid lipid accumulation and attaining morphologic maturity more readily than UCMSCs. In contrast, UCMSCs were far more capable of chondogenic differentiation than adult MSCs (Fig. 3). Grown in pellet cultures with chondrogenic media, the UCMSCs formed tightly compacted spheres with a smooth outer surface. The spheres stained for mucopolysaccharides and collagen II, consistent with chondrogenic differentiation. Staining was more intense than that demonstrated for adult MSCs grown under identical conditions. These findings suggest that UCMSCs may be prime candidates for cartilage repair in future clinical applications.
3.
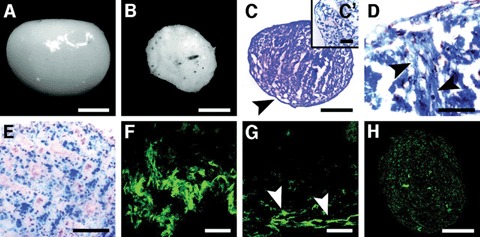
Chondrogenically induced human umbilical cord stromal cells (HUCSCs) formed tiny cell spheres.(A): A shiny-surfaced cell mass. (B): Non-induced cells formed smaller, bulky cell masses. (C): Toluidine blue stain shows the mucopolysaccharide-rich extracellular matrix (pinkish metachromatic areas) and a clear capsule surrounding the entire sphere (arrowhead). (C’): No metachromasia was noted in irregular masses of non-induced cells. (D): In azan-stained cell masses, collagen fibers were clearly distinguished (arrowheads) among many chondrocytes (nuclei in pale red). (E): In cell masses built by the induction of bone marrow MSCs, cells appeared as small groups. Abundant type II (F) and few type I collagen fibers (G) (arrowheads) were detected in chondrogenically induced HUCSCs. (H): Only a few type II collagen fibers were noted in induced bone marrow MSCs. Scale bars = 50 μm (F, G), 100 μm (D, E), s200 μm (C’), and 500 μm (A–C). Reprinted with permission Stem Cells Vol. 25 No. 2 February 2007, pp.319–31.
Intriguingly, type 1 and type 2 UCMSCs differed in their ability to generate non-mesodermal derivatives. Under defined in vitro conditions type 2 differentiated to putative neurons, while type 1 cells did not. Type 2 cells exposed to induction media extended processes and expressed β-III-tubulin, neurofilament-M (NF-M) and NeuN, consistent with early neuronal differentiation. In contrast, type 1 cells remained unaltered, with no significant morphological changes apparent. In these studies neuronal differentiation of type 2 cells was not followed by consolidation of phenotype, and the cells eventually reverted to a more fibroblastic state. Nevertheless, these results suggest divergent plasticity between umbilical cord matrix stem cell populations, and underscore the importance of further analysis and characterization.
In vivo studies involving the transplantation of UCMSCs are limited, but encouraging. UCMSCs transplanted in a mouse model of severe muscle damage survived for at least 2 weeks. The donor cells expressed proteins consistent with skeletal muscle differentiation and enhanced muscle regeneration [36].
Transplantation of UCMSCs to the brain has also been performed. UCMSCs placed in the ischemic rodent brain improved the neurological function of recipients as compared to non-transplant controls. Donor cells showed evidence of differentiation to neurons, glia and vacular endothelial cells, consistent with the multipotency displayed in vitro. Transplantation promoted blood vessel formation and increased local blood flow in the area of the ischemic lesion. Expression of the neurotrophic factors brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) was also enhanced by stem cell transplantation. These studies suggest that transplanted UCMSCs may provide clinical benefit through multiple pathways [39]. Other work has documented the ability of UCMSCs transplanted to the striatum of hemiparkinsonian rats to ameliorated apomorphine-induced rotations. Importantly, there was no evidence of tumor formation up to 12 weeks post-transplantation [35]. UCMSCs may therefore be useful for the treatment of a number of debilitative neurological disorders.
Placenta
The placenta is a large fetomaternal organ that provides nourishment to the developing fetus. The sheer volume of the placenta as compared to other extra-embryonic structures makes this tissue particularly attractive for the isolation of multipotent cells. For example, a full term human placenta on average weighs more than 590 g, almost 15 times the weight of the average umbilical cord [40]. Commercial ventures aimed at exploiting this vast potential reservoir have recently been announced (http://www.celgene.com).
Placental-derived stem cells (PDSCs) can be obtained from dissociated placental tissue based on plastic adherence, a technique widely employed for the isolation of bone marrow mesenchymal cells. Not surprisingly, PDSCs express numerous mesenchymal surface markers, including CD29 and CD44. In vitro, these cells can substitute for bone marrow-derived MSCs, supporting the growth of exogenous HSCs derived from cord blood [41]. PDSCs display fibroblastic morphologies and express the pluripotency markers Oct4 and Rex1. Similar to bone marrow MSCs, the placental-derived cells exist in a multidifferentiated state- simultaneously expressing ectodermal, mesodermal and endodermal genes. PDSCs are highly proliferative and can be maintained for at least 20 passages in culture. Under defined in vitro conditions, PDSCs differentiated to putative osteoblasts and adipocytes. Importantly, clonal lines established from single cells are capable of self-renewal and are multipotent, indicating that PDSCs represent a true stem cell population [42].
In vitro differentiation of PDSCs is not limited to osteoblasts and adipocytes. Differentiation to mesodermal chondrocyte-like, myocyte-like and ectoder-mal neuron-like derivatives has been reported [43]. More recently, differentiation to endodermal hepato-cyte-like cells has been achieved in vitro[44]. In the latter study, PDSCs were isolated from human afterbirths, dissociated and cultured in serum containing media. Flow cytometry identified the isolated cells as CD90+/CD105+ and CD34−, consistent with a PDSC phenotype. Cells cultured in expansion media were uniformly negative for expression of the hepatocyte markers CK18 and albumin. Exposure to hepatocyte growth factor (HGF) and FGF4 up-regulated the expression of these markers within 1 week of culture, and expression was maintained for at least 4 weeks. Interestingly, differentiation was enhanced when the PDSCs were plated on poly-L-lysine coated dishes, suggesting that both soluble and insoluble factors play a role in hepatocyte differentiation.
Functional properties of mature hepatocytes were also present in the differentiated cells, including internalization of low-density lipoprotein (LDL) (Fig. 4). Control cultures of PDSCs not exposed to differentiation cues were incapable of performing this task. Similarly, glycogen storage was only detected in the differentiated cells.
4.
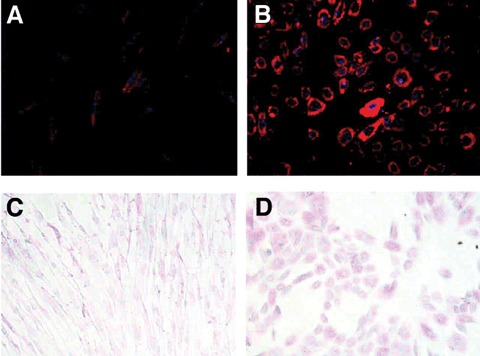
Low-density lipoprotein (LDL) uptake by placenta-derived multipotent cell (PDMC)-derived hepatocyte-like cells. Undifferentiated cells (A) and PDMCs cultured on poly-L-lysine-coated dishes with hepatocyte growth factor (HGF) and fibroblast growth factor-4 (FGF-4) for 14 days (B) were incubated with Dil-acil-LDL. The labeled cells were counter-stained with 4’,6-diamino-2-phenylindole and photographed by fluorescence microscopy (magnification x200). Periodic acid-Schiff (PAS) staining of undifferentiated and differentiated PDMCs. PDMCs were cultured on poly-L-lysine-coated dishes with HGF and FGF-4 for 14 days. PAS staining was described in Materials and methods. (C) Undifferentiated PDMCs. (D) PDMC-derived hepatocyte-like cells (magnification x100). Reprinted with permission Stem Cells Vol. 24 No. 7 July 2006, pp.1759–68.
Together, these results demonstrate PDSC differentiation to mesodermal, ectodermal and endodermal cell types, suggesting plasticity rivaling that of ES cells.
Clinical utility of stem cell populations often implies transplantation of individual cell, either undifferentiated or pre-differentiated in vitro. A complimentary approach is the ex vivo engineering of complex biological structures. PDSCs have been employed in the latter scenario to construct artificial heart valves. PDSCS seeded onto biodegradable scaffolds generated complex structures with mechanical properties similar to native heart valves [45]. Such bioengineering approaches are not unique to PDSCs and could enhance the utility fetal stem cell populations in general.
In vivo studies have shown that PDSCs stereotactically implanted into the intact adult rat brain persisted for up to 3 months, showed migratory activity and assumed typical neuron-like morphologies. Similarly, transplantation to tissues in the periphery resulted in appropriate differentiation to mesodermal bone and cartilage. In complimentary studies, PDSCs infused into fetal sheep in utero resulted in widespread integration into multiple organ systems, including bone marrow, liver and heart. These in vivo results are consistent with the plasticity ascribed in vitro, and provide further evidence of the potency of PDSCs [46].
Amniotic membrane
The amniotic membrane or amnion, delineates the gestational sac, a highly resilient, transparent, fluid-filled cavity that encompasses a developing fetus during gestation. This structure is generated very early in development and is one of the first recognizable tissues derived from the epiblast. The amnion is an avas-cular structure consisting of three discrete layers: an inner epithelial layer, an interposing, acellular basement membrane and an outer layer of mesodermal cells [47]. This relatively simple structure belies the complexity of its origins. In the human, amniotic epithelial cells (AECs) differentiate from the epiblast at the end of the first week of gestation to form the inner layer of the amniotic membrane. In parallel, extra-embryonic somatopleuric mesodermal cells, derived from the caudal end of the epiblast (in the region of the primitive streak), line the outer surface of the mem-brane. Consequently, the amnion is unique: generated early in development from multipotent cells residing in two distinct areas of the developing blastocyst. During subsequent development, the cells of the amnion are not exposed to the same barrage of signals responsible for gradual fate restriction of cells within the embryo proper. It is postulated that stem cell populations sequestered within the amnion might retain the potency of the epiblast cells from which they arose.
Indeed, several multipotent cells have been isolated from the amnion, including AECs, amniotic mesenchymal cells (AMCs) and amnion-derived stem cells (ADSCs). The populations share characteristics, yet differ in many respects. These differences may in turn endow each population with unique therapeutic advantages. Similar to most fetal stem cells, amnion-derived multipotent cells are thought to be immunoprivileged. Intact amniotic membrane has a long history of clinical utility. Amniotic membrane has been extensively used as a biological dressing to treat chemical and thermal burns. The clinical success of amniotic membrane transplantation is due in part to its immunoprivileged characteristics. If this trait is indeed shared by its constituent stem cell populations they may be particularly well suited for allogenic transplantation strategies.
The best-characterized multipotent cells isolated from the amnion are the AECs, residents of the innermost layer of the gestational sac. In the most extensive study to date, Miki et al. isolated AECs from human afterbirths obtained following cesearian section [48]. In vitro, the AECs displayed epithelial morphologies and grew into a tightly packed, cobblestone monolayer in culture. With time, clusters of cells loosely attached to the adherent monolayer became evident. These loosely adherent cell clusters contained high proportions of putative stem cells. Subsets of AECs were positive for the stem cell surface antigens SSEA-3, SSEA-4, TRA-1–60 and TRA-1–81. In addition, the cells expressed Sox2, Oct4, nanog, Rex1 and FGF4, gene products associated with pluripotent ES cells. Expression of telomerase reverse transcriptase (TERT), another ES cell marker, was not detected. This is consistent with the limited in vitro proliferation reported.
The expression of numerous stem cell markers suggests that AECs may be multipotent; a contention supported by in vitro differentiation studies (Fig. 5). To assess capacity for endodermal differentiation, AECs were grown in the presence of nicotimamide for 7 days. Treated cells initiated the expression of multiple pancreatic genes, including the transcription factor Pax-6 and the hormones glucagon and insulin. Different culture conditions encouraged hepatic differentiation, as demonstrated by expression of albumin and α1-antitrypsin.
5.
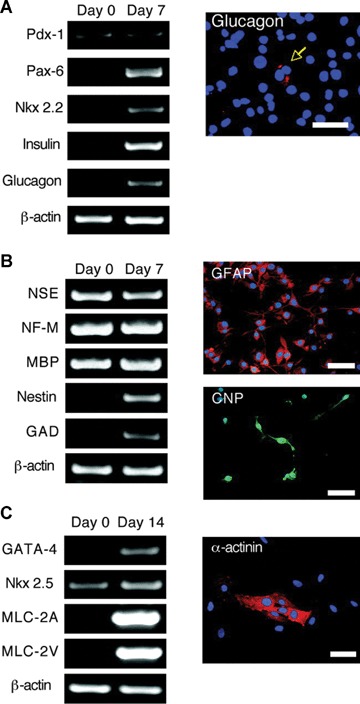
Pancreatic, neural, and cardiac in vitro differentiation of AE cells. (A) Pancreatic differentiation of AE cells. One-step RT-PCR was conducted with the indicated primers on total RNA extracted from cells cultured for 14 days with media supplemented with nicotinamide (10 mM). The expression of the early pancreatic transcription factor PDX-1 and the downstream transcription factors Pax-6 and Nkx 2.2 and the mature hormones insulin and glucagon (Cy3, red) were identified. The photograph shows immunolocalization of glucagon expression with DAPI nuclear counterstaining in blue. Scale bar = 100 μm. (B): Neural differentiation of AE cells. Neural-specific gene expression was examined by one-step RT-PCR. GFAP immunostaining: more than 90% of the cells are GFAP-positive (Cy3, red). Approximately 5–10% of cells are positive for CNP (fluorescein isothiocyanate, green). DAPI nuclear counterstaining (blue). Scale bars = 100 μm. (C) Cardiomyocyte differentiation of AE cells. One-step RT-PCR for cardiomyocyte-specific genes from AE cells cultured for 14 days in basal media supplemented with ascorbic acid 2-phosphate. Immunofluorescent image with an anti-alpha-actinin antibody (Cy-3, red) and DAPI nuclear counterstaining (blue). Scale bar = 50 μm. AE, amniotic epithelial; CNP, cyclic nucleotide phosphodiesterase; DAPI, 4,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; Nkx 2.2, NK2 transcription factor-related locus 2; Pax-6, paired box homeotic gene 6; PDX-1, pancreas duodenum homeobox-1; RT-PCR, reverse transcription-polymerase chain reaction. Reprinted with permission Stem Cells Vol. 23 No. 10 November 2005, pp. 1549–1559.
In vitro differentiation to mesodermal cardiomyocytes was also achieved. Exposure of AECs to ascorbic acid 2-phosphate resulted in the robust expression of the cardiac-specific genes atrial and ventricular myosin light chain 2 (MLC-2A and MLC-2V, respectively).
Lastly, AECs were capable of in vitro differentiation to ectodermal neural cells. Freshly isolated AECs constitutively express a number of neural genes, including neural-specific enolase (NSE), NF-M and myelin basic protein (MBP), perhaps suggesting a predilection for neural differentiation. Exposure to all-trans retinoic acid and FGF4 resulted in adoption of an elongated, neural morphology and enhanced expression of nestin and glutamic acid decarboxylase (GAD). Differentiation to astrocyte-like and oligodendrocyte-like cells was evidenced by expression of glial fibrillary acidic protein (GFAP) and cyclic nucleotide phosphodiesterase (CNP), respectively.
To summarize, the study by Miki et al. strongly suggests that AECs are highly plastic and undergo early stages of differentiation to derivatives of all germ layers. More study is needed to determine whether or not this differentiation can be consolidated, in vitro or in vivo, to render fully functional cells for clinical therapy.
As anticipated, AECs appear to have immunoprivileged characteristics: they do not express HLA-A, B, C and DR antigens, beta 2-microglobulin or MHC Class II antigens [49–51]. In addition, AECs secrete a number of immunosuppressive factors that target the innate and adaptive immune systems, which may support survival following transplantation [52, 53]. Consistent with these observations, donor AECs survive long-term following transplantation to animal models. AECs transplanted to the livers of syngeneic fetal and adult rats integrated and survived for at least 14 days and 30 days, respectively [54, 55]. The focus of these studies was the utility of AECs as vectors to carry therapeutic genes, therefore tissue specific differentiation was not analyzed.
AECs have been examined in particular for their utility in animal models of neurological disease. Encouraged by the inherent synthesis of dopamine by AECs, Kakishita et al. Transplanted cultured human cells into a rat model of Parkinson's disease. Two weeks post-transplantation grafts demonstrated survival without uncontrolled growth. Tyrosine hydroxylase (TH)-immunoreactive cells were present within the grafts and partial amelioration of apomorphine-induced rotations was achieved [56]. In a follow-up study, increased survival of endogenous dopaminer-gic neurons in the midbrain was seen in transplanted animals, suggesting that transplantation of AECs may be a viable approach for the treatment of Parkinson's disease [57]. Transplantation of AECs in animal models of lysosomal storage disease and spinal cord injury has also yielded encouraging results [58, 59].
Amnion mesenchymal cells (AMCs) have also been isolated from the amniotic membrane. These cells arise from the outer layer of the amnion, juxtaposed to the chorion. Similar to AECs, AMCs demonstrate low to no expression of HLA class I and HLA class II, suggesting they would be well tolerated following transplantation. In vitro, AMCs have some features of neuronal progenitor cells, expressing the neural stem cell markers nestin and Musashi1. In vitro differentiation to neuron-like cells may reflect a predisposition for neural differentiation [60]. Other studies have demonstrated that AMCs are capable of differentiation to cardiomyocyte-like cells and putative hepatocytes in vitro and/or in vivo[61].
A recent addition to the mix of multipotent cells derived from the amnion are the ADSCs. These cells were isolated from rat amnion using a tissue explant methodology that discourages the growth of AECs. Cells migrating from the amniotic membrane expressed the cell surface markers CD29 and CD90, suggesting that they originate from the outer somatopleuric layer. Cultured ADSCs express nanog, sox2 and TERT, genes associated with pluripotency and self-renewal. ADSCs can be greatly expanded in vitro and maintained for more than 50 passages. Gene expression analysis demonstrated that populations of ADSCs express a suite of ectodermal, mesodermal and endodermal genes prior to in vitro differ-entiation. When placed in appropriate in vitro conditions, ADSCs are capable of differentiation to osteoblast-, adipocyte-, hepatocyte- and neuron-like cells, derivatives of all three germ layers.
The proliferative capacity of ADSCs has allowed for the establishment of clonal lines derived from established ADSC populations. Clonal ADSC lines express the same combination of cell surface markers as the parental population, and demonstrate robust proliferation in culture. In vitro differentiation of clonal ADSC lines to endodermal, mesodermal and ectodermal derivatives has been achieved. Together these data show that ADSCs are true stem cells, and provide evidence that the amniotic membrane is a rich source of stem cell populations [62].
To date, ADSCs have only been isolated and characterized from rodent sources. Further research is needed to demonstrate that human homologues of ADSCs exist and can be adequately manipulated ex vivo to allow for therapeutic exploitation [62].
Summary and conclusions
Stem cell-based regenerative medicine represents a novel approach to the treatment of disease, potentially revolutionizing medicine. A wide spectrum of disorders affecting diverse organ systems might be targeted through stem cell therapies. While the versatility displayed by adult and ESCs is encouraging, it seems unlikely that any single stem cell population will be ideal for all treatment scenarios. Therefore, a continued effort to identify and characterize novel stem cell populations appears critical for widespread clinical success. In recent years, fetal stem cells isolated from extra-embryonic tissues have been added to the growing list of putative stem cell populations, and may offer some advantages. Isolation from tissues normally discarded at birth facilitates harvest and overcomes ethical concerns. Fetal cells grow well in culture, appear capable of differentiation to multiple cell types and may be less likely to be rejected following transplantation. Additional study is required to fully assess the potential of fetal stem cell populations, however, they seem poised to join embryonic and adult stem cells as participants in the emerging field of regenerative medicine.
References
- 1.Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–4. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 2.Siminovitch L, McCulloch EA, Till JE. The distribution of colony-forming cells among spleen colonies. J Cell Physiol. 1963;62:327–36. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 3.Wu AM, Till JE, Siminovitch L, McCulloch EA. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968;127:455–64. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–9. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 5.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–82. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 6.Stevens LC. Studies on transplantable testicular ter-atomas of strain 129 mice. J Natl Cancer Inst. 1958;20:1257–75. doi: 10.1093/jnci/20.6.1257. [DOI] [PubMed] [Google Scholar]
- 7.Stevens LC. Embryology of testicular teratomas in strain 129 mice. J Natl Cancer Inst. 1959;23:1249–95. [PubMed] [Google Scholar]
- 8.Kleinsmith LJ, Pierce GB., Jr Multipotentiality Of Single Embryonal Carcinoma Cells. Cancer Res. 1964;24:1544–51. [PubMed] [Google Scholar]
- 9.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–9. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 12.Lees JG, Tuch BE. Conversion of embryonic stem cells into pancreatic beta-cell surrogates guided by ontogeny. Regen Med. 2006;1:327–36. doi: 10.2217/17460751.1.3.327. [DOI] [PubMed] [Google Scholar]
- 13.Koller BH, Hagemann LJ, Doetschman T, Hagaman JR, Huang S, Williams PJ, First NL, Maeda N, Smithies O. Germ-line transmission of a planned alteration made in a hypoxanthine phosphoribosyltransferase gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci USA. 1989;86:8927–31. doi: 10.1073/pnas.86.22.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knudtzon S. In vitro growth of granulocytic colonies from circulating cells in human cord blood. Blood. 1974;43:357–61. [PubMed] [Google Scholar]
- 15.Ueno Y, Koizumi S, Yamagami M, Miura M, Taniguchi N. Characterization of hemopoietic stem cells (CFUc) in cord blood. Exp Hematol. 1981;9:716–22. [PubMed] [Google Scholar]
- 16.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–32. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunstein CG, Wagner JE. Umbilical cord blood transplantation and banking. Annu Rev Med. 2006;57:403–17. doi: 10.1146/annurev.med.57.051804.123642. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein G, Toren A, Nagler A. Human umbilical cord blood biology, transplantation and plasticity. Curr Med Chem. 2006;13:1249–59. doi: 10.2174/092986706776872998. [DOI] [PubMed] [Google Scholar]
- 19.Muench MO. In utero transplantation: baby steps towards an effective therapy. Bone Marrow Transplant. 2005;35:537–47. doi: 10.1038/sj.bmt.1704811. [DOI] [PubMed] [Google Scholar]
- 20.Torricelli F, Brizzi L, Bernabei PA, Gheri G, Di Lollo S, Nutini L, Lisi E, Di Tommaso M, Cariati E. Identification of hematopoietic progenitor cells in human amniotic fluid before the 12th week of gestation. Ital J Anat Embryol. 1993;98:119–26. [PubMed] [Google Scholar]
- 21.Cremer M, Schachner M, Cremer T, Schmidt W, Voigtlander T. Demonstration of astrocytes in cultured amniotic fluid cells of three cases with neuraltube defect. Hum Genet. 1981;56:365–70. doi: 10.1007/BF00274694. [DOI] [PubMed] [Google Scholar]
- 22.Macek M, Hurych J, Rezacova D. Letter: Collagen synthesis in long-term amniotic fluid cell cultures. Nature. 1973;243:289–90. doi: 10.1038/243289a0. [DOI] [PubMed] [Google Scholar]
- 23.In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–9. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 24.Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschlager M. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod. 2003;18:1489–93. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- 25.Bossolasco P, Montemurro T, Cova L, Zangrossi S, Calzarossa C, Buiatiotis S, Soligo D, Bosari S, Silani V, Deliliers GL, Rebulla P, Lazzari L. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006;16:329–36. doi: 10.1038/sj.cr.7310043. [DOI] [PubMed] [Google Scholar]
- 26.Tsai MS, Hwang SM, Tsai YL, Cheng FC, Lee JL, Chang YJ. Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol Reprod. 2006;74:545–51. doi: 10.1095/biolreprod.105.046029. [DOI] [PubMed] [Google Scholar]
- 27.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–6. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 28.Kolambkar YM, Peister A, Soker S, Atala A, Guldberg RE. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol. 2007;38:405–13. doi: 10.1007/s10735-007-9118-1. [DOI] [PubMed] [Google Scholar]
- 29.Woodbury D, Kramer BC, Reynolds K, Marcus AJ, Coyne TM, Black IB. Long-term cryopreserved amniocytes retain proliferative capacity and differentiate to ectodermal and mesodermal derivatives in vitro. Mol Reprod Dev. 2006;73:1463–72. doi: 10.1002/mrd.20587. [DOI] [PubMed] [Google Scholar]
- 30.Sartore S, Lenzi M, Angelini A, Chiavegato A, Gasparotto L, De Coppi P, Bianco R, Gerosa G. Amniotic mesenchymal cells autotransplanted in a porcine model of cardiac ischemia do not differentiate to cardiogenic phenotypes. Eur J Cardiothorac Surg. 2005;28:677–84. doi: 10.1016/j.ejcts.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Chiavegato A, Bollini S, Pozzobon M, Callegari A, Gasparotto L, Taiani J, Piccoli M, Lenzini E, Gerosa G, Vendramin I, Cozzi E, Angelini A, Iop L, Zanon GF, Atala A, De Coppi P, Sartore S. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J Mol Cell Cardiol. 2007;42:746–59. doi: 10.1016/j.yjmcc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Cipriani S, Bonini D, Marchina E, Balgkouranidou I, Caimi L, Grassi Zucconi G, Barlati S. Mesenchymal cells from human amniotic fluid survive and migrate after transplantation into adult rat brain. Cell Biol Int. 2007;31:845–50. doi: 10.1016/j.cellbi.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 33.Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319–31. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 35.Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781–92. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 36.Conconi MT, Burra P, Di Liddo R, Calore C, Turetta M, Bellini S, Bo P, Nussdorfer GG, Parnigotto PP. CD105(+) cells from Wharton's jelly show in vitro and in vivo myogenic differentiative potential. Int J Mol Med. 2006;18:1089–96. [PubMed] [Google Scholar]
- 37.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–7. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 38.Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115–24. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 39.Ding DC, Shyu WC, Chiang MF, Lin SZ, Chang YC, Wang HJ, Su CY, Li H. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27:339–53. doi: 10.1016/j.nbd.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Bolisetty S, Koh TH, Hammond S, Panaretto K, Whitehall J. Correlation of umbilical cord weight with birth weight. Arch Dis Child Fetal Neonatal Ed. 2002;86:F140. doi: 10.1136/fn.86.2.F140-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Li C, Jiang X, Zhang S, Wu Y, Liu B, Tang P, Mao N. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol. 2004;32:657–64. doi: 10.1016/j.exphem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–58. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 43.Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, Shun CT, Yen ML, Lee MC, Chen YC. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- 44.Chien CC, Yen BL, Lee FK, Lai TH, Chen YC, Chan SH, Huang HI. In vitro differentiation of human placenta-derived multipotent cells into hepatocyte-like cells. Stem Cells. 2006;24:1759–68. doi: 10.1634/stemcells.2005-0521. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt D, Mol A, Breymann C, Achermann J, Odermatt B, Gossi M, Neuenschwander S, Pretre R, Genoni M, Zund G, Hoerstrup SP. Living autolo-gous heart valves engineered from human prenatally harvested progenitors. Circulation. 2006;114:I125–31. doi: 10.1161/CIRCULATIONAHA.105.001040. [DOI] [PubMed] [Google Scholar]
- 46.Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida-Porada G, Muller HW, Zanjani E, Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoyes AD. Structure and function of the amnion. Obstet Gynecol Annu. 1975;4:1–38. [PubMed] [Google Scholar]
- 48.Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–59. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- 49.Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–5. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- 50.Adinolfi M, Akle CA, McColl I, Fensom AH, Tansley L, Connolly P, Hsi BL, Faulk WP, Travers P, Bodmer WF. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature. 1982;295:325–7. doi: 10.1038/295325a0. [DOI] [PubMed] [Google Scholar]
- 51.Sakuragawa N, Tohyama J, Yamamoto H. Immunostaining of human amniotic epithelial cells: possible use as a transgene carrier in gene therapy for inborn errors of metabolism. Cell Transplant. 1995;4:343–6. doi: 10.1177/096368979500400313. [DOI] [PubMed] [Google Scholar]
- 52.Kamiya K, Wang M, Uchida S, Amano S, Oshika T, Sakuragawa N, Hori J. Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Exp Eye Res. 2005;80:671–9. doi: 10.1016/j.exer.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, Alizadeh H. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:900–7. doi: 10.1167/iovs.04-0495. [DOI] [PubMed] [Google Scholar]
- 54.Nakajima T, Enosawa S, Mitani T, Li XK, Suzuki S, Amemiya H, Koiwai O, Sakuragawa N. Cytological examination of rat amniotic epithelial cells and cell transplantation to the liver. Cell Transplant. 2001;10:423–7. [PubMed] [Google Scholar]
- 55.Takahashi N, Enosawa S, Mitani T, Lu H, Suzuki S, Amemiya H, Amano T, Sakuragawa N. Transplantation of amniotic epithelial cells into fetal rat liver by in utero manipulation. Cell Transplant. 2002;11:443–9. [PubMed] [Google Scholar]
- 56.Kakishita K, Elwan MA, Nakao N, Itakura T, Sakuragawa N. Human amniotic epithelial cells produce dopamine and survive after implantation into the striatum of a rat model of Parkinson's disease: a potential source of donor for transplantation therapy. Exp Neurol. 2000;165:27–34. doi: 10.1006/exnr.2000.7449. [DOI] [PubMed] [Google Scholar]
- 57.Kakishita K, Nakao N, Sakuragawa N, Itakura T. Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6-hydroxydopamine lesions. Brain Res. 2003;980:48–56. doi: 10.1016/s0006-8993(03)02875-0. [DOI] [PubMed] [Google Scholar]
- 58.Kosuga M, Sasaki K, Tanabe A, Li XK, Okawa H, Ogino I, Okuda O, Arai H, Sakuragawa N, Kamata Y, Azuma N, Suzuki S, Yamada M, Okuyama T. Engraftment of genetically engineered amniotic epithelial cells corrects lysosomal storage in multiple areas of the brain in mucopolysaccharidosis type VII mice. Mol Ther. 2001;3:139–48. doi: 10.1006/mthe.2000.0234. [DOI] [PubMed] [Google Scholar]
- 59.Sankar V, Muthusamy R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience. 2003;118:11–7. doi: 10.1016/s0306-4522(02)00929-6. [DOI] [PubMed] [Google Scholar]
- 60.Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- 61.Tamagawa T, Oi S, Ishiwata I, Ishikawa H, Nakamura Y. Differentiation of mesenchymal cells derived from human amniotic membranes into hepatocyte-like cells in vitro. Hum Cell. 2007;20:77–84. doi: 10.1111/j.1749-0774.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 62.Marcus AJ, Coyne TM, Rauch J, Woodbury D, Black IB. Isolation, characterization, and differentiation of stem cells derived from the rat amniotic membrane. Differentiation. 2008;76:130–44. doi: 10.1111/j.1432-0436.2007.00194.x. [DOI] [PubMed] [Google Scholar]


