Abstract
Physiological angiogenesis is essential for development, homeostasis and tissue repair but pathological neovascularization is a major feature of tumours, rheumatoid arthritis and ocular complications. Studies over the last decade have identified γ-secretase, a presenilin-dependent protease, as a key regulator of angiogenesis through: (i) regulated intramembrane proteolysis and transmembrane cleavage of receptors (e.g. VEGFR-1, Notch, ErbB-4, IGFI-R) followed by translocation of the intracellular domain to the nucleus, (ii) translocation of full length membrane-bound receptors to the nucleus (VEGFR-1), (iii) phosphorylation of membrane bound proteins (VEGFR-1 and ErbB-4), (iv) modulation of adherens junctions (cadherin) and regulation of permeability and (v) cleavage of amyloid precursor protein to amyloid-β which is able to regulate the angiogenic process. The γ-secretase-induced translocation of receptors to the nucleus provides an alternative intracellular signalling pathway, which acts as a potent regulator of transcription. γ-secretase is a complex composed of four different integral proteins (presenilin, nicastrin, Aph-1 and Pen-2), which determine the stability, substrate binding, substrate specificity and proteolytic activity of γ-secretase. This seeming complexity allows numerous possibilities for the development of targeted γ-secretase agonists/antagonists, which can specifically regulate the angiogenic process. This review will consider the structure and function of γ-secretase, the growing evidence for its role in angiogenesis and the substrates involved, γ-secretase as a therapeutic target and future challenges in this area.
Keywords: γ-secretase, presenilin, Notch, VEGFR-1, angiogenesis, receptor translocation, amyloid
Introduction
Angiogenesis is one of the most crucial biological processes, which ensures the formation and physiological function of all organs and virtually all tissues in the body [1]. However, aberrant angiogenesis is a major pathological feature of tumours, diabetic retinopathy, rheumatoid arthritis and age-related macular degeneration. Pathology of the vascular system involves the endothelium, which comprises the innermost layer of all blood vessels, cardiac valves and several other body cavities. The endothelial cells are responsible for maintaining vessel patency, preventing thrombosis, and relaxation and contraction of vessels. The migration, proliferation and organization of endothelial cells and their precursors into tubules defines the process of angiogenesis, whether it be during development or during adult reparative or pathological processes [2]. Furthermore, the classic perception that adult angiogenesis develops solely from existing endothelial cells has been challenged by us and others who have shown that hematopoiet-ic stem cells (HSCs) can contribute up to 26% of the endothelial cells in a new vessel [3–5]. It is now quite evident that there is a plethora of pro- and anti-angiogenic factors that regulate the vasculature and are involved in the development and progression of neovascularization [6, 7]. Furthermore, the spatio-temporal balance of these pro- and anti-angiogenic factors is critical in determining whether vascular home-ostasis or pathology occurs [8]. The collective evidence suggests that the vascular endothelial growth factor (VEGF) family is critical for angiogenesis [6, 7, 9]. Increasing VEGF in animal models promotes neovascularization and this can be reversed by neutralizing VEGF or its receptors [10–12]. VEGF is hypoxia-inducible and thus is dramatically up-regulated by the hypoxic environment associated with tumours and diabetic retinopathy [9, 12, 13]. Moreover, treatment of patients with tumours or choroidal neovascularization with VEGF inhibitors such as Avastin or Lucentis significantly reduces the aberrant angiogenesis resulting in the regression of tumours and, in the case of choroidal neovascularization, vessel regression and improved vision [11, 14, 15]. However, many other factors including Delta/Notch, insulin-like growth factor-I (IGF-I), fibroblast growth factor (FGF), angiopoietin and placenta growth factor (PlGF) are also, given the correct tissue environment, strongly proangiogenic. It is also important to note that these factors are constitutively expressed in most tissues under physiological conditions and thus must play an important role in homeostasis. Their pro-angiogenic potential is regulated by a fine balance of anti-angiogenic factors including pigment epithelium-derived factor (PEDF), IGF-binding protein-3 (IGFBP-3) [16] and thrombospondin [2]. When we think of receptor activation by these angiogenic factors we typically associate this with tyrosine kinase activation and downstream intracellular signalling driving a cascade of protein phosphorylation events [17]. However, it has long been recognized, and often dismissed, that full length receptors and receptor fragments are present within the cytosol and nucleus [18, 19]. It is now apparent that these translocated receptors provide an alternative signalling pathway, bind to transcription factors and associate with adherens junctions to regulate intercellular permeability [19]. Furthermore, despite the presence of numerous membrane receptors (e.g. VEGFR-1, VEGFR-2, CXCR4) on HSCs little is known about their down stream signalling [5].
This review will focus on the intracellular translocation of receptors in angiogenesis and will carefully examine the contribution of γ-secretase and its active proteolytic component, presenilin, which plays a critical role in this process.
Regulated intramembrane proteolysis
Over the last decade regulated intramembrane proteolysis (RIP) has been revealed as a novel, but highly conserved mechanism in cell signalling (reviewed in [20–22]). RIP results in the release of extracellular/luminal and/or cytoplasmic domains from transmembrane proteins. These cleaved fragments have been shown to act as biological effectors at other sites within the cell. For example, the intracellular domain of Notch released by RIP translocates to the nucleus where it acts as a transcriptional activator [23] (Fig. 1). RIP is mediated by at least three distinct families of evolutionarily conserved intramembrane proteases, which cleave substrates usually within their transmembrane domains [22]:(1) the presenilin-type aspartyl proteases, including the presenilin-dependent γ-secretase and the signal peptide peptidase (SPP) that cleave tyrosine kinase receptors and the HLA-E epitope [24–25]; (2) the site-2 protease (S2P) family, zinc-metalloproteases that cleave and activate sterol regulatory element binding proteins (SREBPs) [26]; (3) the rhomboid serine proteases that cleave transmembrane ligand substrates including the main EGF ligand Spitz [27]. Presenilin-dependent γ-secretase and rhomboids are believed to cleave only type-I membrane substrates (single pass transmembrane proteins with a cytoplasmic C-terminus and an extracellular or luminal N-terminus), while S2P and SPP cleave type-II membrane proteins (the N- and C-termini in type II proteins are the reverse orientation of type-I proteins). Cleavage of multipass proteins is under debate but there is recent evidence that this might occur with the CXCR4 receptor [28].
1.
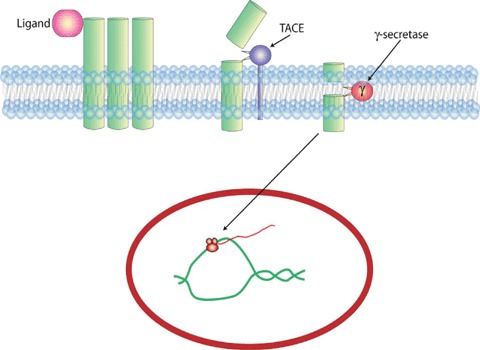
Role of γ-secretase in growth factor receptor signalling. Ligand binding induces ectodomain shedding of the receptor allowing for the second intramembrane cleavage that releases the active cytoplasmic domain, which in the case of Notch translocates to the nucleus. Modified from Landman and Kim [22].
γ-Secretase
Structure
γ-secretase is a complex composed of four different integral membrane proteins: presenilin (PS), nicastrin, Aph-1, and Pen-2 (Fig. 2) [29, 30]. The most studied component of the γ-secretase complex is presenilin, which is an integral enzyme in the cleavage of amyloid precursor protein and contributes to the accumulation of amyloid-β peptide in Alzheimer's disease. Activation of PS is dependent on its endoproteolysis into an N-terminal fragment (NTF) and C-terminal fragment (CTF) [30, 31]. Nicastrin has recently been described as ‘the gatekeeper of the γ-secretase complex’[32]. The extracellular orientated domain of nicastrin is essential for substrate recognition by the γ-secretase complex and nicastrin binding to the substrate is required before presenilin can exert its proteolytic activity. This extracellular domain of nicastrin usually binds to specific amino terminal residues of the transmembrane substrate. Thus nicastrin facilitates presenilin-dependent RIP of the transmembrane fragment [33]. Of the two other proteins, which constitute γ-secretase, Aph-1 is believed to be a scaffolding protein and Pen-2 appears to regulate PS activity. Assembly of the γ-secretase complex begins in the endoplasmic reticulum and is concluded after translocation of the four proteins to the cell membrane [30].
2.
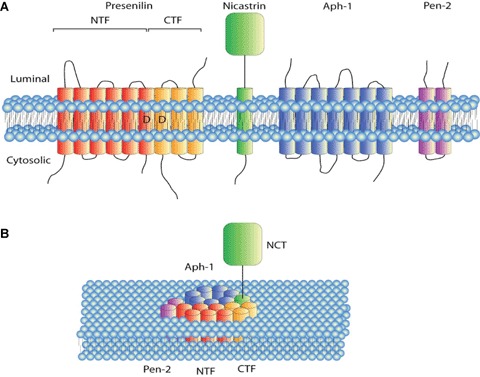
Components and assembly of the γ-secretase complex. (A) γ-secretase is composed of four different integral membrane proteins; presenilin, nicastrin, Aph-1 and Pen-2. Presenilin undergoes endoproteolysis into an N-terminal fragment (NTF) and C-terminal fragment (CTF) that remain associated. (B) Model for how the components of γ-secretase are arranged within the active protease complex. Modified from Wolfe [30].
Although γ-secretase is ubiquitously expressed in a wide range of cell types which themselves express multiple substrates ranging from signalling receptors to junctional complexes there is clear evidence of substrate specificity. However, exactly how this specificity is achieved remains unclear [34]. Six variants of γ-secretase exist based upon different combinations of the two PS and three different Aph-1 proteins. Furthermore, the complex contains a number of different binding, docking and active enzyme sites that exhibit varying degrees of substrate specificity (see Wolfe [30]). There also appears to be allosteric modulation of γ-secretase activity through binding of extrinsic factors to protein components in the complex (see Wolfe [30]). Thus the extrinsic components, in combination with core complexes, design functional variants of γ-secretase that have substrate specificity. Even when active γ-secretase is present in the endothelial cell membrane, transmembrane receptors are not cleaved until they bind to their respective ligand which presumably results in a change in receptor conformation that makes either a docking or cleavage site available on the receptor for γ-secretase.
Although a great deal of progress has been made in identifying the components of the γ-secretase complex, the endogenous regulatory mechanism of γ-secretase expression/activity is only now being elucidated. We have recently demonstrated that the potent anti-angiogenic inhibitor pigment epithelial-derived factor (PEDF) promotes translocation of PS and nicastrin from the cell membrane and up-regulates γ-secretase activity [31]. It is conceivable that this involves kinase-dependent pathways since Kim and colleagues [35] have shown that γ-secretase is endogenously regulated via the extracellular signal regulated MAP kinase (ERK) MAPK pathway. Information on the transcriptional regulation of the proteins of the γ-secretase complex is limited but there is evidence that the PS-1 promoter has Ets binding domains [36], that both PS and Pen-2 are transcriptionally regulated by cyclic AMP-response element binding protein (CREB) [37] and ubiquilin can regulate the post-transcriptional modification of PS, Pen-2 and nicastrin [38].
Substrate cleavage
Presenilin-dependent regulated intramembrane proteolysis has been extensively studied in the context of Notch signalling. Cleavage of transmembrane proteins by presenilin leads to the generation of biologically active protein fragments that signal at various locations within the cell including the nucleus and the adherens junctions (reviewed in [22]). RIP of membrane receptors usually requires two sequential proteolytic cleavages, carried out by different proteases. The first cleavage occurs at the cell surface, and usually leads to the shedding of the protein's extracellular or luminal domain. The initiation of this first cleavage is normally in response to ligand binding to the receptor presumably causing a conformational change and exposing a cleavage site [26, 39, 40]. This cleavage of the ectodomain is typically carried out by proteases of the disintegrin and metalloprotease (ADAM) families, whose active site domain is located in the extracellular/luminal space [22, 41]. This primary cleavage shortens the ectodomain (usually to less than 30 amino acids), which allows the transmembrane cleavage to occur via the action of γ-secretase. The active cytoplasmic domain, which is released subsequently, translocates within the cell where it can either (1) locate to the nucleus where it serves to regulate gene expression through an association with DNA bound cofactors, as reported for Notch and ErbB-4 (reviewed in [42, 43]) or (2) bind to cytsolic proteins and regulate their action (e.g. p75NTR[44] and E-cadherin [45]). Thus, γ-secretase-dependent proteolysis can regulate intracellular signalling pathways via two major mechanisms: elimination of critical domains of the membrane receptors or generation of bioactive intracellular domains. γ-secretase-dependent proteolysis can cleave a number of membrane receptors (see Parks and Curtis) [46]) including ErbB4 [41] to modulate proliferation and differentiation, CD44 that regulates transcriptional activity [47], p75 neurotrophin receptor to regulate cell survival and transcription [48], E-cadherin to change permeability [45], LDL-receptor to regulate transcription [49], VEGFR-1 to regulate angiogenesis [31] and IGF-IR with as yet undetermined functions and Tie 1 [50, 51].
Is there more to γ-secretase than regulated intramembrane proteolysis?
Receptor translocation
Growth factor receptors localized to the plasma membrane are not restricted to the cell surface. Tyrosine kinase receptors can, usually following binding of their concomitant ligand, be rapidly internalized through clathrin-coated pits and enter the endocytic pathway from which they are degraded in lysosomes or be recycled back to the cell surface [19]. However, there is accumulating evidence that membrane bound receptors have an alternative signalling pathway, which involves either the intact full length receptor or a cleaved cytoplasmic domain fragment being translocated to the nucleus. Full length EGFR, FGFR, VEGFR-1 and VEGFR2 appear to accumulate in the nucleus as an intact receptor [18, 31, 52, 53]. Cytosolic fragments of Notch, ErbB-4 and VEGFR-1 have all been localized to the nucleus. γ-secretase appears critical for the transmembrane cleavage of receptors since γ-secretase inhibitors prevent cleavage and subsequent accumulation of receptors in the nucleus. However, we have recently demonstrated that γ-secretase can also regulate the nuclear translocation of intact receptors [31]. Although, the mechanism by which this occurs has yet to be elucidated. The nuclear localization of membrane bound receptors appears to regulate gene expression either by (i) acting directly as a transcription factor (FGFR-1 acts as a transcription factor at the FGF-2 promoter) [54], (ii) binding to and regulating the activity of transcription factors, (iii) induction of early response and cell cycle genes [55] or (iv) co-transport of other molecules into the nucleus (ErbB-1 has been reported to transport the tyrosine phosphorylated transcription factor STAT-3 from the cytosol into the nucleus) [56].
Presenilin-binding proteins
Presenilin/γ-secretase has been shown to date to interact with over 30 proteins but the significance of this association remains unclear (reviewed in [46]. The functions of the diverse range of binding proteins include vesicle trafficking [57], apoptotic regulation and proteosomal degradation [58], ubiquitination [59], endosome recycling [58] and calcium regulation [60]. It is highly likely that many of these proteins will regulate presenilin levels/activity or the stability of the γ-secretase complex. For example, the calcium-binding protein calsenelin can promote γ-secretase-mediated cleavage of Notch and APP [60]. However, it is possible that presenilin binding can lead to a functional change in the bound protein, possibly through phosphorylation.
Phosphorylation
There is some suggestion in the literature that PS regulates the phosphorylation state of a variety of proteins including retinoblastoma protein [61], Src [62], ErbB-4 [63, 64] and VEGFR-1 [31]. Whether this is a direct or indirect phosphorylation is unclear but PS1 is known to be able to activate PI3K leading to downstream phosphorylation/dephosphorylation [65, 66]. There is also evidence that presenilin can bind and phosphorylate proteins both alone and as part of the γ-secretase complex. PS-1 is able to promote cell survival by activating the PI3K/Akt pathway [5, 40], phosphorylating glycogen synthase kinase-3 (GSK-3) and suppressing GSK-3-dependent phosphorylation of tau [65]. The function of PS-1 was not affected by γ-secretase inhibition. However, Cai and colleagues [31] observed that γ-secretase is able to regulate phosphorylation of VEGFR-1. γ-secretase induction greatly reduced VEGFR-1 phosphorylation and phosphorylation was least when γ-secretase was induced in combination with VEGF. This could be blocked by inhibition of γ-secretase. It would thus appear that presenilin can mediate protein function either alone or as part of the γ-secretase complex.
Role of γ-secretase in angiogenesis
We and others have identified a potential role for γ-secretase in vasculogenesis and angiogenesis [31, 67–72]. Abnormal blood vessel development occurs in mice lacking presenilin-1 [68]. Presenilin-1 controls the growth and differentiation of endothelial progenitor cells [73]. An Ets domain is located on the PS promoter and Ets is known to be a key transcriptional regulator of vasculogenesis and angiogenesis [74]. Furthermore, the transcriptional elements Ets and CREB are known to regulate expression of PS and these elements are themselves regulated by a variety of growth factors including VEGF [74, 75]. Aph1A (one of the three Aph1 isoforms that contribute to the stability of the γ-secretase complex) knockout mice failed to develop an organized vascular system [70]. We have shown that γ-secretase is able to regulate VEGFR-2-induced vascular permeability and angiogenesis in retinal microvascular endothelial cells via VEGFR-1 cleavage and translocation of the C-terminal domain and full length VEGFR-1 [31]. A role for γ-secretase in retinal neovascularization is further supported by the observation that PS expression is up-regulated in the OIR model of retinopathy of prematurity [67]. Notch, a γ-secretase regulated receptor, is an important modulator of endothelial behavior. Furthermore, there is crosstalk between Notch and VEGF receptors and Notch is known to regulate both VEGF and VEGFR expression in a variety of vascular and nonvascular cells.
Notch
Notch receptor signalling, unlike many other transmembrane signalling receptors that use protein phosphorylation cascades to transmit intracellular signals, relies on cleavage of its intracellular domain and subsequent translocation to bind directly with downstream transcriptional regulators [20]. Notch undergoes a typical regulated intramembrane proteolysis. Upon ligand binding to Delta or Jagged, the ectodomain is cleaved by TACE/ADAM metalloproteinases. The resultant membrane-anchored c-terminal fragment is subsequently further cleaved by γ-secretase to liberate the Notch intracellular domain, which translocates to the nucleus. The best characterized Notch targets are transcriptional repressors of the Hes and Hey families that act by negatively regulating expression of target genes such as tissue-specific transcriptional activators [71, 76, 77].
Although Notch is classically considered to be a regulator of cell differentiation and pattern formation there is considerable evidence in the literature to support its role in developmental, adult and pathological angiogenesis [71]. Of the Notch receptors, only Notch 1 and Notch 4 are expressed in vascular endothelial cells [78]. There is a close association between Notch and VEGF. Treatment of endothelial cells with VEGF increases γ-secretase activity, Notch 1 cleavage and Hes-1 expression while inhibition of γ-secretase leads to decreased angiogenesis and blocks VEGF-induced endothelial cell proliferation, migration and survival. For example, both Notch and its ligand Delta-like 4 (Dll4) are induced in vascular endothelial cells by VEGF [79], Notch activation down-regulates VEGFR-2 expression on vascular endothelial cells [77] and Notch activation can regulate VEGF expression [80]. γ-secretase is also critical for cell-autonomous notch signalling and regulates endothelial cell branching and proliferation during vascular tubulogenesis [69].
In the last few years, it has become apparent that the activation of Notch by Dll4 regulates the formation of appropriate numbers of tip cells to control vessel sprouting and branching [81]. Inhibition of Notch signalling using γ-secretase inhibitors, inactivation of one allele of Dll4 or genetic deletion of Notch 1 all promoted increased numbers of tip cells. VEGF is essential for the induction of endothelial tip cells and appears to be able to up-regulate Dll4 which is also increased under hypoxia [72]. Furthermore, Dll4 is able to reduce VEGFR-2 expression in endothelial cells thus providing a negative feedback loop for VEGF-induced angiogenesis. Dll4 overexpressing cells show significant induction of Hey-2, which is one of the transcription factors that mediates the Dll4 induced Notch activation [72]. γ-secretase inhibition blocked Dll4 activation of Notch and reconstituted VEGFR-2 expression. It also appears that Dll4/Notch 4 interactions lead to an up-regulation of ephrin B2 (a regulator of cell segregation) and down-regulation of its receptor EphB4, which was blocked by γ-secre-tase inhibition [82]. Presenilin processing of ephrinB itself regulates EphB-induced Src phosphorylation and signalling [62]. It now appears that Dll4 and Notch can regulate multiple angiogenic pathways in HUVEC including VEGF, PlGF, FGF and HGF as well as facilitating up-regulation of VEGFR-1 allowing a continued response to PlGF [83]. Thurston et al. report a paradox for Dll4 activation of Notch, which leads to more tumour vessels but less tumour growth [84]. Thus Dll4, Notch and γ-secretase all appear to play a critical role in VEGF-induced angiogenesis.
Vascular endothelial growth factor receptor-1 (VEGFR-1)
Although VEGFR-2 has long been considered the major effector in VEGF-induced angiogenesis, there is a growing body of evidence that VEGFR-1 is a potent negative regulator of VEGFR-2 [12]. Mice expressing the VEGFR-1 extracellular and transmembrane domains but lacking the tyrosine kinase domain (VEGFR-1 TK(–) mice) develop an essentially normal vasculature [85], which suggests that the VEGFR-1 tyrosine kinase domain appears to be dispensable and that VEGFR-1 may signal via an alternative pathway or pathways. This process may also in part be dependent on the extracellular matrix since Nozaki and colleagues [86] observed that choroidal neovascularization induced by injury was increased by excess VEGF-A before injury but was suppressed by VEGF-A after injury. This antiangiogenic effect was mediated via VEGFR-1 activation that was silenced by secreted protein, acidic and rich in cysteine (SPARC). Thus, the mechanism by which VEGFR-1 supports angiogenesis is complex and likely involves several different mechanisms with different levels of specificity and controls.
We have recently reported that γ-secretase is expressed in retinal microvascular endothelial cells and is able to elicit regulated intramembrane proteolysis of VEGFR-1 as well as translocation and phosphorylation of the full length receptor [31]. The translocation of the cleaved intracellular domain of VEGFR-1 as well as full length VEGFR-1 acts as a potent negative regulator of VEGFR-2 driven neovascularization. Pigment epithelial-derived factor (PEDF), a potent anti-angiogenic factor, induces a greater than 8-fold increase in γ-secretase activity in microvascular endothelial cells. This increase was not due to up-regulated gene expression but reflected mobilization of endogenous γ-secretase constituents to the plasma membrane and an increase in the ratio of the active PS-1 C-terminal fragment (CTF) to inactive full length within 30 min. Once complex formation was complete, both PS-1 and nicastrin co-localized to membrane-bound VEGFR-1 but not VEGFR-2. From studies in other signalling systems, we believe that PS-1 is bound to nicastrin, which in turn binds to VEGFR1. We conclude from these observations that VEGFR-1 will have sequence domains for both nicastrin binding and PS-1 cleavage. Computational modelling studies show that the VEGFR-1 transmembrane domain is highly conserved and contains a valine site (‘cvaatlfwllltlf’), which could serve as a substrate for PS-1.
γ-secretase induction in the presence of VEGF resulted in the appearance of an intracellular cleaved 80 kD C-terminal domain of VEGFR-1 within 1 hr after induction. Interestingly, even though we could induce the formation of an active γ-secretase complex in the plasma membrane, VEGF binding to VEGFR-1 was essential before cleavage occured leading us to hypothesize that a change in VEGFR-1 conformation occurs which in turn exposes the conserved valine residue to PS-1. A challenging thought is that the 80 kD C-terminal fragments of VEGFR-1 released into the cytosol could act to inhibit VEGFR-2 phosphorylation and that some remnant 100 kD N-terminal domains are released extracellularly to involve sequestration of the VEGF similar to the soluble form of VEGFR-1.
Subcellular fractionation detected full length 180 kD VEGFR-1 in the membrane, cytoskeletal and the nuclear fractions plus an additional 80 kD (cleaved) C-terminal domain of VEGFR-1 was observed in the cytosol fraction of γ-secretase-induced cells. The cleavage and translocation of the 80 kD (cleaved) C-terminal domain of VEGFR-1 was blocked by γ-secretase inhibition. The presence of the N-terminal (extra-cellular) domain of VEGFR-1 confirmed the presence of full length VEGFR-1 in membrane, cytoskeletal, and nuclear fractions, but not in the cytosolic fraction.
γ-secretase induction also regulated the phosphorylation state of VEGFR-1 and its translocated domains. Immunoprecipitated phosphorylated proteins and Western blotting for VEGFR-1 demonstrated that VEGF induced an increase in autophosphorylation of VEGFR-1 compared to control with bands at 250 and 180 kD in whole cell lysates. γ-secretase induction greatly reduced VEGFR-1 phosphorylation in both the 250 and 180 kD bands and dephosphorylation was greatest‘when γ-secretase was induced by PEDF in combination with VEGF. Analysis of the subcellular fractions demonstrated that VEGF induced an increase in auto-phosphorylation of full length VEGFR-1 in membrane, cytoskeleton and nuclear fractions. Interestingly, γ-secretase induction almost completely blocked the tyrosine phosphorylation of full length VEGFR-1 in all fractions regardless of whether the endothelial cells were cultured in the presence or absence of VEGF. Phosphorylation of the cleaved 80 kD VEGFR-1 C-terminal domain was not observed. γ-secretase inhibition prevented dephosphorylation confirming a critical role for γ-secretase induction in VEGFR-1 cell signalling. Significant dephosphorylation of both 180 and 250 kD bands occured after 1 hr of γ-secretase induction by PEDF addition. Thus up-regulation of γ-secretase plays a key role in VEGFR-1 negative regulation of VEGFR-2.
Insulin-like growth factor-I receptor (IGF-1R)
IGF-I is a potent pro-angiogenic factor which plays an important role in aberrant neovascularization associated with diabetic retinopathy [6, 9]. Its signalling is primarily via IGF-IR. McElroy and colleagues have recently reported that IGF-IR undergoes regulated intramembrane proteolysis in the human embryonic kidney cell line (HEK 293T) [50]. A metalloproteinase-dependant ectodomain-shedding event generates a ∼52 kD IGF-IR C-terminal domain which is sequentially cleaved by γ-secretase, liberating a ∼50 kD intracellular domain. Inhibition of γ-secretase using Compound E prevented cleavage of the C-terminal domain. Sequence alignments of the transmembrane domains of known γ-secretase substrates and IGF-IR demonstrated conservation of a valine residue at a corresponding position. Although the authors did not undertake functional studies it is reasonable to conclude that this may represent a signalling pathway independent of tyrosine kinase activation and that such a pathway is likely to operate in vascular endothelial cells.
ErbB4
The Erb, or epidermal growth factor receptor, family of transmembrane tyrosine kinase receptors are implicated in a range of cellular responses including the regulation of angiogenesis both in vitro and in vivo[87]. One member of the EGF receptor family, ErbB-4 is known to undergo intramembrane proteolysis to generate an 80 kD intracellular domain which relocates to the nuclear compartment where it regulates gene transcription [88]. It has been elegantly demonstrated that, similar to the Notch system, upon binding to its ligand (heregulin, neuregulin, betacellulin) that the ErbB-4 ectodomain is cleaved by a metalloprotease and that γ-secretase elicits a transmem-brane cleavage thus releasing an intracellular domain [42, 89]. This regulated intramembrane proteolysis and the appearance of a cleaved fragment was blocked by inhibition of γ-secretase or a dominant negative presenilin mutant [42]. The nuclear binding partners for the intracellular domain of ErbB-4 have yet to be fully elucidated. Arasada and Carpenter reported that the cleaved intracellular domain associates with and tyrosine phosphorylates MdM2, a protein that is predominantly localized to the nucleus and regulates p53 levels [63]. Omerovic and colleagues have identified the transcriptional transactivator YAP65 (yes-associated protein) as a binding partner for ErbB-4 using a yeast two hybrid screen [88]. Interestingly, it appears that γ-secretase-dependent processing of β-amyloid precursor protein (see APP section below) can itself regulate EGF receptor expression [90] suggesting a highly context-dependent regulation of the EGF receptor signalling system.
In addition to its more general role in regulating cell proliferation and differentiation it appears that ErbB-4 can play an important role in tumour angiogenesis [87, 91–96]. Activation of ErbB-4 induces its phosphorylation and promotes HUVEC proliferation and migration. Signalling appears to involve the MAPK and PI3K/Akt pathways [93]. Interestingly, ErbB-4 colocalizes with ErbB-1 in HUVEC and aortic endothelial cells which opens the possibility that ErbB-4 may facilitate the entry of ErbB-1 into the nucleus [92]. However, once in the nucleus ErbB-4 locates to the extra-nucleolar region while ErbB-1 is localized to the nucleoli. It is also clear that tumour angiogenesis is likely to be dependent on the differential regulation of distinct ErbB homo- and heterodimers. EGFR/ErbB-4, ErbB-2/ErbB-4 and ErbB-3/ErbB-4 can all induce VEGF expression although this induction is not as great as for EGFR/ErbB-2 and ErbB-2/ErbB-3 [87]. Stimulation of HUVEC with recombinant neuregulin-β (an ErbB-3/ErbB-4 ligand) induces marked in vitro and in vivo angiogenic responses which are independent of VEGF [95]. It has also been shown that ligand binding induces ErbB-3 and ErbB-4 heterdimerization with ErbB-2 which increases secretion of VEGF from breast cancer cells via a p38 MAPK [96]. However, neuregulin-2, a ligand for EGF receptors including ErbB-4, has an inhibitory activity on angiogenesis [94]. While it is clear that ErbB-4 activation can contribute to angiogenesis the contribution of the cleaved intracellular domain versus the classic tyrosine kinase signalling pathway has yet to be elucidated.
Cadherins
Cadherins mediate cell adhesion and participate in the maintenance of cell-cell junctions. In the context of angiogenesis VE-cadherin in vascular endothelial cells plays a critical role in regulating vessel permeability [97–99]. VEGF-induced vascular permeability leads to disassociation of VE-cadherins junctions and vessel leakage. Furthermore, pathologic new vessels are usually fragile and ‘leaky'suggesting that the cadherin junctional complexes are not fully formed. Studies to date have concentrated on the effect of γ-secretase on epithelial (E)- and neuronal (N)-cadherin [100]. Presenilin activation can result in transmembrane cleavage of E-cadherin which dissociates E-cadherin, β-catenin and α-catenin from the cytoskeleton. This leads to dissociation of the E-cadherin-catenin adhesion complex and increases the cytosolic pool of β-catenin, a key regulator of the Wnt signalling pathway [45, 100]. γ-secretase cleavage of N-cadherin is involved in CREB mediated gene expression. While to date there are no reports of γ-secretase cleavage of VE-cadherin it is highly likely that this will occur and will serve as an alternative signalling pathway in endothelial cells undergoing angiogenesis. To support this, Georgakopoulos and colleagues have reported that presenilin-1 is a component of endothelial cell junctions [101].
Amyloid precursor protein (APP)
γ-secretase is traditionally recognized for its contribution to the proteolytic cleavage of amyloid precursor protein (APP) to the amyloid-β peptide which is present in senile plaques in the brain [29]. However, it is now becoming evident that amyloid may be associated with pathological angiogenesis, especially that associated with diabetic retinopathy and age-related macular degeneration (AMD) in the eye. Amyloid is present in Drusen which are a hallmark of AMD [102, 103] and amyloid-β induces a marked increase in VEGF as well as a marked decrease in PEDF production by retinal pigment epithelial (RPE) cells [103] and thus it is likely to affect the balance between pro- and anti-angiogenic factors in the retina. Taken together with our recent observation that γ-secretase is expressed in the RPE this would suggest that the RPE may be the site of cleavage of APP [104] and may be important in the pathogenesis of AMD. There also appears to be a strong link between amyloid-β and proliferative diabetic retinopathy in which new blood vessels grow on the retinal surface. Vitreous fluid from patients showed a significant decrease in amyloid and an increase in levels of the microtubule-associated proteins compared to controls without vascular complications [105]. It is possible that this may be associated with levels of neprilysin (a rate-limiting peptidase involved in the physiological degradation of amyloid-β) [106]. There is a significant increase in neprilysin activity in patients with diabetic retinopathy compared to controls which is consistent with a decrease in amyloid in the vitreous of patients with diabetic retinopathy. This may also correlate with γ-secretase activity since presenilin is significantly up-regulated in oxygen-induced retinopathy in rats [67]. It is likely that APP/amyloid also contributes to angiogenesis outside of the eye since amyloid-β is associated with vasculogenesis in the brain [107], amyloid-β is highly expressed in the endothelium of newly forming vessels and γ-secretase-dependent processing of APP can regulate tyrosine kinase receptor expression [90].
Other substrates
As elegantly highlighted in a recent review by Parks and Curtis at least 25 γ-secretase substrates have been identified to date [46]. These show tremendous diversity and include growth hormone receptor [108], syndecan-3 [109], tyrosinase [110], CD44 [111] and HLA-A2 [112]. The cleaved intracellular domain can regulate transcriptional activity (CD44), protein localization (tyrosinase, syndecan-3), represent degradation (HLA-A2) or function remains unclear (growth hormone receptor). It is almost a certainty that additional γ-secretase substrates will be discovered in the future and that new intracellular pathways for regulation of cellular function will be identified.
γ-Secretase as a therapeutic target
Targetting γ-secretase in the prevention of aberrant angiogenesis is now a real possibility. However, given the ubiquitous expression and diverse substrates for γ-secretase it will be important to develop γ-secretase substrate-specific compounds. Furthermore, in some situations γ-secretase inhibition can prevent angiogenesis while in the case of VEGFR-1 for example it is γ-secretase up-regulation which blocks angiogenesis [31].
Exogenous negative regulation of γ-secretase activity has been extensively studied within the Alzheimers field with a view to therapeutic intervention in the formation of senile plaque [29, 30, 113]. Potent γ -secretase inhibitors have been identified by screening drug libraries or by designing aspartyl protease transition-state analogues based largely on the amyloid precursor protein (APP) substrate cleavage site. Several classes of γ-secretase inhibitors have been developed including small peptidic aldehydes (e.g. MG-132, MDL-28170) that inhibit protease activity, γ-secretase substrate specific inhibitor (e.g. DFK-167) which inhibits aspartyl protease action at the γ-42 cleavage site of APP, transition-state ana-logues (e.g. L685458, DAPT and compound E) with the benzodiazepine compound LY411575 being the most potent γ-secretase inhibitor to date.
Most of these inhibitors are not specific for γ-secretase cleavage of APP, and equally inhibit the processing of other γ-secretase substrates (see Evin et al.[113]). However, some inhibitors show some selectivity for cleavage of amyloid precursor protein relative to Notch. For example, isocoumarin (JLK-6) inhibits amyloid beta degradation but has no effect on Notch. However, selectivity may be cell type dependent since we have shown that in retinal vascular endothelial cells the reportedly non-selective inhibitor L685458 inhibits angiogenesis by blockingγ-secretase modification of VEGFR-1 but does not act via Notch, which is also expressed in these cells. It is clear that as we develop a greater understanding of γ-secretase complex formation, substrate-specific binding and cleavage sites, and transcriptional binding partners we will be able to more accurately targetγ-secretase in the prevention of aberrant angiogenesis. An interesting thought is to develop a mimetic, which will, for example, block the binding of nicastrin to tyrosine kinase receptors and thus prevent substrate-specific cleavage. While many of these inhibitors is relatively well tolerated in vivo there is evidence that the benzodiazepine derivatives can in some situations lead to cell metaplasia [114].
Although, the concept of γ-secretase inhibitors is attractive, it appears that in some facets of angiogenesis an increase in γ-secretase activity acts as a negative regulator of angiogenesis [31]. We have recently shown that PEDF, a non-classic serpin, is a potent up-regulator of γ-secretase activity and acts by promoting translocation of the components of the constituents of the γ-secretase complex to the cell membrane. In addition, an increase in γ-secretase activity has also been reported following activation of the β2-adrenergic receptor [115] and following application of TO-901317, a liver X receptor agonist [116]. Given the potential importance of increasing γ-secretase activity as a therapeutic intervention in pathological angiogenesis, it is likely that the next 5 years will see an explosion of pharmacological γ-secretase activators.
γ-Secretase, what next?
The last decade has seen an explosion in the literature on γ-secretase and its ability to cleave and modulate a wide range of substrates in addition to APP. There is clear evidence to support γ-secretase as a potent regulator of angiogenesis and that this is in part regulated by the VEGF signalling system. Further studies are required to dissect the role of γ-secretase and its substrates in the regulation of angiogenesis and to determination the functional relevance of substrate-dependent and cell-dependent modifications. Emphasis will need to be placed not just on transmembrane cleavage but also on substrate phosphorylation and intracellular translocation to identify potential therapeutic targets. The challenge ahead is to characterize the γ-secretase signalling pathways in angiogenesis and elucidate how these can influence angiogenic outcome.
Acknowledgments
We are extremely grateful for all the excellent previous review articles that have helped shape this review and we apologize to those authors whose primary references were not cited due to space limitations. Our research on γ-secretase and pathological angiogenesis is supported by NIH grants R01 EY018358-01, 2RO1 EY007739-17 and a grant from the Juvenile Diabetes Research Foundation (7-2005-875). Thanks to Lynn Shaw for the art work.
References
- 1.Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci USA. 2002;99:8219–24. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Grant MB, May W, Caballero S, Brown G, Guthrie S, Mames R, Byrne B, Vaught T, Spoerri P, Peck A, Scott E. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–12. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 5.Schatteman GC. Adult bone marrow-derived heman-gioblasts, endothelial cell progenitors, and EPCs. Curr Top Dev Biol. 2004;64:141–80. doi: 10.1016/S0070-2153(04)64007-5. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye. 2002;16:242–60. doi: 10.1038/sj.eye.6700133. [DOI] [PubMed] [Google Scholar]
- 7.Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004;242:91–101. doi: 10.1007/s00417-003-0828-0. [DOI] [PubMed] [Google Scholar]
- 8.Tong JP, Yao YF. Contribution of VEGF and PEDF to choroidal angiogenesis: a need for balanced expressions. Clin Biochem. 2006;39:267–76. doi: 10.1016/j.clinbiochem.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Grant MB, Afzal A, Spoerri P, Pan H, Shaw LC, Mames RN. The role of growth factors in the patho-genesis of diabetic retinopathy. Expert Opin Investig Drugs. 2004;13:1275–93. doi: 10.1517/13543784.13.10.1275. [DOI] [PubMed] [Google Scholar]
- 10.Borgstrom P, Gold DP, Hillan KJ, Ferrara N. Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Res. 1999;19:4203–14. [PubMed] [Google Scholar]
- 11.Van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. JAMA. 2005;293:1509–13. doi: 10.1001/jama.293.12.1509. [DOI] [PubMed] [Google Scholar]
- 12.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 13.Choueiri TK, Bukowski RM, Rini BI. The current role of angiogenesis inhibitors in the treatment of renal cell carcinoma. Semin Oncol. 2006;33:596–606. doi: 10.1053/j.seminoncol.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Rini BI. Vascular endothelial growth factor-targeted therapy in renal cell carcinoma: current status and future directions. Clin Cancer Res. 2007;13:1098–106. doi: 10.1158/1078-0432.CCR-06-1989. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 16.Chang KH, Chang-Ling T, McFarland E, Afzal A, Pan H, Baxter L, Shaw L, Caballero S, Sengupta N, Li Calzi S, Sullivan S, Grant MB. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci USA. 2007;104:10595–600. doi: 10.1073/pnas.0702072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwick E, Bange J, Ullrich A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr Relat Cancer. 2001;8:161–73. doi: 10.1677/erc.0.0080161. [DOI] [PubMed] [Google Scholar]
- 18.Blazquez C, Cook N, Micklem K, Harris AL, Gatter KC, Pezzella F. Phosphorylated KDR can be located in the nucleus of neoplastic cells. Cell Res. 2006;16:93–8. doi: 10.1038/sj.cr.7310012. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter G. Nuclear localization and possible functions of receptor tyrosine kinases. Curr Opin Cell Biol. 2003;15:143–8. doi: 10.1016/s0955-0674(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 20.Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002;3:673–84. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- 21.Heldin CH, Ericsson J. Signal transduction. RIPping tyrosine kinase receptors apart. Science. 2001;294:2111–3. doi: 10.1126/science.1067628. [DOI] [PubMed] [Google Scholar]
- 22.Landman N, Kim TW. Got RIP? Presenilin-dependent intramembrane proteolysis in growth factor receptor signaling. Cytokine Growth Factor Rev. 2004;15:337–51. doi: 10.1016/j.cytogfr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–6. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 24.Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B. Identification of signal peptide pepti-dase, a presenilin-type aspartic protease. Science. 2002;296:2215–8. doi: 10.1126/science.1070925. [DOI] [PubMed] [Google Scholar]
- 25.Xia W, Wolfe MS. Intramembrane proteolysis by pre-senilin and presenilin-like proteases. J Cell Sci. 2003;116:2839–44. doi: 10.1242/jcs.00651. [DOI] [PubMed] [Google Scholar]
- 26.Rawson RB. Regulated intramembrane proteolysis: from the endoplasmic reticulum to the nucleus. Essays Biochem. 2002;38:155–68. doi: 10.1042/bse0380155. [DOI] [PubMed] [Google Scholar]
- 27.Freeman M. Rhomboids. Curr Biol. 2003;13:R586. doi: 10.1016/s0960-9822(03)00519-0. [DOI] [PubMed] [Google Scholar]
- 28.Moninka N, Afzal A, Cai J, Chang K, Shaw L, Grant M, Boulton M. Cleavage and intracellular translocation of the-stromal derived factor -1 (sdf-1) receptor, CXCR4, regulates endothelial cell function. Invest Ophthalmol Vis Sci. 2008;48 ARVO E-Abstract 1724. [Google Scholar]
- 29.Brunkan AL, Goate AM. Presenilin function and gamma-secretase activity. J Neurochem. 2005;93:769–92. doi: 10.1111/j.1471-4159.2005.03099.x. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe MS. The gamma-secretase complex: membrane-embedded proteolytic ensemble. Biochemistry. 2006;45:7931–9. doi: 10.1021/bi060799c. [DOI] [PubMed] [Google Scholar]
- 31.Cai J, Jiang WG, Grant MB, Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J Biol Chem. 2006;281:3604–13. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 32.De Strooper B. Nicastrin: gatekeeper of the gamma-secretase complex. Cell. 2005;122:318–20. doi: 10.1016/j.cell.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Shah S, Lee S, Tabuchi K, Hao Y, Yu C, LaPlant Q, Ball H, Dann C, Sudhof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–47. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Mastrangelo P, Matthews P, Chishti M, Schmidt S, Guy Y, Yang J, Mazella M, Coomaraswamy J, Horne P, Strome B, Pelly H, Levesque G, Ebeling C, Jiang Y, Nixon R, Rozmahel R, Fraser P, George-Hyslop P, Carlson G, Westaway D. Dissociated phenotypes in presenilin transgenic mice define functionally distinct gamma-secretases. Proc Natl Acad Sci USA. 2005;102:8972–7. doi: 10.1073/pnas.0500940102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SK, Park HJ, Hong HS, Baik EJ, Jung MW, Mook-Jung I. ERK1/2 is an endogenous negative regulator of the gamma-secretase activity. FASEB J. 2006;20:157–9. doi: 10.1096/fj.05-4055fje. [DOI] [PubMed] [Google Scholar]
- 36.Pastorcic M, Das HK. Alternative initiation of transcription of the human presenilin 1 gene in SH-SY5Y and SK-N-SH cells. The role of Ets factors in the regulation of presenilin 1. Eur J Biochem. 2004;271:4485–94. doi: 10.1111/j.1432-1033.2004.04453.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang R, Zhang Y, Sun P, Liu R, Zhang X, Xia K, Xia J, Xu H, Zhang Z. Transcriptional regulation of PEN-2, a key component of the gamma-secretase complex, by CREB. Mol Cell Biol. 2006;26:1347–54. doi: 10.1128/MCB.26.4.1347-1354.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massey LK, Mah AL, Monteiro MJ. Ubiquilin regulates presenilin endoproteolysis and modulates gamma-secretase components, Pen-2 and nicastrin. Biochem J. 2005;391:513–25. doi: 10.1042/BJ20050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res. 2003;74:342–52. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- 40.Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–8. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 41.Koike H, Tomioka S, Sorimachi H, Saido T, Maruyama K, Okuyama A, Fujisawa-Sehara A, Ohno S, Suzuki K, Ishiura S. Membrane-anchored metalloprotease MDC9 has an alpha-secretase activity responsible for processing the amyloid precursor protein. Biochem J. 1999;343:371–5. [PMC free article] [PubMed] [Google Scholar]
- 42.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 43.Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–97. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 44.Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci. 2003;23:5425–36. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marambaud P, Shoi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis N. A presenilin-1/gamma-secretase cleavage releases the E-cad-herin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–56. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parks AL, Curtis D. Presenilin diversifies its portfolio. Trends Genet. 2007;23:140–50. doi: 10.1016/j.tig.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Murakami D, Okamoto I, Nagano O, Kawano Y, Tomita T, Iwatsubo T, De Strooper B, Yumoto E, Saya H. Presenilin-dependent gamma-secretase activity mediates the intramembranous cleavage of CD44. Oncogene. 2003;22:1511–6. doi: 10.1038/sj.onc.1206298. [DOI] [PubMed] [Google Scholar]
- 48.Jung KM, Tan S, Landman N, Petrova K, Murray S, Lewis R, Kim PK, Kim DS, Ryu SH, Chao MV, Kim Tw. Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J Biol Chem. 2003;278:42161–9. doi: 10.1074/jbc.M306028200. [DOI] [PubMed] [Google Scholar]
- 49.May P, Reddy YK, Herz J. Proteolytic processing of low density lipoprotein receptor-related protein mediates regulated release of its intracellular domain. J Biol Chem. 2002;277:18736–43. doi: 10.1074/jbc.M201979200. [DOI] [PubMed] [Google Scholar]
- 50.McElroy B, Powell JC, McCarthy JV. The insulin-like growth factor 1 (IGF-1) receptor is a substrate for gamma-secretase-mediated intramembrane proteolysis. Biochem Biophys Res Commun. 2007;358:1136–41. doi: 10.1016/j.bbrc.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 51.Marron M, Singh H, Tahir T, Kavumkal J, Kim H, Koh G, Brindle NP. Regulated proteolytic processing of Tie1 modulates ligand responsiveness of receptor -tyrosine kinase Tie 2. J Biol Chem. 2007;282:30509–17. doi: 10.1074/jbc.M702535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukherjee S, Tessema M, Wandinger-Ness A. Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function. Circ Res. 2006;98:743–56. doi: 10.1161/01.RES.0000214545.99387.e3. [DOI] [PubMed] [Google Scholar]
- 53.Myers JM, Martins GG, Ostrowski J, Stachowiak MK. Nuclear trafficking of FGFR1: a role for the transmembrane domain. J Cell Biochem. 2003;88:1273–91. doi: 10.1002/jcb.10476. [DOI] [PubMed] [Google Scholar]
- 54.Peng H, Moffett J, Myers J, Fang X, Stachowiak E, Maher P, Kratz E, Hines J, Fluharty S, Mizukoshi E, Bloom D, Stachowiak M. Novel nuclear signaling pathway mediates activation of fibroblast growth factor-2 gene by type 1 and type 2 angiotensin II receptors. Mol Biol Cell. 2001;12:449–62. doi: 10.1091/mbc.12.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reilly JF, Maher PA. Importin beta-mediated nuclear import of fibroblast growth factor receptor: role in cell proliferation. J Cell Biol. 2001;152:1307–12. doi: 10.1083/jcb.152.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bild AH, Turkson J, Jove R. Cytoplasmic transport of Stat3 by receptor-mediated endocytosis. EMBO J. 2002;21:3255–63. doi: 10.1093/emboj/cdf351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suga K, Saito A, Tomiyama T, Mori H, Akagawa K. Syntaxin 5 interacts specifically with presenilin holo-proteins and affects processing of betaAPP in neuronal cells. J Neurochem. 2005;94:425–39. doi: 10.1111/j.1471-4159.2005.03210.x. [DOI] [PubMed] [Google Scholar]
- 58.McCarthy JV. Involvement of presenilins in cell-survival signalling pathways. Biochem Soc Trans. 2005;33:568–72. doi: 10.1042/BST0330568. [DOI] [PubMed] [Google Scholar]
- 59.Thomas AV, Herl L, Spoelgen R, Hiltunen M, Jones PB, Tanzi RE, Hyman BT, Berezovska O. Interaction between presenilin 1 and ubiquilin 1 as detected by fluorescence lifetime imaging microscopy and a high-throughput fluorescent plate reader. J Biol Chem. 2006;281:26400–7. doi: 10.1074/jbc.M601085200. [DOI] [PubMed] [Google Scholar]
- 60.Jo DG, Jang J, Kim BJ, Lundkvist J, Jung YK. Overexpression of calsenilin enhances gamma-secre-tase activity. Neurosci Lett. 2005;378:59–64. doi: 10.1016/j.neulet.2004.12.078. [DOI] [PubMed] [Google Scholar]
- 61.Prat MI, Adamo A, Gonzalez S, Affranchino J, Ikeda M, Matsubara E, Shoji M, Smith M, Castano E, Morelli L. Presenilin 1 overexpressions in Chinese hamster ovary (CHO) cells decreases the phosphorylation of retinoblastoma protein: relevance for neurodegeneration. Neurosci Lett. 2002;326:9–12. doi: 10.1016/s0304-3940(02)00298-7. [DOI] [PubMed] [Google Scholar]
- 62.Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/-Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25:1242–52. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arasada RR, Carpenter G. Secretase-dependent tyrosine phosphorylation of Mdm2 by the ErbB-4 intracellular domain fragment. J Biol Chem. 2005;280:30783–7. doi: 10.1074/jbc.M506057200. [DOI] [PubMed] [Google Scholar]
- 64.Maatta JA, Sundvall M, Junttila TT, Peri L, Laine VJ, Isola J, Egeblad M, Elenius K. Proteolytic cleavage and phosphorylation of a tumor-associated ErbB4 isoform promote ligand-independent survival and cancer cell growth. Mol Biol Cell. 2006;17:67–79. doi: 10.1091/mbc.E05-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J. 2004;23:2586–96. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang DE, Yoon IS, Repetto E, Busse T, Yermian N, Ie L, Koo EH. Presenilins mediate phosphatidylinosi-tol 3-kinase/AKT and ERK activation via select signaling receptors. Selectivity of PS2 in platelet-derived growth factor signaling. J Biol Chem. 2005;280:31537–47. doi: 10.1074/jbc.M500833200. [DOI] [PubMed] [Google Scholar]
- 67.Lukiw WJ, Gordon WC, Rogaev EI, Thompson H, Bazan NG. Presenilin-2 (PS2) expression up-regulation in a model of retinopathy of prematurity and pathoangiogenesis. Neuroreport. 2001;12:53–7. doi: 10.1097/00001756-200101220-00019. [DOI] [PubMed] [Google Scholar]
- 68.Nakajima M, Yuasa S, Ueno M, Takakura N, Koseki H, Shirasawa T. Abnormal blood vessel development in mice lacking presenilin-1. Mech Dev. 2003;120:657–67. doi: 10.1016/s0925-4773(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 69.Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, Hughes CC. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J. 2005;19:1027–9. doi: 10.1096/fj.04-3172fje. [DOI] [PubMed] [Google Scholar]
- 70.Serneels L, Dejaegere T, Craessaerts K, Horre K, Jorissen E, Tousseyn T, Hebert S, Coolen M, Martens G, Zwijsen A, Annaert W, Hartmann D, De Strooper B. Differential contribution of the three Aph1 genes to gamma-secretase activity in vivo. Proc Natl Acad Sci USA. 2005;102:1719–24. doi: 10.1073/pnas.0408901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi W, Harris AL. Notch signaling in breast cancer and tumor angiogenesis: cross-talk and therapeutic potentials. J Mammary Gland Biol Neoplasia. 2006;11:41–52. doi: 10.1007/s10911-006-9011-7. [DOI] [PubMed] [Google Scholar]
- 72.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–9. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakajima M, Ogawa M, Shimoda Y, Hiraoka S, Iida M, Koseki H, Shirasawa T, Furukawa K. Presenilin-1 controls the growth and differentiation of endothelial progenitor cells through its beta-catenin-binding region. Cell Biol Int. 2006;30:239–43. doi: 10.1016/j.cellbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Murakami Y, Yamagoe S, Noguchi K, Takebe Y, Takahashi N, Uehara Y, Fukazawa H. Ets-1-dependent expression of vascular endothelial growth factor receptors is activated by latency-associated nuclear antigen of Kaposi's sarcoma-associated herpes virus through Interaction with Daxx. J Biol Chem. 2006;281:28113–21. doi: 10.1074/jbc.M602026200. [DOI] [PubMed] [Google Scholar]
- 75.Mayo LD, Kessler KM, Pincheira R, Warren RS, Donner DB. Vascular endothelial cell growth factor activates CRE-binding protein by signaling through the KDR receptor tyrosine kinase. J Biol Chem. 2001;276:25184–9. doi: 10.1074/jbc.M102932200. [DOI] [PubMed] [Google Scholar]
- 76.Curry CL, Reed LL, Nickoloff BJ, Miele L, Foreman KE. Notch-independent regulation of Hes-1 expression by c-Jun N-terminal kinase signaling in human endothelial cells. Lab Invest. 2006;86:842–52. doi: 10.1038/labinvest.3700442. [DOI] [PubMed] [Google Scholar]
- 77.Taylor KL, Henderson AM, Hughes CC. Notch activation during endothelial cell network formation in vitro targets the basic HLH transcription factor HESR-1 and downregulates VEGFR-2/KDR expression. Microvasc Res. 2002;64:372–83. doi: 10.1006/mvre.2002.2443. [DOI] [PubMed] [Google Scholar]
- 78.Takeshita K, Satoh M, Li M, Silver M, Limbourg F, Mukai Y, Rikitake Y, Radtke F, Gridley T, Losordo D, Liao J. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100:70–8. doi: 10.1161/01.RES.0000254788.47304.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778–84. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- 81.Hellstrom M, Phng L, Hofmann J, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson A, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe M, Kalem M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–80. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 82.Hainaud P, Contreres JO, Villemain A, Liu LX, Plouet J, Tobelem G, Dupuy E. The role of the vascular endothelial growth factor-Delta-like 4 ligand/Notch4-ephrin B2 cascade in tumor vessel remodeling and endothelial cell functions. Cancer Res. 2006;66:8501–10. doi: 10.1158/0008-5472.CAN-05-4226. [DOI] [PubMed] [Google Scholar]
- 83.Harrington LS, Sainson RC, Williams CK, Taylor JM, Shi W, Li JL, Harris AL. Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvasc Res. 2008;75:144–54. doi: 10.1016/j.mvr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 84.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7:327–31. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 85.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA. 1998;95:9349–54. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nozaki M, Sakurai E, Raisler B, Baffi J, Witta J, Ogura Y, Brekken R, Sage E, Ambati B, Ambati J. Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A. J Clin Invest. 2006;116:422–9. doi: 10.1172/JCI26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yen L, Benlimame N, Nie Z, Xiao D, Wang T, Al Moustafa E, Esumi H, Milanini J, Hynes N, Pages G, Alaoui-Jamali Differential regulation of tumor angiogenesis by distinct ErbB homo- and heterodimers. Mol Biol Cell. 2002;13:4029–44. doi: 10.1091/mbc.E02-02-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Omerovic J, Puggioni EM, Napoletano S, Visco V, Fraioli R, Frati L, Gulino A, Alimandi M. Ligand-regulated association of ErbB-4 to the transcriptional co-activator YAP65 controls transcription at the nuclear level. Exp Cell Res. 2004;294:469–79. doi: 10.1016/j.yexcr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 89.Ni CY, Yuan H, Carpenter G. Role of the ErbB-4 car-boxyl terminus in gamma-secretase cleavage. J Biol Chem. 2003;278:4561–5. doi: 10.1074/jbc.M210504200. [DOI] [PubMed] [Google Scholar]
- 90.Zhang YW, Wang R, Liu Q, Zhang H, Liao FF, Xu H. Presenilin/gamma-secretase-dependent processing of beta-amyloid precursor protein regulates EGF receptor expression. Proc Natl Acad Sci USA. 2007;104:10613–8. doi: 10.1073/pnas.0703903104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–80. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- 92.Bueter W, Dammann O, Zscheppang K, Korenbaum E, Dammann CE. ErbB receptors in fetal endothelium–a potential linkage point for inflammation-associated neonatal disorders. Cytokine. 2006;36:267–75. doi: 10.1016/j.cyto.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim HS, Shin HS, Kwak HJ, Cho CH, Lee CO, Koh GY. Betacellulin induces angiogenesis through activation of mitogen-activated protein kinase and phosphatidylinositol 3′-kinase in endothelial cell. FASEB J. 2003;17:318–20. doi: 10.1096/fj.02-0570fje. [DOI] [PubMed] [Google Scholar]
- 94.Nakano N, Higashiyama S, Ohmoto H, Ishiguro H, Taniguchi N, Wada Y. The N-terminal region of NTAK/neuregulin-2 isoforms has an inhibitory activity on angiogenesis. J Biol Chem. 2004;279:11465–70. doi: 10.1074/jbc.M311045200. [DOI] [PubMed] [Google Scholar]
- 95.Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–11. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
- 96.Xiong S, Grijalva R, Zhang L, Nguyen NT, Pisters PW, Pollock RE, Yu D. Up-regulation of vascular endothelial growth factor in breast cancer cells by the heregulin-beta1-activated p38 signaling pathway enhances endothelial cell migration. Cancer Res. 2001;61:1727–32. [PubMed] [Google Scholar]
- 97.Gulino D, Delachanal E, Concord E, Genoux Y, Morand B, Valiron M, Sulpice E, Scaife R, Alemany M, Vernet T. Alteration of endothelial cell monolayer integrity triggers resynthesis of vascular endothelium cadherin. J Biol Chem. 1998;273:29786–93. doi: 10.1074/jbc.273.45.29786. [DOI] [PubMed] [Google Scholar]
- 98.Navarro P, Caveda L, Breviario F, Mandoteanu I, Lampugnani MG, Dejana E. Catenin-dependent and -independent functions of vascular endothelial cadherin. J Biol Chem. 1995;270:30965–72. doi: 10.1074/jbc.270.52.30965. [DOI] [PubMed] [Google Scholar]
- 99.Prandini MH, Dreher I, Bouillot S, Benkerri S, Moll T, Huber P. The human VE-cadherin promoter is subjected to organ-specific regulation and is activated in tumour angiogenesis. Oncogene. 2005;24:2992–3001. doi: 10.1038/sj.onc.1208483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parisiadou L, Fassa A, Fotinopoulou A, Bethani I, Efthimiopoulos S. Presenilin 1 and cadherins: stabilization of cell-cell adhesion and proteolysis-dependent regulation of transcription. Neurodegener Dis. 2004;1:184–91. doi: 10.1159/000080984. [DOI] [PubMed] [Google Scholar]
- 101.Georgakopoulos A, Marambaud P, Friedrich VL, Jr, Shioi J, Efthimiopoulos S, Robakis NK. Presenilin-1: a component of synaptic and endothelial adherens junctions. Ann N Y Acad Sci. 2000;920:209–14. doi: 10.1111/j.1749-6632.2000.tb06924.x. [DOI] [PubMed] [Google Scholar]
- 102.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 103.Yoshida T, Ohno-Matsui K, Ichinose S, Sato T, Iwata N, Saido TC, Hisatomi T, Mochizuki M, Morita I. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J Clin Invest. 2005;115:2793–800. doi: 10.1172/JCI24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Cai J, Afzal A, Grant M, Boulton M. VEGFR-1 is differentially regulated by pigment epithelium-derived factor (PEDF) via presenilin in RPE and retinal endothelial cells. Invest Ophthalmol Vis Sci. 2006;47 ARVO E-Abstract 5333. [Google Scholar]
- 105.Yoneda S, Hara H, Hirata A, Fukushima M, Inomata Y, Tanihara H. Vitreous fluid levels of beta-amyloid((1–42)) and tau in patients with retinal diseases. Jpn J Ophthalmol. 2005;49:106–8. doi: 10.1007/s10384-004-0156-x. [DOI] [PubMed] [Google Scholar]
- 106.Hara H, Oh-hashi K, Yoneda S, Shimazawa M, Inatani M, Tanihara H, Kiuchi K. Elevated neprilysin activity in vitreous of patients with proliferative diabetic retinopathy. Mol Vis. 2006;12:977–82. [PubMed] [Google Scholar]
- 107.Lazarov O, Robinson J, Tang Y, Hairston I, Korade-Mimics Z, Lee V, Hersh L, Sapolsky R, Mimics K, Sisodia S. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–13. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 108.Cowan JW, Wang X, Guan R, He K, Jiang J, Baumann G, Black RA, Wolfe MS, Frank SJ. Growth hormone receptor is a target for presenilin-dependent gamma-secretase cleavage. J Biol Chem. 2005;280:19331–42. doi: 10.1074/jbc.M500621200. [DOI] [PubMed] [Google Scholar]
- 109.Schulz JG, Annaert W, Vandekerckhove J, Zimmermann P, De Strooper B, David G. Syndecan 3 intramembrane proteolysis is presenilin/gamma-secretase-dependent and modulates cytosolic signaling. J Biol Chem. 2003;278:48651–7. doi: 10.1074/jbc.M308424200. [DOI] [PubMed] [Google Scholar]
- 110.Wang R, Tang P, Wang P, Boissy RE, Zheng H. Regulation of tyrosinase trafficking and processing by presenilins: partial loss of function by familial Alzheimer's disease mutation. Proc Natl Acad Sci USA. 2006;103:353–8. doi: 10.1073/pnas.0509822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cui W, Ke JZ, Zhang Q, Ke HZ, Chalouni C, Vignery A. The intracellular domain of CD44 promotes the fusion of macrophages. Blood. 2006;107:796–805. doi: 10.1182/blood-2005-05-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carey BW, Kim DY, Kovacs DM. Presenilin/gamma-secretase and alpha-secretase-like peptidases cleave human MHC Class I proteins. Biochem J. 2007;401:121–7. doi: 10.1042/BJ20060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Evin G, Sernee MF, Masters CL. Inhibition of gamma-secretase as a therapeutic intervention for Alzheimer's disease: prospects, limitations and strategies. CNS Drugs. 2006;20:351–72. doi: 10.2165/00023210-200620050-00002. [DOI] [PubMed] [Google Scholar]
- 114.Milano J, Mckay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, Jacobs R, Zacco A, Greenberg B, Ciaccio P. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–58. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 115.Ni Y, Xhao X, Bao G, Zou L, Teng L, Wang Z, Song M, Xiong J, Bai Y, Pei G. Activation of beta2-adrener-gic receptor stimulates gamma-secretase activity and accelerates amyloid plaque formation. Nat Med. 2006;12:1390–6. doi: 10.1038/nm1485. [DOI] [PubMed] [Google Scholar]
- 116.Czech C, Burns MP, Vardanian L, Augustin A, Jacobsen H, Baumann K, Rebeck GW. Cholesterol independent effect of LXR agonist TO-901317 on gamma-secretase. J Neurochem. 2007;101:929–36. doi: 10.1111/j.1471-4159.2007.04467.x. [DOI] [PubMed] [Google Scholar]


