Abstract
Angiopoietin-1 (Ang-1) is the primary agonist for Tie2 tyrosine kinase receptor (Tie2), and the effect of Ang-1-Tie2 signalling is context-dependent. Deficiency in either Ang-1 or Tie2 protein leads to severe microvascular defects and subsequent embryonic lethality in murine model. Tie2 receptors are expressed in several cell types, including endothelial cells, smooth muscle cells, fibroblasts, epithelial cells, monocytes, neutrophils, eosinophils and glial cells. Ang-1-Tie2 signalling induces a chemotactic effect in smooth muscle cells, neutrophils and eosinophils, and induces differentiation of mesenchymal cells to smooth muscle cells. Additionally, this signalling pathway induces the secretion of serotonin, matrix metalloproteinases (MMPs) and plasmin. Ang-1 inhibits the secretion of tissue inhibitor of matrix metalloproteinase (TIMPs). Aberrant expression and activity of Tie2 in vascular and non-vascular cells may result in the development of rheumatoid arthritis, cancer, hypertension and psoriasis. Ang-1 has an anti-inflammatory effect, when co-localized with vascular endothelial growth factor (VEGF) in the vasculature. Thus, Ang-1 could be potentially important in the therapy of various pathological conditions such as pulmonary hypertension, arteriosclerosis and diabetic retinopathy. In this article, we have summarized and critically reviewed the pathophysiological role of Ang-1-Tie2 signalling pathway.
Keywords: angiopoietin-1(Ang-1), tie2 tyrosine kinase receptor (Tie2), cancer, extracellular matrix (ECM), matrix metalloproteinase (MMP), vascular endothelial growth factor (VEGF)
Introduction
Tie-2 receptor (Tie2), a specific receptor for angiopoietin-1 (Ang-1), is a tyrosine kinase receptor, originally thought to be expressed exclusively on endothelial cells. The role of Ang-1-Tie2 signalling in the regulation of several cellular behaviours has initiated an interest in further in-depth understanding of Ang-1-Tie2 signalling pathway. Ang-1 is an angiogenic growth factor with a central role in promotion of structural integrity in the vasculature [1–4]. Deletion of Ang-1 or Tie2 genes led to severe defects in the vasculature and subsequent lethality, validating their requirement in microvascular development [2, 5–8]. There is a consensus that aberrant angiogenesis due to abnormal levels of Ang-1 and Tie2 plays an important role in the development of rheumatoid arthritis, asthma, cancer and pulmonary hypertension in experimental animal and human [9–14].
Tie2 activates multiple pathways to elicit several downstream effects [15]. Stimulators, such as hypoxia, interleukin (IL)-1β, vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), IL-11 and tumour necrosis factor (TNF)-α, regulate the expression of Tie2 and Ang-1 (Table 1) [16–18]. Discovery of Tie2 expression in non-vascular cells warrants the question of its extravascular involvement in the development of pathological conditions along with its role in angiogenesis. More recent studies have investigated the regulatory role of Ang-1-Tie2 signalling pathway in cellular responses. In this article we critically evaluated the cellular and molecular mechanism underlying Ang-1-Tie2 signalling pathway and its role in health and diseases.
1.
Effect of mediators on the expression of Tie1 and Tie2, and production and secretion of Ang-1 and Ang-2
| Stimulus | Tie1 expression | Tie2 expression | Ang-1 production | Ang-2 production |
|---|---|---|---|---|
| TNF-α | Increase [54, 57] or decrease [56, 59] in cell type dependent manner | Increase in cell type dependent manner [54] | ||
| TGF-β | Increase in rheumatoid synovial fibroblast [54] | Increase in normal fibroblast [54] | ||
| IL-1β | Decrease [56] | |||
| VEGF | Transient acute increase in cell type dependent manner [42] | Increase [62] and transient acute increase in a cell type dependent manner [42], | Increase in cell type dependent manner [42, 60-62] | |
| IL-11 | Increase [16] | |||
| Hypoxia | Increase [41] | Increase [41] and transient acute increase in cell type dependent manner [42], | Transient acute increase in a cell type dependent manner [42] | Increase [55, 60, 61] and increase in a cell type dependent manner [42] |
The numbers in the brackets represent the corresponding citation of the findings.
Background, structural characteristics and distribution of Tie2
Tie1 and Tie2 belong to a distinct family of tyrosine kinase receptors [4, 19, 20]. Both receptors share an extracellular domain similarity of about 33% and an intracellular tyrosine kinase domain similarity of 76%[21, 22]. Although originally discovered in 1993 as an endothelial specific receptor [4, 19, 20], Tie2 receptors are also expressed in non-endothelial cells both in disease and in normal tissue, including carcinoma cells, fibroblast, mural cells, keratinocytes, smooth muscle cells, neutrophils, monocytes, synovial lining cells, eosinophils, ganglion cells, neurons and glial cells [3, 9, 12, 17, 23–26]. Ang-1, 2, 3 and 4 are specific ligands for Tie2, acting as either or both agonists and antagonists. A specific ligand for Tie1 is unknown [1, 27–29]. However, some studies have shown that Ang-1 and 4 can phosphorylate Tie1, suggesting a Tie1 signalling alongside Tie2 signalling [30]. However, Tie1 phosphorylation is dependent on Tie2 activation, suggesting that Tie2 tyrosine kinase domain may be responsible for phosphorylating Tie1 as a result of heterodimerization [31, 32]. Tie1 activation is consequently inhibited when Tie2 expression is down-regulated. Tie1 activation down-regulates Tie2-mediated signalling. Although the underlying mechanism is not clear, Tie1 acts as a regulatory mechanism for Tie2 signalling when co-expressed with Tie2 [31]. Ligand-independent signalling pathways have also been suggested for Tie1 [33–36].
Tie2 is a 140 kD tyrosine kinase receptor with immunoglobulin and epidermal growth factor (EGF) homology domain-2. The extracellular region contains three EGF-like domain modules that are flanked by two immunoglobulin (Ig)-like domains, and three fibronectin type III repeats (Fig. 1) [11, 19]. The two Ig-like domains harbour the Ang-binding site. Investigations have also suggested a third Ig-like domain, which folds together with the three EGFs into a compact, arrowhead-shaped structure [37, 38]. The binding site is contained in the globular head domain. A stalk that acts as a spacer between the head and the cell surface is formed by the three fibronectin type III repeats. The cytoplasmic domain contains tyrosine kinase domains, made up of phos-phorylation and protein interaction sites [11]. New Ets-related factor-2 (NERF2) and E74-like factor-1 (ELF-1), which are members of the Ets family of transcription factors, can bind to the Tie2 gene promoter to induce transcription of the gene [18, 39]. Ang-1 can induce expression of NERF2 under hypoxic condition, although level of Ang-2 is greater than Ang-1 under this condition. Ang-2 can act as a Tie2 agonist under hypoxic conditions and in the presence of VEGF [18, 40]. Other factors, such as IL-11, VEGF and hypoxia, can also regulate Tie1 and Tie2 expression (Table 1) [16–18, 41, 42].
1.
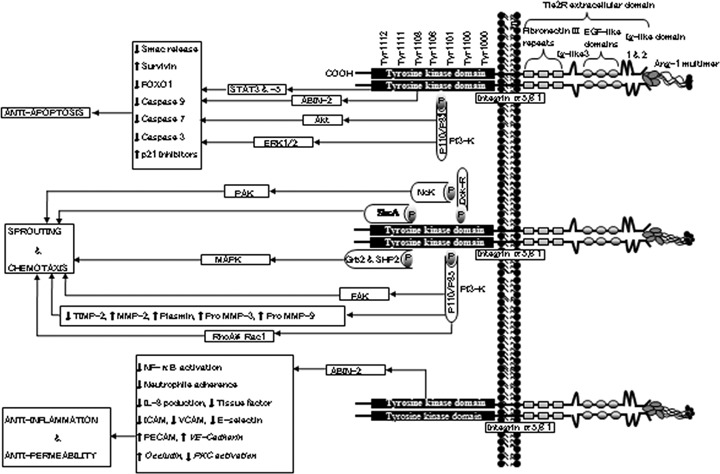
Schematic representation of Tie2 (Tie2 tyrosine kinase receptor) structure and Ang-1-Tie2 signallingsignaling. Ang-1 binding to Tie2 leads to dimerization and autophosphorylation of Tie2. Several effector molecules are recruited and activated, leading to downstream signallingsignaling. Several downstream signallingsignaling molecules have been identified. Tie2 signals primarily through the PI3-kinase (phosphatidylinositol 3-kinase) pathway. However, Tie2 can also activate other molecules, such as Grb2 (growth factor receptor-bound protein 2), ABIN-2 (A20-binding inhibitor of NF-κB-2), SHP2 (Src homology 2 (SH2)-containing protein tyrosine phosphatase), STATs (Signal transducer and activator of transcriptions), Shc-A (Src homology 2 domain containing protein), and Dok-R (Downstream-of-kinase-related protein) to elicit various downstream effects.
Background, structural characteristics and distribution of Ang-1
Ang-1 belongs to an angiopoietin (Ang) family consisting of Ang-1, 2, 3 and 4 [1, 28, 29]. Ang-1 and 2 have indistinguishable binding sites on Tie2 and bind with similar affinity [27, 43, 44]. While Ang-1 is an agonist, Ang-2 could act as antagonist or agonist depending on cell type and microenvironmental conditions [28, 45]. Transgenic overexpression of Ang-2 led to vascular defects similar to Ang-1 or Tie2 deficiency [28]. Ang-3 and Ang-4 are present in murine and humans, respectively. Their signal transduction pathway is less characterized when compared to Ang-1 and 2. While Ang-3 is moderately expressed in multiple mouse tissue, Ang-4 mRNA is abundantly expressed in the lungs. Ang-4 can function as a Tie2 agonist [46]. Ang-3 on the other hand functions as a Tie2 antagonist [29, 30, 40]. However, when using recombinant proteins corresponding to full-length Ang-3, the protein was found to activate Tie2 in a species-dependent manner [27, 47].
Ang-1 can exist as a 70 kD glycoprotein and Ang2 as a 62–70 kD glycoprotein under reduced condition [48]. Ang-1 has a carboxyl-terminal fibrinogen-like domain, a central coiled domain and a short amino-terminal. The carboxyl-terminal is responsible for Tie2 binding, the central coiled domain is required for dimerization of Ang-1 monomers, and the short amino-terminal superclusters these domains, allowing Ang-1 to exist as heterogenous multimers with basic trimeric, tetrameric and pentameric oligomers. Proper oligomerization of Ang-1 having at least four subunits (tetramer) by the intermolecular disulphide linkage involving cysteines 41 and 45 is critical for Tie2 binding and activation (Fig. 1) [27, 43, 48]. Ang-1 dimers and trimers have been shown to be inactive. The cysteine residues at 41 and 54 positions in the superclustering domain of Ang-1 are responsible for higher order multimers of Ang-1 by crosslinking Ang-1 dimers and trimers. Also, very high-order multimeric structures of Ang-1 may be inactive because they may be too big to enter endothelial cell caveolae, where most Tie2 are located. The sizes of higher order multimeric Ang-1 are usually more than 200 nm, compared to the typical 10–150 nm diameter of endothelial caveolae [48]. As a result, these higher order multimeric Ang-1 would not be able to access Tie2 to activate it. This may also explain why recombinant Ang-1 is not always active. So, proper multimerization of the Ang-1 subunits by the disulphite linkage between cysteine 41 and 54 is crucial for access, binding and activation of Tie2. The coiled-coil domain is responsible for Ang-1 dimer and trimer formation. The coiled-coil domain of Ang-1 can bind to other Angs produced by the same cell, rendering them inactive, because it lacks the multimerized-binding domain and is unable to bind Tie2 [48].
After secretion, Ang-1 is incorporated into the extracellular matrix (ECM) [49]. Ang-2 is not associated with the ECM, but rather, it is stored in Weibel–Palade bodies predominantly in the cytoplasm of endothelial cells and quickly secreted when needed [50]. Ang-3, on the other hand is tethered on the cell's surface via heparin sulphate proteoglycans [51]. While Ang-2 is mainly expressed in endothelial cells and located at sites of vascular remodelling, Ang-1 is expressed by several cell types in various tissues [27, 28]. AP-1 and ESE-1 have binding sites on the Ang-1 promoter and are presumably involved in the transactivation of the Ang-1 gene [52]. Activator protein-1 (AP-1) is also involved in Ang-2 gene transcription [53]. Epithelium-specific Ets-1 (ESE-1) is said to be particularly involved in Ang-1 gene activation in inflammatory conditions [52]. Several stimulators including IL-1β, TNF-α, TGF-β, IL-10, VEGF and hypoxia regulate Ang-1 and Ang-2 expression in various cell types (Table 1) [14, 40, 42, 54–62].
Transmembrane signalling pathway in Ang-1-Tie2-response coupling
Ang-1 binding to Tie2 leads to receptor dimerization. Following the dimerization, the kinase domain is activated and autophosphorylation of specific tyrosine residues take place [63]. These phosphorylated sites act as binding sites for a number of effector molecules. Effector molecules are recruited to these sites and their interaction with the Tie2 phosphorylated sites leads to the initiation of various signalling cascades. These downstream signalling pathways ultimately lead to cellular responses, such as differentiation, survival, proliferation and ECM interaction (Fig. 1) [25, 64–68]. The p85 subunit of phosphatidyli-nositol 3 kinase (PI3K) interacts with tyrosine-1101 of Tie2 via Src homology 2 (SH2) or phosphotyrosine-binding (PTB) domain. Downstream activation of the PI3K-Akt in endothelial cells leads to a survival pathway and cell chemotaxis [25, 66, 69]. This pathway inhibits Smac release from mitochondria and up-regulates expression of survivin protein [69]. Ang-1 can also elicit anti-apoptotic effect in endothelial cells by inhibiting forkhead transcription factor FKHR (FOXO1) through Akt activation [70]. The Akt phosphorylates the FOXO1 at three conserved sites, resulting in inhibition and promotion of nuclear export of the FOXO1 protein [70, 71]. The p110α subunit of PI3K can function as an upstream regulator of Tie2 expression [72].
Tie2 also interacts with Dok-R. Dok-R/Dok-2 association with Tie2 leads to tyrosine phosphorylation of Dok-R and subsequent recruitment of Nck and p21-activating kinase (PAK) to the activated receptor. The ability of Dok-R to bind to Nck is required for maximum PAK activation, which can lead to Ang-1-mediated endothelial cell migration [73, 74]. Tie2 also interacts with Src homology domain containing adapter proteins, Grb 2, 7, 14 and tyrosine phos-phatase, Shp2, which can activate Ras-Raf-mitogen-activated protein kinase (MAPK) pathway [75]. Ang-1 may also regulate MAPK signalling by modulating phosphorylation of ERK1/2 and p38MAPK by PI3K [76]. The MAPK has a role in Ang-1-mediated cell survival and migration [65, 76, 77]. Ang-1-Tie2 signalling is able to simultaneously activate both pro-apoptotic p38MAPK and anti-apoptotic PI3K pathways. However, the anti-apoptotic pathways are stronger than the pro-apoptotic pathway, resulting in net anti-apoptotic effect [76].
Similar to Ang1, Ang2 can activate both p38 MAPK pro-apoptotic and ERK1/2 anti-apoptotic path-way. While both Ang1 and Ang2 induced similar levels of p38 MAPK phosphorylation, Ang2-induced activation of ERK1/2 was considerably weaker than Ang1. Also unlike Ang1, Ang2 attenuated VEGF-induced ERK1/2 pathway [78]. Since there was no inhibitory interaction between Ang2 and VEGF to activate p38 MAPK, combined stimulation with Ang2 and VEGF may skew balance towards a p38 MAPK signalling, which is pro-apoptotic in nature. This is consistent with the model that in the presence of VEGF, Ang2 destabilizes the blood vessels, making the endothelial cells more susceptible to VEGF stimulation [78]. Ang2 induced an anti-apoptotic effect through Akt and ERK1/2 survival pathway. So, similar to VEGF and Ang1, Ang2 activation of survival pathway supersedes the activation of pro-apoptotic pathway [76, 79]. Although some studies have shown that Ang2 does not induce a chemotactic effect in human umbilical vein endothelial cells (HUVECs), another study demonstrated that Ang-2-induced chemotaxis in murine brain capillary endothelial cells through c-Fes and c-Fyn. This suggests that Ang-2 may induce different effects in different tissues [78, 80, 81].
Ang-1 can activate nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, resulting in the production of reactive oxygen species (ROS). The production of ROS promotes endothelial cell migration [82]. Tie2 also interacts with human A20-binding inhibitor of NF-κB activation-2 (ABIN-2), a key regulator of inflammatory response [65]. ABIN-2 can also inhibit endothelial apoptosis [83]. ShcA adaptor proteins can interact with activated Tie2 and have been implicated in the transactivation of the Ras-Raf-MAPK pathway, leading to cell migration and sprouting [75, 84]. Ang-1 can also activate signal transducers and activators of transcription factor-3 and 5 (STAT3 and STAT5) leading to increased expression of p21 [85]. Ang-1-Tie2 signalling can activate focal adhesion kinase, which produces simultaneous phosphorylation of paxillin and may be involved in endothelial cells sprouting [86]. Although several studies have reported that Ang-1 does not have a mitogenic effect on endothelial cells, Ang-1 can exert a mitogenic effect on endothelial cells through the activation of both MAPK and p70S6K [87].
A crosstalk between Tie2 and integrin α5β1 in endothelial cells results in engagement of fibronectin and increased sensitivity of Tie2 receptors to Ang-1 activation [88]. In Tie2-negative cardiac and skeletal myocytes, Ang-1 promoted cell adhesion and survival via a pathway mediated by integrins [89]. Recombinant truncated and monomeric Ang-1 variants bind Tie2 but do not activate the receptor [90]. However, the same protein binds with similar affinity to integrin α5β1 and activates a downstream signalling similar to that activated via Tie2 by a full-length Ang-1 protein [90]. There is a suggestion that this downstream effect may have been achieved through integrin dimerization [90]. In Tie2 negative fibroblast cells, direct integrin-mediated cell adhesion to Ang-1 has been reported [91]. These integrins may compete with Tie2 for available Ang-1. This information suggests a possible Tie2-independent Ang-1 signalling pathway. On the other hand, mutation in the kinase domain of Tie2 results in ligand-independent activation [92]. This phenomenon is seen in vascular anomalies in conditions such as multiple cutaneous mucosal venous malformations and intramuscular hemangiomas [11].
Regulation of Tie2 signalling
Tie2 signalling can be regulated in several ways. Ang-1 activation of Tie2 can lead to subsequent Tie2 internalization and degradation [93]. With half-life of about 9 hrs in unstimulated cells, Ang-1 reduces the half-life of Tie2 to about 3 hrs. However, the rate of Tie2 synthesis remained unchanged with or without Ang-1, resulting in reduction of Tie2 overtime. This could be a pre-emptive action in preventing multiple and at times contradicting signalling through this pathway. After activating Tie2, Ang-1 was not internalized but was released from the cell surface into the surrounding medium [93]. Tie2 can be inhibited by soluble Tie2s (sTie2Fc) and antagonists such as Ang-2 and Ang-1cc. sTie2Fc is a soluble form of the extracellular domain of Tie2 and has been detected in the plasma of healthy individuals [5, 40, 80]. This suggests that sTie2Fc may be a physiological regulatory mechanism of Tie2 [94]. Elevated levels of sTie2Fc have been reported in pathological conditions such as coronary heart diseases and diabetic retinopathy [95]. Several stimulators, such as VEGF, phorbol 12-myristate 13-acetate (PMA) and basic fibroblast growth factor (FGF) have been implicated in this shedding process of Tie2 that results in sTie2Fc [94, 96]. Because of this shedding process, Tie2 protein expression could be decreased without any effect on the Tie2R mRNA [94]. sTie2Fc could be as a tumour marker [97]. However, serum level of sTie2Fc do not provide any further information on tumour behaviour [98]. Ang-1-Tie2 chemotactic effect was blocked by sTie2Fc but not sTie1Fc, suggesting that sTie1Fc may not be an effective regulator of Ang-1-Tie2 signalling [80]. Tie2 expression can be regulated by Ang-1 through a feedback mechanism [99]. Tie2 also associates with a vascular endothelial protein tyrosine phosphatase, which can regulate the tyrosine kinase activity (Fig. 2) [100]. Tie2 carboxyl terminal can also participate as a negative regulator of Tie2 signalling [101]. Although Tie2 mRNA and Ang-1 expression are down-regulated under hypoxic condition, Tie2 translation is maintained [102]. This suggests a translational mechanism that proceeds despite unfavourable conditions [102].
2.
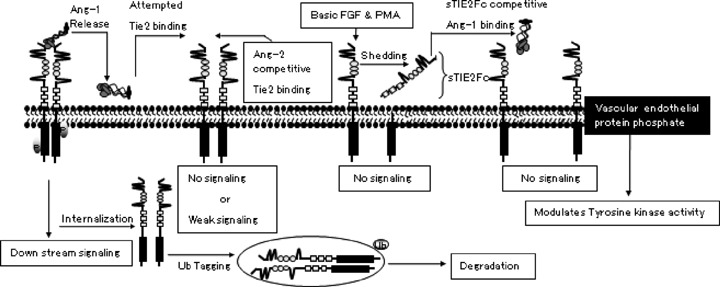
Schematic depiction of Tie2 signallingsignaling regulation. Ang-1 activation of Tie2 subsequently leads to Tie2 internalization and degradation. Tie2 activation can also be inhibited by Ang-2 competitive binding. Stimulator such as PMA (phorbol 12-myristate 13-acetate) and basic FGF (basic fibroblast growth factor) can induce Tie2R shedding, leaving the extracellular domain (sTie2RFc) in the area. sTie2Fc can compete with Tie2 for Ang-1 binding. Vascular endothelial protein phosphatase associates with Tie2 and can also regulate the activity of the tyrosine kinase domain.
Physiological roles
Gene deletion and mutation have made it possible to study the function of Ang-1-Tie2 signalling. Tie2 deficiency or Ang-1 deficiency resulted in embryonic lethality in murine model by day 13. Defects have been seen in the form of poor association between endothelial cells and ECM in the basement membrane, lack of myocardial trabecular projection and poor smooth muscle cell association with vessels [5–7,27]. Unlike Ang-1 deficient mice, Ang-2 deficient mice develop normally, with the exception of mice with C129 genetic background, which develop chylous ascites and die within 14 days. This suggests that Ang-2 is dispensable for proper embryonic development [22]. Embryonic deficiency of Tie1 led to impaired structural integrity of vascular endothelial cells, resulting in oedema and subsequent localized haemorrhage [7,103]. Ang-1-Tie2 has an anti-apoptotic effect in endothelial cells [13]. Ang-1 supports the localization of cell adhesion molecules in cell junctions in endothelial cells, thereby supporting the stabilization of the vessels [64]. Ang-1 regulates endothelial cell recruitment of smooth muscle cells to stabilize the newly formed vessels [5, 17]. Ang-1 has a chemotactic effect on endothelial cells with little to no effect on endothelial cell proliferation [80]. However, some studies have shown mitogenic effect on endothelial cells [87, 104]. Ang-1 also promotes the differentiation of mesenchymal cells to vascular smooth muscle cells [52]. Apart from its involvement in embryogenesis, Ang-1-Tie2 signalling is also involved in the postnatal vascularization and maintenance of mature quiescent vasculature [32]. There is a constitutive expression of Tie2 in the endothelium. This expression is essential in the maintenance of vascular integrity [105, 106].
Ang-1-Tie2 signalling is required during postnatal bone marrow haematopoiesis. Tie2 and Tie1 expression have been found in some haematopoietic cells [107–111]. Tie2 deficient mice exhibit severely impaired haematopoiesis. On the contrary, some studies showed that Tie2 is not required for embryonic haematopoiesis [109, 112–114]. Ang-1-Tie2 plays a role in maintaining haematopoietic stem cells in the quiescent state in the bone marrow. This role is associated with increased adhesion of Tie2-positive stem cells to Ang-1 expressing osteoblast [109, 115]. Ang-1 and 2 activated growth and migration in Tie2 positive trophoblast during placental development [116]. Tie2 expression is also localized to primary cilia of the surface epithelium of the ovary, bursa and extrao-varian rete duct, as well as to motile cilia of the oviduct, suggesting a role for Tie2 signalling in these cells [117]. A subset of Tie2 expressing monocytes accounts for 2–7% of mononuclear cells in a healthy person. An in vitro study demonstrated Ang-2-mediated migration of this subset of monocytes [118]. Ang-1-Tie2 signalling promotes neural outgrowth from dorsal root ganglion cells. Tie2 expression has been discovered in certain neuronal cells. Tie2 signal transduction elicits neuroprotective effects and a mitogenic effect in vitro (Fig. 3) [23, 119–121]. Overexpression of Ang-1 in the forebrain led to increased vascularization as well as changes in dendrite organization of the neurons [122]. Because Tie2 expression has not been observed in vivo, it is that Ang-1 effects might be integrin mediated. This is consistent with the evidence that Ang-1 is able to inter act with certain integrins to activate downstream signalling [88, 89].
3.
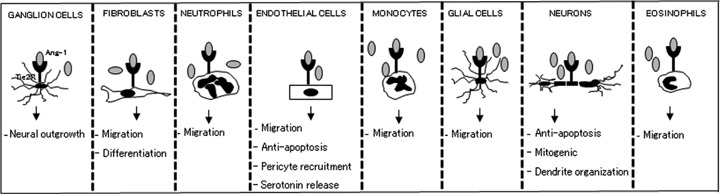
Schematic depiction of Ang-1-Tie2 effect on various cell types. Several cells are capable expression Ang-1 and Tie2 receptors. Ang-1 is activates Tie2 receptors on these cells, leading to downstream signallingsignaling and ultimately regulation of cellular behavioursbehaviors. Some of the various known roles of Ang-1-Tie2 effects in vascular and extravascular cells have been illustrated.
Pathophysiological role
There is sufficient evidence to suggest that both beneficial and deleterious effects can be exerted through the activation of Tie2. Within the scope of this article, summary of the role of Ang-1-Tie2 signalling pathway in rheumatoid arthritis, tumour progression, and asthma is presented in the following section (Fig. 4).
4.
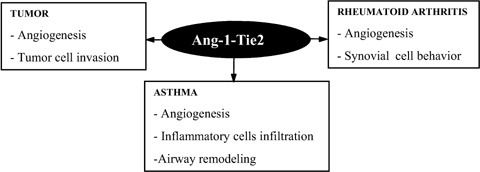
Illustration of the relationship between Ang-1-Tie2 signallingsignaling and pathological conditions. Ang-1-Tie2 signallingsignaling induces various responses in different cells and these responses contribute to the development of various pathological conditions.
Ang-1-Tie2R and rheumatoid arthritis
Ang-1 and Tie2 is up-regulated in cells in rheumatoid arthritis [123]. Ang-1 and Tie2 receptors are expressed on synovial lining cells, endothelial cells, smooth muscle cells and fibroblast in synovial tissue in rheumatoid arthritis patients [12, 13]. In normal specimen, Tie2 expression is limited to the capillary endothelium [12, 124]. In synovial lining cells, Tie2 expression was observed mainly in the basal layer and it was frequently co-localized with vimentin and proliferating nuclear antigen (PCNA). In rheumatoid arthritis, Tie2 positive cells often overexpressed PCNA [12, 13]. However stimulation with Ang-1 did not alter DNA synthesis, so Ang-1 may not be directly involved in fibroblastic synoviocyte proliferation [13]. Inflammatory cytokines, such as TNF-α and TGF-β up-regulate the expression of Tie2 in endothelial cells, and also increase the expression of Ang-1 in other cells in rheumatoid arthritis [54, 58, 123]. Interaction between synoviocytes and endothelial cells led to paracrine regulation of angiogenesis [123]. A recent study showed that osteoblast from rheumatoid arthritis patients constitutively secreted significant amount of Ang-1. Ang-1 production was inhibited by co-stimulation with TNF-α and IFN-γ[59]. Ang-1 induced a chemotactic effect on fibroblastic synoviocyte in rheumatoid arthritis and fibroblast stably transfected with Tie2 [13, 80]. Collagen-induced arthritis was ameliorated after blocking Tie2 signalling [125]. In a recent study, the direct effect of Ang-1-Tie2 signalling on the progression of rheumatoid arthritis was demonstrated [126]. Ang-1 and matrix metalloproteinases (MMP3) were strongly expressed in vivo at the invasive front of the active rheumatoid pannus extensively invading the cartilaginous matrix [126]. Ang-1 induced the activation of NF-κB via the Akt pathway to promote cell proliferation and also inhibited apoptosis via ERK and Akt pathway. Ang-1 activation of ERK and Akt also led to extension of synovial cells. Ang-1 also induced proMMP-3 secretion from synovial cells at levels comparable to those induced by TNF-α, which resulted in direct degradation of the cartilaginous matrix [126]. All of these Ang-1-induced effects validate the role of Ang-1-Tie2 signalling in the destruction of arthritic joint in rheumatoid arthritis.
Ang-1-Tie2 and cancer
Although Tie2 plays a significant role in tumour angiogenesis, its expression is not limited to vascular cells but is also expressed in cells that constitute the origin of the tumour [3, 107, 127–129]. The fact that several tumour cells express high levels of Ang-1 would suggest an autocrine/paracrine loop of Ang-1-Tie2 signalling in the tumour [107, 127]. Also in gastric carcinoma, Ang-2 was expressed not only in endothelial cells but also in cancer cells, suggesting an Ang-2-Tie2 extravascular signalling together with Ang-1-Tie2 signalling [130]. Tie1 is expressed and constitutively phosphorylated to induce downstream signals in cancer cells of the breast, thyroid and gastrointestinal tract and in tumour cell line [131]. There is very little information on Ang-1-Tie2 signalling in cancer, outside the vasculature. Ang-1 has anti-apoptotic effect in neuroblastoma cell [23]. It would be interesting to know if Ang-1-Tie2 signalling would induce anti-apoptotic effect in other tumour cells that express Tie2. A subset of monocytes distinct from inflammatory monocytes expresses Tie2. In cancer patients, this subset of Tie2 expressing monocytes represented the main monocyte population within tumour, distinct from tumour-associated monocytes. Elevated levels of Ang-2 have been observed in tumour. An in vitro study reported Ang-2-mediated migration of these monocytes, suggesting a homing mechanism for these monocytes to tumour sites [118]. Hypoxia stimulates the release of Ang-2 from tumour blood vessel cells, which in turn stimulates the recruitment of Tie2 expressing monocytes from the blood into the tumour and modulate mediator release [55]. Tie2 expression has been reported on subpopulation of glioma cells. The expression of Tie2 correlated with level of tumour progression. Ang-1 stimulation of these Tie2+ glioma cells resulted in an increased adherence of these cells to ECM protein [127]. There was also a correlation between Tie2 activation and up-regulation of inte-grin β1 and phosphorylation of focal adhesion kinase (FAK), and formation of focal adhesions [127]. Integrin β1 is directly involved in the escape of cancer cells from immunological surveillance [132]. By modulating the adhesive properties of tumour cells, Tie2 signalling might be contributing to the invasive properties of the tumour cells. Interaction between tumour cells and ECM is important in tumour development including proliferation, survival and tumour migration [127]. Similar to Ang-1, Ang-2 can also promote tumour metastasis through activation of ανβ1-mediated signalling pathway, leading to increased expression of MMP2 [133].
New blood vessel growth provides the much-needed nutrients for these cells to proliferate. Overexpression of Ang-1 inhibits angiogenesis and tumour growth in different types of cancer, possibly by recruitment of mural cells to become associated with endothelial cells [115, 134]. Paradoxically, inhibition of Ang-1 expression in tumour cells have also been shown to inhibit tumour growth [8, 64, 135]. Contrary to some other reports, Ang-1 can induce a mitogenic effect on endothelial cells in vitro[87]. This may explain why Ang-1 induces tumour angiogenesis in certain contexts. Ang-2 on the other hand is up-regulated by hypoxia and tumour-derived VEGF. Ang- 2 destabilizes the vasculature, supporting initiation of angiogenesis [136]. The role of Ang-1 in tumour angiogenesis is controversial, since some studies have reported Ang-1 as anti-angiogenic and others have reported it as pro-angiogenic [8]. None-the-less, the above information suggests that Tie2 signalling may control critical aspects of tumour behaviours.
Ang-1-Tie2R and asthma
There are elevated levels of Ang-1, VEGF and Ang-2 in induced sputum of asthmatic patients, and this increase correlates with the degree of airway obstruction [137]. Ang-1 levels inversely correlate with vascular permeability index, while Ang-2 and VEGF levels positively correlate with vascular permeability index. Reduction in vascular permeability index by corticosteroids correlates particularly with reduction in VEGF and Ang-2 [137–139]. This suggests that the ratio of localized Ang-1 to Ang-2, and not increase or decrease of Ang-1 and Ang-2, determines the predominant signalling pathway. It would be interesting to examine the effect of specifically blocking Ang2 and VEGF on vascular permeability, as similar studies conducted in cancer research have yielded promising results [140–144]. Severe infiltration of inflammatory cells and hyperplasia of smooth muscle cells may create a state of hypoxia, and it is known that Ang2 is up-regulated under this condition [55, 145]. It would be interesting to find out the levels and origin of these two proteins in the asthmatic lung tissue, as this would help to further elucidate their roles in asthma.
During inflammation, there is a transition of the endothelium from a resting anti-adhesive state to active adhesive state. So, endothelium activation is not only involved in the initiation of angiogenesis, it is also crucial for initiation of inflammation. Ang-1-Tie2 signalling keeps the vasculature in a quiescent state, and is inhibited by the up-regulation of Ang-2 [2, 28]. The antagonistic effect of Ang-2 leads to destabilized endothelial cell junction and pericyte dropout leaving the endothelial cells exposed and also making them more susceptible to activation by mediators, such as VEGF. VEGF induces increased permeability and initiates angiogenesis [28, 145, 146]. VEGF is significantly more potent than Ang-2 at interrupting endothelial tight junction. However, combined stimulation with Ang-2 and VEGF is several times more potent at increasing endothelial cell layer permeability than addition of individual stimulatory effect [147]. Thus, Ang-2 plays a complementary role in VEGF-induced permeability. Also, in the presence of VEGF, Ang-2 promotes angiogenesis, while leading to vessel regression in the absence of VEGF [148]. Ang-2 also promotes adhesion of inflammatory cells by sensitizing endothelial cells towards TNF-α-induced expression of adhesion molecules, leading to increased extravasation of inflammatory cells [21]. Ang-2 deficient mice cannot elicit an inflammatory response in thioglycolate-induced or staphylococcus aureus-induced peritonitis [21, 149], suggesting the critical role of Ang-2-Tie2 signalling in the initiation of inflammatory response.
Balance and sequential expression of Ang-1 and VEGF is necessary for proper and functional formation of new blood vessels [8]. In asthma, there is elevated plasma baseline leakage, suggesting that Ang-1-Tie2 signalling has been compromised and is not balanced with VEGF–VEGFR signalling [149]. Ang-1 can induce anti-permeability effects, as well as inhibit TNF-α and VEGF-induced expression of adhesion molecules such as VCAM-1. Ang-1 also supports the localization of platelet endothelial cell adhesion molecule-1 (PECAM-1) into endothelial cell junctions, while also decreasing the phosphorylation of PECAM-1 and vascular endothelial cadherin. Both of these cell adhesion molecules strengthen the cells junction and supports anti-leakage properties [139, 150–153]. Ang-1 blocked VEGF and TNF-α induced expression of tissue factor in endothelial cells [121]. Ang-1 also blocked NF-kB responsive genes, such as intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1 and E-selectins that are induced by VEGF and TNF-α in endothelial cells [65, 83, 154]. This suggests that Ang-1 may be a potential therapeutic candidate in asthma. However, one has to consider its potential pro-inflammatory and vasoconstrictory effects, as discussed in the following section.
Ang-1-Tie2 signalling is also involved in several pro-inflammatory activities. Ang-1 reduced endothelin-1 expression in endothelial cells in vitro and also blocked TNF-α and lipopolysaccharide (LPS) mediated endothelin-1 expression [67, 155–157]. On the contrary, Ang-1 stimulation of pulmonary endothelial cells from patients with idiopathic pulmonary arterial hypertension increased expression of endothelin-1 and serotonin [158]. Endothelin-1 is a bronchocon-strictory agent with inflammatory properties [158]. This suggests that an Ang-1-based therapy may potentially lead to pulmonary hypertension. Ang-1 blocks Ca+ entry, RhoA activation induced by VEGF, platelet-activating factor (PAF), histamine, bradykinin and thrombin [152, 159–161]. However, Ang-1 can also activate P-selectin translocation and neutrophil adhesion to endothelial cells [162]. Ang-1 by itself has a chemotactic effect on neutrophils and eosinophils, potentially contributing to the inflammatory process. Interestingly, Ang-1 can block VEGF-induced neutrophil and eosinophil chemoattraction [25, 26]. Ang-1 has been shown to induce secretion of plasmin [163]. Ang-1 can also induce the secretion of MMP2 and inhibited the secretion of TIMP-2. Ang-1 also induces expression of proMMP-3 and 9, albeit in small amounts [24, 86, 164]. Secreted MMPs could then be activated by inflammatory cytokines such as TGF-β, suggesting a possible link between Ang-1-Tie2 signalling and exacerbation of subepithelial fibrosis in chronic asthma [14, 165].
Therapeutic implications
Since Ang-1-Tie2 signalling plays a critical role in several pathological conditions, it is a logical target for therapeutic purposes. In physiological processes, there are naturally occurring Tie2 inhibitors that regulate Tie2R functions. sTie2Fc inhibits Tie2 activation and Ang-2 can also inhibit Ang-1-mediated Tie2 activation. In an attempt to control the aberrant angiogenesis that contributes to the pathology of various diseases, development of a therapeutic Tie2 inhibitor has been generating a lot of interest in recent years. In 2001, Abbot Laboratories announced the development of 5-arylpyrrolo[2,3-d]pyrimidin-4-amine derivative, a potent and selective Tie2 inhibitor. Glaxo Smith-Kline company has also developed a Tie2 inhibitor in the form of derivative of 3-arylpyrazo-lo[3,4-d]pyrimidine-6-amine core . More recently, an alkynylpyrimidine amide derivative and 2-(pyridin-2-yl)-1,3,5-triasine have been added to this list of Tie2 inhibitors [166]. Although these inhibitors have been proven effective in murine angiogenesis model, their efficacy in human subjects still needs to be determined. While several studies have shown that Ang-1 may be therapeutic in slowing down angiogenesis, one has to be cautious since Ang-1 could also elicit pro-angiogenic effects [64, 167]. In cell-based Ang-1 gene transfer, there was improvement in morphological, biochemical and molecular parameters of lung injury and inflammation, suggesting a therapeutic role for Ang-1 signalling in treating lung injury [8]. Apart from anti-angiogenic therapy, Ang-1-Tie2 signalling properties have been explored in the development of therapeutic neovascularization. Combined administration of adeno-associated virus-2 vector simultaneously encoding human VEGF (165) and Ang-1, in combination with endothelial progenitor cells have shown considerable efficacy as therapy for ischaemic diseases [168]. Development of RNA apt-mer, antibody and peptide-Fc fusion protein that block binding of Ang-2 to Tie2 have been proven effective as an anti-angiogenic therapy [142, 169]. The ineffectiveness of some of the treatment for several pathological conditions, underscores the need for novel therapy. Probing into various Tie2 signalling pathways, suggests a broad physiological function for Tie2 signalling in different cell types. However, the question is whether or not the therapy using these proteins as target would work without interfering with normal angiogenesis and other physiological functions.
Conclusions
In summary, Ang-1-Tie2 could be potentially important in the development of therapy for numerous pathological conditions. The Ang-1-Tie2 signalling pathway seems to depend on time, dose, source and modification of the Ang-1 protein used in the study. Thus, the biological effect of Ang-1-Tie2 signalling could be tissue specific and dependent on developmental stage. Several seemingly contradictory reports on Tie2 signalling reflect the complexity and perhaps lack of detailed understanding of the Ang-1-Tie2 signalling pathway. Further elucidation of Ang-1-Tie2 and Ang-2-Tie2 signalling would open the door to the emergence of effective therapeutic modalities in various inflammatory diseases.
Acknowledgments
This work was supported by a grant from the Nebraska Cancer and Smoking-Related Diseases Program (LB506) of Nebraska Department of Health (to D.K.A.), and by NIH grants R01HL070885 and R01HLL073349 (both to D.K.A.).
References
- 1.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–9. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 2.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama T, Hatachi G, Wen CY, Yoshizaki A, Yamazumi K, Niino D, Sekine I. Expression and significance of Tie-1 and Tie-2 receptors, and angiopoietins-1, 2 and 4 in colorectal adenocarcinoma: Immunohistochemical analysis and correlation with clinicopathological factors. World J Gastroenterol. 2005;11:964–9. doi: 10.3748/wjg.v11.i7.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnurch H, Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993;119:957–68. doi: 10.1242/dev.119.3.957. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascu-larization. Circ Res. 1998;83:233–40. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 6.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 7.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–4. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 8.Stoeltzing O, Ahmad SA, Liu W, McCarty MF, Parikh AA, Fan F, Reinmuth N, Bucana CD, Ellis LM. Angiopoietin-1 inhibits tumour growth and ascites formation in a murine model of peritoneal car-cinomatosis. Br J Cancer. 2002;87:1182–7. doi: 10.1038/sj.bjc.6600598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voskas D, Jones N, Van Slyke P, Sturk C, Chang W, Haninec A, Babichev YO, Tran J, Master Z, Chen S, Ward N, Cruz M, Jones J, Kerbel RS, Jothy S, Dagnino L, Arbiser J, Klement G, Dumont DJ. A cyclosporine-sensitive psoriasis-like disease produced in Tie2 transgenic mice. Am J Pathol. 2005;166:843–55. doi: 10.1016/S0002-9440(10)62305-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan CC, Du L, Chu D, Cho AJ, Kido M, Wolf PL, Jamieson SW, Thistlethwaite PA. Induction of pulmonary hypertension by an angiopoietin 1/TIE2/serotonin pathway. Proc Natl Acad Sci USA. 2003;100:12331–6. doi: 10.1073/pnas.1933740100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macdonald PR, Progias P, Ciani B, Patel S, Mayer U, Steinmetz MO, Kammerer RA. Structure of the extracellular domain of Tie receptor tyrosine kinases and localization of the angiopoietin-binding epitope. J Biol Chem. 2006;281:28408–14. doi: 10.1074/jbc.M605219200. [DOI] [PubMed] [Google Scholar]
- 12.Uchida T, Nakashima M, Hirota Y, Miyazaki Y, Tsukazaki T, Shindo H. Immunohistochemical local-isation of protein tyrosine kinase receptors Tie-1 and Tie-2 in synovial tissue of rheumatoid arthritis: correlation with angiogenesis and synovial proliferation. Ann Rheum Dis. 2000;59:607–14. doi: 10.1136/ard.59.8.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahara K, Iioka T, Furukawa K, Uchida T, Nakashima M, Tsukazaki T, Shindo H. Autocrine/paracrine role of the angiopoietin-1 and -2/Tie2 system in cell proliferation and chemotaxis of cultured fibroblastic synoviocytes in rheumatoid arthritis. Hum Pathol. 2004;35:150–8. doi: 10.1016/j.humpath.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Makinde T, Murphy RF, Agrawal DK. Immunomodulatory role of vascular endothelial growth factor and angiopoietin-1 in airway remodeling. Curr Mol Med. 2006;6:831–41. doi: 10.2174/156652406779010795. [DOI] [PubMed] [Google Scholar]
- 15.Homer JJ, Greenman J, Drevs J, Marme D, Stafford ND. Soluble Tie-2 receptor levels independently predict locoregional recurrence in head and neck squamous cell carcinoma. Head Neck. 2002;24:773–8. doi: 10.1002/hed.10123. [DOI] [PubMed] [Google Scholar]
- 16.Wen CY, Ito M, Wang H, Chen LD, Xu ZM, Matsuu M, Shichijo K, Nakayama T, Nakashima M, Sekine I. IL-11 up-regulates Tie-2 expression during the healing of gastric ulcers in rats. World J Gastroenterol. 2003;9:788–90. doi: 10.3748/wjg.v9.i4.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metheny-Barlow LJ, Tian S, Hayes AJ, Li LY. Direct chemotactic action of angiopoietin-1 on mesenchy-mal cells in the presence of VEGF. Microvasc Res. 2004;68:221–30. doi: 10.1016/j.mvr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Dube A, Thai S, Gaspar J, Rudders S, Libermann TA, Iruela-Arispe L, Oettgen P. Elf-1 is a transcriptional regulator of the Tie2 gene during vascular development. Circ Res. 2001;88:237–44. doi: 10.1161/01.res.88.2.237. [DOI] [PubMed] [Google Scholar]
- 19.Runting AS, Stacker SA, Wilks AF. tie2, a putative protein tyrosine kinase from a new class of cell surface receptor. Growth Factors. 1993;9:99–105. [PubMed] [Google Scholar]
- 20.Anghelina M, Moldovan L, Moldovan NI. Preferential activity of Tie2 promoter in arteriolar endothelium. J Cell Mol Med. 2005;9:113–21. doi: 10.1111/j.1582-4934.2005.tb00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–9. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 22.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–23. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 23.Kosacka J, Figiel M, Engele J, Hilbig H, Majewski M, Spanel-Borowski K. Angiopoietin-1 promotes neurite outgrowth from dorsal root ganglion cells positive for Tie-2 receptor. Cell Tissue Res. 2005;320:11–9. doi: 10.1007/s00441-004-1068-2. [DOI] [PubMed] [Google Scholar]
- 24.Iurlaro M, Scatena M, Zhu WH, Fogel E, Wieting SL, Nicosia RF. Rat aorta-derived mural precursor cells express the Tie2 receptor and respond directly to stimulation by angiopoietins. J Cell Sci. 2003;116:3635–43. doi: 10.1242/jcs.00629. [DOI] [PubMed] [Google Scholar]
- 25.Brkovic A, Pelletier M, Girard D, Sirois MG. Angiopoietin chemotactic activities on neutrophils are regulated by PI-3K activation. J Leukoc Biol. 2007;81:1093–101. doi: 10.1189/jlb.0906580. [DOI] [PubMed] [Google Scholar]
- 26.Feistritzer C, Mosheimer BA, Sturn DH, Bijuklic K, Patsch JR, Wiedermann CJ. Expression and function of the angiopoietin receptor Tie-2 in human eosinophils. J Allergy Clin Immunol. 2004;114:1077–84. doi: 10.1016/j.jaci.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 27.Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–41. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 29.Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, McClain J, Copeland NG, Gilbert DJ, Jenkins NA, Huang T, Papadopoulos N, Maisonpierre PC, Davis S, Yancopoulos GD. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci USA. 1999;96:1904–9. doi: 10.1073/pnas.96.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saharinen P, Kerkela K, Ekman N, Marron M, Brindle N, Lee GM, Augustin H, Koh GY, Alitalo K. Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2. J Cell Biol. 2005;169:239–43. doi: 10.1083/jcb.200411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan HT, Venkatesha S, Chan B, Deutsch U, Mammoto T, Sukhatme VP, Woolf AS, Karumanchi SA. Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J. 2007;21:3171–83. doi: 10.1096/fj.07-8487com. [DOI] [PubMed] [Google Scholar]
- 32.Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res. 1997;81:567–74. doi: 10.1161/01.res.81.4.567. [DOI] [PubMed] [Google Scholar]
- 33.Yabkowitz R, Meyer S, Yanagihara D, Brankow D, Staley T, Elliott G, Hu S, Ratzkin B. Regulation of tie receptor expression on human endothelial cells by protein kinase C-mediated release of soluble tie. Blood. 1997;90:706–15. [PubMed] [Google Scholar]
- 34.Yabkowitz R, Meyer S, Black T, Elliott G, Merewether LA, Yamane HK. Inflammatory cytokines and vascular endothelial growth factor stimulate the release of soluble tie receptor from human endothelial cells via metalloprotease activation. Blood. 1999;93:1969–79. [PubMed] [Google Scholar]
- 35.Chen-Konak L, Guetta-Shubin Y, Yahav H, Shay-Salit A, Zilberman M, Binah O, Resnick N. Transcriptional and post-translation regulation of the Tie1 receptor by fluid shear stress changes in vascular endothelial cells. FASEB J. 2003;17:2121–3. doi: 10.1096/fj.02-1151fje. [DOI] [PubMed] [Google Scholar]
- 36.Tsiamis AC, Morris PN, Marron MB, Brindle NP. Vascular endothelial growth factor modulates the Tie-2: Tie-1 receptor complex. Microvasc Res. 2002;63:149–58. doi: 10.1006/mvre.2001.2377. [DOI] [PubMed] [Google Scholar]
- 37.Barton WA, Tzvetkova-Robev D, Miranda EP, Kolev MV, Rajashankar KR, Himanen JP, Nikolov DB. Crystal structures of the Tie2 receptor ectodomain and the angiopoietin-2-Tie2 complex. Nat Struct Mol Biol. 2006;13:524–32. doi: 10.1038/nsmb1101. [DOI] [PubMed] [Google Scholar]
- 38.Shewchuk LM, Hassell AM, Ellis B, Holmes WD, Davis R, Horne EL, Kadwell SH, McKee DD, Moore JT. Structure of the Tie2 RTK domain: self-inhibition by the nucleotide binding loop, activation loop, and C-terminal tail. Structure. 2000;8:1105–13. doi: 10.1016/s0969-2126(00)00516-5. [DOI] [PubMed] [Google Scholar]
- 39.Dube A, Akbarali Y, Sato TN, Libermann TA, Oettgen P. Role of the Ets transcription factors in the regulation of the vascular-specific Tie2 gene. Circ Res. 1999;84:1177–85. doi: 10.1161/01.res.84.10.1177. [DOI] [PubMed] [Google Scholar]
- 40.Yamakawa M, Liu LX, Belanger AJ, Date T, Kuriyama T, Goldberg MA, Cheng SH, Gregory RJ, Jiang C. Expression of angiopoietins in renal epithelial and clear cell carcinoma cells: regulation by hypoxia and participation in angiogenesis. Am J Physiol Renal Physiol. 2004;287:F649–57. doi: 10.1152/ajprenal.00028.2004. [DOI] [PubMed] [Google Scholar]
- 41.Christensen RA, Fujikawa K, Madore R, Oettgen P, Varticovski L. NERF2, a member of the Ets family of transcription factors, is increased in response to hypoxia and angiopoietin-1: a potential mechanism for Tie2 regulation during hypoxia. J Cell Biochem. 2002;85:505–15. doi: 10.1002/jcb.10148. [DOI] [PubMed] [Google Scholar]
- 42.Park YS, Kim NH, Jo I. Hypoxia and vascular endothelial growth factor acutely up-regulate angiopoietin-1 and Tie2 mRNA in bovine retinal pericytes. Microvasc Res. 2003;65:125–31. doi: 10.1016/s0026-2862(02)00035-3. [DOI] [PubMed] [Google Scholar]
- 43.Procopio WN, Pelavin PI, Lee WM, Yeilding NM. Angiopoietin-1 and -2 coiled coil domains mediate distinct homo-oligomerization patterns, but fibrinogen-like domains mediate ligand activity. J Biol Chem. 1999;274:30196–201. doi: 10.1074/jbc.274.42.30196. [DOI] [PubMed] [Google Scholar]
- 44.Davis S, Papadopoulos N, Aldrich TH, Maisonpierre PC, Huang T, Kovac L, Xu A, Leidich R, Radziejewska E, Rafique A, Goldberg J, Jain V, Bailey K, Karow M, Fandl J, Samuelsson SJ, Ioffe E, Rudge JS, Daly TJ, Radziejewski C, Yancopoulos GD. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat Struct Biol. 2003;10:38–44. doi: 10.1038/nsb880. [DOI] [PubMed] [Google Scholar]
- 45.Sato A, Iwama A, Takakura N, Nishio H, Yancopoulos GD, Suda T. Characterization of TEK receptor tyrosine kinase and its ligands, Angiopoietins, in human hematopoietic progenitor cells. Int Immunol. 1998;10:1217–27. doi: 10.1093/intimm/10.8.1217. [DOI] [PubMed] [Google Scholar]
- 46.Brown LF, Dezube BJ, Tognazzi K, Dvorak HF, Yancopoulos GD. Expression of Tie1, Tie2, and angiopoietins 1, 2, and 4 in Kaposi's sarcoma and cutaneous angiosarcoma. Am J Pathol. 2000;156:2179–83. doi: 10.1016/S0002-9440(10)65088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HJ, Cho CH, Hwang SJ, Choi HH, Kim KT, Ahn SY, Kim JH, Oh JL, Lee GM, Koh GY. Biological characterization of angiopoietin-3 and angiopoietin- 4. FASEB J. 2004;18:1200–8. doi: 10.1096/fj.03-1466com. [DOI] [PubMed] [Google Scholar]
- 48.Kim KT, Choi HH, Steinmetz MO, Maco B, Kammerer RA, Ahn SY, Kim HZ, Lee GM, Koh GY. Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. J Biol Chem. 2005;280:20126–31. doi: 10.1074/jbc.M500292200. [DOI] [PubMed] [Google Scholar]
- 49.Xu Y, Yu Q. Angiopoietin-1, unlike angiopoietin-2, is incorporated into the extracellular matrix via its linker peptide region. J Biol Chem. 2001;276:34990–8. doi: 10.1074/jbc.M103661200. [DOI] [PubMed] [Google Scholar]
- 50.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–6. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Liu YJ, Yu Q. Angiopoietin-3 is tethered on the cell surface via heparan sulfate proteoglycans. J Biol Chem. 2004;279:41179–88. doi: 10.1074/jbc.M400292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown C, Gaspar J, Pettit A, Lee R, Gu X, Wang H, Manning C, Voland C, Goldring SR, Goldring MB, Libermann TA, Gravallese EM, Oettgen P. ESE-1 is a novel transcriptional mediator of angiopoietin-1 expression in the setting of inflammation. J Biol Chem. 2004;279:12794–803. doi: 10.1074/jbc.M308593200. [DOI] [PubMed] [Google Scholar]
- 53.Ye FC, Blackbourn DJ, Mengel M, Xie JP, Qian LW, Greene W, Yeh IT, Graham D, Gao SJ. Kaposi's sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and Ets1. J Virol. 2007;81:3980–91. doi: 10.1128/JVI.02089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott BB, Zaratin PF, Colombo A, Hansbury MJ, Winkler JD, Jackson JR. Constitutive expression of angiopoietin-1 and -2 and modulation of their expression by inflammatory cytokines in rheumatoid arthritis synovial fibroblasts. J Rheumatol. 2002;29:230–9. [PubMed] [Google Scholar]
- 55.Lewis CE, Hughes R. Inflammation and breast cancer. Microenvironmental factors regulating macrophage function in breast tumours: hypoxia and angiopoietin-2. Breast Cancer Res. 2007;9:209. doi: 10.1186/bcr1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan F, Stoeltzing O, Liu W, McCarty MF, Jung YD, Reinmuth N, Ellis LM. Interleukin-1beta regulates angiopoietin-1 expression in human endothelial cells. Cancer Res. 2004;64:3186–90. doi: 10.1158/0008-5472.can-03-0407. [DOI] [PubMed] [Google Scholar]
- 57.Gravallese EM, Pettit AR, Lee R, Madore R, Manning C, Tsay A, Gaspar J, Goldring MB, Goldring SR, Oettgen P. Angiopoietin-1 is expressed in the synovium of patients with rheumatoid arthritis and is induced by tumour necrosis factor alpha. Ann Rheum Dis. 2003;62:100–7. doi: 10.1136/ard.62.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott BB, Zaratin PF, Gilmartin AG, Hansbury MJ, Colombo A, Belpasso C, Winkler JD, Jackson JR. TNF-alpha modulates angiopoietin-1 expression in rheumatoid synovial fibroblasts via the NF-kappa B signalling pathway. Biochem Biophys Res Commun. 2005;328:409–14. doi: 10.1016/j.bbrc.2004.12.180. [DOI] [PubMed] [Google Scholar]
- 59.Kasama T, Isozaki T, Odai T, Matsunawa M, Wakabayashi K, Takeuchi HT, Matsukura S, Adachi M, Tezuka M, Kobayashi K. Expression of angiopoietin-1 in osteoblasts and its inhibition by tumor necrosis factor-alpha and interferon-gamma. Transl Res. 2007;149:265–73. doi: 10.1016/j.trsl.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem. 1999;274:15732–9. doi: 10.1074/jbc.274.22.15732. [DOI] [PubMed] [Google Scholar]
- 61.Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res. 1998;83:852–9. doi: 10.1161/01.res.83.8.852. [DOI] [PubMed] [Google Scholar]
- 62.Hangai M, Murata T, Miyawaki N, Spee C, Lim JI, He S, Hinton DR, Ryan SJ. Angiopoietin-1 upregulation by vascular endothelial growth factor in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42:1617–25. [PubMed] [Google Scholar]
- 63.Murray BW, Padrique ES, Pinko C, McTigue MA. Mechanistic effects of autophosphorylation on receptor tyrosine kinase catalysis: enzymatic characterization of Tie2 and phospho-Tie2. Biochemistry. 2001;40:10243–53. doi: 10.1021/bi010959e. [DOI] [PubMed] [Google Scholar]
- 64.Stoeltzing O, Ahmad SA, Liu W, McCarty MF, Wey JS, Parikh AA, Fan F, Reinmuth N, Kawaguchi M, Bucana CD, Ellis LM. Angiopoietin-1 inhibits vascular permeability, angiogenesis, and growth of hepatic colon cancer tumors. Cancer Res. 2003;63:3370–7. [PubMed] [Google Scholar]
- 65.Hughes DP, Marron MB, Brindle NP. The antiinflammatory endothelial tyrosine kinase Tie2 interacts with a novel nuclear factor-kappaB inhibitor ABIN-2. Circ Res. 2003;92:630–6. doi: 10.1161/01.RES.0000063422.38690.DC. [DOI] [PubMed] [Google Scholar]
- 66.Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86:4–5. doi: 10.1161/01.res.86.1.4. [DOI] [PubMed] [Google Scholar]
- 67.McCarter SD, Lai PF, Suen RS, Stewart DJ. Regulation of endothelin-1 by angiopoietin-1: implications for inflammation. Exp Biol Med. 2006;231:985–91. [PubMed] [Google Scholar]
- 68.Maulik N, Das DK. Potentiation of angiogenic response by ischemic and hypoxic preconditioning of the heart. J Cell Mol Med. 2002;6:13–24. doi: 10.1111/j.1582-4934.2002.tb00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harfouche R, Hassessian HM, Guo Y, Faivre V, Srikant CB, Yancopoulos GD, Hussain SN. Mechanisms which mediate the antiapoptotic effects of angiopoietin-1 on endothelial cells. Microvasc Res. 2002;64:135–47. doi: 10.1006/mvre.2002.2421. [DOI] [PubMed] [Google Scholar]
- 70.Daly C, Wong V, Burova E, Wei Y, Zabski S, Griffiths J, Lai KM, Lin HC, Ioffe E, Yancopoulos GD, Rudge JS. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18:1060–71. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA. 2004;101:2975–80. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lelievre E, Bourbon PM, Duan LJ, Nussbaum RL, Fong GH. Deficiency in the p110alpha subunit of PI3K results in diminished Tie2 expression and Tie2(-/-)-like vascular defects in mice. Blood. 2005;105:3935–8. doi: 10.1182/blood-2004-10-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones N, Dumont DJ. The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene. 1998;17:1097–108. doi: 10.1038/sj.onc.1202115. [DOI] [PubMed] [Google Scholar]
- 74.Master Z, Jones N, Tran J, Jones J, Kerbel RS, Dumont DJ. Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak. EMBO J. 2001;20:5919–28. doi: 10.1093/emboj/20.21.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones N, Master Z, Jones J, Bouchard D, Gunji Y, Sasaki H, Daly R, Alitalo K, Dumont DJ. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem. 1999;274:30896–905. doi: 10.1074/jbc.274.43.30896. [DOI] [PubMed] [Google Scholar]
- 76.Harfouche R, Gratton JP, Yancopoulos GD, Noseda M, Karsan A, Hussain SN. Angiopoietin-1 activates both anti- and proapoptotic mitogen-activated protein kinases. FASEB J. 2003;17:1523–5. doi: 10.1096/fj.02-0698fje. [DOI] [PubMed] [Google Scholar]
- 77.Fujikawa K, De Aos Scherpenseel I, Jain SK, Presman E, Christensen RA, Varticovski L. Role of PI 3-kinase in angiopoietin-1-mediated migration and attachment-dependent survival of endothelial cells. Exp Cell Res. 1999;253:663–72. doi: 10.1006/excr.1999.4693. [DOI] [PubMed] [Google Scholar]
- 78.Harfouche R, Hussain SN. Signaling and regulation of endothelial cell survival by angiopoietin-2. Am J Physiol Heart Circ Physiol. 2006;291:H1635–45. doi: 10.1152/ajpheart.01318.2005. [DOI] [PubMed] [Google Scholar]
- 79.Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa WC. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J Biol Chem. 2001;276:30359–65. doi: 10.1074/jbc.M009698200. [DOI] [PubMed] [Google Scholar]
- 80.Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem. 1998;273:18514–21. doi: 10.1074/jbc.273.29.18514. [DOI] [PubMed] [Google Scholar]
- 81.Mochizuki Y, Nakamura T, Kanetake H, Kanda S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci. 2002;115:175–83. doi: 10.1242/jcs.115.1.175. [DOI] [PubMed] [Google Scholar]
- 82.Harfouche R, Malak NA, Brandes RP, Karsan A, Irani K, Hussain SN. Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. FASEB J. 2005;19:1728–30. doi: 10.1096/fj.04-3621fje. [DOI] [PubMed] [Google Scholar]
- 83.Tadros A, Hughes DP, Dunmore BJ, Brindle NP. ABIN-2 protects endothelial cells from death and has a role in the antiapoptotic effect of angiopoietin-1. Blood. 2003;102:4407–9. doi: 10.1182/blood-2003-05-1602. [DOI] [PubMed] [Google Scholar]
- 84.Audero E, Cascone I, Maniero F, Napione L, Arese M, Lanfrancone L, Bussolino F. Adaptor ShcA protein binds tyrosine kinase Tie2 receptor and regulates migration and sprouting but not survival of endothelial cells. J Biol Chem. 2004;279:13224–33. doi: 10.1074/jbc.M307456200. [DOI] [PubMed] [Google Scholar]
- 85.Korpelainen EI, Karkkainen M, Gunji Y, Vikkula M, Alitalo K. Endothelial receptor tyrosine kinases activate the STAT signaling pathway: mutant Tie-2 causing venous malformations signals a distinct STAT activation response. Oncogene. 1999;18:1–8. doi: 10.1038/sj.onc.1202288. [DOI] [PubMed] [Google Scholar]
- 86.Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, Ahn BC, Koh GY. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000;86:952–9. doi: 10.1161/01.res.86.9.952. [DOI] [PubMed] [Google Scholar]
- 87.Kanda S, Miyata Y, Mochizuki Y, Matsuyama T, Kanetake H. Angiopoietin 1 is mitogenic for cultured endothelial cells. Cancer Res. 2005;65:6820–7. doi: 10.1158/0008-5472.CAN-05-0522. [DOI] [PubMed] [Google Scholar]
- 88.Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170:993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ Res. 2005;96:e8–24. doi: 10.1161/01.RES.0000158285.57191.60. [DOI] [PubMed] [Google Scholar]
- 90.Weber CC, Cai H, Ehrbar M, Kubota H, Martiny-Baron G, Weber W, Djonov V, Weber E, Mallik AS, Fussenegger M, Frei K, Hubbell JA, Zisch AH. Effects of protein and gene transfer of the angiopoietin-1 fibrinogen-like receptor-binding domain on endothelial and vessel organization. J Biol Chem. 2005;280:22445–53. doi: 10.1074/jbc.M410367200. [DOI] [PubMed] [Google Scholar]
- 91.Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276:26516–25. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- 92.Wang H, Zhang Y, Toratani S, Okamoto T. Transformation of vascular endothelial cells by a point mutation in the Tie2 gene from human intramuscular haemangioma. Oncogene. 2004;23:8700–4. doi: 10.1038/sj.onc.1208006. [DOI] [PubMed] [Google Scholar]
- 93.Bogdanovic E, Nguyen VP, Dumont DJ. Activation of Tie2 by angiopoietin-1 and angiopoietin-2 results in their release and receptor internalization. J Cell Sci. 2006;119:3551–60. doi: 10.1242/jcs.03077. [DOI] [PubMed] [Google Scholar]
- 94.Reusch P, Barleon B, Weindel K, Martiny-Baron G, Gödde A, Siemeister G, Marmé D. Identification of a soluble form of the angiopoietin receptor TIE-2 released from endothelial cells and present in human blood. Angiogenesis. 2001;4:123–31. doi: 10.1023/a:1012226627813. [DOI] [PubMed] [Google Scholar]
- 95.Lip PL, Chatterjee S, Caine GJ, Hope-Ross M, Gibson J, Blann AD, Lip GY. Plasma vascular endothelial growth factor, angiopoietin-2, and soluble angiopoietin receptor tie-2 in diabetic retinopathy: effects of laser photocoagulation and angiotensin receptor blockade. Br J Ophthalmol. 2004;88:1543–6. doi: 10.1136/bjo.2004.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Findley CM, Cudmore MJ, Ahmed A, Kontos CD. VEGF Induces Tie2 Shedding via a Phosphoinositide 3-Kinase/Akt-Dependent Pathway to Modulate Tie2 Signaling. Arterioscler Thromb Vasc Biol. 2007;27:2619–26. doi: 10.1161/ATVBAHA.107.150482. [DOI] [PubMed] [Google Scholar]
- 97.Chin KF, Greenman J, Reusch P, Gardiner E, Marme D, Monson J. Changes in serum soluble VEGFR-1 and Tie-2 receptors in colorectal cancer patients following surgical resections. Anticancer Res. 2004;24:2353–7. [PubMed] [Google Scholar]
- 98.Schimming R, Gellrich NC, Eyrich G. Markers in patients with squamous cell carcinoma of the oral cavity. Expression and long-term follow-up of VEGF, FLT-1 and Tie2 in serum. Hno. 2004;52:235–41. doi: 10.1007/s00106-003-0915-x. [DOI] [PubMed] [Google Scholar]
- 99.Hashimoto T, Wu Y, Boudreau N, Li J, Matsumoto M, Young W. Regulation of tie2 expression by angiopoietin–potential feedback system. Endothelium. 2004;11:207–10. doi: 10.1080/10623320490512417. [DOI] [PubMed] [Google Scholar]
- 100.Fachinger G, Deutsch U, Risau W. Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2. Oncogene. 1999;18:5948–53. doi: 10.1038/sj.onc.1202992. [DOI] [PubMed] [Google Scholar]
- 101.Niu XL, Peters KG, Kontos CD. Deletion of the car-boxyl terminus of Tie2 enhances kinase activity, signaling, and function. Evidence for an autoinhibitory mechanism. J Biol Chem. 2002;277:31768–73. doi: 10.1074/jbc.M203995200. [DOI] [PubMed] [Google Scholar]
- 102.Park EH, Lee JM, Blais JD, Bell JC, Pelletier J. Internal translation initiation mediated by the angiogenic factor Tie2. J Biol Chem. 2005;280:20945–53. doi: 10.1074/jbc.M412744200. [DOI] [PubMed] [Google Scholar]
- 103.Koblizek TI, Runting AS, Stacker SA, Wilks AF, Risau W, Deutsch U. Tie2 receptor expression and phosphorylation in cultured cells and mouse tissues. Eur J Biochem. 1997;244:774–9. doi: 10.1111/j.1432-1033.1997.00774.x. [DOI] [PubMed] [Google Scholar]
- 104.Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmén C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–8. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 105.Pfaff D, Fiedler U, Augustin HG. Emerging roles of the Angiopoietin-Tie and the ephrin-Eph systems as regulators of cell trafficking. J Leukoc Biol. 2006;80:719–26. doi: 10.1189/jlb.1105652. [DOI] [PubMed] [Google Scholar]
- 106.Niu Q, Perruzzi C, Voskas D, Lawler J, Dumont DJ, Benjamin LE. Inhibition of Tie-2 signaling induces endothelial cell apoptosis, decreases Akt signaling, and induces endothelial cell expression of the endogenous anti-angiogenic molecule, throm-bospondin-1. Cancer Biol Ther. 2004;3:402–5. doi: 10.4161/cbt.3.4.735. [DOI] [PubMed] [Google Scholar]
- 107.Kukk E, Wartiovaara U, Gunji Y, Kaukonen J, Bühring HJ, Rappold I, Matikainen MT, Vihko P, Partanen J, Palotie A, Alitalo K, Alitalo R. Analysis of Tie receptor tyrosine kinase in haemopoietic progenitor and leukaemia cells. Br J Haematol. 1997;98:195–203. doi: 10.1046/j.1365-2141.1997.1732989.x. [DOI] [PubMed] [Google Scholar]
- 108.Iwama A, Hamaguchi I, Hashiyama M, Murayama Y, Yasunaga K, Suda T. Molecular cloning and characterization of mouse TIE and TEK receptor tyrosine kinase genes and their expression in hematopoietic stem cells. Biochem Biophys Res Commun. 1993;195:301–9. doi: 10.1006/bbrc.1993.2045. [DOI] [PubMed] [Google Scholar]
- 109.Takakura N, Huang XL, Naruse T, Hamaguchi I, Dumont DJ, Yancopoulos GD, Suda T. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9:677–86. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- 110.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 111.Shaw JP, Basch R, Shamamian P. Hematopoietic stem cells and endothelial cell precursors express Tie-2, CD31 and CD45. Blood Cells Mol Dis. 2004;32:168–75. doi: 10.1016/j.bcmd.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 112.Puri MC, Bernstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc Natl Acad Sci USA. 2003;100:12753–8. doi: 10.1073/pnas.2133552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Partanen J, Puri MC, Schwartz L, Fischer KD, Bernstein A, Rossant J. Cell autonomous functions of the receptor tyrosine kinase TIE in a late phase of angiogenic capillary growth and endothelial cell survival during murine development. Development. 1996;122:3013–21. doi: 10.1242/dev.122.10.3013. [DOI] [PubMed] [Google Scholar]
- 114.Hamaguchi I, Morisada T, Azuma M, Murakami K, Kuramitsu M, Mizukami T, Ohbo K, Yamaguchi K, Oike Y, Dumont DJ, Suda T. Loss of Tie2 receptor compromises embryonic stem cell-derived endothelial but not hematopoietic cell survival. Blood. 2006;107:1207–13. doi: 10.1182/blood-2005-05-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian S, Hayes AJ, Metheny-Barlow LJ, Li LY. Stabilization of breast cancer xenograft tumour neovasculature by angiopoietin-1. Br J Cancer. 2002;86:645–51. doi: 10.1038/sj.bjc.6600082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dunk C, Shams M, Nijjar S, Rhaman M, Qiu Y, Bussolati B, Ahmed A. Angiopoietin-1 and angiopoietin-2 activate trophoblast Tie-2 to promote growth and migration during placental development. Am J Pathol. 2000;156:2185–99. doi: 10.1016/S0002-9440(10)65089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Teilmann SC, Christensen ST. Localization of the angiopoietin receptors Tie-1 and Tie-2 on the primary cilia in the female reproductive organs. Cell Biol Int. 2005;29:340–6. doi: 10.1016/j.cellbi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 118.Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–85. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 119.Poncet S, Gasc JM, Janzer RC, Meyer S, Juillerat-Jeanneret L. Expression of Tie-2 in human peripheral and autonomic nervous system. Neuropathol Appl Neurobiol. 2003;29:361–9. doi: 10.1046/j.1365-2990.2003.00472.x. [DOI] [PubMed] [Google Scholar]
- 120.Valable S, Bellail A, Lesne S, Liot G, Mackenzie ET, Vivien D, Bernaudin M, Petit E. Angiopoietin-1-induced PI3-kinase activation prevents neuronal apoptosis. FASEB J. 2003;17:443–5. doi: 10.1096/fj.02-0372fje. [DOI] [PubMed] [Google Scholar]
- 121.Kim I, Oh JL, Ryu YS, So JN, Sessa WC, Walsh K, Koh GY. Angiopoietin-1 negatively regulates expression and activity of tissue factor in endothelial cells. FASEB J. 2002;16:126–8. doi: 10.1096/fj.01-0556fje. [DOI] [PubMed] [Google Scholar]
- 122.Ward NL, Putoczki T, Mearow K, Ivanco TL, Dumont DJ. Vascular-specific growth factor angiopoietin 1 is involved in the organization of neuronal processes. J Comp Neurol. 2005;482:244–56. doi: 10.1002/cne.20422. [DOI] [PubMed] [Google Scholar]
- 123.DeBusk LM, Chen Y, Nishishita T, Chen J, Thomas JW, Lin PC. Tie2 receptor tyrosine kinase, a major mediator of tumor necrosis factor alpha-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum. 2003;48:2461–71. doi: 10.1002/art.11213. [DOI] [PubMed] [Google Scholar]
- 124.Shahrara S, Volin MV, Connors MA, Haines GK, Koch AE. Differential expression of the angiogenic Tie receptor family in arthritic and normal synovial tissue. Arthritis Res. 2002;4:201–8. doi: 10.1186/ar407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen Y, Donnelly E, Kobayashi H, Debusk LM, Lin PC. Gene therapy targeting the Tie2 function ameliorates collagen-induced arthritis and protects against bone destruction. Arthritis Rheum. 2005;52:1585–94. doi: 10.1002/art.21016. [DOI] [PubMed] [Google Scholar]
- 126.Hashiramoto A, Sakai C, Yoshida K, Tsumiyama K, Miura Y, Shiozawa K, Nose M, Komai K, Shiozawa S. Angiopoietin 1 directly induces destruction of the rheumatoid joint by cooperative, but independent, signaling via ERK/MAPK and phosphatidylinositol 3-kinase/Akt. Arthritis Rheum. 2007;56:2170–9. doi: 10.1002/art.22727. [DOI] [PubMed] [Google Scholar]
- 127.Lee OH, Xu J, Fueyo J, Fuller GN, Aldape KD, Alonso MM, Piao Y, Liu TJ, Lang FF, Bekele BN, Gomez-Manzano C. Expression of the receptor tyrosine kinase Tie2 in neoplastic glial cells is associated with integrin beta1-dependent adhesion to the extracellular matrix. Mol Cancer Res. 2006;4:915–26. doi: 10.1158/1541-7786.MCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 128.Yu Y, Varughese J, Brown LF, Mulliken JB, Bischoff J. Increased Tie2 expression, enhanced response to angiopoietin-1, and dysregulated angiopoietin-2 expression in hemangioma-derived endothelial cells. Am J Pathol. 2001;159:2271–80. doi: 10.1016/S0002-9440(10)63077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakayama T, Yoshizaki A, Kawahara N, Ohtsuru A, Wen CY, Fukuda E, Nakashima M, Sekine I. Expression of Tie-1 and 2 receptors, and angiopoietin-1, 2 and 4 in gastric carcinoma; immunohisto-chemical analyses and correlation with clinicopathological factors. Histopathology. 2004;44:232–9. doi: 10.1111/j.0309-0167.2004.01817.x. [DOI] [PubMed] [Google Scholar]
- 130.Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145–53. [PubMed] [Google Scholar]
- 131.Rees KA, Singh H, Brindle NP. The receptor tyrosine kinase Tie1 is expressed and activated in epithelial tumour cell lines. Int J Oncol. 2007;31:893–7. [PubMed] [Google Scholar]
- 132.Yasuda M, Tanaka Y, Tamura M, Fujii K, Sugaya M, So T, Takenoyama M, Yasumoto K. Stimulation of beta1 integrin down-regulates ICAM-1 expression and ICAM-1-dependent adhesion of lung cancer cells through focal adhesion kinase. Cancer Res. 2001;61:2022–30. [PubMed] [Google Scholar]
- 133.Hu B, Jarzynka MJ, Guo P, Imanishi Y, Schlaepfer DD, Cheng SY. Angiopoietin 2 induces glioma cell invasion by stimulating matrix metalloprotease 2 expression through the alphavbeta1 integrin and focal adhesion kinase signaling pathway. Cancer Res. 2006;66:775–83. doi: 10.1158/0008-5472.CAN-05-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hayes AJ, Huang WQ, Yu J, Maisonpierre PC, Liu A, Kern FG, Lippmann ME, McLeskey SW, Li LY. Expression and function of angiopoietin-1 in breast cancer. Br J Cancer. 2000;83:1154–60. doi: 10.1054/bjoc.2000.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beecken WD, Kramer W, Jonas D. New molecular mediators in tumor angiogenesis. J Cell Mol Med. 2000;4:262–9. doi: 10.1111/j.1582-4934.2000.tb00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang L, Yang N, Park JW, Katsanos D, Fracchioli S, Cao G, O’Brien-Jenkins A, Randall TC, Rubin SC, Coukos G. Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res. 2003;63:3403–12. [PubMed] [Google Scholar]
- 137.Kanazawa H, Nomura S, Asai K. Roles of angiopoietin-1 and angiopoietin-2 on airway microvascular permeability in asthmatic patients. Chest. 2007;131:1035–41. doi: 10.1378/chest.06-2758. [DOI] [PubMed] [Google Scholar]
- 138.Feltis BN, Wignarajah D, Zheng L, Ward C, Reid D, Harding R, Walters EH. Increased vascular endothelial growth factor and receptors: relationship to angiogenesis in asthma. Am J Respir Crit Care Med. 2006;173:1201–7. doi: 10.1164/rccm.200507-1105OC. [DOI] [PubMed] [Google Scholar]
- 139.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 140.Sarraf-Yazdi S, Mi J, Moeller BJ, Niu X, White RR, Kontos CD, Sullenger BA, Dewhirst MW, Clary BM. Inhibition of in vivo tumor angiogenesis and growth via systemic delivery of an angiopoietin 2-specific RNA aptamer. J Surg Res. 2008;146:16–23. doi: 10.1016/j.jss.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 141.White RR, Shan S, Rusconi CP, Shetty G, Dewhirst MW, Kontos CD, Sullenger BA. Inhibition of rat corneal angiogenesis by a nuclease-resistant RNA aptamer specific for angiopoietin-2. Proc Natl Acad Sci USA. 2003;100:5028–33. doi: 10.1073/pnas.0831159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oliner J, Min H, Leal J, Yu D, Rao S, You E, Tang X, Kim H, Meyer S, Han SJ, Hawkins N, Rosenfeld R, Davy E, Graham K, Jacobsen F, Stevenson S, Ho J, Chen Q, Hartmann T, Michaels M, Kelley M, Li L, Sitney K, Martin F, Sun JR, Zhang N, Lu J, Estrada J, Kumar R, Coxon A, Kaufman S, Pretorius J, Scully S, Cattley R, Payton M, Coats S, Nguyen L, Desilva B, Ndifor A, Hayward I, Radinsky R, Boone T, Kendall R. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6:507–16. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 143.Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodés J, Friedman SL, Carmeliet P, Jiménez W, Morales-Ruiz M. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–26. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 144.Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber HP, Ferrara N, Fuh G. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281:951–61. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 145.Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos S, Armaganidis A, Papapetropoulos A, Roussos C. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med. 2007;35:199–206. doi: 10.1097/01.CCM.0000251640.77679.D7. [DOI] [PubMed] [Google Scholar]
- 146.Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T, Renner O, Breier G, Brownlee M, Deutsch U. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53:1104–10. doi: 10.2337/diabetes.53.4.1104. [DOI] [PubMed] [Google Scholar]
- 147.Peters S, Cree IA, Alexander R, Turowski P, Ockrim Z, Patel J, Boyd SR, Joussen AM, Ziemssen F, Hykin PG, Moss SE. Angiopoietin modulation of vascular endothelial growth factor: Effects on retinal endothelial cell permeability. Cytokine. 2007;40:144–50. doi: 10.1016/j.cyto.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 148.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 149.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- 150.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 151.Baffert F, Le T, Thurston G, McDonald DM. Angiopoietin- decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am J Physiol Heart Circ Physiol. 2006;290:H107–18. doi: 10.1152/ajpheart.00542.2005. [DOI] [PubMed] [Google Scholar]
- 152.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87:603–7. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 153.Kim I, Moon SO, Park SK, Chae SW, Koh GY. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res. 2001;89:477–9. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 154.Jeon BH, Khanday F, Deshpande S, Haile A, Ozaki M, Irani K. Tieing the antiinflammatory effect of angiopoietin-1 to inhibition of NF-kappaB. Circ Res. 2003;92:586–8. doi: 10.1161/01.RES.0000066881.04116.45. [DOI] [PubMed] [Google Scholar]
- 155.Finsnes F, Christensen G, Lyberg T, Sejersted OM, Skjonsberg OH. Increased synthesis and release of endothelin-1 during the initial phase of airway inflammation. Am J Respir Crit Care Med. 1998;158:1600–6. doi: 10.1164/ajrccm.158.5.9707082. [DOI] [PubMed] [Google Scholar]
- 156.Finsnes F, Lyberg T, Christensen G, Skjonsberg OH. Effect of endothelin antagonism on the production of cytokines in eosinophilic airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2001;280:L659–65. doi: 10.1152/ajplung.2001.280.4.L659. [DOI] [PubMed] [Google Scholar]
- 157.Finsnes F, Skjonsberg OH, Lyberg T, Christensen G. Endothelin-1 production is associated with eosinophilic rather than neutrophilic airway inflammation. Eur Respir J. 2000;15:743–50. doi: 10.1034/j.1399-3003.2000.15d19.x. [DOI] [PubMed] [Google Scholar]
- 158.Dewachter L, Adnot S, Fadel E, Humbert M, Maitre B, Barlier-Mur AM, Simonneau G, Hamon M, Naeije R, Eddahibi S. Angiopoietin/Tie2 pathway influences smooth muscle hyperplasia in idiopathic pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1025–33. doi: 10.1164/rccm.200602-304OC. [DOI] [PubMed] [Google Scholar]
- 159.Jho D, Mehta D, Ahmmed G, Gao XP, Tiruppathi C, Broman M, Malik AB. Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2 influx. Circ Res. 2005;96:1282–90. doi: 10.1161/01.RES.0000171894.03801.03. [DOI] [PubMed] [Google Scholar]
- 160.Li X, Hahn CN, Parsons M, Drew J, Vadas MA, Gamble JR. Role of protein kinase Czeta in thrombin-induced endothelial permeability changes: inhibition by angiopoietin-1. Blood. 2004;104:1716–24. doi: 10.1182/blood-2003-11-3744. [DOI] [PubMed] [Google Scholar]
- 161.Pizurki L, Zhou Z, Glynos K, Roussos C, Papapetropoulos A. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br J Pharmacol. 2003;139:329–36. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lemieux C, Maliba R, Favier J, Theoret JF, Merhi Y, Sirois MG. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. 2005;105:1523–30. doi: 10.1182/blood-2004-09-3531. [DOI] [PubMed] [Google Scholar]
- 163.Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol. 2003;9:1144–55. doi: 10.3748/wjg.v9.i6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Das A, Fanslow W, Cerretti D, Warren E, Talarico N, McGuire P. Angiopoietin/Tek interactions regulate mmp-9 expression and retinal neovascularization. Lab Invest. 2003;83:1637–45. doi: 10.1097/01.lab.0000097189.79233.d8. [DOI] [PubMed] [Google Scholar]
- 165.Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol Cell Biol. 2007;85:348–56. doi: 10.1038/sj.icb.7100044. [DOI] [PubMed] [Google Scholar]
- 166.Cee VJ, Albrecht BK, Geuns-Meyer S, Hughes P, Bellon S, Bready J, Caenepeel S, Chaffee SC, Coxon A, Emery M, Fretland J, Gallant P, Gu Y, Houdous BL, Hoffman D, Johnson RE, Kendall R, Kim JL, Long AM, McGowan D, Morrison M, Olivieri PR, Patel VF, Polverino A, Powers D, Rose P, Wang L, Zhao H. Alkynylpyrimidine amide derivatives as potent, selective, and orally active inhibitors of Tie-2 kinase. J Med Chem. 2007;50:627–40. doi: 10.1021/jm061112p. [DOI] [PubMed] [Google Scholar]
- 167.Hodous BL, Geuns-Meyer SD, Hughes PE, Albrecht BK, Bellon S, Bready J, Caenepeel S, Cee VJ, Chaffee SC, Coxon A, Emery M, Fretland J, Gallant P, Gu Y, Hoffman D, Johnson RE, Kendall R, Kim JL, Long AM, Morrison M, Olivieri PR, Patel VF, Polverino A, Rose P, Tempest P, Wang L, Whittington DA, Zhao H. Evolution of a highly selective and potent 2-(pyridin-2-yl)-1,3,5- triazine Tie-2 kinase inhibitor. J Med Chem. 2007;50:611–26. doi: 10.1021/jm061107l. [DOI] [PubMed] [Google Scholar]
- 168.McCarter SD, Mei SH, Lai PF, Zhang QW, Parker CH, Suen RS, Hood RD, Zhao YD, Deng Y, Han RN, Dumont DJ, Stewart DJ. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med. 2007;175:1014–26. doi: 10.1164/rccm.200609-1370OC. [DOI] [PubMed] [Google Scholar]
- 169.Chen F, Tan Z, Dong CY, Li X, Xie Y, Wu Y, Chen X, Guo S. Combination of VEGF(165)/Angiopoietin-1 gene and endothelial progenitor cells for therapeutic neovascularization. Eur J Pharmacol. 2007;568:222–30. doi: 10.1016/j.ejphar.2007.04.047. [DOI] [PubMed] [Google Scholar]


