Abstract
We recently demonstrated original anti-tumor effects of zoledronic acid (Zol) on osteosarcoma cell lines independently of their p53 and Rb status. The present study investigated the potential Zol-resistance acquired by osteosarcoma cells after prolonged treatment. After 12 weeks of culture in the presence of 1 μm Zol, the effects of high doses of Zol (10–100 μm) were compared between the untreated rat (OSRGA, ROS) and human (MG63, SAOS2) osteosarcoma cells and Zol-pretreated cells in terms of cell proliferation, cell cycle analysis, migration assay and cytoskeleton organization. Long-term treatment with 1 μm Zol reduced the sensitivity of osteosarcoma cells to high concentrations of Zol. Furthermore, the Zol-resistant cells were sensitive to conventional anti-cancer agents demonstrating that this resistance process is independent of the multidrug resistance phenotype. However, as similar experiments performed in the presence of clodronate and pamidronate evidenced that this drug resistance was restricted to the nitrogen-containing bisphosphonates, we then hypothesized that this resistance could be associated with a differential expression of farnesyl diphos-phate synthase (FPPS) also observed in human osteosarcoma samples. The transfection of Zol-resistant cells with FPPS siRNA strongly increased their sensitivity to Zol. This study demonstrates for the first time the induction of metabolic resistance after prolonged Zol treatment of osteosarcoma cells confirming the therapeutic potential of Zol for the treatment of bone malignant pathologies, but points out the importance of the treatment regimen may be important in terms of duration and dose to avoid the development of drug metabolic resistance.
Keywords: osteosarcoma, bisphosphonate, zoledronic acid, metabolic resistance, farnesyl diphosphate synthase
Introduction
Osteosarcoma is the most frequent malignant primary bone tumor that develops mainly in the young, the median age of diagnosis being 18 years [1]. Despite recent improvements in chemotherapy and surgery, the problem of non-response to chemotherapy remains. Thus, current strategies for the treatment of high-grade osteosarcoma fail to improve its prognosis [2, 3], mainly because of chemotherapy resistance. This poor prognosis of osteosarcoma warrants new therapeutic strategies to improve the overall rate of survival.
Bisphosphonates (BPs) are stable synthetic analogues deriving from endogenous pyrophosphate (PPi) [4]. Various side chains can be added to the central carbon atom, thus producing a range of BPs with differential clinical activity and potency [5]. The most common therapeutic application of BPs is osteoporosis, and their use has been extended to the treatment of malignant osteolysis and hypercalcemia. Two groups of BPs can be identified including non-nitrogen-containing and nitrogen-containing BPs. The BPs that lack a nitrogen atom, closely related to PPi (such as clodronate, etidronate and tilu-dronate) are metabolized intracellularly to cytotoxic analogues of ATP and decrease osteoclast survival [5]. In contrast, nitrogen-containing BPs (such as pamidronate, alendronate, risedronate, ibandronate and zoledronate) induce apoptosis of osteoclasts by inhibiting enzymes of the mevalonate pathway, especially farnesyl diphosphate synthase (FPPS) [6, 7]. FPPS prevents the biosynthesis of cholesterol and isoprenoid lipids (FPP and geranylgeraniol diphos-phate) which are required for the prenylation of small GTPases (i.e. Ras, Rho and Rac), a biochemical reaction essential for the anchorage of small GTPases to cell membranes and to protein-protein interactions [8]. In addition to their powerful anti-bone resorption effects, recent in vitro studies evidenced a direct anti-tumor activity exerted by zoledronic acid (Zol) on several cancer cells (myeloma, carcinoma and sarcoma) [9, 10]. Preclinical data confirmed the Zol anti-tumor activity in experimental models of bone tumors. Among these studies, we reported recently the enhancement of tumor regression and tissue repair when Zol is combined with ifosfamide in rat osteosarcoma [11] and that Zol suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice [12]. Furthermore, recent clinical trials in patients suffering from malignant bone diseases demonstrated that Zol was safe and well tolerated at the approved dose of 4 mg i.v. every 3–4 weeks [4]. Because the main difficulty encountered in treating cancer relates to mutations carried by many tumor cells in key genes such as p53, Rb or proteins affecting caspase signalling, we demonstrated selective and original anti-tumor effects of Zol on several osteosarcoma cell lines independently of their p53 and Rb status [13]. Indeed, Zol inhibited osteosarcoma cell proliferation through a cell cycle arrest in S and G2/M phases and induced atypical apoptosis independent of caspase activation, characterized by the translocation of Apoptosis Inducing Factor and Endonuclease-G [13]. These data now allow to consider these molecules as potential therapeutic agents in clinical trials of tumor bone pathologies independently of the p53 and Rb status of the tumor.
The optimization and increase in specificity of cancer treatments has improved their efficacy and reduced the associated adverse effects, but unfortunately has not yet resulted in a cure for the majority of patients. Studies of the mechanisms by which tumor cells escape treatment is essential to circumvent drug resistance in cancer cells and to design new therapeutic protocols that are not subject to these drug-resistances [14]. Two types of resistance mechanism have been identified [15]. The first one results in resistance restricted to a specific drug or limited to a small number of related drugs, which can be bypassed by modification of the chemotherapeutic agent. The second mechanism confering multi-resistance to many unrelated drugs, is called mul-tidrug resistance (MDR) and is responsible for many failures of cancer treatment [16]. The most common mechanisms responsible for the various forms of resistance are the overexpression of efflux pumps, inhibition of apoptosis, increased repair of DNA damage, mutations in key cell cycle checkpoint genes and increased or altered drug targets [14]. Similar to non-osseous malignancies, osteosarcomas frequently exhibit a MDR phenotype explaining why patient survival has not improved since the mid-1980s despite advances in anticancer therapies. Because Zol represents a potential novel anti-neoplastic agent for the therapy of osteosarcoma, the present study investigated the potential development of innate and/or acquired resistance to Zol and the molecular mechanisms involved in this phenomenon.
Material and methods
Patients
This study included seven patients (three females aged 41–93 years, four males aged 16–79 years) that were referred to our institution for the treatment of osteosarcoma. All cases were diagnosed as osteogenic osteosarcoma based on histological samples obtained by open biopsies. The experimental procedures followed in the present study were in accordance with the ethical standards of the responsible institutional committee on human experimentation and with Helsinki Declaration of 1975, revised in 1983. The study was approved by the institutional ethic committee.
Cells, culture conditions and establishment of Zol-resistant cell lines
The rat osteosarcoma OSRGA cell line was initially established from a radio-induced osteosarcoma [17, 18]. The rat ROS17/2.8 cell line was kindly provided by Prof. H.J. Donahue (Penn State University, USA), and the human MG63 and SAOS2 cell lines were purchased from ATCC (USA). These cell lines were cultured in DMEM (BioWhittaker, Belgium) supplemented with 5% Fetal Calf Serum (Hyclone, France) and 2 mM L-glutamine (BioWhittaker). Rat and human osteosarcoma cell lines resistant to Zol (MG53res, SAOS2res, ROSres, OSRGAres) were established by 3 months of continuous treatment with 1 μm Zol.
Cell growth and viability
Cell growth and viability were determined by a cell proliferation reagent assay kit using sodium 3′[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene sul-fonic acid hydrate (XTT) (Roche Molecular Biomedicals, Germany). Two thousand cells/well were plated into 96-well plates and cultured for 72 hrs in culture medium in the presence or the absence of 10−12–10−4 M Zol. Zol was provided by Novartis Pharma AG (Basel, Switzerland) as the disodium hydrate form. In another set of experiments, cells were treated for 72 hrs in the presence or the absence of 10−9–10−6 M methotrexate (Sigma, France), doxorubicine (Sigma) and 1–50 μg/ml mafosfamide (Baxter, France), 10–1000 μm clodronate (Sigma), 1–500 μm pamidronate (Sigma) and 5 μm verapamil (Sigma). After the culture period, XTT reagent was added to each well and incubated for 5 hrs at 37°C, the corresponding absorbance was then determined at 490 nm. Cell viability was also assessed by trypan blue exclusion and live and dead cells were scored manually. Cell death was also monitored microscopically after Hoechst n°33258 staining (Sigma). In this experiment, cells were seeded at 104 cells/well in a 24-well plate and treated or not with 10 μm Zol for 48 hrs or 100 nm staurosporine (Sigma) for 16 hrs, stained by 10 μg/ml Hoechst reagent for 30 min at 37°C and then observed under UV microscopy (DMRXA, Leica, Germany). Statistical evaluation of the data was performed using the ANOVA test.
Western blot analysis
Zol-treated cells were lysed in RIPA buffer (150 mM NaCl, 5% Tris pH 7.4, 1% NP-40, 0.25% Na deoxycholate, 1 mM Na3VO4, 0.5 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin). Protein concentration was determined by the BCA kit (Pierce Chemical, USA). A total of 50 μg of total cell lysate protein were run on SDS-PAGE, electrophoretically transferred to Immobilon-P membrane (Millipore, MA, USA). The membrane was blotted with antibodies anti-p-Rb (Ser 807/811), -p-cdc2 (tyr15), -actin (Cell Signaling Technologies, USA), -p21WAF1(BD Biosciences, USA) and the unprenylated form of Rap1A (Santa Cruz, USA) to indirectly quantified FPPS enzymatic activity, in PBS, 0.05% Tween 20 and 3% bovine serum albumin (BSA). The membrane was washed and probed with the secondary antibody coupled to horseradish peroxidase. Antibody binding was then visualized with the enhanced chemoluminescence system (ECL Kit; Roche Molecular Biomedicals). The band densities were measured using the GeneTools computer software program (SynGene).
Caspase -1,-3 and -8 activities
Caspase -1, -3 and -8 activities were assessed on 10 μl of total Zol-treated or not cell lysates using the kit CaspACE™ Assay System, ‘Fluorometric’ (Promega, USA) following the manufacturer's recommendations. Cells treated with UV light for 30 sec 24 hrs before harvesting were used as a positive control. Results were expressed in arbitrary units referred to the total protein content.
Cell cycle analysis
OSRGA, MG63 and SAOS2 cells were incubated in the absence or the presence of 10 μm Zol for 48 hrs, trypsinized, washed twice and incubated in PBS containing 0.12% Triton X-100, 0.12 mM EDTA and 100 μg/ml ribonuclease A. Then 50 μg/ml propidium iodide were added to each sample for 20 min at 4°C. Cell cycle distribution was analyzed by flow cytometry (FAC Scan), based on 2N and 4N DNA content.
Time-lapse microscopy and confocal microscopic analysis
For time-lapse experiments, cells were seeded at 5 × 104 cells/well and cultured in 6-multiwell plates in the absence or the presence of 10 μm Zol. Phase-contrast photographs (Leica) were taken every 10 min during 60 hrs and edited using the Metamorph™ software. Cell divisions and apop-totic cells were then manually scored. To study cell migration, cells plated in 6-well plates and cultured until confluence were treated or not with 10 μm Zol for 24 hrs before a slit was made in the cell monolayer. Actin filament detection was performed after cell treatment with or without 10 μm Zol fixed in 4% paraformaldehyde and stained with FITC-conjugated phalloidin (0.25 μg/ml; Sigma). Cover glasses were fitted with the Long Pro Kit (Molecular probes). Images were collected on a Leica TCS-SP1 con-focal microscope with 63/1.4x oil immersion lens. The digital images were visualized with a 24-bit imaging system including Leica's TCS-NT software and projections were generated from z-stacks.
siRNA gene silencer
The FPPS gene expression was knocked down using specific human and rat FPPS siRNA (Ambion, France) and the INTERFERin™ transfection reagent (Polyplus transfection, France). Cells were seeded at 40% confluency in a 24-well plate 1 day before transfection. In each well 10 nm siRNA duplexes diluted in serum-free medium were incubated with 2 μl of INTERFERin™ for 30 min at room temperature. Then, 100 μl mixture per well were added onto the cells and incubated at 37°C. The 72 hrs-Zol treatment started 24 hrs after siRNA transfection. For each condition tested, a negative siRNA control was used (Santa Cruz biotechnology, Germany). Additional experiments were performed in the presence of geranylgeraniol (GGO) (Sigma, France).
RT-PCR analysis
Total RNA was isolated from cultured OSRGA, MG63 and SAOS2 cells using the TRIzol reagent (Invitrogen, France). First, RNA was reversed-transcribed (RT), using 400 U MMLV-RT from Invitrogen, then 2 μl of the RT reaction mixture were subjected to PCR using upstream and downstream primers to determine the expression of rat and human FPPS [Human FPPS sense: AGATCTGTGGGGGTCTTCCT, anti sense: TCCCGGAATGCTACTACCAC; Rat FPPS sense: AGTACAATCGGGGTCTGACG, anti sense: CGCGATAGGCAGGTAGAAAG] and 0.25 μl of 5 U/μl Taq polymerase (Eurobio, France). After the number of PCR cycles was increased, a plot was done for each sample, the cycle values corresponding to the linear part of the amplification curve were then determined (28 cycles, Tm = 58°C) and used to quantify the message versus the 18S signal determined in the same way. The PCR products were electrophoresed in 1% agarose gel-containing ethidium bromide. The band densities were measured using the GeneTools computer software pro-gram. Three independent experiments were performed for each gene and a representative experiment is shown in the Results section.
Results
Osteosarcoma cell lines develop Zol-resistance after long-term continuous treatment with low-dose Zol
Consistent with previous results [11, 12, 19], Zol treatment of Zol-sensitive rat ROS, OSRGA (Fig. 1A) and human MG63, SAOS2 (Fig. 1B) osteosarcoma cells strongly reduced their proliferation and induced the cell death without any caspase 1, 3 or 8 activation (data not shown). Thus, 0.1–100 μM Zol decreased the viable cell number in a dose-dependent manner (IC50: 1–8 μM) as revealed by the XTT assay. After 3 month continuous treatment with 1 μm Zol, rat and human osteosarcoma cells became less sensitive to Zol and resistant cell lines (OSRGAres, ROSres, MG63res, SAOS2res) were then progressively established (Fig. 1A). Indeed, the potency of Zol to affect cell proliferation was strongly reduced on human resistant cell lines and Zol was ineffective on rat resistant cell lines (Fig. 1A).
1.
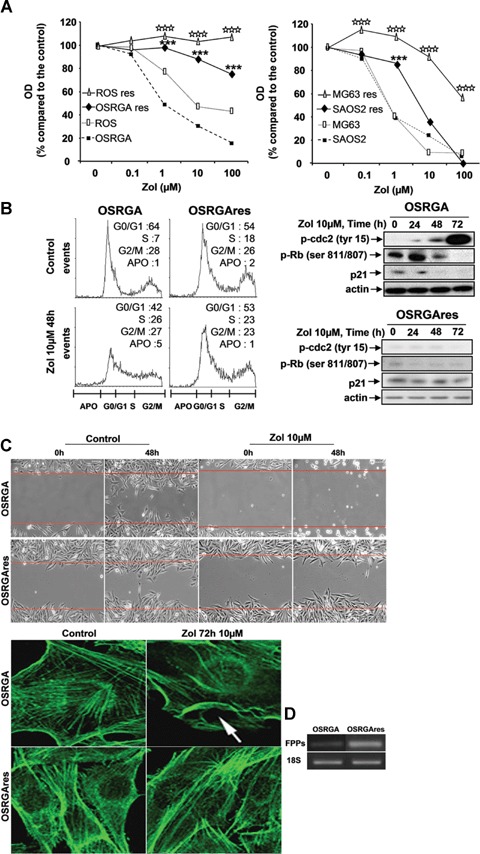
Osteosarcoma cell lines develop Zol-resistance after long-term of continuous treatment with low doses of Zol. (A) rat (OSRGA, ROS) and human (MG63, SAOS2) sensitive and resistant (corresponding cell Name-res) osteosarcoma cell lines were treated with increasing concentrations of Zol (0.1–100 μm) for 72 hrs. The number of viable cells was then measured using the XTT assay. Graphs represent the mean values of three independent experiments performed in triplicate. ***P < 0.001. Statistical evaluation of the data was performed using the ANOVA test. (B) Cell cycle distribution of OSRGA and OSRGAres, treated or not with 10 μm Zol for 48 hrs was analyzed by propidium iodide staining and FACS analysis. G1/S and G2/M DNA checkpoints were analyzed by western blot and compared between sensitive and resistant OSRGA osteosarcoma cell lines in the presence or absence of 10 μm Zol for 24, 48 and 72 hrs. All experiments were repeated three times and a representative blot is shown. (C) Zol effects on organization of actin stress fibres were observed by confocal microscopy after phalloïdine staining. The actin network reorganization was associated with membrane ruffling (white arrow) in Zol-sensitive OSRGA cell line (Original magnification: × 1000). Zol effects on cell migration were also analyzed by time-lapse microscopy. The horizontal bars represent the limit of the slit cut performed on the cell monolayer at the start of the experiment (Original magnification: × 100). (D) Farnesyl diphosphate synthase (FPPS) transcription level was determined by semi quantitative RT-PCR in OSRGA sensitive and resistant cell lines. The 18S was used as a control.
The influence of this resistance process was also assessed on the other known activities of Zol on tumor cells [cell cycle (Fig. 1B), DNA checkpoints (Fig. 1B), cytoskeleton (Fig. 1C), cell migration (Fig. 1C) [13]). Cell cycle analysis was performed after 48 hrs of 10 μm Zol-treatment. The results obtained confirmed that 48 hrs of Zol-treatment induced a strong cell cycle arrest in S and G2/M phases in Zol-sensitive OSRGA cells (Fig. 1B, [13]) and showed that Zol-treatment did not modulate the cell cycle in OSRGAres cells (Fig. 1B). Indeed, the number of cells in S, G2/M phases strongly increased from 35% to 53% for OSRGA cells in the presence of 10 μm Zol concomitantly with a decrease of cells in G0/G1 phase: 42%versus 64% (Fig. 1B). A similar phenomenon was observed in human osteosarcoma cell lines (data not shown). We therefore investigated by western blot whether the DNA checkpoint proteins were involved in the cell cycle blockade observed in the presence of Zol. Thus, the treatment of sensitive OSRGA cells by 10 μm Zol increased the inactive form of cdc2 (p-cdc2, Tyr15) after 72 hrs of treatment. Simultaneously, Zol strongly reduced p21 expression and transiently upregulated Rb phosphorylation (Ser 807 and 811) after 24 hrs of Zol treatment (Fig. 1B). No modulation of p-cdc2, Rb and p21 was observed in OSRGAres cells regardless of the duration of Zol treatment (Fig. 1B).
As Zol has been shown to disturb cytoskeletal organization and to inhibit cell migration [13], we wondered whether Zol could alter such parameters in OSRGAres cells. Confocal microscopy revealed a major disorganization of the actin stress fibres associated with membrane ruffling in sensitive OSRGA cells treated with 10 μm Zol for 72 hrs, this was never observed in OSRGAres cells (Fig. 1C). Moreover, as shown by the time-lapse assay, 10 μm Zol totally blocked the migration of sensitive OSRGA cells but was not able to abolish migration of OSRGAres cells (Fig. 1C).
The molecular mechanism involved in the reduced-Zol sensitivity is not associated with a multidrug resistance (MDR) phenotype and is restricted to the nitrogen-containing bisphosphonates
The potential role of the MDR phenotype in the Zol resistance phenomenon was assessed by XTT assays. The MDR phenotype is conventionally defined as the resistance of cells to conventional chemotherapeutic agents such as mafosfamide, methotrexate and doxorubicin [20, 21]. The XTT assays revealed that OSRGAres cells were still always sensitive to increasing doses of mafosfamide, methotrexate and doxorubicin (Fig. 2). Furthermore, 5 μm verapamil, a P-gp pump inhibitor [22] was not able to abolish the Zol resistance (Fig. 2). Overall, these data demonstrate that the Zol resistance was not associated with MDR phenotype. In addition, similar experiments performed in the presence of clodronate, a non-nitrogen containing-BP [4], revealed that OSRGAres are as sensitive to clo-dronate as they are to lower concentrations of Zol (Fig. 2). When, osteosarcoma cells were treated with another nitrogen-containing BP, pamidronate that also targets FPPS, it significantly reduced Zol-sensitive OSRGA proliferation in contrast to OSRGAres cells, which are also resistant to pamidronate (Fig. 2). Similar results have been obtained with the osteosarcoma cell lines MG63 and SAOS2 (data not shown). These experiments demonstrated that the Zol-resistance phenomenon in osteosarcoma cells appears to be MDR-independent and is apparently restricted to nitrogen-containing BPs.
2.
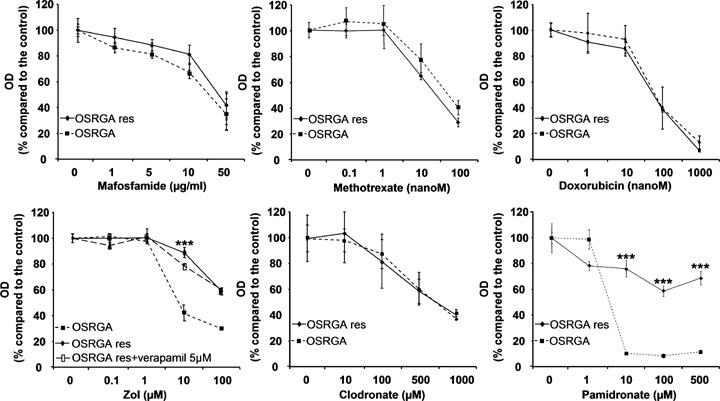
The molecular mechanism involved in the reduced-Zol sensitivity is not associated with a multidrug resistance (MDR) phenotype and is restricted to the nitrogen-containing bisphosphonates. OSRGA and OSRGAres sensitivity to conventional anti-cancer agents mafosfamide, methotrexate, doxorubicin and sensitivity to Zol in the presence or absence of a P-gp pump inhibitor (5 μm verapamil) was analyzed by the XTT assay. Similar experiments were performed in the presence of clodronate and pamidronate. Graphs represent the mean values of three independent experiments performed in triplicate. Error bars represent the standard deviation. ***P < 0.001. Statistical evaluation of the data was performed using the ANOVA test.
Farnesyl diphosphate synthase (FPPS) is implicated in the Zol-resistance mechanism of osteosarcoma cell lines
FPPS being the main molecular target of nitrogen containing BPs [23], the FPPS transcript expression was analyzed by RT-PCR and compared in sensitive OSRGA and OSRGAres cells (Fig. 1D). Thus, the Zol-resistant cells expressed a higher level of FPPS mRNA than the sensitive cells. To further determine the involvement of FPPS in the Zol-resistance mechanism of human and rat osteosarcoma cells, the effect of Zol on OSRGA, OSRGAres, MG63 and SAOS2 was analyzed after transfection with FPPS siRNA. Semi-quantitative RT-PCR analysis was used to evaluate the efficacy of FPPS siRNA on FPPS mRNA expression. In all experiments, FPPS mRNA levels were significantly decreased in FPPS siRNA-transfected cell lines compared to the siRNA control (Fig. 3A). Inhibition of FPPS activity was then assessed indirectly by the expression of the unpreny-lated form of the small GTPase Rap1A (unRAP1A) that is expressed after inhibition of FPPS [24, 25] (Fig. 3B). The transfection of Zol-sensitive cells with FPPS siRNA strongly increased their sensitivity to Zol in all osteosarcoma cell lines studied. Indeed, FPPS siRNA transfection modified the unRAP1A expression kinetic in OSRGA, MG63 and SAOS2 cells. In the presence of FPPS siRNA, unRAP1A expression was strongly induced by 1 μm Zol treatment for 24 hrs whereas its expression was only observed with 10 μm Zol treatment for 48 hrs in control siRNA transfected cells (Fig. 3B). In OSRGAres cells, a very weak expression of unRAP1A was observed after Zol treatment. Interestingly, FPPS siRNA re-induced the sensitivity to Zol treatment in these resistant cells to a level comparable to parental OSRGA cells transfected with FPPS siRNA. Thus, the unRAP1A expression was observed after 24 hrs treatment with 1 μm Zol in FPPS siRNA-OSRGAres transfected (Fig. 3B). Similarly, micoscopic observations confirmed the FPPS siRNA effects on the sensitization of osteosarcoma cells to Zol treatment (Fig. 3C). Thus, an increase of floating cell number associated with an inhibition of cell proliferation was observed after transfection of all osteosarcoma cell lines with FPPS siRNA (Fig. 3C).
3.
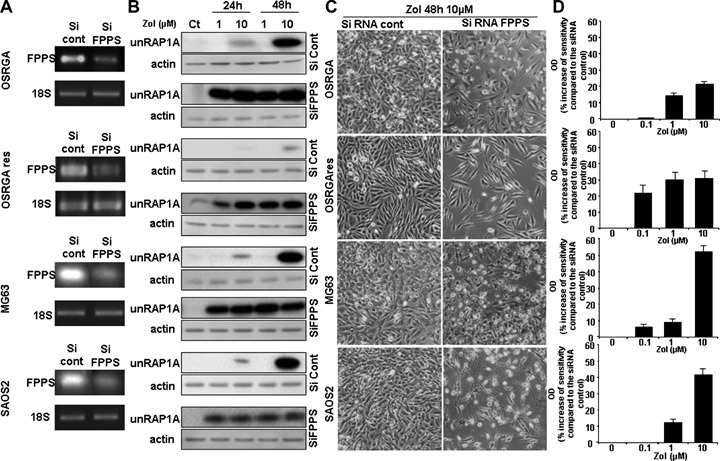
Involvement of farnesyl diphosphate synthase (FPPS) in the Zol-induced resistance mechanism in osteosarcoma. (A) Farnesyl diphosphate synthase (FPPS) transcription level was determined by semi quantitative RT-PCR in FPPS siRNA transfected cell lines compared to the siRNA control cells. The 18S was used as a control. (B) Western blot analysis of unprenylated RAP1A (unRAP1A) from OSRGA cell lines transfected with FPPS siRNA and control siRNA, treated 24 and 48 hrs with 1 and 10 μm Zol. All experiments were repeated three times, and a representative blot is shown. (C) Photomicrographs of FPPS siRNA transfected cells after 48 hrs with 10 μm Zol compared to control siRNA. Original magnification: × 100. (D) Rat (OSRGA, OSRGAres) and human (MG63, SAOS2) osteosarcoma cell lines were transfected with FPPS siRNA and treated after 24 hrs of culture by increasing concentrations of Zol (0.1–10 μm) for 72 hrs. The number of viable cells was then determined using the XTT assay. Histograms represent the percentage of the increased sensitivity to Zol in the presence of FPPS siRNA compared to control siRNA. Values are mean of three independent experiments performed in triplicate. Error bars represent the standard deviation.
XTT analyses were performed to determine the impact of FPPS siRNA on Zol activity (Fig. 3D). Transfection with FPPS siRNA significantly increased the sensitivity to Zol treatment of all osteosarcoma cell lines analyzed (Fig. 3D). The sensitivity to 10 μm Zol was up-modulated by 22%, 31%, 53% and 42% in OSRGA, OSRGAres MG63 and SAOS2, respectively, in the presence of FPPS siRNA compared to the control siRNA (Fig. 3D). Furthermore, the efficacy of FPPS siRNA occured for lower doses of Zol in OSRGAres compared to OSRGA cells (respectively 22% and 1% increase of sensitivity in the presence of 0.1 μm Zol) (Fig. 3D).
siRNA FPPS increases the Zol-induced blockade of the cell cycle in S, G2/M phases in osteosarcoma cell lines
We previously demonstrated that Zol induces osteosarcoma cell cycle arrest in S, G2/M phases in OSRGA sensitive cells [13]. To determine whether FPPS siRNA could modulate this sensitivity, the cell cycle of FPPS siRNA-transfected osteosarcoma cells was analyzed by flow cytometry. Figure 4 reveals that FPPS siRNA accentuates the Zol-induced effects observed on cell-cycle distribution, leading to a significant increase of cells blocked in S phase compared to the control siRNA. Indeed, the number of cells in S phase increased from 26% to 30% for OSRGA, from 20% to 26% for MG63, from 34% to 46% for SAOS2 and from 23% to 38% for OSRGAres cells in the presence of FPPS siRNA compared to the control siRNA after 48 hrs of treatment with 10 μm Zol (Fig. 4). Furthermore, these observations were concomitant with a significant reduction of the cell number in G0/G1 phase:35%versus 42% for OSRGA, 61%versus 69% for MG63, 36%versus 57% for SAOS2 and 41%versus 53% for OSRGAres.
4.
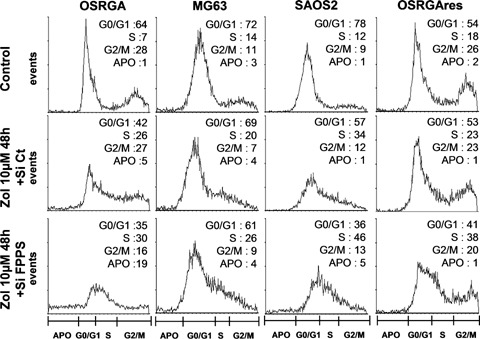
FPPS siRNA increases the Zol-induced blockade of the cell cycle in S phases in osteosarcoma cell lines. Cell cycle distribution of osteosarcoma cell lines (FPPS siRNA versus control siRNA) treated or not treated with 10 μm Zol for 48 hrs were analyzed by propidium iodide staining and FACS analysis.
Geranyl geraniol (GGO) reversed the FPPS siRNA effects in osteosarcoma cell lines
To determine whether the effects previously demonstrated for the FPPS siRNA in osteosarcoma cells are reversible, FPPS siRNA transfected cells treated with increasing doses of Zol were cultured in the presence of 25 μm geranylgeraniol, the FPPS metabolic product (Fig. 5). GGO protected rat and human osteosarcoma cell lines from the effects of Zol in the FPPS siRNA-transfected cells and totally reversed FPPS siRNA effects (Fig. 5A). We therefore investigated by western blot the expression kinetic of unRAP1A in the presence of 25 μm GGO in FPPS siRNA-transfected cells (Fig. 5B). GGO totally abolished unRAP1A expression similar to what had been observed in Zol-resistant cell lines (Fig. 3B). Overall, these data then strengthen our conclusion that FPPS is involved in the Zol-resistance mechanism.
5.
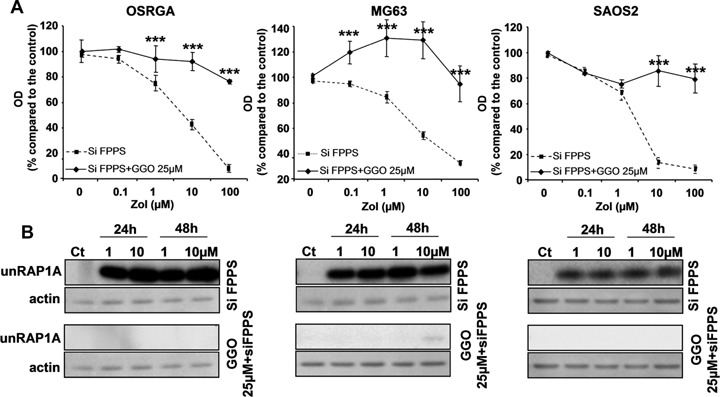
Geranylgeraniol (GGO) reverses FPPS siRNA effects in osteosarcoma cell lines. (A) Rat (OSRGA) and human (MG63, SAOS2) osteosarcoma cell lines were transfected with FPPS siRNA and treated 24 hrs after with increasing concentrations of Zol (0.1–100 μm) for 72 hrs in the presence or not of 25 μm GGO. The number of viable cells was then determined using the XTT assay. Graphs represent the mean values of three independent experiments performed in triplicate. Error bars represent the standard deviation.***P < 0.001. Statistical evaluation of the data was performed using the ANOVA test.(B) Western blot analysis of unprenylated RAP1A (unRAP1A) form. Cells transfected with control siRNA or with FPPS siRNA combined with 25 μm GGO were treated with 1 and 10 μm Zol for 24 and 48 hrs. All experiments were repeated three times, and a representative blot is shown.
Dual origin of Zol resistance: innate and/or acquired
To explain the origin of the Zol-resistance observed in osteosarcoma cell lines, two hypotheses can be proposed: (i) an innate resistance mechanism linked to differential levels of FPPS expression and associated with selection of a sub-population of cells expressing a higher FPPS activity, (ii) an acquired resistance mechanism linked to an increased FPPS transcription level as a feedback response to long-term, low-dose Zol treatment. To distinguish between these two hypotheses, OSRGA osteosarcoma cell lines were treated with low Zol concentrations (1–104 pM) for 72 hrs (Fig. 6A). Low concentrations of Zol induced a 60% increase of viable cells and up-modulated the expression of FPPS mRNA in a dose-dependent manner (Fig. 6A), these results support acquired resistance to Zol. Since a potential mechanism of innate resistance could be also envisaged, OSRGA cell line was cloned by limiting dilution and the expression of FPPS was analyzed by semi-quantitative RT-PCR (Fig. 6B). Several clones were isolated with heterogenous sensitivity to Zol treatment (Fig. 6B). Furthermore, the isolated clones expressed differential levels of FPPS related to their sensitivity to Zol treatment, these results support innate resistance to Zol (Fig. 6B). Similarly, we analyzed the transcriptional expression of FPPS in seven human osteosarcoma samples analyzed by semi-quantitative RT-PCR before any chemotherapy (Fig. 6C). The results revealed that a very high heterogeneity of FPPS expression inthese patients strengthening the hypothesis of innate resistance to Zol.
6.
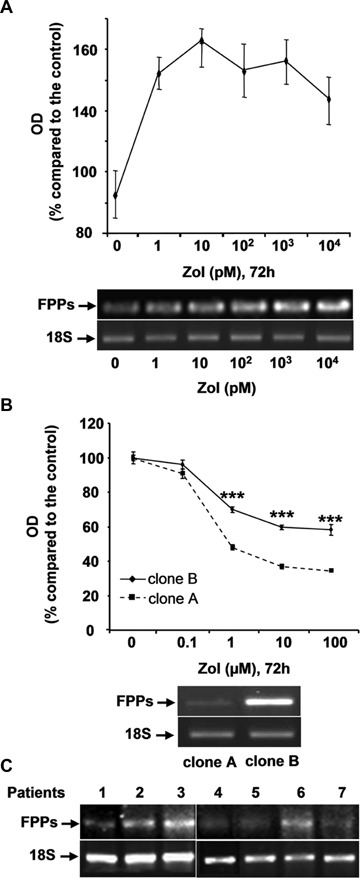
Dual origin of Zol-resistance: innate and/or acquired. (A) OSRGA osteosarcoma cell lines were treated with increasing low concentration of Zol (1–104 pM) for 72 hrs. The number of viable cells was then determined using a XTT assay. Graphs represent the average values of three independent experiments performed in triplicate. Error bars represent the standard deviation. Farnesyl diphosphate synthase (FPPS) transcription level was determined by semi-quantitative RT PCR under the same conditions of Zol treatment. The 18S was used as a control. (B) Similar experiments were performed with higher concentrations of Zol (0.1–100 μM) in two OSRGA clones obtained by limiting dilution.***P < 0.001. Statistical evaluation of the data was performed using the ANOVA test. (C) Transcriptional analysis of FPPS in seven human osteosarcoma samples analyzed by semi-quantitative RT-PCR.
Discussion
The first effects of BPs on calcium metabolism were discovered over 30 years ago, and these drugs have become the most widely used agents in the treatment of bone diseases associated with excessive resorption (osteoporosis, malignant osteolysis, etc). The recent evidences of an anti-tumor effect of nitrogen-containing BPs have led to investigation of the potential-acquired resistance mechanism. Indeed, failure of anti-cancer therapies often occur from innate or acquired drug resistance of the tumor cells to the chemotherapeutic agents [26]. In this context, the elucidation of potential resistance mechanisms to the Zol will allow adaptation of the treatment regimen in terms of duration and dose to avoid the development of drug resistance. The present study demonstrated that after 3 months of continuous treatment with 1 μm Zol, osteosarcoma cell lines became less sensitive to Zol inhibition and resistant cell lines were then progressively established. Furthermore, this resistance appeared to be independent of the MDR phenotype and was clearly related to a differential expression of FPPS.
To exert its activities, Zol must be internalized by cells. Although the mode of Zol internalization is still controversial, two mechanisms have been proposed: first, cellular uptake of Zol may require fluid-phase endocytosis in osteoclasts [27]; in the second case, integrins located at the cell membrane could represent a binding site for Zol which could explain why Zol is able to inhibit cell adhesion and that RGD pep-tide prevents the Zol effects on osteosarcoma cell lines [13]. However, it remains unclear whether cell types other than osteoclasts can internalize BPs [27]. Recently, Notarnicola et al. demonstrated that high FPPS activity level correlates to a stronger inhibition of cellular apoptosis in colorectal cancer cells [28]. Similarly, Ortiz-Gomez et al. demonstrated that over-expression of FPPS confers resistance to rise-dronate in Leishmania major and that the degree of resistance was correlated with an increase in this enzymatic activity [29]. These data strongly support our present results and strengthen the pivotal function of FPPS in the Zol-resistance mechanism. Although FPPS is considered as the main target of nitrogen-containing-BPs, the inhibition of prenylation being the most likely explanation for their biological effects, van Beek et al. evidenced that undetermined additional mechanisms could be involved which may be also proposed for specific resistance mechanisms in certain specific cell types [30].
In the present study, we wondered what could be the origin of the Zol-induced resistance mechanism: an innate or an acquired resistance mechanism? In fact, the results did not allow us to distinguish between these two hypotheses. The main argument in favor of an innate resistance mechanism is the differential FPPS expression of OSRGA osteosarcoma sub-clones composing the heterogenous ‘parental’ OSRGA cell line. Indeed, Zol treatment exerts a selective inhibitory effect on cancer cells expressing less FPPS and after several weeks of culture, FPPS overexpressing cells become predominant and emerge from the parental population (Fig. 6B) [28]. Interestingly, we observe the same kind of heterogeneity in patients. We haven't yet correlated this with sensitivity to Zol treatment but it will be performed in a clinical trial and we could expect using FPPs expression level as a prognosis factor of Zol efficacy. On the other hand, the effect of Zol treatment on FPPS expression is in favor of an acquired resistance mechanism. Indeed, 72 hrs treatment with low doses of Zol (1–104 pM) increased FPPS expression in OSRGA cells (Fig. 6A) inducing the development of FPPS overexpressing tumor cells (Fig. 1D). Similar involvement has been envisaged in myeloma cells [31]. This hypothesis was also strengthened by Ortiz-Gomez et al. who obtained resistant cell lines by stepwise selection in the presence of risedronate, resulting in the development of resistant promastigotes exhibiting increased levels of FPPS at the transcriptional and the translational levels [29]. These authors considered that as a result of drug pressure, cells overcame the effects of rise-dronate by overexpressing the target protein. Such modification has been already observed in osteosarcoma patients treated with chemotherapy. Indeed, after comparison of primary biopsy tissue with that removed after metastasectomy, genetic changes acquired by the tumors have been demonstrated [32, 33]. An acquired resistance to BPs was also reported by Papapoulos et al. in Paget's disease [34]. These authors argued that resistance to the action of BPs in Paget's disease is caused by disease-related factors rather than decreased responsiveness of the molecular target in contrast to the present data. They supported this hypothesis with studies using statins that target the same intracellular biochemical pathway upstream of FPPS, these studies showed no evidence of development of resistance to their action [35, 36]. They also presented data suggesting that acquired resistance is specific for pamidronate and does not extend to other nitrogen-containing BPs. In summary, various and concomitant resistance mechanisms cannot be excluded: direct or indirect effects on FPPS, innate and/or acquired mechanisms.
Chemotherapy resistance in osteosarcoma is well documented [37]. Osteosarcoma cells are subjected to genetic disturbances such as alterations in the tumor suppressor pathways centered on p53 and Rb [38, 39], changes in oncogenes/anti-oncogenes such as deletions in p16INK4A (cyclin-dependent kinase inhibitor 2A), c-fos overexpression and amplification of cyclin-dependent kinase 4 [40–42]. These genetics instabilities lead to heterogenic cell populations within the same tumor and to the emergence of resistant tumor cells. The most described resistance phenomena concern widely used chemotherapeutic agents such as cisplatin, doxorubicin or methotrexate. In these cases, the resistance mechanisms involved are mutation of the drug target, up- or down-regulation of the drug target, decreased drug uptake, drug inactivation, increased drug elimination and increased DNA repair [43–45]. Multidrug resistance phenotype (MDR), due to P-gp or related protein overexpression is the most reported resistance mechanism. In osteosarcoma, MDR1 [46] or P-gp [47] expression could be used as a prognostic marker for sensitivity to chemotherapy, allowing the selection of patients for whom alternative treatments may be considered. Recently, other prognostic factors have been described, such as the expression level of clusterin/apolipoprotein J [48] or expression of a preg-nane xenobiotic receptor (PXR), a major inducer of cytochrome P450 3A4 [49]. Therefore, these factors may also represent predictive markers correlating with the response of cancer cells to chemotherapy.
We described in ostesarcoma a Zol-resistance mechanism specific to nitrogen-containing BPs which did not confer simultaneous resistance to other unrelated drugs. In this context, drug resistance could be circumvented using multiple drugs with different cellular targets and different mechanisms of action. For instance, when Zol is associated with ifosfamide in rat osteosarcoma, enhanced tumor regression and tissue repair have been observed [11]. In the future, Zol could be combined with other chemotherapeutic agent to increase therapeutic efficacy and avoid the emergence of resistance mechanism [50].
Acknowledgments
Zoledronic acid was kindly provided by Novartis Pharma AG and masfosamide by Baxter Oncology (Dr Martinez, France). We thank Dr. Jonathan Green for helpful discussions, Caroline Colombeix from the confocal microscopy platform (Institut Fédératif de Recherche 26, Nantes) and Dr Philippe Juin (INSERM U601, Nantes) for help in time-lapse microscopy. This work was supported by INSERM and the Région des Pays de la Loire. Benjamin ORY received a fellowship from INSERM and the Région des Pays de la Loire.
References
- 1.Link MP, Goorin AM, Horowitz M, Meyer WH, Belasco J, Baker A, Ayala A, Shuster J. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop Relat Res. 1991:8–14. [PubMed] [Google Scholar]
- 2.Rosen G, Murphy ML, Huvos AG, Gutierrez M, Marcove RC. Chemotherapy, en bloc resection, and prosthetic bone replacement in the treatment of osteogenic sarcoma. Cancer. 1976;37:1–11. doi: 10.1002/1097-0142(197601)37:1<1::aid-cncr2820370102>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, Yunis EJ, Huvos AG, Betcher DL, Baum ES, Kisker CT, Miser JS. Treatment of nonmetastat-ic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's Cancer Group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 4.Heymann D, Ory B, Gouin F, Green JR, Redini F. Bisphosphonates: new therapeutic agents for the treatment of bone tumors. Trends Mol Med. 2004;10:337–43. doi: 10.1016/j.molmed.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–78. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9:2643–58. doi: 10.2174/1381612033453640. [DOI] [PubMed] [Google Scholar]
- 7.Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119:S150–62. doi: 10.1542/peds.2006-2023H. [DOI] [PubMed] [Google Scholar]
- 8.Coxon FP, Helfrich MH, Van't Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ. Protein ger-anylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res. 2000;15:1467–76. doi: 10.1359/jbmr.2000.15.8.1467. [DOI] [PubMed] [Google Scholar]
- 9.Mackie PS, Fisher JL, Zhou H, Choong PF. Bisphosphonates regulate cell growth and gene expression in the UMR 106-01 clonal rat osteosarco-ma cell line. Br J Cancer. 2001;84:951–8. doi: 10.1054/bjoc.2000.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonnemann J, Eckervogt V, Truckenbrod B, Boos J, Winkelmann Wvan Valen F. The bisphosphonate pamidronate is a potent inhibitor of human osteosar-coma cell growth in vitro. Anticancer Drugs. 2001;12:459–65. doi: 10.1097/00001813-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Heymann D, Ory B, Blanchard F, Heymann MF, Coipeau P, Charrier C, Couillaud S, Thiery JP, Gouin F, Redini F. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone. 2005;37:74–86. doi: 10.1016/j.bone.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Ory B, Heymann MF, Kamijo A, Gouin F, Heymann D, Redini F. Zoledronic acid suppresses lung metas-tases and prolongs overall survival of osteosarcoma-bearing mice. Cancer. 2005;104:2522–9. doi: 10.1002/cncr.21530. [DOI] [PubMed] [Google Scholar]
- 13.Ory B, Blanchard F, Battaglia S, Gouin F, Redini F, Heymann D. Zoledronic acid activates the DNA S-phase checkpoint and induces osteosarcoma cell death characterized by apoptosis-inducing factor and endonuclease-G translocation independently of p53 and retinoblastoma status. Mol Pharmacol. 2007;71:333–43. doi: 10.1124/mol.106.028837. [DOI] [PubMed] [Google Scholar]
- 14.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman MM, Ambudkar SV, Ni B, Aran JM, Sugimoto Y, Cardarelli CO, Pastan I. Exploiting multidrug resistance to treat cancer. Cold Spring Harb Symp Quant Biol. 1994;59:677–83. doi: 10.1101/sqb.1994.059.01.078. [DOI] [PubMed] [Google Scholar]
- 16.Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett. 2006;580:2903–9. doi: 10.1016/j.febslet.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Jasmin C, Allouche M, Jude JG, Klein B, Thiery JP, Perdereau B, Gongora R, Gongora G, Mazabraud A. An experimental model of osteosarco-mas in rats. Sem Hop. 1982;58:1684–9. [PubMed] [Google Scholar]
- 18.Klein B, Pals S, Masse R, Lafuma J, Morin M, Binart N, Jasmin JR, Jasmin C. Studies of bone and soft-tissue tumours induced in rats with radioactive cerium chloride. Int J Cancer. 1977;20:112–9. doi: 10.1002/ijc.2910200118. [DOI] [PubMed] [Google Scholar]
- 19.Evdokiou A, Labrinidis A, Bouralexis S, Hay S, Findlay DM. Induction of cell death of human osteogenic sarcoma cells by zoledronic acid resembles anoikis. Bone. 2003;33:216–28. doi: 10.1016/s8756-3282(03)00223-0. [DOI] [PubMed] [Google Scholar]
- 20.Biedler JL, Riehm H. Cellular resistance to actino-mycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970;30:1174–84. [PubMed] [Google Scholar]
- 21.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 22.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cyto-toxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41:1967–72. [PubMed] [Google Scholar]
- 23.Gibbs JB, Oliff A. The potential of farnesyltrans-ferase inhibitors as cancer chemotherapeutics. Annu Rev Pharmacol Toxicol. 1997;37:143–66. doi: 10.1146/annurev.pharmtox.37.1.143. [DOI] [PubMed] [Google Scholar]
- 24.Suri S, Monkkonen J, Taskinen M, Pesonen J, Blank MA, Phipps RJ, Rogers MJ. Nitrogen-containing bisphosphonates induce apoptosis of Caco-2 cells in vitro by inhibiting the mevalonate pathway: a model of bisphosphonate-induced gastrointestinal toxicity. Bone. 2001;29:336–43. doi: 10.1016/s8756-3282(01)00589-0. [DOI] [PubMed] [Google Scholar]
- 25.Reszka AA, Halasy-Nagy J, Rodan GA. Nitrogen-bis-phosphonates block retinoblastoma phosphorylation and cell growth by inhibiting the cholesterol biosynthetic pathway in a keratinocyte model for esophageal irritation. Mol Pharmacol. 2001;59:193–202. doi: 10.1124/mol.59.2.193. [DOI] [PubMed] [Google Scholar]
- 26.Kruh GD. Introduction to resistance to anticancer agents. Oncogene. 2003;22:7262–4. doi: 10.1038/sj.onc.1206932. [DOI] [PubMed] [Google Scholar]
- 27.Coxon FP, Thompson K, Rogers MJ. Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol. 2006;6:307–12. doi: 10.1016/j.coph.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Notarnicola M, Messa C, Cavallini A, Bifulco M, Tecce MF, Eletto D, Di Leo A, Montemurro S, Laezza C, Caruso MG. Higher farnesyl diphosphate synthase activity in human colorectal cancer inhibition of cellular apoptosis. Oncology. 2004;67:351–8. doi: 10.1159/000082918. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz-Gomez A, Jimenez C, Estevez AM, Carrero-Lerida J, Ruiz-Perez LM, Gonzalez-Pacanowska D. Farnesyl diphosphate synthase is a cytosolic enzyme in Leishmania major promastigotes and its overex-pression confers resistance to risedronate. Eukaryot Cell. 2006;5:1057–64. doi: 10.1128/EC.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Beek ER, Cohen LH, Leroy IM, Ebetino FH, Lowik CW, Papapoulos SE. Differentiating the mechanisms of antiresorptive action of nitrogen containing bisphosphonates. Bone. 2003;33:805–11. doi: 10.1016/j.bone.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Salomo M, Jurlander J, Nielsen LB, Gimsing P. How myeloma cells escape bisphosphonate-mediated killing: development of specific resistance with preserved sensitivity to conventional chemothera-peutics. Br J Haematol. 2003;122:202–10. doi: 10.1046/j.1365-2141.2003.04437.x. [DOI] [PubMed] [Google Scholar]
- 32.Ifergan I, Meller I, Issakov J, Assaraf YG. Reduced folate carrier protein expression in osteosarcoma: implications for the prediction of tumor chemosensi-tivity. Cancer. 2003;98:1958–66. doi: 10.1002/cncr.11741. [DOI] [PubMed] [Google Scholar]
- 33.Zhou H, Randall RL, Brothman AR, Maxwell T, Coffin CM, Goldsby RE. Her-2/neu expression in osteosarcoma increases risk of lung metastasis and can be associated with gene amplification. J Pediatr Hematol Oncol. 2003;25:27–32. doi: 10.1097/00043426-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Papapoulos SE, Eekhoff EM, Zwinderman AH. Acquired Resistance to Bisphosphonates in Paget's Disease of Bone. J Bone Miner Res. 2006;21:P88–91. doi: 10.1359/jbmr.06s216. [DOI] [PubMed] [Google Scholar]
- 35.Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569–82. [PubMed] [Google Scholar]
- 36.Doggrell SA. Statins in the 21st century: end of the simple story? Expert Opin Investig Drugs. 2001;10:1755–66. doi: 10.1517/13543784.10.9.1755. [DOI] [PubMed] [Google Scholar]
- 37.Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther. 2006;6:1075–85. doi: 10.1586/14737140.6.7.1075. [DOI] [PubMed] [Google Scholar]
- 38.Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–52. doi: 10.1056/NEJM199907293410507. [DOI] [PubMed] [Google Scholar]
- 39.Sandberg AA, Bridge JA. Updates on the cytoge-netics and molecular genetics of bone and soft tissue tumors: osteosarcoma and related tumors. Cancer Genet Cytogenet. 2003;145:1–30. [PubMed] [Google Scholar]
- 40.Benassi MS, Molendini L, Gamberi G, Ragazzini P, Sollazzo MR, Merli M, Asp J, Magagnoli G, Balladelli A, Bertoni F, Picci P. Alteration of pRb/p16/cdk4 regulation in human osteosarcoma. Int J Cancer. 1999;84:489–93. doi: 10.1002/(sici)1097-0215(19991022)84:5<489::aid-ijc7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 41.David JP, Mehic D, Bakiri L, Schilling AF, Mandic V, Priemel M, Idarraga MH, Reschke MO, Hoffmann O, Amling M, Wagner EF. Essential role of RSK2 in c-Fos-dependent osteosarcoma development. J Clin Invest. 2005;115:664–72. doi: 10.1172/JCI22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei G, Lonardo F, Ueda T, Kim T, Huvos AG, Healey JH, Ladanyi M. CDK4 gene amplification in osteosarcoma: reciprocal relationship with INK4A gene alterations and mapping of 12q13 amplicons. Int J Cancer. 1999;80:199–204. doi: 10.1002/(sici)1097-0215(19990118)80:2<199::aid-ijc7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Grem JL, King SA, Wittes RE, Leyland-Jones B. The role of methotrexate in osteosarcoma. J Natl Cancer Inst. 1988;80:626–55. doi: 10.1093/jnci/80.9.626. [DOI] [PubMed] [Google Scholar]
- 44.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 45.Beretta GL, Gatti L, Tinelli S, Corna E, Colangelo D, Zunino F, Perego P. Cellular pharmacology of cis-platin in relation to the expression of human copper transporter CTR1 in different pairs of cisplatin-sensitive and -resistant cells. Biochem Pharmacol. 2004;68:283–91. doi: 10.1016/j.bcp.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Gomes CM, Van Paassen H, Romeo S, Welling MM, Feitsma RI, Abrunhosa AJ, Botelho MF, Hogendoorn PC, Pauwels E, Cleton-Jansen AM. Multidrug resistance mediated by ABC transporters in osteosarcoma cell lines:mRNA analysis and functional radiotracer studies. Nucl Med Biol. 2006;33:831–40. doi: 10.1016/j.nucmedbio.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Serra M, Pasello M, Manara MC, Scotlandi K, Ferrari S, Bertoni F, Mercuri M, Alvegard TA, Picci P, Bacci G, Smeland S. May P-glycoprotein status be used to stratify high-grade osteosarcoma patients? Results from the Italian/Scandinavian Sarcoma Group 1 treatment protocol. Int J Oncol. 2006;29:1459–68. [PubMed] [Google Scholar]
- 48.Lourda M, Trougakos IP, Gonos ES. Development of resistance to chemotherapeutic drugs in human osteosarcoma cell lines largely depends on up-regulation of Clusterin/Apolipoprotein J. Int J Cancer. 2007;120:611–22. doi: 10.1002/ijc.22327. [DOI] [PubMed] [Google Scholar]
- 49.Mensah-Osman EJ, Thomas DG, Tabb MM, Larios JM, Hughes DP, Giordano TJ, Lizyness ML, Rae JM, Blumberg B, Hollenberg PF, Baker LH. Expression levels and activation of a PXR variant are directly related to drug resistance in osteosarcoma cell lines. Cancer. 2007;109:957–65. doi: 10.1002/cncr.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ory B, Moriceau G, Redini F, Heymann D. mTOR inhibitors (rapamycin and their derivatives) and nitrogen bisphosphonates: bi-functional conpounds for the treatment of bone tumors. Curr Med Chem. 2007;14:1381–7. doi: 10.2174/092986707780831159. [DOI] [PubMed] [Google Scholar]


