Dear Editor:
It has been extensively documented that osteoprotegerin (OPG), a soluble member of the TNF receptor superfamily, inhibits osteoclastogenesis by binding to receptor activator of NF-kB ligand (RANKL) and preventing interaction with its cognate transmembrane receptor RANK [reviewed in 1]. However, OPG can also interact with, and neutralize, TNF-related apoptosis inducing ligand (TRAIL), whose extracellular domain shares a 30% homology with the extracellular domain of RANKL [reviewed in 2]. Although some inconsistencies on the differential binding affinity of OPG for RANKL versus TRAIL were present in initial studies [2, 3], it has been recently demonstrated that the affinity of native OPG for native TRAIL is comparable to that for RANKL (45 nM versus 23 nM, respectively) at 37°C, as determined by plasmon surface resonance analysis [4]. Consistently with this biochemical study, OPG has been shown to act in a paracrine and autocrine manner by binding TRAIL and promoting the survival of multiple myeloma [5], prostate cancer [6], ameloblastoma cells [7] and synovial fibroblasts [8]. Interestingly, previous data from different groups have shown that recombinant TRAIL modulates the differentiation of erythroid and myeloid precursors [9–10], while inhibits both human and mouse osteoclastogenesis when added to pre-osteoclast cultures induced to differentiate with recombinant macrophage colony-stimulating factor (M-CSF) +RANKL as well as to mature osteoclasts [11–14]. On the other hand, a couple of studies suggested that recombinant soluble TRAIL might promote osteoclastogenesis [4, 15], and the proposed molecular mechanism to explain such observation was a competition between TRAIL and RANKL for OPG binding. However, it should be noticed that Vitovsky et al. used much higher concentrations of TRAIL (500 ng/ml) than RANKL (30 ng/ml) or OPG (50 ng/ml) and more importantly used mouse bone marrow pre-osteoclasts [4]. In this respect, it has been clearly shown that mouse pre-osteoclasts only express TRAIL-R2 [2], while human peripheral blood-derived pre-osteoclasts express both death receptors TRAIL-R1 and TRAIL-R2 as well as TRAIL-R4 [11–14].
Therefore, to further elucidate the important issue of the interplay between RANKL, TRAIL and OPG in human osteoclastogenesis, we have cultured adherent PBMC with M-CSF+RANKL for 14 days in the absence or presence of recombinant OPG and recombinant TRAIL, prepared as previously described [16]. TRAIL and OPG were added alone or in combination. Importantly, all cytokines were used at the same concentration (50 ng/ml). As expected [11–14], the addition of TRAIL to M-CSF+RANKL significantly (P < 0.05) inhibited osteoclast formation (Fig. 1A and B). Of note, recombinant OPG completely abrograted (P < 0.05) osteoclast formation irrespectively of the presence of recombinant TRAIL in culture (Fig. 1A and B). The anti-osteoclastic activity of OPG could not be ascribed to a low affinity of OPG for TRAIL since OPG (50 ng/ml) efficiently inhibited the apoptosis induced by TRAIL (50 ng/ml) in HL-60 leukemic cells (data not shown).
1.
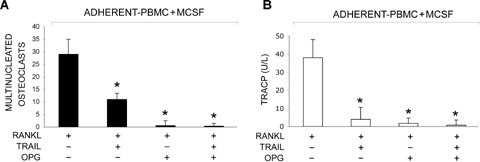
Effect of combined treatment of RANKL, TRAIL and OPG on osteoclastic differentiation. Adherent PBMC were cultured with M-CSF alone for 6 days. Then cells were cultured for additional 14 days with the addition of RANKL in the absence or presence of TRAIL and/or OPG, as indicated. Cultures were analysed for osteoclastic differentiation by scoring the number of TRAP+ multinucleated cells (A), and measuring the levels of TRAP 5b (B), a specific marker of resorption activity, in culture supernatants by ELISA. Data represent the means ±SD of three different experiments performed in duplicate.
Our current observations on one hand confirm that TRAIL has anti-osteoclastic activity and on the other hand indicate that it does not affect the potent anti-osteoclastic activity of OPG at least in the simplified model of osteoclastogenesis represented by human PBMC induced to differentiate by M-CSF+RANKL. Taken together with previous studies [4, 11–15], these data also suggest that the relative concentrations of TRAIL, RANKL and OPG in the local microenvironment are likely key determinant for the regulation of osteoclastogenesis.
References
- 1.Boyle WJ, Scott SW, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 2.Zauli G, Secchiero P. The role of theTRAIL/TRAIL-receptors system in hematopoiesis and endothelial cell biology. Cytokine Growth Factor Rev. 2006;17:245–57. doi: 10.1016/j.cytogfr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Truneh A, Sharma S, Silverman C, Khandekar S, Reddy MP, Deen KC, McMaughlin MM, Srinivasula SM, Livi GP, Marshall LA, Alnemri ES, Williams WV, Doyle ML. Temperature-sensitive differential affinity of TRAIL for its receptors. J Biol Chem. 2000;275:23319–25. doi: 10.1074/jbc.M910438199. [DOI] [PubMed] [Google Scholar]
- 4.Vitovski S, Phillips JS, Sayers J, Croucher PI. Investigating the interaction between osteoprotegerin and RANKL or TRAIL: evidence for a pivotal role for osteoprotegerin in regulating two distinct pathways. J Biol Chem. 2007;282:31601–9. doi: 10.1074/jbc.M706078200. [DOI] [PubMed] [Google Scholar]
- 5.Shipman CM, Croucher PI. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003;63:912–6. [PubMed] [Google Scholar]
- 6.Nyambo R, Cross N, Lippitt J, Holen I, Bryden G, Hamdy FC, Eaton CL. Human bone marrow stromal cells protect prostate cancer cells from TRAIL-induced apoptosis. J Bone Miner Res. 2004;19:1712–21. doi: 10.1359/JBMR.040703. [DOI] [PubMed] [Google Scholar]
- 7.Sandra F, Hendarmin L, Nakamura S. Osteoprotegerin (OPG) binds with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL): suppression of TRAIL-induced apoptosis in ameloblastomas. Oral Oncol. 2006;42:415–20. doi: 10.1016/j.oraloncology.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Miyashita T, Kawakami A, Nakashima T, Yamasaki S, Tamai M, Tanaka F, Kamachi M, Ida H, Migita K, Origuchi T, Nakao K, Eguchi K. Osteoprotegerin (OPG) acts as an endogenous decoy receptor in tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis of fibroblast-like synovial cells. Clin Exp Immunol. 2004;137:430–6. doi: 10.1111/j.1365-2249.2004.02534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Secchiero P, Melloni E, Heikinheimo M, Mannisto S, Di Pietro R, Icone A, Zauli G. TRAIL regulates normal erythroid maturation through an ERK-dependent pathway. Blood. 2004;103:517–22. doi: 10.1182/blood-2003-06-2137. [DOI] [PubMed] [Google Scholar]
- 10.Secchiero P, Gonelli A, Mirandola P, Melloni E, Zamai L, Celeghini C, Milani D, Zauli G. Tumor necrosis factor-related apoptosis-inducing ligand induces monocytic maturation of leukemic and normal myeloid precursors through a caspase-dependent pathway. Blood. 2002;100:2421–9. doi: 10.1182/blood-2002-01-0047. [DOI] [PubMed] [Google Scholar]
- 11.Zauli G, Rimondi E, Nicolin V, Melloni E, Celeghini C, Secchiero P. TNF-related apoptosis inducing lig-and (TRAIL) blocks osteoclastic differentiation induced by RANKL+M-CSF. Blood. 2004;104:2044–50. doi: 10.1182/blood-2004-03-1196. [DOI] [PubMed] [Google Scholar]
- 12.Roux S, Lambert-Comeau P, Saint-Pierre C, Lepine M, Sawan B, Parent JL. Death receptors, Fas and TRAIL receptors, are involved in human osteoclast apoptosis. Biochem Biophys Res Commun. 2005;333:42–50. doi: 10.1016/j.bbrc.2005.05.092. [DOI] [PubMed] [Google Scholar]
- 13.Colucci S, Brunetti G, Cantore FP, Oranger A, Mori G, Pignataro P, Tamma R, Grassi FR, Zallone A, Grano M. The death receptor DR5 is involved in TRAIL-mediated human osteoclast apoptosis. Apoptosis. 2007;12:1623–32. doi: 10.1007/s10495-007-0095-3. [DOI] [PubMed] [Google Scholar]
- 14.Zauli G, Rimondi E, Stea S, Baruffaldi F, Stebel M, Zerbinati C, Corallini F, Secchiero P. TRAIL inhibits osteoclastic differentiation by counteracting RANKL-dependent p27Kip1 accumulation in pre-osteoclast precursors. J Cell Physiol. 2008;214:117–25. doi: 10.1002/jcp.21165. [DOI] [PubMed] [Google Scholar]
- 15.Colucci S, Brunetti G, Rizzi R, Zonno A, Mori G, Colaianni G, Del Prete D, Faccio R, Liso A, Capalbo S, Liso V, Zallone A, Grano M. T cells support osteoclastogenesis in an in vitro model derived from human multiple myeloma bone disease: the role of the OPG/TRAIL interaction. Blood. 2004;104:3722–30. doi: 10.1182/blood-2004-02-0474. [DOI] [PubMed] [Google Scholar]
- 16.Zauli G, Pandolfi A, Gonelli A, Di Pietro R, Guarnieri S, Ciabattoni G, Rana R, Vitale M, Secchiero P. Tumor necrosis factor-related apopto-sis-inducing ligand (TRAIL) sequentially upregulates nitric oxide and prostanoid production in primary human endothelial cells. Circ Res. 2003;92:732–40. doi: 10.1161/01.RES.0000067928.83455.9C. [DOI] [PubMed] [Google Scholar]


