Abstract
Magnesium is associated with several important cardiovascular diseases. There is an accumulating body of evidence verifying the important roles of Mg2+-permeable channels. In the present study, we estimated the intracellular free Mg2+ concentration ([Mg2+]i) using 31P-nuclear magnetic resonance (31P-NMR) in porcine carotid arteries. pHi and intracellular phosphorus compounds were simultaneously monitored. Removal of extracellular divalent cations (Ca2+ and Mg2+) in the absence of Na+ caused a gradual decrease in [Mg2+]i to ∼60% of the control value after 125 min. On the other hand, the simultaneous removal of extracellular Ca2+ and Na+ in the presence of Mg2+ gradually increased [Mg2+]i in an extracellular Mg2+-dependent manner. 2-aminoethoxydiphenyl borate (2-APB) attenuated both [Mg2+]i load and depletion caused under Na+- and Ca2+-free conditions. Neither [ATP]i nor pHi correlated with changes in [Mg2+]i. RT-PCR detected transcripts of both TRPM6 and TRPM7, although TRPM7 was predominant. In conclusion, the results suggest the presence of Mg2+-permeable channels of TRPM family that contribute to Mg2+ homeostasis in vascular smooth muscle cells. The low, basal [Mg2+]i level in vascular smooth muscle cells is attributable to the relatively low activity of this Mg2+ entry pathway.
Keywords: magnesium, vascular smooth muscle, 2-aminoethoxydiphenyl borate, ATP, NMR
Introduction
From both clinical and epidemiologic aspects, Mg2+-deficiency is related to cardiovascular diseases [1–3]. More Mg2+ intake is recommended to prevent arteriosclerosis and hypertension. It is important, however, to point out that the serum Mg2+ level does not reflect Mg2+-deficiency of the entire body [4]. Cellular Mg2+-deficiency is caused by a malfunction of Mg2+ transporters across the plasma membrane, as well as a fall in the extracellular Mg2+ concentration. It is therefore of great interest to investigate mechanisms driving Mg2+ transport into the cell, especially into vascular smooth muscle cells.
[Mg2+]i is believed to be regulated by two transmembrane Mg2+ pathways: the Na+–Mg2+ exchange driven by the Na+-gradient, and the Na+-independent ‘passive’ Mg2+ transport via Mg2+-permeable channels [5–8]. Since the molecular identification of the latter Mg2+ pathway, such as melastatin-type transient receptor potential (TRPM) homologue channels, there has been an accumulating body of evidence for the crucial role that this pathway plays in Mg2+ homeostasis [9–11]. Also, TRPM homologue channels are bifunctional proteins, which contain a kinase domain in the C-terminus.
[Mg2+]i is known to change slowly, and thereby act as a chronic regulator. In addition, changes in the intracellular milieu, such as the intracellular pH (pHi) and [ATP]i can affect [Mg2+]i regulation. However, the importance of TRPM homologues in [Mg2+]i regulation during relatively short durations has been assessed using fluorescent Mg2+ indicators. In the present study, we thus utilized 31P-NMR to estimate slow changes in [Mg2+]i over several hours in carotid arteries, which are now frequently used as a model to evaluate arteriosclerotic changes, and assessed the contribution of TRPM-like Mg2+-permeable channels.
Materials and methods
Preparation
Porcine carotid arteries were collected at an abattoir. The arteries were stripped of fat and connective tissue, and cut into segments of approximately 30 mm in length. The lumen was exposed by cutting the artery segments into two strips along the longitudinal direction. The endothelium was removed by scratching with a cotton-tipped stick. The resultant pig carotid artery strips (∼2 g wet weight) were mounted in a sample tube of 10 mm in diameter. This study was approved by the institutional committee of animal experiments.
31P-NMR
The methods employed for the 31P-NMR measurements were essentially the same as those previously described [12]. NMR spectrometers (GSX270W: JEOL, Tokyo, Japan; UNITY-500plus: Varian, Tokyo, Japan) were operated at 109.4 and 202.3 MHz, respectively. The temperature of the sample was maintained at 32°C. Radio frequency pulses corresponding to a flip angle of 30 were applied every 0.6 sec. 31P-NMR spectra were obtained by accumulating 2500 signals (free induction decays) over 25 min. Before Fourier transformation, a broadening factor of 20 Hz was applied to enhance the signal-to-noise ratio. Spectral peak resonances (frequencies) were measured relative to that of phosphocreatine (PCr) in p.p.m.
Control spectra were acquired in the absence of Ca2+. Then, experiments were carried out in the absence of extracellular Na+ to rule out the contribution of Na+-coupled Mg2+ transport, that is, Na+–Mg2+ exchange. Six major peaks were observed (Fig. 1): phosphomonoesters (PME), inorganic phosphate (Pi), PCr and the γ-, α- and β-phosphorus atoms of ATP (γ-, α- and β-ATP).
1.

Changes in the 31P-NMR spectrum during exposures to a divalent-cation-free, Na+-free solution. After acquiring the control spectrum in a Ca2+-free solution (a), extracel-lular Mg2+ and Na+ were simultaneously removed (0 Ca2+, 0 Mg2+, 0 Na+: K+ substitution) for 125 min. The spectra (b) and (c) were obtained during 25–50 min and 100–125 min periods, respectively. Each spectrum was obtained with 2500 signals accumulated over 25 min. The whole spectrum is shown in (A), and the β-ATP peaks are shown expanded in (B). The vertical line indicates the initial chemical shift of the β-ATP peak. The explanations are the same for the spectra in (C) and (D), but the divalent cation-free, Na+-free solution (0 Ca2+, 0 Mg2+, 0 Na+: K+ substitution) contained 150 μM 2-APB.
Concentrations of phosphorus compounds were estimated by integrating the peak areas (Scion image; Scion Corp., Fredrick, MA, U.S.A.) and by correcting with their saturation factors (Pi, 1.60; PCr, 1.36; β-ATP, 1.07).
Estimation of [Mg2+]i and pHi
Intracellular pH (pHi) was estimated from the chemical shift observed for the Pi peak (δo(PI)), using a Henderson–Hasselbalch type equation:
| Eq(1) |
where pKa is the negative logarithm of the dissociation constant of Pi (= 6.70), and δp(Pi) and δd(Pi) are the chemical shifts for H2PO4− (= 3.15 p.p.m.) and HPO4− (= 5.72 p.p.m.), respectively.The pHi value was used to correct the [Mg2+]i estimation.
Mg2+ usually binds to ATP as a 1 to 1 complex. [Mg2+]i was thus estimated from the chemical shift observed for the β-ATP peak (γoβ) using the following equation [13,14]:
| Eq(2) |
where δfβ and δbβ are the chemical shifts of metal-free and Mg2+-bound forms of β-ATP, respectively. We have previously shown that KD“MgATP”δfβ and δbβ can be described as functions of pH [15] (See, Supplementary Material S1: Details of estimation procedures). KD“MgATP”(pHi) was derived from quadratic pH-functions for KD“MgATP” at 25 and 37°C [16], using the van't Hoff isochore. The pH–functions of δfβ and δbβ were constructed by fitting the data points of model solutions with sigmoid curves [17]. Thus, Eq(2) can be rewritten as:
| Eq(3) |
In Table 2, [Mg2+]i and pHi values were also estimated, from the chemical shifts of β- and γ-ATP [12,17]. For the chemical shift of γ-ATP, an equation analogous to Eq(3) can be written:
2.
[Mg2+]i and pHi values estimated using two methods: (1) from the chemical shifts of β-and γ-ATP or 2) from the chemical shifts of β-ATP and Pi.(See Materials and methods for details). [Mg2+]i and pHi values during [Mg2+]i deple- tion were compared in A and B. In C and D, [Mg2+]i and pHi values were during the elevation of [Mg2+]i. Single (*) and double asterisks (**) indicate P<0.05 and P<0.01 versus control, respectively.
| (A) Exposure to a divalent cation- and Na+-free solution (n = 7). | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1) from β- and γ-ATP | 2) from β-ATP and Pi | ||||||||||||
| [Mg2+]i (mM) | pHi | [Mg2+]i (mM) | pHi | ||||||||||
| Control (0 Ca2+) | 0.70±0.13 | 7.20±0.11 | 0.75±0.09 | 7.09±0.05 | |||||||||
| 0 Ca2+, 0 Mg2+, 0 Na+ (K+) 25–50 min | 0.61±0.07** | 7.09±0.09** | 0.62±0.04** | 7.06±0.04** | |||||||||
| 0 Ca2+, 0 Mg2+, 0 Na+ (K+) 100–125 min | 0.43±0.04** | 7.01±0.08** | 0.46±0.05** | 6.92±0.05** | |||||||||
| (B) Exposure to a divalent cation- and Na+-free solution containing 2-APB (n = 7). | |||||||||||||
| 1) from β- and γ-ATP | 2) from β-ATP and Pi | ||||||||||||
| [Mg2+]i (mM) | pHi | [Mg2+]i (mM) | pHi | ||||||||||
| Control (0 Ca2+) | 0.68±0.04 | 7.21±0.05 | 0.74±0.05 | 7.09±0.04 | |||||||||
| 0 Ca2+, 0 Mg2+, 0 Na+ (K+) +150μM 2-APB 25–50 min | 0.65±0.08* | 7.09±0.08** | 0.68±0.07* | 7.03±0.04** | |||||||||
| 0 Ca2+, 0 Mg2+, 0 Na+ (K+) +150μM 2-APB 100–125 min | 0.60±0.12** | 7.00±0.11** | 0.62±0.08** | 6.94±0.05** | |||||||||
| (C) Exposure to a Ca2+- and Na+-free, high Mg2+ (6.0 mM) solutions (n = 7). | |||||||||||||
| 1) from β- and γ-ATP | 2) from β-ATP and Pi | ||||||||||||
| [Mg2+]i (mM) | pHi | [Mg2+]i (mM) | pHi | ||||||||||
| Control (0 Ca2+) | 0.72±0.10 | 7.20±0.09 | 0.78±0.08 | 7.08±0.12 | |||||||||
| 0 Ca2+, 6.0 Mg2+, 0 Na+ (K+) 25–50 min | 1.11±0.28** | 7.12±0.10** | 1.18±0.28** | 7.03±0.05** | |||||||||
| 0 Ca2+, 6.0 Mg2+, 0 Na+ (K+) 100–125 min | 1.63±0.23** | 6.99±0.11** | 1.79±0.18** | 6.92±0.04** | |||||||||
| (D) Exposure to a Ca2+- and Na+-free, high Mg2+ (6.0 mM) solution containing 2-APB (n = 6). | |||||||||||||
| 1) from β- and γ-ATP | 2) from β-ATP and Pi | ||||||||||||
| [Mg2+]i (mM) | pHi | [Mg2+]i (mM) | pHi | ||||||||||
| Control (0 Ca2+) | 0.70±0.09 | 7.20±0.11 | 0.75±0.04 | 7.10±0.03 | |||||||||
| 0 Ca2+, 6.0 Mg2+, 0 Na+ (K+) +150μM 2-APB 25–50 min | 0.91±0.10** | 7.06±0.07** | 0.93±0.06** | 7.02±0.02** | |||||||||
| 0 Ca2+, 6.0 Mg2+, 0 Na+ (K+) +150μM 2-APB 100–125 min | 1.10±0.08** | 6.99±0.04** | 1.14±0.08** | 6.95±0.03** | |||||||||
| Eq(4) |
δfγ and δbγ are the chemical shifts of metal-free and Mg2+-bound forms of γ-ATP, respectively, and these parameters are also expressed as pH-functions. [Mg2+]i and pHi were estimated by solving the Eq(3) and Eq(4) simultaneously.
Similar to the effect of Mg2+, Ca2+-binding to ATP changes the chemical shifts of the ATP peaks. However, basal [Ca2+]i is maintained at ∼100 nM in smooth muscle cells under physiological conditions. Furthermore, experiments in the present study were carried out mainly under Ca2+-free conditions. Therefore, the effect of Ca2+-binding to ATP is considered negligible.
Solutions and chemicals
The extracellular solution used for the ‘normal'solution had the following composition in mM: NaCl, 137.9; KHCO3 5.9; CaCl2 2.4; MgCl2 1.2; glucose 11.8; HEPES 5 (pH adjusted to 7.4–7.5 at 32°C). The ionic composition was modified iso-osmotically. Also, divalent cation-free solutions contained 1 mM EDTA. The solutions used for 31P-NMR measurements were normally aerated with 95% O2/5%CO2. 2-aminoethoxydiphenyl borate (2-APB) was purchased from Calbiochem (San Diego, CA, USA).
RT-PCR
The procedures for RT-PCR were essentially the same as previously described [18]. Total RNA was extracted from porcine carotid arteries. After treatment with RQ1 DNase (Promega, Madison, WI, USA), the total RNA was subjected to an RT (reverse-transcription) reaction. RT was performed using a random hexamer (12 pmol/reaction) and Moloney murine leukemia virus (MMLV) reverse transcriptase 5 (100 U/reaction) according to the manufacture's instructions (Gibco-BRL, Rockville MD, U.S.A.). The cycling condition was 3 min of initial denaturation at 95°C followed by 35 cycles of 95°C for 30 sec, 54°C for 30 sec and 72°C for 35 sec. The RT sample was then used as a template for the PCR reaction. The amplicons (5 μl were run on a 2.5% agarose gel and stained with ethidium bromide. Because the cDNA sequences for porcine TRP (transient receptor potential) homologue cation channels have not been published, the PCR primers were designed by using the conserved sequences in humans and mice. The primers used are listed in Table 1.
1.
PCR primers. PCR primers for porcine sample were designed by using the conserved sequences in humans and mice
| Clones | Primer Sequence (+): Sense, (−): Antisense | Primer site (in human clones) | Accession No (human) (mouse) | |||
|---|---|---|---|---|---|---|
| TRPM2 | (+): 5′-TTC CAG GAG ATG TTT GAG AC-3′ | 1604-1938 | NM_003307 | |||
| (−): 5′-TCA GGC TTG TTG GAG ATG AG-3′ | NM_138301 | |||||
| TRPM4 | (+): 5′-GTG GGA GGG ACT GGA ATT GA-3′ | 863-1263 | NM_017636 | |||
| (−): 5′-AGC TCA TCC AGG TAG GCT GA-3′ | NM_175130 | |||||
| TRPM6 | (+): 5′-TGT TGG TGG AGA TGC AGC C-3′ | 2862-3174 | NM_017662 | |||
| (−): 5′-CCT GCA TGT TGA TTC ACA GC-3′ | NM_153417 | |||||
| TRPM7 | (+): 5′-GAT GCC CTC AAA GAA CAT GC-3′ | 794-1269 | NM_017672 | |||
| (−): 5′-GGC TCT GCT GCA TCA GGA AG-3′ | NM_021450 | |||||
Statistics
Numerical data are expressed as the mean (S.D. Differences between groups with different experimental protocols were evaluated by use of ANOVA for repeated measures. When a significant difference was identified between the groups (P<0.05), individual comparisons at the same time point were performed using an unpaired t-test.
Results
Depletion of [Mg2+]ivia Na+-independent Mg2+ pathways
31P-NMR was used to continuously measure phosphorus compounds in porcine carotid artery smooth muscle (Fig. 1). In this study, we mainly estimated [Mg2+]i from the chemical shift of the β-ATP peak, and correction was made by pHi estimated from the chemical shift of the Pi peak.
During exposure to a divalent cation-free solution (i.e. 0 Ca2+, 0 Mg2+), [Mg2+]i decreased from from 0.74±0.11 to 0.49±0.08 mM (n = 7; Fig. 2A, ▪) after 125 min, while pHi did not change significantly (Fig. 2B, •). Essentially the same decrease in [Mg2+]i (from 0.75±0.09 to 0.46±0.05 mM; n = 7; Fig. 2A, □) was observed in the absence of Na+, suggesting that Mg2+-permeable channels make a major contribution to the changes in [Mg2+]i under divalent cation-free conditions. On the other hand, pHi decreased from 7.09 ±0.05 to 6.92 ±0.05 (n = 7) after 125 min in the absence of Na+ (Fig. 2B, ○), presumably due to the inhibition of Na+-coupled pHi regulatory mechanisms, such as Na+-H+ exchange and Na+-HCO3− co-transport.
2.
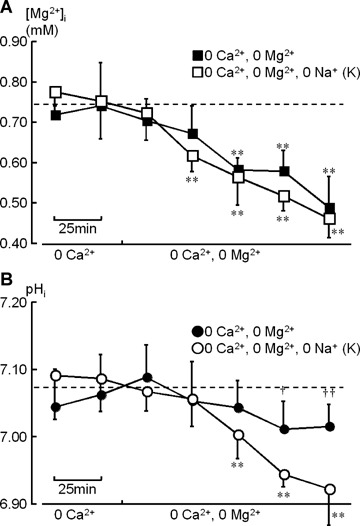
Time course of changes in [Mg2+]i (A: squares) and pHi (B: circles) during exposure to divalent cation-free solutions. After control measurements in a Ca2+-free solution (control), extracellular Mg2+ was also removed for 125 min. Each point was obtained from the accumulation of NMR signals over 25 min. The open (□, ○) and filled symbols (▪, •) represent the data obtained under exposures to Na+-free (0 Ca2+, 0 Mg2+, 0 Na+: K+ substitution; n = 7) and Na+-containing solutions (0 Ca2+, 0 Mg2+; n = 7), respectively. The spectra shown in Fig. 1A and B correspond to this Na+-free experiment. The dotted lines indicate the mean value of [Mg2+]i and pHi before exposure to the divalent cation-free solutions (n= 14 for all preparations shown in this figure). Vertical bars represent S.D. values. Asterisks indicate statistically significant differences compared to the [Mg2+]i and pHi values before removal of extracellu-lar Mg2+ (*, P<0.05; **, P<0.01). Crosses on filled symbols, in the presence of Na+, indicate statistically significant differences compared to the open symbols, in the absence of Na+, at the same time point (†, P<0.05; ††, P<0.01).
Effects of 2-APB in Na+-independent depletion of [Mg2+]i
2-APB is known to block TRPM7 [11]. To substantiate the involvement of analogous Mg2+-permeable channels in vascular muscle cells, the effect of 2-APB was examined (Fig. 1C and D). As shown in Figure 3, application of 150 μM 2-APB to the divalent cation-and Na+-free solution significantly attenuated the depletion of [Mg2+]i (from 0.74 ± 0.05 to 0.62 ± 0.08 mM after 125 min; Fig. 3A, ▪). This inhibitory effect of 2-APB was concentration-dependent (Fig. 3C). The decrease in [Mg2+]i after 125 min was –0.27 ± 0.11 mM at 15 μM (n = 5), (0.22 ± 0.07 mM at 50 μM (n = 5) and –0.12±0.08 mM at 150 μM (n = 7). On the other hand, this drug had little effect on the changes in pHi (unpaired t-test, P >0.05, n = 7; Fig. 3B, •).
3.
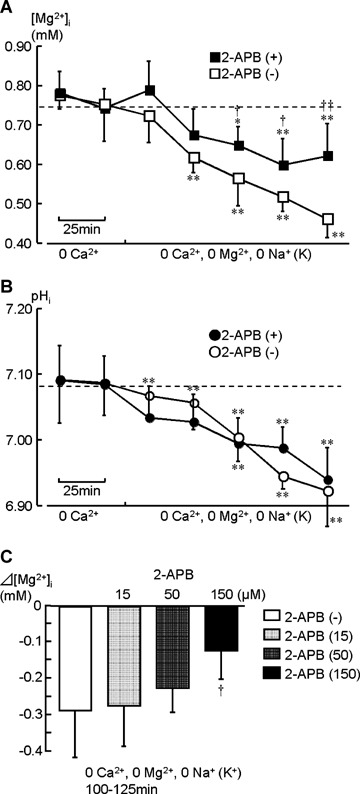
The effect of 2-APB application during exposure to divalent cation- and Na+-free solutions. (0 Ca2+, 0 Mg2+, 0 Na+). Changes in [Mg2+]i and pHi are plotted in (A; ▪) and (B; •), respectively. After acquiring the control data in a Ca2+-free solution, extracellular Mg2+ and Na+ were simultaneously removed, and 150 μM 2-APB was added (n = 7). The spectra in Figures 1C and D correspond to this experiment. The data obtained in the absence of 2-APB (open symbols: □, ○; the same data shown in Fig. 2, n= 7)are also plotted to clearly show the inhibitory effect of 2-APB. Asterisks indicate statistically significant differences compared to the [Mg2+]i and pHi values before removal of extracellular Na+ (*, P<0.05;**, P<0.01). Crosses on filled symbols indicate statistically significant differences compared to the open symbols at the same time point (†, P<0.05; ††, P<0.01). Bar graphs in (C) indicate effects of 15, 50 and 150 μM 2-APB during 125–150 min (n= 7 in (–) and 150 μM 2-APB; n= 5 in 15 and 50 μM 2-APB).
Increase in [Mg2+]ivia Na+-independent Mg2+ pathways
Next, to demonstrate the increase in [Mg2+]ivia transmembrane Mg2+-permeable channels, the effect of Na+ removal was examined in the presence of Mg2+ (Supplementary Fig. S1, A). Extracellular Ca2+ was again removed to potentiate Mg2+ transport, i.e. to reduce competition between divalent cations at the channel pore.
Changes in [Mg2+]i and pHi during exposure to Ca2+- and Na+-free solutions are shown in Figures 4A and B, respectively. In the presence of 1.2 mM Mg2+ (the ‘normal’Mg2+ concentration in extracellular medium), [Mg2+]i increased from 0.73±0.07 to 1.01±0.09 mM after 125 min (▪; n= 5; P<0.01). When the concentration of extracellular Mg2+ was increased to 6.0 mM, [Mg2+]i increased from 0.78±0.08 to 1.79 ± 0.18 mM after 125 min (□; n= 7; P<0.01). The [Mg2+]i rise was clearly enhanced by raising the extracellular Mg2+ concentration (unpaired t-test, P<0.01).
4.

Changes in [Mg2+]i (A) and pHi (B) during exposures to Ca2+- and Na+-free solutions containing Mg2+. After the carotid artery preparations were superfused with a Ca2+-free solution containing 1.2 mM Mg2+, extra-cellular Na+ was removed for 125 min (filled symbols: ▪, •). In the experiments indicated by open symbols (□, ○), extracellular Mg2+ was increased to 6.0 mM during Na+ removal. Asterisks indicate statistically significant differences compared to the [Mg2+]i and pHi values before removal of extracellular Mg2+ (*, P<0.05; **, P<0.01). Crosses on open symbols indicate statistically significant differences compared to the filled symbols at the same time point (†, P<0.05;††, P<0.01).
Effects of 2-APB on the increase in [Mg2+]i
To assess whether the same Mg2+-permeable channels contributed to the inward and outward transport of Mg2+ under Na+-free conditions, we examined the effect of 2-APB in the presence of Mg2+. 2-APB (150 μM) was applied to a Ca2+- and Na+-free solution containing 6.0 mM Mg2+ (Supplementary Fig. S1, B). After 125 min, [Mg2+]i increased from 0.75±0.04 to 1.14±0.08 mM (Fig. 5A, ▪; n = 7; P<0.01), but this increase in [Mg2+]i was significantly smaller than without 2-APB (unpaired t-test, P<0.01). On the other hand, pHi with and without 2-APB, was comparable throughout experiments (not shown).
5.

The inhibitory effect of 2-APB on [Mg2+]i rise in Mg2+-containing solutions. In (A), after acquiring the control data in a Ca2+-free solution, the extracellular Mg2+ was increased to 6.0 mM, Na+ was removed, and 150 μM 2-APB was added in the extracellular solution. The data indicated by open symbols (□) represent experiments without 2-APB (the same data shown in Fig. 4A, □). In (B), extracellular Na+ was substituted with NMDG. Crosses on filled symbols (□, ○) indicate statistically significant differences compared to the open symbols at the same time point (†, P<0.05;††, P<0.01). Bar graphs in (C) indicate effects of 15, 50 and 150 μM 2-APB during 125–150 min in the presence of Ca2+ (n= 4 for each experiment).
A gradual, and slow increase in [Mg2+]i was caused by substituting extracellular Na+ with N-methyl-D-glucamine (NMDG), even in the presence of Ca2+ (Fig. 5B, □; n= 4). Application of 150 μM 2-APB again attenuated the increase in [Mg2+]i significantly (Fig. 5B, ▪; n= 4; P<0.01), suggesting that TRPM7-like Mg2+-permeable channels sensitive to 2-APB play a crucial role in Mg2+ regulation (i.e. uptake) under physiological conditions, in which Ca2+ is present. Lower concentrations (15 and 50 μM) of 2-APB only attenuated the increase in [Mg2+]i slightly (P >0.05; Fig.5C).
In the present experiments, Na+ was frequently substituted with equimolar K+. To rule out that Na+–Mg2+ exchangers coupled Mg2+ transport with K+ under Na+-free conditions, we examined the effect of amiloride, which is known to inhibit a broad range of Na+-coupled transporters [19], including Na+–Mg2+ exchange [12, 20]. Application of amiloride (1 mM) had little effect on the increase in [Mg2+]i caused by exposure to a Ca2+- and Na+-free solution containing 6.0 mM Mg2+ (unpaired t-test, P>0.05; see Supplementary Fig.S2).
Estimation of [Mg2+]i and pHi from the β and γ-ATP peaks
To confirm the changes in [Mg2+]i and pHi estimated above, we used a different procedure; specifically, the chemical shifts of β- and γ-ATP were used to solve simultaneous equations for [Mg2+]i and pHi (see, Material and methods). The changes in pHi, as well as [Mg2+]i, were comparable between the two analyses (Table 2). [Mg2+]i estimated from β- and γ-ATP were slightly smaller because of the higher pHi estimated; i.e. the apparent dissociation constant for MgATP is smaller in a higher pH.
High-energy phosphates
ATP is known to affect the activity of TRPM7 and to act as an important intracellular Mg2+ buffer. Correlation analysis revealed that [ATP]i and [Mg2+]i are not correlated regardless of the presence of extracellular Mg2+ (Fig. 6). Also, [PCr]i did not change significantly throughout (Supplementary Table S1).
6.
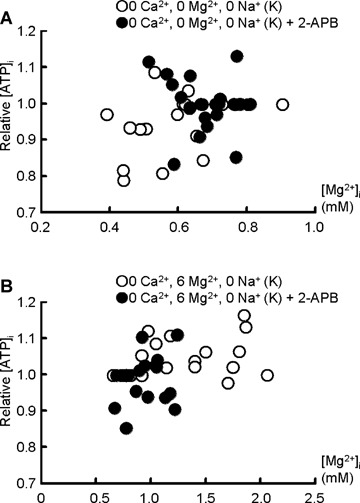
Correlation between [ATP]i and [Mg2+]i in the absence (0 Mg2+; A) and presence of extracellular Mg2+ (6 mM Mg2+; B). Each open () and filled circle () represents a 31P-NMR data point (spectrum accumulated over 25 min) obtained in the absence and presence of 2-APB, respectively. [ATP]i is expressed relative to that in the control. Data points in (A) are from experiments shown in Fig. 3, while those in (B) are from Fig. 5A.
RT-PCR
To confirm the transcription of genes for Mg2+-permeable channels, RT-PCR was performed. Because the cDNA sequences for porcine TRP (transient receptor potential) homologue cation channels have not been published, the PCR primers were designed by using the conserved sequences from humans and mice. Of TRPM2, 4, 6 and 7, the TRPM7 was predominant. TRPM6 was also detectable under the same PCR condition (Fig. 7; see also Supplementary Fig. S3).
Discussion
The present 31P-NMR measurements revealed several features of [Mg2+]i modulation via transmem-brane Mg2+-permeable channels, including sensitivity to 2-APB. Simultaneous removal of extracellular Mg2+ and Ca2+ reduced [Mg2+]i to approximately 60% of the control after 125 min even in the absence of Na+ (Fig. 2), under which conditions Na+-coupled Mg2+ transporters i.e. Na+–Mg2+ exchange, do not operate. In addition, the removal of extracellular Na+ in the presence of extracellular Mg2+ increased [Mg2+]i in an extracellular Mg2+-concentration-dependent manner (Fig. 4), and this effect was enhanced by the simultaneous removal of Ca2+. Altogether, these results suggested an important role of transmembrane Mg2+-permeable channels in regulating [Mg2+]i in vascular smooth muscle cells.
As shown in Fig. 5B, the activity of Mg2+-permeable channels are attenuated by extracellular Ca2+, but Mg2+ entry still occurred. In these experiments, extracellular Na+ was substituted with NMDG, and this procedure maintained the negative membrane potential, unlike Na+ substitution with K+, which was used in other experiments. Furthermore, simultaneous removal of extracellular divalent cations, that is, Ca2+ and Mg2+, did not significantly decrease [Mg2+]i when extracellular Na+ was substituted with NMDG (data not shown). Therefore, it is considered that, although Mg2+ loss can be caused via Mg2+-permeable channels under extreme conditions, this mechanism mainly contributes to Mg2+ uptake under physiological conditions, in which the negative resting membrane potential is preserved (probably several tens of mV).
TRPM6 and TRPM7 are known Mg2+-permeable non-selective cation channels. It has been shown that TRPM6 is abundant in the kidney and small intestine, while TRPM7 is expressed ubiquitously in numerous tissues and organs [9, 21, 22]. It is thought that the former and latter are responsible for Mg2+ regulation at the organ and cellular levels, respectively. In line with this notion, RT-PCR examinations revealed that TRPM7 was a major component of TRPM homologues in porcine carotid arteries, but TRPM6 was also detectable (Fig. 7). Previous patch clamp studies have shown that the removal of extracellular divalent cations facilitates TRPM7-like current [9, 11, 23], a finding that is in good agreement with our [Mg2+]i measurements, that is Figure 5.
7.
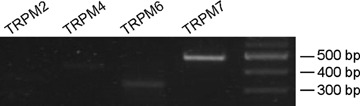
RT-PCR detection of TRPM channel members in porcine carotid arteries. The PCR amplification was performed by 35 cycles. A 100-bp molecular weight marker was used (right column). The size of each PCR product is as expected from the mouse and human sequences.
2-APB is known to block store-operated Ca2+ release-activated Ca2+ (CRAC) channels as well as Mg2+-permeable channels [24]. However, Mg2+ transport via CRAC channels is considered negligible, because these channels have even greater Ca2+ selectivity than voltage-sensitive Ca2+ channels [25]. In the present study, Mg2+ transport via Mg2+-permeable channels demonstrated as Na+-independent [Mg2+]i changes, was not completely suppressed by 2-APB at <150 μM, which is three times greater than the IC50 (∼50 μM) reported for TRPM7-like cation currents [11, 23]. This discrepancy may be explained as follows: (1) The IC50 of 2-APB in TRPM7 may be altered by the intracellular milieu, namely, [ATP]i is maintained in our 31P-NMR measurements, while no ATP was added in the intracellular solution of the previous patch clamp experiments in which the effect of 2-APB was examined [11, 23]; (2) Native TRP channels may act as multimeric proteins of complex compositions [26]; 3) some other, as yet unknown Mg2+ transport mechanisms operate in parallel. Recently, it has been shown that the application of 2-APB does not block, but rather facilitates TRPM6 [27], which is contained as a minor component in porcine carotid arteries. In future studies, it would thus be interesting to examine the effects of drugs, such as 2-APB in TRPM6/7 knockdown cells. NMR measurements provide us with abundant information on [Mg2+]i regulation, that is pHi and [ATP]i which affect intracellular Mg2+-buffering, but require a considerable amount of vascular smooth muscle tissue to achieve reasonable time-resolution. For tissue level experiments, gene-targeting techniques, for example, RNA interference, remain to be improved.
The TRPM homologue channels consist of channel pore and kinase domains. Thus, these channels are referred to as ‘chanzymes’. It has been reported that Mg2+ and MgATP regulate the activity of TRPM7 via the kinase domain [9–11, 28]. In addition to this mechanism, intracellular ATP itself acts as an important Mg2+ buffer. With respect to the inhibitory effect of 2-APB on [Mg2+]i rise in the absence of extracellu-lar Ca2+ and Na+ (in the presence of 6 mM Mg2+; Fig. 4), one may suspect that 2-APB interacts with the kinase domain to elevate its sensitivity to [Mg2+]i. If this mechanism operates, increase in [Mg2+]i would be slowed at around the [Mg2+]i which significantly reduces the open probability of the Mg2+-permeable channels. The present 31P-NMR measurements, however, revealed that changes in [Mg2+]i were suppressed throughout the application of 2-APB (Fig. 6; Supplementary Material IV, Supplementary Table S1). Therefore, the present results suggest that 2-APB directly blocks Mg2+-permeable channels at the channel pore.
Reported values for [Mg2+]i in smooth muscle cells are lower than found in the other two muscle types, that is, skeletal and cardiac muscles. Furthermore, among smooth muscles distributed in numerous tissues and organs, we estimated [Mg2+]i in vascular smooth muscle cells to be the lowest after correcting for the pHi and temperature [8]:(0.8-0.85 mM in taenia caeci; 0.7–0.9 mM in the uterus; 0.8 mM in the urinary bladder and 0.65–0.75 mM in the carotid artery. Under divalent cation-free conditions, [Mg2+]i decreased to ∼60% of the control value after 125 min in porcine carotid arteries, however, the decrease in [Mg2+]i was much slower than that previously observed in intestinal smooth muscle cells (a decrease to tens of μM after 100 min) [6, 17]. The difference in the rate of [Mg2+]i depletion under Na+-free conditions suggests that the activity of Mg2+-permeable channels is lower in vascular smooth muscle cells. This hypothesis may account for the fact that the lowest basal [Mg2+]i level has been estimated in vascular smooth muscle cells and may contribute to our understanding of Mg2+-dependent cellular mechanisms. For example, the physiological significance of Ca2+-induced Ca2+ release from ryanodine receptors in vascular smooth muscle could be attributed to the low [Mg2+]i[29]. Also, Mg2+-permeable non-selective cation channels, such as TRPM7, have been recently shown to play a crucial role in generating spontaneous rhythmicity in pacemaker interstitial cells of intestinal smooth muscle tissues [30, 31]. Taken together, these facts warrant systematic investigation into cell- and tissue-specific roles of TRPM channels in numerous smooth muscle tissues containing pacemaker-like interstitial cells [32–35].
Functional mutations of ion channels are known to cause numerous diseases. Functional mutations of TRPM7 result in a significant effect on cellular Mg2+ homeostasis. It has been experimentally shown that mutations of the kinase domain in TRPM7 modulate the sensitivity to [Mg2+]i. Furthermore, knockout of TRPM7 causes intracellular Mg2+-deficiency in cultured cells [36]. In clinical fields, it has long been recognized that Mg2+ is associated with several important diseases, e.g. diabetes mellitus, hypertension, and cardiovascular and cerebrovascular diseases [37, 38]. Subgroups of these diseases may involve functional mutations of Mg2+-permeable channels contributing to passive Mg2+ transport via the electrochemical gradient. Similarly to the studies on TRPM6 [39, 40], future genetic analyses to clarify the contribution of TRPM7-like Mg2+-permeable channels to cardiovascular diseases will be of great interest. Indeed, a TRPM7 variant has been recently reported for two Guamanian neurodegenerative disorders [41].
In conclusion, the present 31P-NMR data have suggested the existence of Mg2+-permeable channels of TRPM homologues, contributing to [Mg2+]i regulation in vascular smooth muscle cells. The fact that [Mg2+]i decreases slowly under divalent cation-free conditions, presumably corresponding to the low expression level and/or activity of Mg2+-permeable channels, may account for the low basal [Mg2+]i in vascular smooth muscle cells. From the inhibitory effect of 2-APB and the results of RT-PCR, TRPM7 appears to play a predominant role in the passive Mg2+ transport.
Acknowledgments
The authors are grateful to Dr Lorraine M. Smith (Edinburgh University, U.K.) for useful discussions and improvement in the manuscript, and to Nagoya Meat Hygiene Laboratory (Nagoya, Japan) for continuous supply of porcine carotid arteries.
Supporting Information
Details of estimation procedures.
Example spectra for changes in the β-ATPpeak during exposures to a Ca2+- andNa+-free, high (6.0 mM)Mg2+ solution.
The effect of amiloride on[Mg2+]i.
Tests of primers.
Changes in the concentration of high-energy phosphates.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.McGuigan JAS, Elder HY, Günzel D, Schlue W-R. Magnesium homeostasis in heart: a critical reappraisal. J Clin Basic Cardiol. 2002;5:5–22. [Google Scholar]
- 2.Vaskonen T. Dietary minerals and modification of cardiovascular risk factors. J Nutr Biochem. 2003;14:492–506. doi: 10.1016/s0955-2863(03)00074-3. [DOI] [PubMed] [Google Scholar]
- 3.Touyz RM. Magnesium in clinical medicine. Front Biosci. 2004;9:1278–93. doi: 10.2741/1316. [DOI] [PubMed] [Google Scholar]
- 4.Kisters K, Tepel M, Spieker C, Zidek W, Barenbrock M, Tokmak F, Kosch M, Hausberg M, Rahn KH. Decreased membrane Mg2+ concentrations in a subgroup of hypertensives: membrane model for the pathogenesis of primary hypertension. Am J Hypertens. 1998;11:1390–3. doi: 10.1016/s0895-7061(98)00169-1. [DOI] [PubMed] [Google Scholar]
- 5.Flatman PW, Smith LM. Magnesium transport in ferret red cells. J Physiol. 1990;431:11–25. doi: 10.1113/jphysiol.1990.sp018318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakayama S, Tomita T. Regulation of intracellular free magnesium concentration in the taenia isolated from guinea-pig caecum. J Physiol. 1991;435:559–72. doi: 10.1113/jphysiol.1991.sp018525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handy RD, Gow IF, Ellis D, Flatman PW. Na-dependent regulation of intracellular free magnesium concentration in isolated rat ventricular myocytes. J Mol Cell Cardiol. 1996;28:1641–51. doi: 10.1006/jmcc.1996.0154. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama S, Clark JF. Smooth muscle and NMR review: An overview of smooth muscle metabolism. Mol Cell Biochem. 2003;244:17–30. [PubMed] [Google Scholar]
- 9.Nadler MJS, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Strokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–5. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 10.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–7. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 11.Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A. Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol. 2002;539:445–58. doi: 10.1113/jphysiol.2001.013361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uetani T, Matsubara T, Nomura H, Murohara T, Nakayama S. Ca2+-dependent modulation of intracellular Mg2+ concentration with amiloride and KB-R7943 in pig carotid artery. J Biol Chem. 2003;278:47491–7. doi: 10.1074/jbc.M307898200. [DOI] [PubMed] [Google Scholar]
- 13.Dillon PF. 31P Nuclear magnetic resonance spectroscopy. In: Bárány M, editor. Biochemistry of Smooth Muscle Contraction. San Diego: Academic Press; 1996. pp. 393–404. [Google Scholar]
- 14.Kushmerick MJ, Dillon PF, Meyer RA, Brown TR, Krisanda JM, Sweeney HL. 31P NMR spectroscopy, chemical analysis, and free Mg2+ of rabbit bladder and uterine smooth muscle. J Biol Chem. 1986;261:14420–9. [PubMed] [Google Scholar]
- 15.Nakayama S, Nomura H, Smith LM, Clark JF. Simultaneous estimation of intracellular free Mg2+ and pH using a new pH-dependent dissociation constant of MgATP. Jpn J Physiol. 2002;52:323–6. doi: 10.2170/jjphysiol.52.323. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Truttmann AC, Lüthi D, McGuigan JAS. Apparent Mg2+-adenosine 5′ -triphosphate dissociation constant measured with Mg2+ macroelectrodes under conditions pertinent 31P-NMR ionized magnesium determinations. Anal Biochem. 1997;251:246–50. doi: 10.1006/abio.1997.2238. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama S, Nomura H, Smith LM, Clark JF, Uetani T, Matsubara T. Mechanisms for monovalent cation-dependent depletion of intracellular Mg2+ Na+-independent Mg2+ pathways in guinea-pig smooth muscle. J Physiol. 2003;551:843–53. doi: 10.1113/jphysiol.2003.047795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kajioka S, Nakayama S, Asano H, Brading AF. Involvement of ryanodine receptors in muscarinic-receptor-mediated membrane current oscillation in urinary bladder smooth muscle. Am J Physiol. 2005;288:C100–8. doi: 10.1152/ajpcell.00161.2004. [DOI] [PubMed] [Google Scholar]
- 19.Simchrowitz L, Kleyman TR, Cragoe EJ., Jr . An overview of the structure-activity relations in the amiloride series. In: Cragoe EJ Jr, Kleyman TR, Simchowitz L, editors. Amiloride and Its Analogues. New York: VCH Publishers; 1992. pp. 9–24. [Google Scholar]
- 20.Nakayama S, Nomura H. Mechanisms of intracellular Mg2+ regulation affected by amiloride and ouabain in the guinea-pig taenia caeci. J Physiol. 1995;488:1–12. doi: 10.1113/jphysiol.1995.sp020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voets T, Nilius B, Hoefs S, Van Der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 22.Montell C. Mg2+ homeostasis: the Mg2+ nificent TRPM chanzymes. Curr Biol. 2003;13:R799–801. doi: 10.1016/j.cub.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 23.Prakriya M, Lewis RS. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J Gen Physiol. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bootman MD, Collins TJ, Mackenzie H, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–50. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 25.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 26.Schaefer M. Homo- and heteromeric assembly of TRP channel subunits. Pflügers Arch. 2005;451:35–42. doi: 10.1007/s00424-005-1467-6. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–37. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demeuse P, Penner R, Fleig A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol. 2006;127:421–34. doi: 10.1085/jgp.200509410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa Y, Murayama T, Kurebayashi N. Ryanodine receptor isoforms of non-mammalian skeletal muscle. Front Biosci. 2002;7:d1187–94. doi: 10.2741/A832. [DOI] [PubMed] [Google Scholar]
- 30.Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS, So I, Stanfield PR, Kim KW. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterol. 2005;129:1504–17. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama S, Kajioka S, Goto K, Takaki M, Liu H-N. Calcium-associated mechanisms in gut pacemaker activity. J Cell Mol Med. 2007;11:958–68. doi: 10.1111/j.1582-4934.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huizinga JD, Faussome-Pellegrini MS. About the presence of interstitial cells of Cajal outside the musculature of the gastrointestinal tract. J Cell Mol Med. 2005;9:468–73. doi: 10.1111/j.1582-4934.2005.tb00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harhun MI, Pucovsky V, Povstyan OV, Gordienko DV, Bolton TB. Interstitial cells in the vasculature. J Cell Mol Med. 2005;9:232–43. doi: 10.1111/j.1582-4934.2005.tb00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popescu LM, Ciontea SM, Cretoiu D. Interstitial Cajal-like cells in human uterus and fallopian tube. Ann N Y Acad Sci. 2007;1101:139–65. doi: 10.1196/annals.1389.022. [DOI] [PubMed] [Google Scholar]
- 35.Brading AF, McCloskey KD. Mechanisms of Disease: specialized interstitial cells of the urinary tract—an assessment of current knowledge. Nat Clin Pract Urol. 2005;2:546–54. doi: 10.1038/ncpuro0340. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 37.Paolisso G, Barbagallo M. Hypertension, diabetes mellitus, and insulin resistance: the role of intracellular magnesium. Am J Hypertens. 1997;10:368–70. doi: 10.1016/s0895-7061(96)00342-1. [DOI] [PubMed] [Google Scholar]
- 38.Yang C-Y. Calcium and magnesium in drinking water and risk of death from cerebrovascular disease. Stroke. 1998;29:411–4. doi: 10.1161/01.str.29.2.411. [DOI] [PubMed] [Google Scholar]
- 39.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–70. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 40.Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–4. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 41.Hermosura MC, Nayakanti H, Dorovkov MV, Calderon FR, Ryazanov AG, Haymer DS, Garruto RM. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc Natl Acad Sci USA. 2005;102:1510–5. doi: 10.1073/pnas.0505149102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of estimation procedures.
Example spectra for changes in the β-ATPpeak during exposures to a Ca2+- andNa+-free, high (6.0 mM)Mg2+ solution.
The effect of amiloride on[Mg2+]i.
Tests of primers.
Changes in the concentration of high-energy phosphates.


