Abstract
Transforming growth factor-β3 (TGF-β3), a multi-functional growth modulator of embryonic development, tissue repair and morphogenesis, immunoregulation, fibrosis, angiogenesis and carcinogenesis, is the third mammalian isoform of the TGF-β subfamily of proteins. The pleiotropism of the signalling proteins of the TGF-β superfamily, including the TGF-β proteins per se, are highlighted by the apparent redundancy of soluble molecular signals initiating de novo endochondral bone induction in the primate only. In the heterotopic bioassay for bone induction in the subcutaneous site of rodents, the TGF-β3 isoform does not initiate endochondral bone formation. Strikingly and in marked contrast to the rodent bioassay, recombinant human (h)TGF-β3, when implanted in the rectus abdominis muscle of adult non-human primates Papio ursinus at doses of 5, 25 and 125 μg per 100 mg of insoluble collagenous matrix as carrier, induces rapid endochondral bone formation resulting in large corticalized ossicles by day 30 and 90. In the same animals, the delivery of identical or higher doses of theTGF-β3 protein results in minimal repair of calvarial defects on day 30 with limited bone regeneration across the pericranial aspect of the defects on day 90. Partial restoration of the bone induction cascade by the hTGF-β3 protein is obtained by mixing the hTGF-β3 device with minced fragments of autogenous rectus abdominis muscle thus adding responding stem cells for further bone induction by the hTGF-β3 protein. The observed limited bone induction in hTGF-β3/treated and untreated calvarial defects in Papio ursinus and therefore by extension to Homo sapiens, is due to the influence of Smad-6 and Smad-7 down-stream antagonists of the TGF-β signalling pathway. RT-PCR, Western and Northern blot analyses of tissue specimens generated by the TGF-β3 isoform demonstrate robust expression of Smad-6 and Smad-7 in orthotopic calvarial sites with limited expression in heterotopic rectus abdominis sites. Smad-6 and -7 overexpression in hTGF-β3/treated and untreated calvarial defects may be due to the vascular endothelial tissue of the arachnoids expressing signalling proteins modulating the expression of the inhibitory Smads in pre-osteoblastic and osteoblastic calvarial cell lines controlling the induction of bone in the primate calvarium.
Keywords: transforming growth factor-β3, TGF-β superfamily members, primates, redundancy, bone induction, inhibitory Smad proteins, responding stem cells
Introduction
Reconstruction of large craniofacial and appendicular skeletal defects in humans requires the harvesting of living autogenous bone from a distant donor site, most often the iliac crest [1]. The harvesting of fresh living bone is associated with harvest-related morbidity [1, 2]. A further limitation is the finite volume of bone available from any one donor site [1, 2]. Adapting the donor bone to fit the shape of the recipient defect is the final challenge to autogenous bone grafting in clinical contexts [1–3].
The seminal experiments of Levander, Huggins, Urist, Sampath and Reddi [4 for review] have indicated that the extracellular matrix of mineralized tissues is the repository of differentiating morphogens (firstly defined by Turing as ‘form generating substances’[5]), tightly bound to the mineralized matrix [4]. The induction of bone formation, that is, the de novo endochondral bone formation in heterotopic extraskeletal sites of animal models, can be initiated by partially extracted and intact demineralized extra-cellular matrices of bone and dentine [6–13].
A critical contribution to the mechanistic understanding of the phenomenon of bone: formation by autoinduction [10], has been achieved by the dissociative extraction and reconstitution of the bone matrix components [14]. Purification to homogeneity of the chromatographed and geleluted proteins [15] was followed by expression cloning of the recombinant human bone morphogenetic/osteogenic proteins (BMPs/OPs) [16–18]. These experiments have lead to the initiation of clinical trials which have culminated in the use of recombinant human osteogenic protein-1 (hOP-1) and human bone morphogenetic protein-2 (hBMP-2) in clinical contexts [19–21].
Until 1993, the characteristic and discriminatory osteogenic function of the osteogenic proteins of the TGF-β superfamily was conferred only to the BMPs/OPs [21]. It was then shown that recombinant Drosophila melanogaster decapentaplegic and 60A proteins induce endochondral bone formation in mammals [22]. This has indicated that a phylogenetically ancient signalling process deployed in dorso-ventral patterning in the fruit fly D. melanogaster also operates to produce the unique vertebrate trait of the induction of bone and skeletogenesis and thus the emergence of the vertebrates [21–23]. The apparent redundancy of molecular signals initiating endochondral bone induction in heterotopic extraskeletal sites is further emphasized by the capacity of the signalling proteins of the TGF-β subfamily to initiate the osteogenic cascade in primates [21]. In the rodent bioassay, the mammalian TGF-β isoforms do not initiate endochondral bone formation [24]. Strikingly, however, the TGF-β1 and TGF-β2 isoforms are powerful inducers of endochondral bone when implanted in the rectus abdominis muscle of the non-human primate Papio ursinus at doses of 5, 25 and 125 μg per 100 mg of insoluble collagenous matrix as carrier [21, 25–29].
The mature, bioactive form of the third mammalian TGF-β isoform, that is, the TGF-β3 protein, shares 80% amino acid sequence identity with TGF-β1 and TGF-β2, respectively [30–32]. Using the rectus abdo-minis muscle and the calvarium of Papio ursinus as a model for tissue induction and morphogenesis, we now show the previously unreported and novel endochon-dral osteoinductivity of the TGF-β3 protein. We also show that the newly formed tissue constructs in both heterotopic and orthotopic calvarial sites are modulated by invocation of a regenerative response tightly regulated by the TGF-β inducible intracellular antagonists of the TGF-β signalling cascade, the inhibitory Smad-6 and -7 proteins [33–35]. We show that partial restoration of the bone induction cascade in cal-varial defects by the hTGF-β3 protein is obtained by mixing the hTGF-β3 device with minced fragments of autogenous rectus abdominis muscle thus adding responding stem cells for further bone induction by the hTGF-β3 protein.
We further show that theTGF-β3 isoform at the doses tested and delivered by the insoluble collage-nous bone matrix (ICBM) as carrier is the most powerful osteogenic protein of the TGF-β superfamily so far tested in the rectus abdominis muscle of Papio ursinus. The induction of large mineralized and corti-calized ossicles by hTGF-β3 in Papio ursinus holds great promise for the use of hTGF-β3 singly or in synergistic combination with hBMPs/OPs for novel molecular therapeutics for regenerative medicine and osteogenesis in clinical contexts.
Materials and methods
Preparation and doses of the hTGF-β3 osteogenic devices
Mature recombinant hTGF-β3, a glycosylated 25-kDa homodimer with a C-terminal domain of 112 amino acids with nine cysteine residues [30–32], was obtained from Novartis Pharma AG, Basel, Switzerland. Stock solutions of the morphogen were prepared by aliquoting the required amounts in a liquid vehicle (35% ethanol, 0.1% HCl).
Demineralized bone matrix, prepared from diaphyseal segments of baboon cortical bone, was dissociatively extracted in 4 M guanidinium-HCl containing protease inhibitors [14]. The resulting ICBM, inactive after extraction of osteogenic proteins [14], was washed three times with distilled water, dehydrated in ethanol and ether and used as carrier for the hTGF-β3 isoform. Collagenous bone matrix is an optimal substratum for cell attachment, proliferation and differentiation [14, 36, 37] resulting in significant osteoinduction in primate models using both TGF-βs and BMPs/OPs [25–27, 38–40].
For the preparation of samples suitable for heterotopic implantation in the rectus abdominis muscle of the baboon, implants were prepared in sterile 10 ml polypropylene tubes by adding 5, 25 and 125 μg of hTGF-β3 in 100 μl of liquid vehicle to 100 mg of insoluble collagenous matrix [25–27].
Implants for orthotopic calvaria implantation were prepared in sterile 50 ml Falcon tubes with 1 g of lyophilized collagenous matrix combined with 5, 25, 125 and 250 μg of hTGF- β3 in 500 μl of liquid vehicle as described [38–40].
Primate models for tissue induction
Twenty-three clinically healthy adult Chacma baboons Papio ursinus, with a mean weight of 19.1±5.5 kg, were selected from the primate colony of the University of the Witwatersrand, Johannesburg. Comparative histomorpho-metric studies between iliac crest biopsy specimens of human and Papio ursinus show a remarkable degree of similarity [41]. This makes adult Papio ursinus species ideally suited for the study of comparative bone physiology and repair with relevance to man [41]. Criteria for selection, housing conditions and diets were as described [42]. Research protocols were approved by the Animal Ethics Screening Committee of the university, and conducted according to the Guidelines for the Care and Use of Experimental Animals prepared by the university, and in compliance with the National Code for Animal Use in Research, Education and Diagnosis in South Africa[43].
The heterotopic rectus abdominis and orthotopic calvarial models of tissue induction and morphogenesis by recombinant proteins in the adult baboon Papio ursinus have been described in detail [21, 25–27, 38–40, 44]. Lyophilized pellets of 100 mg of collagenous matrix combined with relevant doses of the hTGF-β3 isoform were implanted bilaterally in duplicate or quadruplicate in 12–16 ventral intramuscular pouches created by sharp and blunt dissection in the rectus abdominis muscle of each animal [25–27, 40, 44]. Pellets of collagenous matrix with liquid vehicle but without hTGF-β3 were implanted as a control. After heterotopic implantation, the calvariae were exposed and, on each side of the calvaria two full-thickness defects 25 mm in diameter, each separated by 2.5–3 cm of intervening calvarial bone, were created with a craniotome under saline irrigation [38–40, 44]. Latin square block designs were used to allocate the position of the TGF-β3 osteogenic devices in 72 calvarial defects in 23 adult baboons [38–40, 44]. Number of heterotopic and ortho-topic implanted specimens per treatment modality and time periods are presented in Table 1.
1.
Overview of number of recombinant human of transforming growth factor-β3 (hTGF-B3) specimens implanted hetero-topically (A) and orthotopically (B) in a cohort of 23 adult Chacma baboons Papio ursinus and harvested at different time points
| A Heterotopic implants | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hTGF-β3 | 0 μg | 5 μg | 25 μg | 125 μg | |||||||||||||||||||||||
| Time periods | 30 days | 90 days | 30 days | 90 days | 30 days | 90 days | 20 days | 30 days | 90 days | ||||||||||||||||||
| # Animals | 7 | 3 | 9 | 6 | 10 | 7 | 4 | 10 | 8 | ||||||||||||||||||
| Delivery system | ICBM | ICBM | ICBM | ICBM | ICBM | ICBM | ICBM | ICBM | ICBM | ||||||||||||||||||
| # Implants | 5 | 3 | 12 | 10 | 11 | 8 | 7 | 9 | 7 | ||||||||||||||||||
| Total no/dose | 8 | 24 | 25 | 23 | |||||||||||||||||||||||
| (B) Orthotopic calvarial implants | |||||||||||||||||||||||||||
| hTGF-β3 | 0 μg | 25 μg | 125 μg | 250 μg | |||||||||||||||||||||||
| Time periods | 30 days | 90 days | 30 days | 90 days | 20 days | 30 days | 90 days | 20 days | 30 days | 90 days | |||||||||||||||||
| Animals | 8 | 8 | 4 | 8 | 2 | 10 | 10 | 2 | 5 | 4 | |||||||||||||||||
| Delivery system | ICBM | ICBM | ICBM | ICBM | ICBM | ICBM | ICBM | ICBM | ICBM | ICBM | |||||||||||||||||
| Implants | 7 | 7 | 9 | 9 | 4 | 9 | 9 | 6 | 3 | 2 | |||||||||||||||||
| 9+ramcs | 9+ramcs | 2+ramcs | 2+ramcs | 4+ramcs | 4+ramcs | 3+ramcs | 2+ramcs | ||||||||||||||||||||
| Total no/dose | 32 | 22 | 30 | 16 | |||||||||||||||||||||||
ICBM, insoluble collagenous bone matrix; ramcs, rectus abdominis cells
Reconstitution of the hTGF-β3 osteogenic device with fragments of minced rectus abdominis muscle
Orthotopic tissue sections prepared from calvarial specimens harvested on day 90 occasionally showed mineralized bone across the defect confined to the pericranial aspect (Fig. 4A). The presence of inhibitory binding proteins and/or the expression of inhibitory Smads has been suggested by the morphological analysis of calvarial specimens treated with varying doses of the TGF-β3 combined with ICBM as carrier, that is, the TGF-β3 osteogenic device. Morphological analysis showed that bone formed essentially at the pericranial surface of the implant below the temporalis muscle on day 90. Moreover, the bone that had formed at both interfacial regions seemed to be, at least morphologically, inhibited to proceed centripetally with the generation of a substantial fibrogenic response between the inactive particles of the collagenous matrix subjacent to the newly formed bone pericranially (see Fig. 4B and D). It was thus mandatory to study the expression of the inhibitory Smad-6 and -7 proteins in orthotopic calvarial defects to mechanistically unravel the segregated osteogenic induction by the hTGF-β3 isoform in calvarial defects.
4.
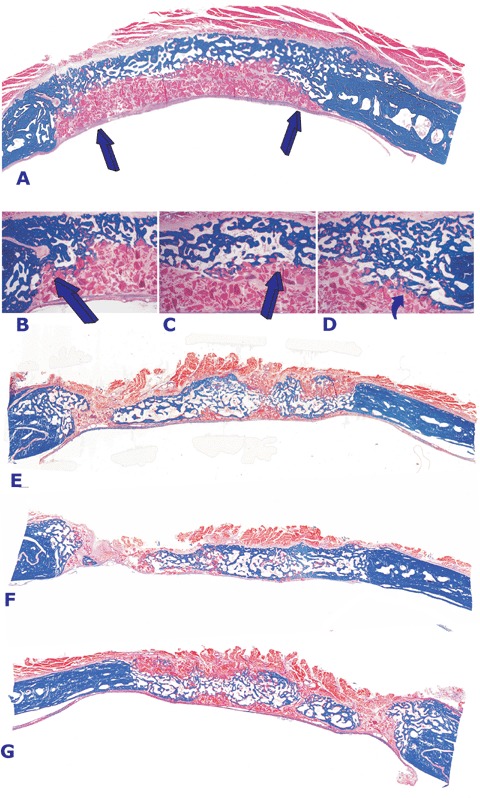
Morphology of calvarial regeneration and induction of bone formation in calvarial defects implanted with doses of the transforming growth factor-β3 (hTGF-β3) osteogenic device without (A) and with minced fragments of autogenous rectus abdominis muscle (E, F and G) harvested 90 days post-implantation. (A) Induction of bone in a calvarial defect implanted with 125 μg of the hTGF-β3 osteogenic device showing bone formation predominantly on the pericranial aspect of the specimen with lack of bone formation at the endocranial dural aspect of the specimen (arrows). Arrows in B, C and D point to the inhibition of bone formation within the fibrogenic collagenous matrix facing the newly formed bone pericranially.(E, F and G). Addition to the hTGF-β3 osteogenic device of autogenous minced fragments of rectus abdominis muscle induces partial restoration of the biological activity of 125 μg of the hTGF-β3 osteogenic device with large islands of newly formed bone in specimens harvested on day 90 after implantation. Undecalcified sections cut at 5 μm stained free-floating with Goldner's trichrome. (A, E, F and G) original magnification ×1.8; (B, C and D) original magnification ×7.
At the same time, and importantly for regenerative medicine, the presence of newly induced mineralized bone albeit much thinner than the normal calvaria on day 90, pointed to the potential role of the pericranium and temporalis muscle as sources of responding stem cells inducible by the hTGF-β3 protein thus responsible for the partial and/or complete restoration of the bone induction cascade in the treated cal-varial defects. In an additional set of experiments, it was therefore decided to harvest fragments of autogenous rectus abdominis muscle which were minced into multiple fragments with both fine scissors and scalpels on pre-cooled sterile surfaces, then added to and mixed with the hTGF-β3 osteogenic device just before implantation in calvarial defects (Table 1).
Tissue harvest, histology and histomorphometry
Anaesthetized animals were killed with an intravenous overdose of sodium pentobarbitone on day 12, 20, 30 and 90, two, three or four animals per time period (Table 1). After harvesting of heterotopic and orthotopic tissues for reverse transcription-polymerase chain reaction (RT-PCR), Northern and Western blot analyses, anaesthetized animals were subjected to bilateral buffered saline carotid perfusion and harvest of specimens with surrounding calvaria as described [38–40, 44]. Specimen blocks were cut along the sagittal one-third of the implanted defects, fixed and dehydrated in ascending grades of ethanol, and embedded, undecalcified in a methyl-methacrylate plastic embedding system (K-Plast; Dia Tec Diagnostic Systems, Germany). Heterotopic specimens were fixed, processed and embedded as described above.
Serial sections, cut at 6 μm (Leica SM2500 Polycut-S; Reichert, Heidelberg, Germany), were stained, free-floating with modified Goldner's trichrome. Sections were examined with a Provis AX70 research microscope (Olympus Optical Co., Japan) equipped with a calibrated Zeiss Integration Platte II (Oberkochem, Germany) with 100 lattice points for determination by the point-counting technique of mineralized bone, osteoid and residual collagenous matrix volumes (in %) [45, 46].
Calvarial sections were analysed at 40x, superimposing the Zeiss graticule over five sources [46] selected for histomorphometry and defined as follows: anterior and posterior interfacial regions (AIF and PIF), anterior and posterior internal regions (AIN and PIN) and a central region (CEN) [38–40, 44]. This technique allows for the histomorphome-tric evaluation of the distribution of bone regeneration across the defects [39, 44]. Each source represented a field of 7.84 mm2. Undecalcified sections generated from heterotopic specimens were evaluated by the point-counting technique for mineralized bone, osteoid and residual collagenous matrix volumes (in %) [46] superimposing the Zeiss graticule over corticalized outer levels, and traversing to internal regions of the ossicles [26, 27, 40].
Semi-quantitative reverse transcription-PCR analyses of TGF-β ligands, receptors and antagonists
Total RNA was isolated from tissues harvested from both heterotopic and orthotopic sites after 30 and 90 days implantation using a TriPure™ isolation kit according to the manufacturer's protocol (Roche Molecular Biochemicals, Germany). RNA concentration was determined spec-trophotometrically at 260 nm. The quality and integrity was confirmed by the A260/A280 absorbance ratio and the ribosomal RNA bands visualized on agarose gels [26, 27, 40]. Equivalent amounts of RNA (0.5 μg) were initially reverse-transcribed for first strand cDNA synthesis using gene specific forward and reverse primers (synthesized by Integrated DNA Technologies, Coralville, IA, USA) in a total reaction volume of 25 μl containing dNTPs (0.2 mM), MgCl2 (1.5 mM), Taq DNA polymerase (0.625 U) in buffer (pH 8.5; PCR Master Mix, Promega, Madison, WI, USA). The gene-specific oligonucleotide sequences used primer concentration, the generated PCR product size and the cycling conditions are shown in Table 2[47–51]. A variable number of cycles were permutated to ensure amplification was in the linear phase to avoid any between-experiment variation in PCR efficiency. Positive and negative controls were included to confirm the PCR product size and that no genomic DNA was present in the RT samples. PCR products were resolved on 2% agarose gels prepared with 1x tris acetate EDTA (TAE), containing ethidium bromide (0.5 μg/ml) and visualized using UV transillumination. Product sizes were confirmed using a 1500–100 bp DNA molecular marker (Promega, Madison, WI, USA). Semi-quantitation of each PCR product was performed by densitometric scanning (Gene Tools, Syngene) normalizing values to those of glyceraldehyde 3-phosphate dehydroge-nase (GADP) [26, 27].
2.
Primer sequences and cycles used for the RT-PCR
| Gene | Sequence (5′→ 3′) | Cycling conditions | Amplicon | Ref.: |
|---|---|---|---|---|
| TGF-β1 | F:GCTGCGCTTGCAGAGATTAAA | 40 cycles; 94°C 30 sec, 58°C 30 sec and 72°C 45 sec. | 552 bp | (47) |
| R: TTGCTGTACTGTGTGTCCAG | ||||
| BMP-3 | F: CGCCAGGAGATACCTCAAGGTAGA | 35 cycles; One cycle of 93°C 5 min then 64°C 45 sec, 72°C 45 sec and 93°C 45 sec. | 330 bp | (48) |
| R: TCAAATGAGTTCTTTGCCAGGTTATC | ||||
| OP-1 | F: TTTTCCTTTCGCACAGACACC | 37 cycles; 94°C 30 sec, 58°C 30 sec and 72°C 45 sec. | 313 bp | (49) |
| R: TTCCCCTCCCTATCCCCAACTTT | ||||
| TβR I | F: TCGTCTGCATCTCACTCAT | 35 cycles; 94°C 30 sec, 55°C 30 sec and 72°C 45 sec. | 342 bp | (50) |
| R: GATAAATCTCTGCCTCACG | ||||
| TβR II | F: GCACGTTCAGAAGTCGGTTA | 35 cycles; 94°C 30 sec, 58°C 30 sec and 72°C 45 sec. | 493 bp | (50) |
| R: GCGGTAGCAGTAGAAGATGA | ||||
| TβR III | F: AATCTGGGCCATGATGCAG | 40 cycles; 94°C 30 sec, 55°C 30 sec and 72°C 45 sec. | 286 bp | (50) |
| R: ACTGCTGTTTTCCGAGGCT | ||||
| Smad-6 | F: TGAATTCTCAGACGCCAGCA | 34 cycles; 94°C 30 sec, 55°C 30 sec and 72°C 45 sec. | 386 bp | (50) |
| R: GCTCGAAGTCGAACACCTT | ||||
| Smad-7 | F: GCCCTCTCTGGATATCTTCT | 29 cycles; 94°C 30 sec, 58°C 30 sec and 72°C 45 sec. | 320 bp | (47) |
| R: GCTGCATAAACTCGTGGTCA | ||||
| GAPDH | F: CCCTTCATTGACCTCAACTACATG | 27 cycles; One cycle of 90°C 5 min and one cycle of 60°C 5 min followed by 72°C 1 min, 90°C 1 min, 60°C 1 min and 72°C 10 min | 587 bp | (51) |
| R: GACTTGCCCTTCGAGTGACCGTAC | ||||
Western blot analyses of Smad-6 and Smad-7
Bone and/or soft tissue samples were homogenized (6000 rpm UltraTurax) and resuspended in protein extraction buffer (8.76 g/l NaCl, 0.252 g/l NaHCO3). Following cen-trifugation steps, protein concentrations were determined in relation to bovine serum albumin (BSA) standards [26, 27]. Samples corresponding to equal protein concentrations were resolved by SDS gel electrophoresis and Western blotting performed. After blocking the membrane overnight at 4°C with 5% BSA, the nitrocellulose membrane was incubated with polyclonal antibody directed against goat Smad-6 or Smad-7 (Santa Cruz Biotechnology, CA, USA). The membrane was subsequently washed with tris-buffered saline tween-20 (TBST) and incubated with horseradish peroxidase conjugated donkey anti-goat antibody (Santa Cruz Biotechnology, CA, USA. The secondary antibody was detected by chemiluminescence (Pierce, Rockford, IL, USA). Immunoreactive polypeptide bands visualized on X-ray film (Protea Medical Services, Sandton, South Africa) were quantified as relative intensities by image analysis using Gene Tools (UVP, San Gabriel, CA, USA). Molecular weights of the proteins were verified in relation to a biotinylated protein ladder (Cell Signalling Technology, Beverly, MA). Statistical analysis was done using Costat software (CoHort Software, Berkely, CA, USA).
Statistical analyses
Histomorphometric data of heterotopic and orthotopic tissue sections were analysed using Graph Pad Prism software with one way analysis of variance procedure using Bonferroni's multiple comparison test and are presented in Tables 3 and 4.
3.
Effect of hTGF-β3 doses on key parameters of heterotopic of bone induction on day 30. Histomorphometric results of induced mineralized bone (md bone), osteoid, matrix and fibrovascular tissue (fva) volumes (in %)
| hTGF-β3 | Bone | md bone | Osteoid | Matrix | fva |
|---|---|---|---|---|---|
| μg | (%) | (%) | (%) | (%) | (%) |
| 5 | 28.8±6.0 | 21.5±4.2 | 7.3±2.7 | 22.7±9.1 | 49.6±7.0 |
| 25 | 27.2±1.7 | 19.6±2.0 | 7.7±1.9 | 1.0±2.0 | 70.1±4.7 |
| 125 | 33.4±5.5 | 21.0±4.6 | 12.5±3.1 | 1.7±4.0 | 64.0±10.7 |
Doses of recombinant hTGF-β3 in 100 μl of liquid vehicle were added to 100 mg of allogeneic insoluble collagenous bone matrix as carrier. Lyophilized pellets were implanted in the rectus abdominis muscle of the baboon. Specimens were harvested on day 30 after implantation and processed for undecalcified histology. Serial undecalcified sections, cut at 7 μm, were analysed by histomorphometry. Volume fractions of tissue components (in %) were calculated superimposing the Zeiss Integration Platte II with 100 lattice points over corticalized outer levels and transversing into internal regions of the induced ossicles. Bone refers to mineralized bone (md bone) plus osteoid. Matrix refers to the residual collagenous carrier used for local delivery of hTGF-β3. Values are mean ±standard deviation.
4.
Effect of fragmented rectus abdominis muscle added to hTGF-β3 doses on key parameters of orthotopic bone induction in calvarial defects on day 30 and 90. Histomorphometric results of induced mineralized bone (md bone), osteoid, matrix and fibrovascular tissue (fva) volumes (in %)
| Day 30 hTGF-β3without muscle cells | Bone | md bone | Osteoid | Matrix | fva |
|---|---|---|---|---|---|
| μg | (%) | (%) | (%) | (%) | (%) |
| 25 | 0.5±1.2 | 0.26±0.65 | 0.23±0.57 | 50.3±16.2 | 39.5±11.0 |
| 125 | 1.2±2.94 | 0.76±1.87 | 0.43±1.06 | 48.2±17.0 | 40.7±10.8 |
| 250 | 2.5±3.5 | 1.2±1.7 | 1.3±1.8 | 52.25±6.0 | 40.2 ±2.5 |
| hTGF-β3with muscle cells | Bone | md bone | Osteoid | Matrix | fva |
| μg | (%) | (%) | (%) | (%) | (%) |
| 25 | 7.8±1.6 | 5.3±0.77 | 2.5±077 | 32.7±7.1 | 54.2±7.7 |
| 125 | 10.85±4.4 | 7.45±3.89 | 7.4±3.89 | 28.2±3.18 | 60.9±1.27 |
| 250 | 10.4±1.1 | 6.6±0.7 | 3.8±0.36 | 11.5±1.38 | 78.0±2.12 |
| Day 90 hTGF-β3without muscle cells | Bone | md bone | Osteoid | Matrix | fva |
| μg | (%) | (%) | (%) | (%) | (%) |
| 25 | 12.8±9.5 | 10.3±7.4 | 4.9±3.6 | 40.86±10.5 | 40.5±5.0 |
| 125 | 29.1±1.8 | 23.1±2.96 | 6.0±1.13 | 26.7±0.42 | 44.2±2.26 |
| 250 | 20.7±5.7 | 17.0±4.7 | 3.7±2.8 | 29.7±6.7 | 49.6±7.7 |
| hTGF-β3with muscle cells | Bone | md bone | Osteoid | Matrix | fva |
| μg | (%) | (%) | (%) | (%) | (%) |
| 25 | 24.6±1.04 | 195±1.01 | 5.13±0.46 | 31.0±4.9 | 44.3±3.9 |
| 125 | 29.6±6.75 | 23.3±6.06 | 6.25±1.59 | 15.1±1.0 | 55.4±6.4 |
| 250 | 19.7±6.55 | 15.9±6.04 | 3.84±0.56 | 21.5±8.62 | 58.8±5.67 |
Doses of recombinant hTGF-β3 in 100 μl of liquid vehicle were added to 1 g of allogeneic insoluble collagenous bone matrix as carrier. At time of surgery after implantation of doses of hTGF-β3 in the rectus abdominis muscle, fragments of the muscle were also harvested, fragmented, minced and added to doses of the TGF- β3 proteins. After mixing with sterile spatulas, treated collagenous matrices were implanted in calvarial defects of the baboon. Specimens were harvested on day 20, 30 and 90 after implantation and processed for undecalcified histology. Serial undecalcified sections, cut at 7 μm, were analysed by histomorphometry. Volume fractions of tissue components (in %) were calculated superimposing a Zeiss Integration Platte II with 100 lattice points over five sources in each of the four sagittal sections used for analysis as described in Methods. Bone refers to mineralized bone (md bone) plus osteoid. Matrix refers to the residual collagenous carrier used for local delivery of hTGF-β3. Values are mean±standard deviation.
Results
Induction of bone formation by the hTGF-β3 osteogenic device in the rectus abdominis muscle of Papio ursinus
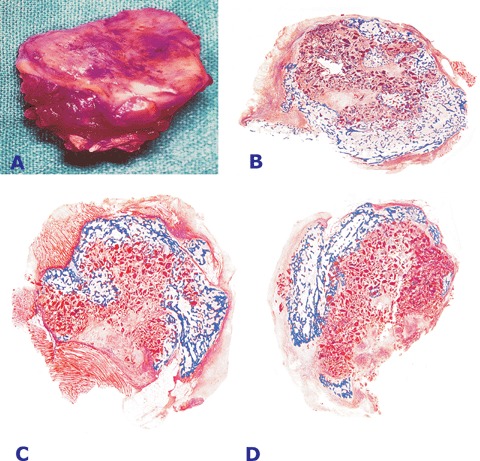
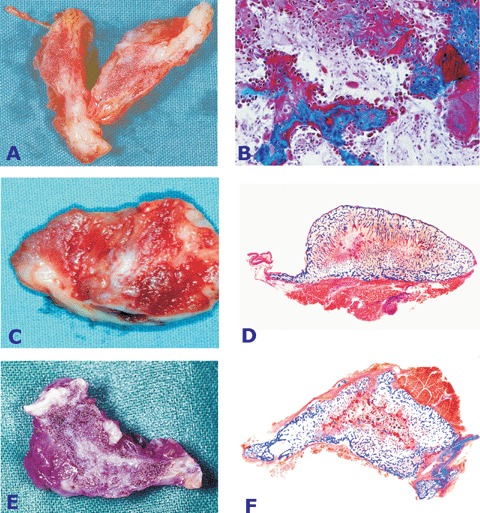
Implantation of 5 and 25 μg of the hTGF-β3 protein in the rectus abdominis muscle resulted in the induction of large corticalized heterotopic ossicles by day 20 and 30 after implantation (Fig. 1). Doses of 125 μg hTGF-β3 induced large corticalized ossicles embedded within the rectus abdominis muscle (Fig. 2). The large ossicles showed a planar convex geometry extending for several centimetres preferentially along the longitudinal plane of the fascia (Figs. 1A, 2A, C, E). Cut surfaces showed mineralization of the external cortex and were macroscopically brownish-red in colour indicating the induction of bone marrow with endosteal vascularization.
1.
Heterotopic induction of bone formation by doses of the recombinant human transforming growth factor-β3 (hTGF-β3) osteogenic device in the rectus abdominis of adult baboons Papio ursinus.(A) Induction of a large ossicle after intramuscular implantation of 25 μg hTGF-β3 delivered by the insoluble collagenous matrix as carrier. (B) Corticalization of the ossicle as shown in A by newly formed and mineralized bone in blue surrounding scattered remnants of the collagenous matrix embedded within prominent fibrovascular invasion. (C, D) Histological images of mineralized and corticalized ossicles induced by the 5 μg dose of the hTGF-β3 osteogenic device in the rectus abdominis muscle showing mineralization of the newly formed bone by induction in the rectus abdominis muscle of Papio ursinus. Undecalcified sections cut at 5 βm and stained free-floating with Goldner's trichrome (A, B, C and D, original magnification ×2.8).
2.
Tissue induction and morphogenesis of large corticalized ossicles (A, C and E) induced by the 125 μg dose of the hTGF-β3 osteogenic device implanted in the rectus abdominis muscle of adult non-human primates Papio ursinus and harvested on day 30. (B) Histological detail of the ossicle shown in (A) with mineralized newly formed bone (in blue) surfaced by osteoid seams populated by contiguous osteoblasts facing hyper cellular vascular connective tissue. (D and F) Prominent corticalization of the newly formed ossicles with mineralized bone (in blue) surrounding scattered remnants of the collagenous matrix as carrier facing newly formed trabeculae of osteoid embedded within a prominent angiogenic fibrovascular connective tissue matrix. Undecalcified sections cut at 5 βm and stained free-floating with Goldner's trichrome (A and E) original magnification ×1.3; (C) ×1.8; (B), original magnification ×175; (D and F) ×1.3.
Ossicles showed peripheral corticalization, whilst scattered remnants of the collagenous matrix occupied the centre of the specimens together with a highly vascular invading connective tissue (Figs. 1B, C, 2B, D, F). Histological analysis on undecalcified sections showed the presence of newly formed mineralized bone covered by osteoid seams populated by contiguous osteoblasts (Figs. 1 and 2). Doses of 125 μg hTGF-β3 induced prominent angiogenesis and numerous trabeculae of osteoid as yet to be mineralized extending toward the centre of the specimens (Figs. 2D and F). In summary, morphological and histological analyses of the ossicles induced by the hTGF-β3 osteogenic device showed prominent osteoblastic cell differentiation, osteoid synthesis and between the trabeculae of newly formed and mineralized bone a highly vascular hypercellular stroma (Figs. 2B and D).
Morphology of calvarial regeneration by the hTGF-β3 osteogenic device
On day 20 and 30 after implantation, control calvarial defects treated with collagenous matrix without hTGF-β3 showed minimal osteogenesis confined to the margins of the defects only (Fig. 3A). Doses of the recombinant hTGF-β3 protein (25, 125 and 250 μg) also failed to regenerate bone in the treated calvarial defects (Figs. 3C, E, G and I). Newly formed bone was strictly confined to the margins of the craniotomies (Fig. 3D) occasionally extending pericranially (Figs. 3C and D). A single specimen treated with 125 μg hTGF-β3 showed an island of newly formed mineralized bone just below the temporalis muscle on day 30 (Fig. 3G).
3.
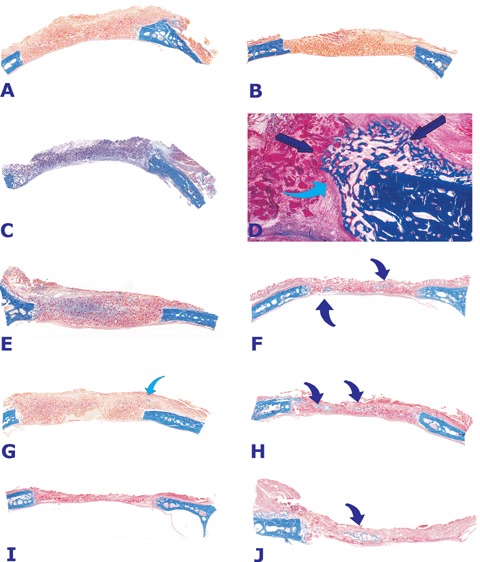
Morphology of calvarial repair and induction of bone formation by doses of the hTGF-β3 osteogenic device without (left column) and with (right column) minced fragments of autogenous rectus abdominis muscle harvested 30 days after implantation.(A, C, E, G and I) Lack of bone induction and differentiation in calvarial defects harvested on day 30 after implantation of 25 (A), 125 (C, E, G) and 250 (I) μg of the hTGF-β3 osteogenic device without the addition of minced cellular fragments of autogenous rectus abdominis muscle. Blue arrow in (G) indicates a very mall island of mineralized bone (in blue) located in the pericranial area of the specimen.(B) Lack of bone differentiation in a calvarial defect implanted with insoluble collagenous bone matrix solo as control 30 days after implantation.(D) Higher power view of (C) illustrating an interfacial region with newly formed mineralized bone at the level of the craniotomy only (dark blue arrows) blending into the remnants of the collagenous matrix. A prominent fibrous layer (light blue arrow) inhibits the induction of bone formation from the margin of the craniotomy.(F, H, J) Calvarial defects harvested on day 30 after implantation of 125 (F and H) and 250 (J) μg the hTGF-β3 osteogenic device with the addition of minced fragments of autogenous rectus abdominis muscle. Partial restoration of the biological activity and induction of islands of newly formed mineralized bone in blue (arrows) within the implanted hTGF-β3 osteogenic device. Undecalcified sections cut at 5 μm stained free-floating with Goldner's trichrome.(A, B, C, E, F, G, H, I, J) original magnification ×1.2 (D) original magnification ×7.
On day 90, bone formation in calvarial defects treated with the hTGF-β3 osteogenic device remained limited, with scattered areas of osteogenesis below the pericranium and the temporalis muscle. Few specimens showed the induction of bone pericranially albeit to a limited extent across the treated defects (Fig. 4A). Morphological analysis showed a recurrent pattern of histological features extending from the pericranial to the endocranial surfaces of the specimens with particular reference to the interfacial regions (Figs. 4A, B and D). The pericranial aspect of a particular specimen showed newly formed mineralized bone across the defect (Fig. 4A). Below the newly formed mineralized bone there was loose fibrovascular tissue with scattered remnants of the residual and inactive collagenous matrix resting on the dural layer (Fig. 4A). The presence of inhibiting binding proteins and/or the expression of inhibitory Smad proteins is highly suggested by the morphological analyses of calvarial specimens treated by the hTGF-β3 osteogenic device in which the bone, that had formed at the interfacial regions (Figs. 4A, B and D), seemed to be inhibited, at least morphologically, to further growth with centripetal extension resulting in limited bone formation across the pericranial aspect of the defects with lack of bone formation adjacent to the dura (Figs. 4A, B, C and D).
Minced fragments of rectus abdominis muscle with responding cells enhance bone induction by hTGF-β3
Partial restoration of the bone induction cascade by the hTGF-β3 osteogenic device is obtained by combining the device with minced fragments of autoge-nous rectus abdominis muscle prior to implantation in calvarial defects. On day 20 and 30, histological analyses of calvarial specimens treated with minced fragments of autogenous rectus abdominis muscle showed that the addition of responding stem cells partially restored the osteogenic activity of doses of the hTGF-β3 osteogenic device yielding islands of mineralized newly formed bone (Figs. 3F, H and J). Histological examination of treated specimens showed large trabeculae of mineralized bone covered by thick osteoid seams populated by contiguous osteoblasts.
On day 90, the addition of minced fragments of autogenous rectus abdominis muscle to the TGF-β3 osteogenic device induced solid blocks of mineralized bone across the defects with newly formed trabeculae covered by osteoid seams facing highly cellular diploic lacunae (Fig. 4). Doses of 25 and 125 μg of the TGF-β3 protein induced mineralized trabeculae of newly formed bone with haematopoietic bone marrow (Figs. 4E, F and G). The addition of autogenous rectus abdominis responding cells also induced large islands of chondrogenic differentiation in treated calvarial defects which were evident on day 90 post-implantation (Fig. 5).
5.
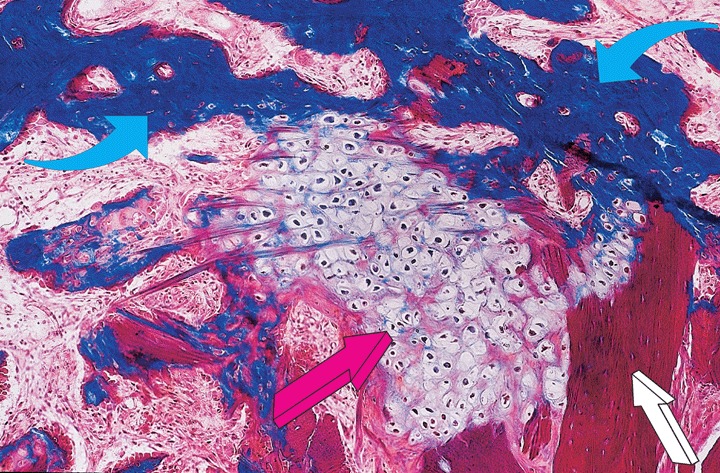
High power view of a section prepared from a specimen of the 125 μg hTGF-β3 osteogenic device additionally pre-treated with autogenous fragments of rectus abdominis muscle showing the induction of chondrogenesis (lilac arrow) between mineralized bone in blue (light blue arrows) and residual collagenous matrix as carrier (white arrow). The addition of morcellized fragments of autogenous rectus abdominis muscle engineers endochondral bone formation with large islands of chondrogenesis as a recapitulation of embryonic development 90 days after implantation. Undecalcified section cut at 5 μm stained free-floating with Goldner's trichrome. Original magnification ×125.
Histomorphometric analyses of heterotopic specimens induced by the hTGF-β3 osteogenic device
Volume fractions of tissue components of heterotopic specimens on day 30 treated with doses of hTGF-β3 delivered by 100 mg of allogeneic ICBM as carrier are presented in Table 3. Doses of the hTGF-β3 isoform induced substantial osteoid volume particularly the 125 μg dose of the osteogenic device (P < 0.05 versus the 5 and 25 μg doses of the protein [Table 3]).
Morphometric analyses of calvarial specimen: effect of doses and minced fragments of rectus abdominis muscle of the hTGF-β3 osteogenic device
Volume fractions of tissue components of mineralized bone and osteoid in calvarial defects treated with doses of the hTGF-β3 protein with or without minced fragments of autogenous rectus abdominis muscle are presented in Table 4.
On day 30, doses up to 250 μg hTGF-β3 did not result in the induction of bone formation, yielding minimal amounts of mineralized bone at the interfaces of the calvarial defects only (Table 4 and Fig. 3). Addition of minced fragments of rectus abdominis muscle to the hTGF-β3 osteogenic device significantly raised the amount of osteoid and mineralized bone within the additionally treated calvarial defects (Table 4, Fig. 3). Restoration of the osteoinductive activity of the hTGF-β3 osteogenic device with the addition of minced fragments of rectus abdominis muscle was particularly evident on day 30 as evaluated both morphologically (Fig. 3) and histomorphometrically (Table 4). On day 90, the inductive effect of the minced fragments of the rectus abdominis muscle was less evident with the exclusion of the 25 μg hTGF-β3 osteogenic device which doubled the amount of bone formation with the addition of minced fragments of rectus abdominis muscle as compared to untreated osteogenic devices of 25 μg hTGF-β3 (P < 0.01, Table 4). Occasionally, sections of 125 μg hTGF-β3 showed a remarkable restoration of the osteoinductive activity when additionally treated with minced fragments of autogenous rectus abdominis muscle (Figs. 4E–G).
Reverse transcription PCR analyses
Smad-6 and Smad-7 expression
On day 30 (Fig. 6A), heterotopic samples showed no or limited expression of Smad-6 and -7 as compared to significantly elevated expression in orthotopic tissue samples (Fig. 6A). Tissue samples of insoluble collagenous matrix without hTGF-β3 as control also resulted in relative expression of Smad-6 and -7 in orthotopic sites (Fig. 6A).
6.
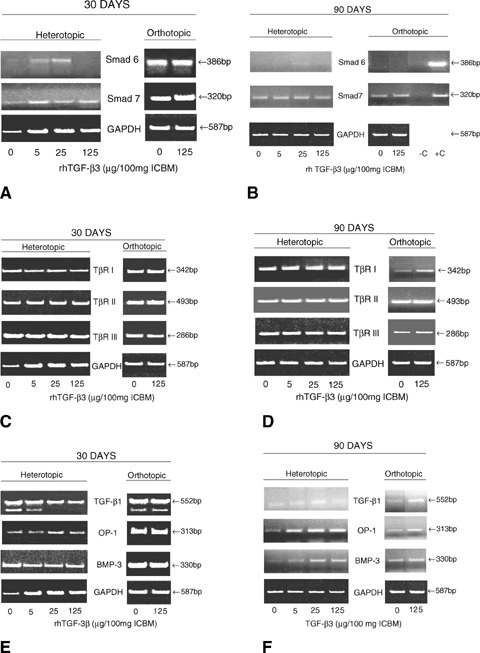
Expression of the inhibitory Smad-6 and -7 on day 30 (A) and 90 (B), TGF- β1, OP-1 and BMP-3 on day 30 (C) and 90 (D), and expression patterns of TβR I, TβR II, and TβR III on day 30 (E) and 90 (F) after calvarial implantation of inactive collagenous matrix solo as control (0 μg hTGF-β3) and 5, 25 and 125 μg doses of the h hTGF-β3 osteogenic device additionally treated without or with autogenous cellular fragments (B) of rectus abdominis muscle (−C and/or +C). Total RNA from heterotopic or orthotopic samples was reverse-transcribed and amplified with the respective primers. GAPDH expression was assayed as a control. Experiments were performed twice with similar results and representative data shown.
On day 90 (Fig. 6B), the elevated expression of Smad-6 and -7 in orthotopic samples seen on day 30 (Fig. 6A) was not observed (Fig. 6B). Treatment with the 125 μg dose of the hTGF-β3 resulted in decreased expression of Smad-6 and -7; interestingly, samples of the hTGF-β3 osteogenic device with the addition of minced rectus abdominis muscle did enhance Smad-6 and -7 expression as compared to samples without the addition of minced rectus abdominis muscle (Fig. 6B). There was limited expression of Smad-6 and -7 (particularly Smad-6) in control orthotopic samples without hTGF-β3 (Fig. 6B). The relative reduction of expression of both Smad-6 and -7 in calvarial sites as shown on day 90 correlates with the induction of bone formation pericranially on day 90 (Fig. 4A), particularly when using the 125 μg doses of hTGF-β3 protein combined with minced fragments of rectus abdominis muscle (Figs. 4E–G).
The addition of multiple responding cells in the form of minced fragments of autogenous rectus abdominis muscle resulted, on one hand, in greater bone deposition by induction in calvarial sites but, on the other hand, on greater expression of the inhibitory Smad-6 and -7 (Fig. 6B).
TGF-β1, OP-1 and BMP-3 expression
On day 30 (Fig. 6E), TGF-β1, OP-1 and BMP-3 expression was found to be elevated in the orthotopic calvarial samples. In heterotopic samples, treatment with hTGF-β3 led to a slight decrease in OP-1 expression as compared to calvarial samples.
On day 90 (Fig. 6F), TGF-β1, OP-1 and BMP-3 transcripts expression in the heterotopic specimens of the hTGF-β3 osteogenic device was reduced as compared to day 30 particularly BMP-3 and TGF-β1 expression (Fig. 6F). Calvarial samples also showed a reduction of expression of TGF-β1, OP-1 and BMP-3 as compared to day 30 (Figs. 6E and F). Tissue samples of insoluble collagenous matrix without hTGF-β3 as control also showed some degree of TGF-β1, OP-1 and BMP-3 expression particularly on day 30 (Fig. 6E).
TβR-I, TβR-II and TβR-III expression
On day 30 (Fig. 6C), heterotopic and orthotopic calvarial samples with and without hTGF-β3 expressed relative high levels of TβR-I, TβR-II and TβR-III transcripts.
On day 90 (Fig. 6D), heterotopic samples showed expression of the three receptors; orthotopic calvarial samples showed limited TβR-I, TβR-II and TβR-III expression as compared to heterotopic samples as evaluated on day 30. Control samples of ICBM without hTGF-β3 also showed relative expression of the three Tβ receptors particularly in heterotopic sites (Fig. 6D).
Western blot analysis
Orthotopic calvarial samples showed accumulation of Smad-6 and -7 as compared to heterotopic samples evaluated on day 30 (not shown). On day 90, treatment with the hTGF-β3 osteogenic device implanted in orthotopic calvarial sites decreased Smad-6 accumulation, as well as some Smad-7 accumulation as compared to untreated orthotopic samples (not shown).
Discussion
The transforming growth factor-β3 mammalian isoform is a member of a superfamily of functionally diverse, but molecularly and structurally conserved morphogens that regulate cell proliferation, differentiation, chemotaxis and chemochinesis in a cell and context-specific manner [31, 52]. The TGF-β3 isoform is a multifunctional pleiotropic cytokine that has a fundamental role in the spatial-temporal organization of developing tissues and has been shown to be expressed primarily in embryonic tissues [30–32, 52].
In the context of the heterotopic extraskeletal microenvironment of the rectus abdominis muscle of Papio ursinus, the TGF-β3 osteogenic device induces significant amounts of endochondral bone formation culminating in large mineralized and corticalized ossicles by day 20, 30 and 90 after implantation.
In marked contrast, in the context of a calvarial bone defect, the hTGF-β3 osteogenic device does not induce bone formation as evaluated morphologically and morphometrically 20 and 30 days post-implantation. Molecular analyses by RT-PCR showed a complex pattern of expression of the regulating intracellular inhibitory Smad-6 and -7 proteins modulating TGF-β signalling [33–35]. RT-PCR also showed the expression of TGF-β1, BMP-3, OP-1 and Tβ receptors during the induction of endochondral bone in heterotopic and orthotopic calvarial sites after the application of doses of the TGF-β3 protein as well as ICBM without hTGF-β3 as control in orthotopic calvarial defects.
The complex results as shown by the RT-PCR analyses reflect the pleiotropism of the TGF-β3 isoform [30–32] in the context of cell proliferation, differentiation and induction of tissue morphogenesis of primate cells and tissues ultimately resulting in contradictory findings both molecularly and morphologically. On day 30, Smad-6 and -7 are expressed in tissue specimens harvested from the orthotopic calvarial sites generated by doses of the hTGF-β3 protein. Control specimens of ICBM solo similarly show Smad-6 and -7 expression equivalent to tissue samples generated by the hTGF-β3 osteogenic device. Morphologically thus both treatments show limited bone formation
On day 90, expression of Smad-6 and -7 is consistently low and/or absent in heterotopic specimens; PCR data show a significant reduction of Smad-6 and -7 expression in calvarial sites as well as in control specimens of collagenous matrix solo. Further, the analysis shows expression of Smad-6 and to a lesser degree of Smad-7 in orthotopic calvarial sites implanted with the TGF-β3 osteogenic device pretreated with minced fragments of autogenous rectus abdominis muscle.
Smad-6 and -7 expression in calvarial sites implanted with collagenous matrix solo as control reflects the lack of substantial bone formation as seen morphologically in control defects whereas the expression of TGF-β1 and related OP-1 and BMP-3 may simply reflect the vigorous osteogenic cell differentiation at the margins of the surgically created calvarial defects. The increased presence of Smad-6 and -7 in calvarial tissue samples explain the limited de novo bone induction following hTGF-β3 treatment as well as the lack of bone formation in control defects.
Smad-6 and -7 have been shown to inhibit the effects of pathway-restricted Smad proteins by competing for binding to activated type I receptors [53]. Smad-6 has also been shown to compete with Smad-4 for complex formation with phosphorylated Smad-1 [54]. The inhibitory Smads are potently induced by TGF-β family members and may thus participate in a negative feedback loop to control the intensity and duration of TGF-β signalling [53–55].
Together these findings indicate that regenerative phenomena in untreated and hTGF-β3– treated calvarial defects are modulated by invocation of a regenerative response tightly controlled by the expression of the inhibitory Smad-6 and -7 proteins. An inhibitory response that also modulates the expression of the inhibitory Smad proteins in calvarial specimens implanted with the hTGF-β3 osteogenic device additionally pre-treated with minced autogenous fragments of the rectus abdominis muscle with partial morphological restoration of the biological activity of the hTGF-β3 osteogenic device. Potentially, the inhibitory Smad response as shown in Figure 6B after adding minced fragments of the rectus abdominis muscle, may be the result of activating proteins released by the vascular arachnoids just below the dura [56] in an attempt to further and significantly inhibit the restored or partially restored biological activity of the hTGF-β3 osteogenic device as shown in Figures 3 and 4.
The multi-functional character of the hTGF-β3 protein reflects the pleiotropic activity of the TGF-β superfamily and the tight control of its activities with both positive and negative feedbacks. These regulate multiple biological activities in relation to different biological niches and microenvironments, responding cells and different site/tissue locations for biological activities in vivo[57]. As a net result, results obtained are often divergent and contradictory.
The contradictory and divergent results in orthotopic calvarial sites are further highlighted by the implantation of the hTGF-β3 osteogenic device in non-healing mandibular defects of Papio ursinus (Carlo Ferretti and Bone Research Unit, unpublished data). Implantation of 125 μg of the hTGF-β3 osteogenic device per g of ICBM as carrier yields rapid and prominent induction of bone formation with corticalization of the newly formed mandibular buccal and lingual plates as early as 30 days after implantation (Bone Research Unit, unpublished data).
The hTGF-β3 is a potent regulator of functions associated with angiogenesis and osteogenesis. It is three- to fivefold more potent than hTGF-β1 in mitogenesis, collagen synthesis and alkaline phosphatase activity as shown in osteoblastenriched bone cell cultures [31]. The higher receptorbinding affinity of TGF-β3 compared with TGF-β1 correlates well with the relatively greater biological activity of TGF-β3[31].
Differences in tissue distribution and gene regulation also suggest distinct pleiotropic biological activities amongst the mammalian TGF-β isoforms [57, 59]. Expression of TGF-β3 mRNA is more restricted than in the case of the other more ubiquitous mammalian isoforms; TGF-β3 mRNA is mainly expressed in cell lines from mesenchymal origin, suggesting a biological role different from the other mammalian TGF-β isoforms [52]. Comparison with the precursor sequences of TGF-β1 and -β2 indicates a strong conservation of the mature sequences, but a relaxed homology in the precursor segments [52].
The above suggests superior mesenchymal and vascular cell recruitment which combined with superior chemotaxis would explain the rapid induction of mineralized ossicles with haematopoietic marrow as shown heterotopically and orthotopically after adding minced autogenous fragments of the rectus abdominis muscle to the TGF-β3 osteogenic device. The profound and incisive endochondral bone formation by induction in heterotopic extraskeletal sites of the rectus abdominis muscle microenvironment is predated molecularly by the lack of expression of the inhibitory Smad-6 and -7 proteins. Ultimately, the application of the TGF-β3 osteogenic device in calvarial defects resulting in minimal bone formation does not increase the expression of the inhibitory Smad-6 and -7 as compared to calvarial defects implanted with collagenous bone matrix solo as control. Importantly, this indicates that the intrinsic spontaneous healing capacity of a calvarial defect in non-human primates is tightly regulated by the expression of the inhibitory Smad-6 and -7 proteins resulting in minimal bone formation at the defect margins only.
Since the discovery of the TGF-β isoforms in the extracellular matrix of bone, several manuscripts have provocatively suggested that the TGF-β proteins do induce endochondral bone formation [60–63]. When discussing about osteoinduction it is important to properly define the terminology related to the induction of bone formation [5, 10–13]. The acid test of the induction of bone formation is the de novo generation of heterotopic bone after extraskeletal implantation of an osteogenic soluble molecular signal of the TGF-β supergene family [21, 28]. A protein labelled as osteoinductive must thus be endowed with the striking prerogative of initiating endochondral bone formation in heterotopic extraskeletal sites of animal models [21, 28, 36]. The heterotopic implantation site avoids the ambiguities of the orthotopic site where some degree of bone formation by conduction may occur from the viable bone interfaces [5, 10, 36] particularly when using osteophilic porous substrata or smart biomimetic matrices [21, 23, 63] as bone repair materials.
Importantly and conclusively in the bioassay for bone induction in rodents, the TGF-β isoforms do not initiate the induction of bone formation [5, 24, 36]. In marked contrast, however, the mammalian TGF-β isoforms and in this study the TGF-β3 protein (and even the amphibian TGF-β5 isoform) [23] do induce rapid and substantial endochondral bone formation when implanted in heterotopic extraskeletal sites of Papio ursinus[5, 21, 25–29]. In the primate and in the primate only, the TGF-β isoforms may act upstream to the BMPs/OPs and may induce the induction of heterotopic bone by expressing BMPs/OPs related gene products resulting in the cascade of bone differentiation by induction. Indeed, heterotopic implantation of the three mammalian TGF-β isoforms results in expression of BMP-3 and OP-1 as evaluated by Northern analyses [26, 27] and RT-PCR in this study. Alternatively, the TGF-β isoforms in the primate deploy a bone induction mechanism via different and unique pathways that result in the induction of large masses of endochondral bone. To combine noggin, a negative regulator and inhibitor of BMPs/OPs [65, 66], to osteogenic devices reconstituted with the TGF-β3 isoform would highlight the endochondral osteoinductivity of the TGF-β protein in the primate and it is currently under investigation.
The precise activities, relationships and interactions of different morphogens and growth regulators in bone formation by induction, maintenance and homeostasis such as the TGF-β isoforms, fibroblast growth factors and more specifically the BMPs/OPs are still poorly understood and need to be assigned [21, 25, 31]. To date, the mammalian TGF-β isoforms tested in Papio ursinus are potent inducers of heterotopic endochondral bone formation when implanted in the rectus abdominis muscle [28].
BMPs/OPs and TGF-β isoforms are abundant in the extracellular matrix of bone, indicating that both families are critical for bone physiology, homeostasis and bone regeneration [36, 55, 57, 59]. In addition, the presence of multiple molecular forms raises the biological significance of this apparent redundancy and indicates functional interactions and synergistic activities during both embryonic bone development and bone regeneration in postnatal life. The induction of bone formation in postnatal life recapitulates embryonic development that can be exploited as a template for regenerative medicine [21, 23].
To date, more than 15 related proteins with BMP/OP-like sequences and activity have been cloned, but little is known about their interaction during the cascade of bone formation by induction, or about the biological and therapeutic significance of this apparent redundancy [21]. All recombinantly produced hBMPs/OPs tested are capable of singly initiating bone formation in vivo[67]. Similarly, though so far in heterotopic sites of Papio ursinus only, recombinant human TGF-β1, -β2 and now the β3 isoform, initiate endochondral bone formation by induction. The TGF-β3 mammalian isoform is a further welcome addition to the family of the osteogenic proteins endowed with the striking prerogative of initiating de novo bone formation by induction [28].
The data presented show that the recombinant hTGF-β3 osteogenic device is now ready to be used in clinical contexts. The endochondral osteoinductivity initiated by doses of the hTGF-β3 has yielded the most significant heterotopic ossicles in the history of bone induction in Papio ursinus, with trabeculae of newly formed and mineralized bone covered by thick osteoid seams as early as 20 days post-implantation in the rectus abdominis muscle. The presence of multiple molecular forms with bone inductive activity points to synergistic interactions during endochondral bone formation [28]. Indeed, a potent and accelerated synergistic induction of endochondral bone formation has been shown by the binary application of recombinant or native TGF-β1 with hOP-1 in both heterotopic and orthotopic sites of Papio ursinus[25, 26, 28].
The striking pleiotropic effects of the BMPs/OPs and TGF-βs spring from minor amino acid sequence variations in the carboxy terminal region of the proteins, as well as in the transduction of distinct signalling pathways by Smad proteins after transmembrane serinethreonine kinase receptor activation and expression of the down-stream antagonists Smad-6 and Smad-7 [21]. Amino acid sequence variations in the active carboxyterminal domain of the protein confer specialized activities to a BMP/OP and TGF-β isoform, the molecular basis that determines the structure/activity profile of the osteogenic proteins of the TGF- β superfamily [21, 23, 28]. Previous experimentation has predicted that in vivo studies should now design therapeutic approaches based on information about gene regulation initiated by osteogenic proteins of the TGF-β superfamily [28, 40]. The induction of TGF-β mRNAs in both heterotopic and orthotopic sites mechanistically explain the synergistic induction of bone formation when binary applications of molecularly different osteogenic proteins are implanted in Papio ursinus[40]. Similarly, doses of the hTGF-β3 osteogenic device also induce the expression of mRNA of BMP-3 and OP-1 further raising the bone induction cascade activity.
The extrapolation of data from animal models including non-human primate species is difficult since dosage is species-specific [5, 21, 23]. The induction of bone in human patients has dramatically shown that regenerative medicine in clinical contexts is on a different scale altogether when compared to animal models including non-human primates [4, 23]. No matter how the hTGF-β3 isoform does initiate the robust induction of endochondral bone formation in Papio ursinus, the results of the RT-PCR analyses as well as previous studies demonstrating expression of TGF-β mRNAs by a single recombinant osteogenic protein [40] clearly indicate that therapeutic success in clinical contexts will rest on the binary application of a hTGF-β isoform with a recombinant osteogenic protein. The binary application of selected morphogens recapitulating embryonic development (as shown by the induction of organized pseudoepiphyseal structures in the rectus abdominis muscle of adult Papio ursinus) [5, 25] is the osteogenic drive for the induction of bone formation in clinical contexts. The hTGF-β3 isoform synergistically acting with a selected hBMP/OP to induce bone formation is a realistic alternative to hBMPs/OPs doses that are several hundredfold greater than the doses suggested by results in animal trials and the concentration of BMPs/OPs in mammalian bone [19, 20].
The rapid sculpting of mineralized tissue constructs in the rectus abdominis muscle by the hTGF-β3 isoform solo is a novel source of developing bone for autologous transplantation in clinical contexts. Conceivably, the rapid induction of endochondral bone by the TGF-β3 isoform could be utilized for the generation of large ossicles in the rectus abdominis of human patients. Thirty days after heterotopic implantation, generated ossicles could be harvested and prepared fragments transplanted into bony defects in an autogenous fashion to treat defects of either the axial and craniofacial skeletons including periodontal osseous defects [68]. The rapidity of tissue morphogenesis and the induction of bone formation complete with mineralization of the outer cortex of the ossicles and bone marrow formation by day 30 is of particular importance for repair and regeneration of bone in the elderly, where repair phenomena are temporally delayed and healing progresses slower than in younger patients [21, 25]. In addition, fragments of autogenously induced heterotopic bone could be prepared from ossicles induced in the rectus abdominis by binary applications of hOP-1 and relatively low doses of hTGF-β3, a synergistic strategy known to yield massive mineralized ossicles with large seams of osteoid populated by contiguous osteoblasts by day 15 after heterotopic implantation [21, 25–26].
The results of this study have shown significant bone induction by the TGF-β3 mammalian isoform in Papio ursinus. The rapid induction of endochondral bone formation by the hTGF-β3 osteogenic device together with TGF-β1, BMP-3 and OP-1 mRNA expression, hypercellular osteoblastic activity, osteoid synthesis, angiogenesis and capillary sprouting are the novel molecular and morphological basis for the induction of bone formation in clinical contexts to regenerate the bone/bone marrow organ in man [69].
Acknowledgments
Supported by the South African Medical Research Council, the University of the Witwatersrand, Johannesburg, the National Research Foundation and ad hoc grants of the Bone Research Unit. We thank Kohei Miyazono and Takeshi Imamura for Smad-6 and Smad-7 cDNAs, Janet Patton and Karolina Kuun for the molecular work of the polymerase chain reaction, Northern and Western blot analyses, the Central Animal Services of the University for the help with primate experimentation, Novartis AG Basel for the recombinant human transforming growth factor-β3, Laura Roden for critical reading of the manuscript, and Kathleen Ripamonti for understanding and support.
References
- 1.Habal MB. Bone grafting in craniofacial surgery. Clin Plast Surg. 1994;21:349–63. [PubMed] [Google Scholar]
- 2.Burchardt H. Biology of bone transplantation. Orthop Clin North Am. 1987;18:187–201. [PubMed] [Google Scholar]
- 3.Friedlaender GE. Bone grafts. The basic science rationale for clinical applications. J Bone Joint Surg. 1987;69A:786–97. [PubMed] [Google Scholar]
- 4.Ripamonti U, Ferretti C, Heliotis M. Soluble and insoluble signals and the induction of bone formation: molecular therapeutics recapitulating development. J Anat. 2006;209:447–68. doi: 10.1111/j.1469-7580.2006.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turing AM. The chemical basis of morphogenesis. Philos Trans Roy Soc Lond. 1952;237:37. [Google Scholar]
- 6.Levander G. A study of bone regeneration. Surg Obster. 1938;67:705–14. [Google Scholar]
- 7.Levander G, Willestaedt H. Alcoholsoluble osteogenetic substance from bone marrow. Nature. 1946;3992:587. doi: 10.1038/157587b0. [DOI] [PubMed] [Google Scholar]
- 8.Moss ML. Extraction of an osteogenic inductor factor from bone. Science. 1958;127:755–6. doi: 10.1126/science.127.3301.755. [DOI] [PubMed] [Google Scholar]
- 9.Trueta J. The role of the vessels in osteogenesis. J Bone Joint Surg. 1963;45B:402–18. [Google Scholar]
- 10.Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 11.Reddi AH, Huggins CB. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci USA. 1972;69:1601–5. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddi AH. Cell biology and biochemistry of endochondral bone development. Coll Relat Res. 1981;1:209–26. doi: 10.1016/s0174-173x(81)80021-0. [DOI] [PubMed] [Google Scholar]
- 13.Urist MR, DeLange RJ, Finnerman GAM. Bone cell differentiation and growth factors. Science. 1993;220:680–6. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- 14.Sampath TK, Reddi AH. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci USA. 1981;78:7599–603. doi: 10.1073/pnas.78.12.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang EA, Rosen V, Cordes P, Hewick RM, Kriz MJ, Luxenberg DP, Sibley BS, Wozney JM. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci USA. 1988;85:9484–8. doi: 10.1073/pnas.85.24.9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 17.Özkaynak E, Rueger DC, Drier EA, Corbett C, Ridge RJ, Samapth T, Oppermann H. OP-1 cDNA encodes an osteogenic protein in the TGF-β family. Embo J Org. 1990;9:2085–93. doi: 10.1002/j.1460-2075.1990.tb07376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampath TK, Maliakal JC, Hauschka PV, Jones WK, Sasak H, Tucker RF, White KH, Coughlin JE, Tucker MM, Pang RHL, Corbett C, Özkaynak E, Oppermann H, Rueger DC. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267:20352–62. [PubMed] [Google Scholar]
- 19.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83A:151–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Börner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, Van Der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Rüter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T BMP-2 Evaluation in Surgery for Tibial Trauma (BESTT) Study Group. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg. 2002;84A:2123–34. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Ripamonti U. Soluble osteogenic molecular signals and the induction of bone formation. Biomaterials. 2006;27:807–22. doi: 10.1016/j.biomaterials.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Sampath TK, Rashka KE, Doctor JS, Tucker RF, Hoffmann FM. Drosophila TGF-β superfamily proteins induce endochondral bone formation in mammals. Proc Natl Acad Sci USA. 1993;90:6004–8. doi: 10.1073/pnas.90.13.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ripamonti U. Embedding molecular signals in biomimetic matrices for regenerative medicine. S Afr J Sci. 2006;102:211–6. [Google Scholar]
- 24.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Fauci AS. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–71. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ripamonti U, Duneas N, Van Den Heever B, Bosch C, Crooks J. Recombinant transforming growth factor-β1 induces endochondral bone in the baboon and synergizes with recombinant osteogenic protein-1 (bone morphogenetic protein-7) to initiate rapid bone formation. J Bone Miner Res. 1997;2:1584–95. doi: 10.1359/jbmr.1997.12.10.1584. [DOI] [PubMed] [Google Scholar]
- 26.Duneas N, Crooks J, Ripamonti U. Transforming growth factor-ß1: Induction of bone morphogenetic protein genes expression during endochondral bone formation in the baboon, and synergistic interaction with osteogenic protein-1 (BMP-7) Growth Factors. 1998;15:259–77. doi: 10.3109/08977199809017482. [DOI] [PubMed] [Google Scholar]
- 27.Ripamonti U, Crooks J, Matsaba T, Tasker J. Induction of endochondral bone formation by recombinant human transforming growth factor-β2 in the baboon (Papio ursinus) Growth Factors. 2000;17:269–85. doi: 10.3109/08977190009028971. [DOI] [PubMed] [Google Scholar]
- 28.Ripamonti U. Osteogenic proteins of the TGF-β superfamily. In: Henry HL, Norman AW, editors. Encyclopedia of Hormones. Austin Academic Press; 2004. pp. 80–6. [Google Scholar]
- 29.Ripamonti U. Soluble, insoluble and geometric signals sculpt the architecture of mineralized bone. J Cell Mol Med. 2004;8:169–80. doi: 10.1111/j.1582-4934.2004.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ten Dijke P, Hansen P, Iwata KK, Pieler C, Foulkes JG. Identification of another member of the transforming growth factor type ß gene family. Prof Natl Acad Sci USA. 1988;85:4715–9. doi: 10.1073/pnas.85.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ten Dijke P, Iwata KK, Goddard C, Pieler C, Canalis E, McCarthy TL, Centrella M. Recombinant transforming growth factor type β3: biological activities and receptor-binding properties in isolated bone cells. Mol Cell Biol. 1990;10:4473–9. doi: 10.1128/mcb.10.9.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ten Dijke P, Iwata KK, Thorikay M, Schwedes J, Stewart A, Pieler C. Molecular characterization of transforming growth factor type β3. Ann NY Acad Sci. 1990;593:26–42. doi: 10.1111/j.1749-6632.1990.tb16097.x. [DOI] [PubMed] [Google Scholar]
- 33.Imamura T, Takase M, Nishihara A, Oeda E, Hanai J-I, Kawabata M, Miyazono K. Smad6 inhibits signalling by the TGF-β superfamily. Nature. 1997;389:622–6. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 34.Nakao A, Afrakhte M, Morén, Nakayama T, Christian JL, Heuchel R, Itoh, Kawabata M, Heldin N-E, Heldin C-H, Ten Dijke P. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature. 1997;389:631–5. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 35.Hanyu A, Ishidou Y, Ebisawa T, Shimanuki T, Imamura T, Miyazono K. The N domain of Smad7 is essential for spcific inhibition of transforming growth factor-β signaling. J Cell Biol. 2001;155:1017–27. doi: 10.1083/jcb.200106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddi AH. Morphogenesis and tissue engineering of bone and cartilage: inductive signals stem cells, and biomimetic biomaterials. Tissue Eng. 2000;6:351–9. doi: 10.1089/107632700418074. [DOI] [PubMed] [Google Scholar]
- 37.Sampath TK, Reddi AH. Homology of bone-inductive proteins from human, monkey, bovine and rat extracellular matrix. Proc Natl Acad Sci USA. 1983;80:6591–5. doi: 10.1073/pnas.80.21.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ripamonti U, Van Den Heever B, Sampath TK, Tucker MM, Rueger DC, Reddi AH. Complete regeneration of bone in the baboon by recombinant human osteogenic protein-1 (hOP-1, bone morphogenetic protein-7) Growth Factors. 1996;13:273–89. doi: 10.3109/08977199609003228. [DOI] [PubMed] [Google Scholar]
- 39.Ripamonti U, Van Den Heever B, Crooks J, Rueger DC, Reddi Long-term evaluation of bone formation by osteogenic protein-1 in the baboon and relative efficacy of bone-derived bone morphogenetic proteins delivered by irradiated xenogeneic collagenous matrices. J Bone Miner Res. 2000;15:1798–809. doi: 10.1359/jbmr.2000.15.9.1798. [DOI] [PubMed] [Google Scholar]
- 40.Ripamonti U. Bone induction by recombinant human osteogenic protein-1 (hOP-1, BMP-7) in the primate Papio ursinus with expression of mRNA of gene products of the TGF-β superfamily. J Cell Mol Med. 2005;9:911–28. doi: 10.1111/j.1582-4934.2005.tb00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnitzler CM, Ripamonti U, Mesquita JM. Histomorphometry of iliac crest trabecular bone in adult male baboons in captivity. Calcif Tissue Int. 1993;52:447–54. doi: 10.1007/BF00571335. [DOI] [PubMed] [Google Scholar]
- 42.Ripamonti U. Bone induction in non-human primates. An experimental study on the baboon Papio ursinus. Clin Orthop Rel Res. 1991;269:284–94. [PubMed] [Google Scholar]
- 43.Public Service Department. National Code for Animal Use in Research, Education, Diagnosis and Testing of Drugs and Related Substances in South Africa. Pretoria, South Africa: Public Service Department; 1990. [Google Scholar]
- 44.Ripamonti U, Ramoshebi LN, Matsaba T, Tasker J, Crooks J, Teare J. Bone induction by BMPs/OPs and related family members in primates. The critical role of delivery systems. J Bone Joint Surg. 2001;83-A(S1):116–27. [PubMed] [Google Scholar]
- 45.Parfitt AM. Stereologic basis of bone histomorphometry; theory of quantitative microscopy and reconstruction of the third dimension. In: Recker RR, editor. Bone Histomorphometry: Techniques and Interpretation. Boca Raton, FL, USA: CRC Press; 1983. pp. 53–87. [Google Scholar]
- 46.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 47.Machida H, Ogawa K, Funaba M, Mizutani T, Tsujimoto M. mRNA expression of type I and type II receptors for activin, transforming growth factor-β, and bone morphogenetic protein in the murine erythroleukemic cell line, F5–5.fl. Eur J Endocrinol. 2000;143:705–10. doi: 10.1530/eje.0.1430705. [DOI] [PubMed] [Google Scholar]
- 48.Bentley H, Hamdy FC, Hart KA, Seid JM, Williams JL, Johnstone D, Russell RG. Expression of bone morphogenetic proteins in human prostatic adenocarcinoma and and benign prostatic hyperplasia. Br J Cancer. 1992;66:1159–63. doi: 10.1038/bjc.1992.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chubinskaya S, Merrihew C, Cs-Szabó G, Mollenhauer J, McCartney J, Rueger DC, Kuettner KE. Human articular chondrocytes express osteogenic protein-1. J Histochem Cytochem. 2000;48:239–50. doi: 10.1177/002215540004800209. [DOI] [PubMed] [Google Scholar]
- 50.Xu G, Chakraborty C, Lala PK. Expression of TGF-beta signaling genes in the normal, premalignant, and malignant human trophoblast: loss of smad3 in choriocarcinoma cells. Biochem Biophys Res Commun. 2001;287:47–55. doi: 10.1006/bbrc.2001.5533. [DOI] [PubMed] [Google Scholar]
- 51.Sabath D, Broome HE, Prystowsky MB. mRNA is a major interleukin 2-induced transcript in a cloned T-helper lymphocyte. Gene. 1990;91:185–91. doi: 10.1016/0378-1119(90)90087-8. [DOI] [PubMed] [Google Scholar]
- 52.Derynck R, Lindquist PB, Lee A, Wen D, Tamm J, Graycar JL, Rhee L, Mason AJ, Miller DA, Coffey RJ, Moses HL, Chen EY. A new type of transforming growth factor-β, TGF-β3. The EMBO J. 1998;7:3737–43. doi: 10.1002/j.1460-2075.1988.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 54.Ten Dijke P, Korchynskyi L, Valdimrsdottir G, Goumans M-J. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105–13. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 55.Sakou T, Onishi T, Yamamoto T, Nagamine T, Sampath T, Ten Dijke P. Localization of Smads, the TGF-beta family intracellular signaling components during endochondral ossification. J Bone Miner Res. 1999;14:1145–52. doi: 10.1359/jbmr.1999.14.7.1145. [DOI] [PubMed] [Google Scholar]
- 56.Topper JN, Cai J, Qiu Y, Anderson KR, Xu Y-Y, Deeds JD, Feeley R, Gimeno C, Woolf EA, Tayber O, Mays GG, Sampson BA, Schoen FJ, Gimbrone MA, Falb D. Vascuar MADs: Two novel MAD-related genes selectively inducible by flow in human vascular endothelium. Proc Natl Acad Sci USA. 1997;94:9314–9. doi: 10.1073/pnas.94.17.9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piek E, Heldin C-H, Ten Dijke P. Specificity, diversity, and regulation in TGF-β superfamily signaling. Faseb J. 1999;13:2105–24. [PubMed] [Google Scholar]
- 58.Ripamonti U, Bosch C, Van Den Heever B, Duneas N, Melsen B, Ebner R. Limited chondroosteogenesis by recombinant human transforming growth factor-β1 in calvarial defects of adult baboons (Papio ursinus. J Bone Miner Res. 1996;11:938–45. doi: 10.1002/jbmr.5650110710. [DOI] [PubMed] [Google Scholar]
- 59.Sporn MB, Roberts AB, Wakefield M, De Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. Cell Biol. 1987;105:1039–45. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosen DM, Nathan R, Armstrong R, Bentz H, Thompson A, De Leon E, Buckman E, Fiedler L, Seyedin S. Bone induction and transforming growth factor-β. Ann NY Acad Sci. 1990;593:98–106. doi: 10.1111/j.1749-6632.1990.tb16103.x. [DOI] [PubMed] [Google Scholar]
- 61.Noda N, Camilliere J. In vivo stimulation of bone formation by transforming growth factor-β. Endocrinology. 1989;124:2991–4. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- 62.Joyce ME, Roberts AB, Sporn MB, Bolander ME. Transforming growth factor-β and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110:2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kibblewhite DJ, Bruce AG, Strong DM, Ott SM, Purchio AF, Larrabee WF. Transforming growth factor-β accelerates osteoinduction in a craniofacial onlay model. Growth Factors. 1993;9:185–93. [PubMed] [Google Scholar]
- 64.Ripamonti U, Richter PW, Thomas ME. Self-inducing shape memory geometric cues embedded within smart hydroxyapatite-based biomimetic matrices. Plast Recontsr Surg. 2007;120:1–12. doi: 10.1097/01.prs.0000287133.43718.89. [DOI] [PubMed] [Google Scholar]
- 65.Gazzerro E, Canji V, Canalis E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest. 1998;102:2106–14. doi: 10.1172/JCI3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides AR, Kwiatkowski W, Baban K, Affolter M, Vale WW, Belmonte JCI, Choe S. Structural basis of BMP signalling inhibition by noggin, a novel twelve-membered cystine knot protein. J Bone Joint Surg. 2003;85-A:52–8. doi: 10.2106/00004623-200300003-00010. [DOI] [PubMed] [Google Scholar]
- 67.Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, Haydon RC, He T-C. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Therapy. 2004;11:1312–20. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 68.Teare J, Ramoshebi LN, Ripamonti U. Periodontal tissue regeneration by recombinant human transforming growth factor-β3 in Papio ursinus. J Periodont Res. 2007 doi: 10.1111/j.1600-0765.2007.00987.x. doi:DOI: 10.1111/j.1600--0765.2007.00987.x. [DOI] [PubMed] [Google Scholar]
- 69.Urist MR. The reality of a nebulous, enigmatic myth. Clin Orthop. 1968;59:243–83. [Google Scholar]


