The observation that ~ 30% of dilated cardiomyopathy (DCM) is genetic in origin represents one of the most important advances in modern cardiology and has transformed our view of this common disorder1. Equally surprising is the more recent finding that sarcomeric genes previously linked to the development of Hypertrophic Cardiomyopathy (HCM) were also causative in the pathogenesis of DCM2. Mutations in most of the protein components of the cardiac sarcomere have now been linked to DCM, many of them arising in close proximity to known HCM mutations, and provide a unique challenge, how can such divergent patterns of ventricular remodeling arise from such closely related structural changes? While it may appear to be straightforward that discrete alterations in sarcomeric function caused by gene mutations could eventually lead to either LV dilation or hypertrophy, our lack of understanding regarding the earliest clinical stages of DCM before the effects of secondary changes are manifest have precluded mechanistic insight. In this issue of Circulation Genetics, Lakdawala, et al utilized a unique and well-characterized sarcomeric DCM cohort to directly determine whether abnormal systolic function can be detected in genotype positive patients with normal LV EF and size. Their robust approach, including determination of echo strain and systolic myocardial tissue velocity and comparisons among sublinical DCM, overt DCM and control (genotype negative) family members across four independent mutation groups revealed a significant decrease in systolic function in the subclinical group. These results compliment their previous findings that gene mutations linked to HCM cause diastolic dysfunction in subclinical cohorts and fully establish that the primary mechanisms driving diverse ventricular remodeling in sarcomeric cardiomyopathies are tightly coupled to the underlying changes in sarcomere dynamics3.
Since the publication of the first linkage studies in 1990, over one thousand mutations in genes encoding the protein components of the cardiac sarcomere have been associated with the development of cardiomyopathic remodeling (reviewed in3). While the disorder is unique in that the overall structure and function of the affected proteins are well-established, this extensive biophysical understanding has not yet translated to patient care, and the lack of genotype – phenotype correlation has hindered the use of genotype to predict prognosis and direct clinical management. Indeed, due in part to the characteristic phenotypic heterogeneity in patients with sarcomeric cardiomyopathies, it has been suggested that the goal of genotype-driven patient management may not be attained4. Two major observations have refocused efforts to establish robust genotype – phenotype relationships in HCM and DCM. First, the biophysical basis of the clinical disorder was established by the overall concordance among the in vitro studies in identifying precise alterations in sarcomeric function coupled to the persistent finding in animal models that contractile dysfunction preceded histopathology. Second, longitudinal studies of genotyped cohorts have begun to establish the progressive nature of the ventricular remodeling caused by sarcomeric mutations5, 6. These findings mirrored those in animal models where initial abnormalities in sarcomeric function led to activation of multiple downstream myocellular signaling pathways that drove cardiac remodeling. The latter observation is similar to the difficulties in determining etiology in congestive heart failure patients, in that end-stage, remodeled tissue is temporally removed from the initial pathogenic process. Thus, in order to mechanistically couple a primary biophysical abnormality to a specific pattern of ventricular remodeling, it is imperative to identify and study the earliest stages of the cardiomyopathy, exactly the approach taken in the current study.
To directly address this central question the authors have assembled a unique clinical cohort drawn from 5 independent families carrying mutations in three sarcomeric proteins previously linked to DCM (MYH7, TMP1 and TNNT2). While the statistical power was insufficient to elucidate mutation-specific information and the reliance on families for recruitment may limit the ability to address some questions raised by the data (for example the intriguing sex differences), the multigenerational cohort yielded three well-defined groups for study. The main subgroup (“subclinical DCM”) was comprised of twelve genotype – positive patients with baseline clinical and standard echo parameters largely indistinguishable from genotype-negative controls, including normal LV size and EF. Of note, many of these parameters exhibited clear differences when compared to the genotype-positive subgroup with overt DCM. Application of more sensitive global systolic echo methodologies including tissue Doppler and strain imaging revealed striking differences in nearly all indices within the subclinical group as compared to controls. As noted by the authors, the decreases in global peak systolic myocardial tissue velocity, longitudinal peak systolic strain and strain rates all remained significant after controlling for LV geometry and EF. While subsequent tests for the predictive power of these indices to differentiate genotype positive from genotype negative individuals again revealed significant differences, the aggregate data did not support the use of these metrics for driving clinical management. This limitation does not, however, diminish the potential use of these indices for longitudinal study of disease progression, a clear future step. Finally, application of these approaches to a previously published preclinical HCM cohort did not reveal systolic dysfunction in any measured parameter while diastolic indices were preserved in the subclinical DCM group. Thus, in the context of largely preserved LV geometry, the earliest stages of sarcomeric DCM and HCM are distinguishable and indeed can be defined by the degree of impairment in high resolution systolic and diastolic indices respectively. This robust coupling between cardiac dysfunction in early disease states and the eventual clinical phenotype is not only clinically relevant, it again reinforces the importance of elucidating the primary biophysical mechanisms whereby individual mutations cause these precise pathophysiologic responses in cardiac muscle.
Given the relatively small size of the subclinical DCM group the authors were reasonably circumspect in the extrapolation of their results to the broader questions of genotype – phenotype correlation and eventual patient management in genetic cardiomyopathies. Nonetheless, the current study raises important questions that will spur both basic and clinical investigations. As noted in an extensive recent review by Moore, et al, HCM-linked myosin mutations tend to enhance contractility while the DCM-linked subset decrease contractile function7. Though biophysical data are limited (especially for the myosin motor mutations where the challenge of exogenously expressing myosin is a significant limitation) the mutations studied in the current report generally fit within this paradigm. Both of the S532P myosin and del210K cTnT mutations have been modeled in mice and, in both cases, homozygous animals not only recapitulated the DCM phenotype but deficits in contractile function were detected prior to overt histopathology, mirroring the clinical findings in the current study8, 9. Analysis of molecular motor function for the S532P myosin isolated from homozygous animals revealed significant decreases in actin sliding velocity and ATPase activity, in direct contrast to previous results from the HCM-linked R403Q myosin mice10. The combination of the accumulating evidence from animal models and in vitro studies provides strong support for the hypothesis that HCM and DCM mutations cause differential disease states via precise (and opposite) modulation of sarcomeric efficiency. The link between these changes, however, and the diverse ventricular remodeling patterns remains unclear.
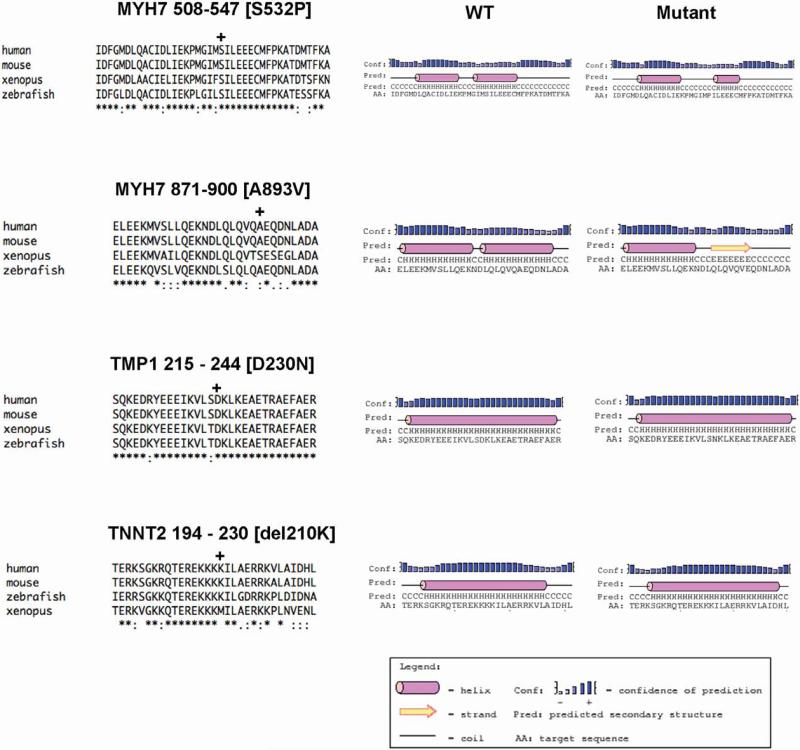
From the standpoint of sarcomeric dynamics, structure and function, the varied functional roles of the four independent protein mutations are intriguing. Why would four such disparate mutations cause such a similar clinical phenotype? All four mutations are located within highly conserved regions of the affected proteins (see Figure). The MYH7 mutations S532P and A893V occur in the globular N-terminal domain of the myosin motor. Specifically the S532P mutation is within the actin-myosin interface while the A893V mutation is immediately distal to the Regulatory Light Chain (RLC) binding domain in close proximity to the transition between the neck and hinge11. While no biophysical studies have been performed on the A893V mutation, this unique linker region of the motor is thought to, in part, modulate intermolecular interactions between the two myosin molecules12 . Either of these domains could thus alter motor efficiency. Comparing the effects of either mutation on predicted secondary structure using the PSIPRED algorithm reveals potentially significant effects on local structure (See Figure)13. At residue 532 in the actin binding domain the substitution of the non-polar Phe residue for Ser is predicted to decrease the overall helicity of the region and lengthen the linker domain. The relatively conservative Ala to Val substitution at residue 893 also significantly decreases the helical nature of the region, in this case, appearing to favor a structure more consistent with a beta-sheet. Either of these structural changes would be predicted to alter both local and distant inter and intramolecular protein interactions and contribute to a decrease in motor efficiency. Computational modeling of protein dynamics would provide additional mechanistic detail14. While it is relatively straightforward to envision the role of structural changes in myosin as a cause of altered motor function, the molecular effects of the thin filament mutations TMP1 D230N and TNNT2 del210K are less clear. The regulatory thin filament directly determines the access of actin and myosin in part by allosterically modulating the position of tropomyosin in response to calcium binding to cTnC. Until recently, the complexity of the protein-protein interactions within the complex and the lack of high-resolution structure for the N-terminal domain of cTnT has complicated disease insight for thin filament mutations. Transgenic mouse models of HCM-linked cTnT mutations have demonstrated both significantly altered energetics and inefficient ATP utilization that can be partially rescued by genetically switching the myosin isoform in vivo15, 16. These results support a direct role for the troponin complex in modulating crossbridge dynamics that may, in part, explain how thin filament mutations can cause similar clinical phenotypes as compared to thick filament mutations. Interestingly, the local structural and functional effects are likely to be different than the myosin mutations, even though both the TMP1 and TNNT2 mutations in the current study also occur in highly helical domains. This is illustrated in the secondary structure predictions (Figure) where no change in overall helicity is observed for either mutation. Depending on the position of the mutated residue within the helical array (for example, D230N faces away from the inner coiled-coil structure) the effects of the mutations are likely to influence protein-protein interactions within the complex via changes in electrostatics and/or flexibility. Again, Molecular Dynamics will be a useful approach in determining the molecular mechanisms.
Figure.
Homology Alignments and Secondary Structure Plots for Mutation Sites and Immediate Flanking Regions
Like all benchmark translational studies, the current work both raises the bar and generates a new framework for future studies on both the biophysical and clinical sides of the genetic cardiomyopathy divide. The early onset of systolic dysfunction in the preclinical DCM cohort establishes the both the primary role of the biophysical changes in sarcomeric function that determines disease onset and lends further support to the emerging consensus that deficits in contractile function drive the development of DCM. While at present the high resolution echo techniques employed in the current study are not sufficiently predictive to be used to identify relatives at risk, that is likely to change as larger patient cohorts are obtained. This latter point is key as this study illustrates the way forward for future work, with a focus on genotyped, multigenerational cohorts and careful longitudinal characterization of clinical phenotypes from the earliest stages of disease. The techniques described here will clearly be useful in following the progression of the cardiac dysfunction, a crucial next step in developing a more robust understanding of the natural history of HCM and DCM. The demonstration that diverse protein mutations can cause similar patterns of early ventricular remodeling will help focus efforts to develop more functionally driven molecular studies where the structural and dynamic effects on single proteins can be better integrated into multi-protein in silico and in vitro approaches and finally provide insight as to how mutations in proteins of the cardiac sarcomere cause distinct patterns of ventricular remodeling. The eventual results will be fully translational in that they will identify unique points of therapeutic intervention and move us closer to the goal of using genotype to inform clinical management in this not uncommon disorder.
Acknowledgments
Funding Sources: This work is supported by grants from the National Institutes of Health (5R01HL107046-02, 5R01HL075619-8) and the Children's Cardiomyopathy Foundation. The author is also supported by the Gootter Chair for the Prevention of Sudden Cardiac Death.
Footnotes
Conflict of Interest Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dellefave L, McNally EM. The genetics of dilated cardiomyopathy. Curr Opin Cardiol. 2010;25:198–204. doi: 10.1097/HCO.0b013e328337ba52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000;343:1688–1696. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- 3.Seidman CE, Seidman JG. Identifying sarcomere gene mutations in hypertrophic cardiomyopathy: A personal history. Circ Res. 2011;108:743–750. doi: 10.1161/CIRCRESAHA.110.223834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Driest SL, Ackerman MJ, Ommen SR, Shakur R, Will ML, Nishimura RA, et al. Prevalence and severity of “benign” mutations in the beta-myosin heavy chain, cardiac troponin t, and alpha-tropomyosin genes in hypertrophic cardiomyopathy. Circulation. 2002;106:3085–3090. doi: 10.1161/01.cir.0000042675.59901.14. [DOI] [PubMed] [Google Scholar]
- 5.Revera M, van der Merwe L, Heradien M, Goosen A, Corfield VA, Brink PA, et al. Long-term follow-up of r403wmyh7 and r92wtnnt2 hcm families: Mutations determine left ventricular dimensions but not wall thickness during disease progression. Cardiovasc J Afr. 2007;18:146–153. [PMC free article] [PubMed] [Google Scholar]
- 6.Lakdawala NK, Dellefave L, Redwood CS, Sparks E, Cirino AL, Depalma S, et al. Familial dilated cardiomyopathy caused by an alpha-tropomyosin mutation: The distinctive natural history of sarcomeric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55:320–329. doi: 10.1016/j.jacc.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore JR, Leinwand L, Warshaw DM. Understanding cardiomyopathy phenotypes based on the functional impact of mutations in the myosin motor. Circ Res. 2012;111:375–385. doi: 10.1161/CIRCRESAHA.110.223842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt JP, Debold EP, Ahmad F, Armstrong A, Frederico A, Conner DA, et al. Cardiac myosin missense mutations cause dilated cardiomyopathy in mouse models and depress molecular motor function. Proc Natl Acad Sci U S A. 2006;103:14525–14530. doi: 10.1073/pnas.0606383103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, et al. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007;101:185–194. doi: 10.1161/CIRCRESAHA.106.146670. [DOI] [PubMed] [Google Scholar]
- 10.Debold EP, Schmitt JP, Patlak JB, Beck SE, Moore JR, Seidman JG, et al. Hypertrophic and dilated cardiomyopathy mutations differentially affect the molecular force generation of mouse alpha-cardiac myosin in the laser trap assay. Am J Physiol Heart Circ Physiol. 2007;293:H284–291. doi: 10.1152/ajpheart.00128.2007. [DOI] [PubMed] [Google Scholar]
- 11.Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, et al. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993;261:58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- 12.Craig R, Woodhead JL. Structure and function of myosin filaments. Curr Opin Struct Biol. 2006;16:204–212. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- 14.Sweeney HL, Houdusse A. Structural and functional insights into the myosin motor mechanism. Annu Rev Biophys. 2010;39:539–557. doi: 10.1146/annurev.biophys.050708.133751. [DOI] [PubMed] [Google Scholar]
- 15.He H, Hoyer K, Tao H, Rice R, Jimenez J, Tardiff JC, et al. Myosin-driven rescue of contractile reserve and energetics in mouse hearts bearing FHC-associated mutant Troponin T is mutation-specific. J Physiol. 2012 Aug 20; doi: 10.1113/jphysiol.2012.234252. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He H, Javadpour MM, Latif F, Tardiff JC, Ingwall JS. R-92l and r-92w mutations in cardiac troponin t lead to distinct energetic phenotypes in intact mouse hearts. Biophys J. 2007;93:1834–1844. doi: 10.1529/biophysj.107.107557. [DOI] [PMC free article] [PubMed] [Google Scholar]