Abstract
Autoimmune diseases currently affect 5–7% of the world's population; in most diseases there are circulating autoantibodies. Brain-reactive antibodies are present in approximately 2–3% of the general population but do not usually contribute to brain pathology. These antibodies penetrate brain tissue only early in development or under pathologic conditions. This restriction on their pathogenicity and the lack of correlation between serum titers and brain pathology have, no doubt, contributed to a delayed appreciation of the contribution of autoantibodies in diseases of the central nervous system. Nonetheless, it is increasingly clear that antibodies can cause damage in the brain and likely initiate or aggravate multiple neurologic conditions; brain-reactive antibodies contribute to symptomatology in autoimmune disease, infectious disease, and malignancy.
Keywords: autoimmunity, molecular mimicry, blood-brain barrier
INTRODUCTION
An increased awareness of the contribution of brain-reactive antibodies to human pathobiology has been propelled by new approaches to both animal models of disease and clinical investigation. In fact, a large part of the difficulty in recognizing brain-focused autoimmunity has arisen from imprecise definitions of autoimmune disease and from the models traditionally used to study autoimmunity. The classification of a disease as autoimmune first requires the exclusion of other etiologic explanations. Once other causes have been ruled out, additional criteria must be met: (a) for antibody-mediated diseases, maternal transmission of disease symptoms to the neonate; (b) transfer of pathology by antibodies or lymphocytes from an affected individual to a laboratory animal or to cells in culture; (c) development of an animal model in which disease can be transferred to syngeneic hosts via antibodies or lymphocytes; and (d) the presence of lymphocytes or antibodies in affected tissue.
It is obvious that several of these criteria are not easily met in studies of brain pathology. Even the most straightforward model—that of maternal antibody–mediated alteration in fetal brain development—becomes difficult to entertain, let alone prove. Abnormalities in brain development may not be detected until months to years after birth. At that time, the possible causes of brain damage are legion. Furthermore, the mother herself is unlikely to display untoward effects of brain-reactive antibody production due to the integrity of her blood-brain barrier (BBB), which limits the access of serum proteins to brain parenchyma. Thus, an autoimmune explanation of brain pathology in the child may well not arise, and there is often no investigation of maternal antibody.
Other forms of evidence are also difficult to obtain. Transfer of brain disease from a human patient to a laboratory animal via serum or purified antibodies does not occur unless there is a breach in the recipient's BBB integrity; thus, the pathogenicity of many brain-reactive antibodies is obscured unless an insult to BBB integrity is imposed on the recipient animal. Ex vivo studies may also be uninformative. Because so much brain function requires cell-to-cell communication, pathology may be overlooked in preparations in which antibodies are added directly to cells in culture. The development of an animal model of autoantibody-driven brain disease is also difficult, as such a model must include a mechanism for antibody to gain access to brain tissue. Sometimes the mechanism occurs as part of the immune process itself, as in experimental autoimmune encephalitis (EAE), a mouse model of multiple sclerosis (MS) in which activated T cells can disrupt the BBB (1). However, to model autoantibody-mediated diseases, it is often necessary to compromise BBB integrity through a process that is distinct from the generation of autoantibody (2). Finally, evidence that the human disease is immune mediated may also be difficult to obtain. Brain biopsy is a relatively rare clinical procedure; premortem observation of brain tissue is therefore limited, although newer neuroimaging tools promise to improve the analysis of living brains. Postmortem tissue is of lesser value, because terminal illness often includes many breaches of BBB integrity with ensuing pathologic processes in the brain that may not have been ongoing prior to the terminal event. For that reason, postmortem data must be interpreted cautiously. All these difficulties in analyzing brain tissue and developing appropriate animal or cell culture models have delayed our appreciation of autoimmune diseases of the brain.
The most common approach to implicating antibodies in brain disease is to find them in cerebrospinal fluid (CSF) of an affected individual. Yet the presence of autoantibodies in CSF is not sufficient evidence to incriminate them as a pathogenic agent. Cessation of neuropsychiatric symptomatology once antibodies are no longer present in CSF may suggest that antibodies are pathogenic. However, it is possible for such symptoms to persist: In the brain, as in other tissues, antibodies may trigger irreversible damage. Moreover, even when CSF antibody titers correlate with symptoms, antibody need not be the causative agent for the symptoms; it may be a marker of disease but not a contributor to pathogenesis. It is often the case that anti-brain antibodies are found in the serum but not in CSF. Indeed, many brain-reactive antibodies that have been implicated in disease pathogenesis are made within lymphoid organs, are present in serum, and are not necessarily present in CSF.
If the antibody is the culprit in the disease process and not merely an epiphenomenon, then the targeted antigen should be present in a functional pathway that is relevant to disease symptoms. As described above, the antibody should transmit the relevant symptoms to an experimental animal model by passive transfer or by active immunization strategies. An additional test for attributing pathogenesis to the functional properties of antibody includes the demonstration that immunomodulation or removal of antibody diminishes symptomatology; this again assumes that autoantibody-mediated damage is reversible.
Importantly, not all brain-reactive antibodies are likely to cause disease even if they access brain parenchyma. As with autoantibodies targeted to other organs, it is possible to have elevated serum titers and no evidence of disease. This may reflect insufficient titers or a requirement for a particular fine specificity or affinity. Alternatively, it may reflect the lack of a susceptible target organ because of diminished antigen display or a lack of a tissue inflammatory response. All these considerations, as well as the need to penetrate the BBB, also apply to brain-reactive antibodies. Nonetheless, multiple investigations now demonstrate a contribution of brain-reactive antibodies to clinical disease. In this review, we discuss brain-specific issues in the diagnosis and pathology of antibody-mediated diseases, focusing on illustrative examples.
INDUCTION OF BRAIN-REACTIVE ANTIBODIES
Brain-reactive antibodies arise in three situations. First, individuals with autoimmune disease may produce brain-reactive antibodies (Table 1). In these situations, one might expect to observe features common to many known autoimmune diseases, such as a human leukocyte antigen (HLA) association and additional genetic susceptibility including other autoimmune risk alleles. Neuropsychiatric lupus represents this type of disease. The disease is associated with particular HLA haplotypes, and affected individuals harbor other autoimmune risk alleles as well. Anti-DNA antibodies and anti–ribosomal P autoantibodies are part of the pathologic reaction of the immune system and have been shown to cross-react with neuronal antigens (see below). Similarly, neuromyelitis optica (NMO) is an autoimmune disease in which pathology is antibody dependent, with autoantibodies selectively targeting the astrocytic aquaporin-4 (AQP4) water channel protein. Importantly, the immune responses that trigger the generation of these autoantibodies may be initiated by microbial or other exogenous antigens, but in the autoimmune host they continue after the exogenous antigen is no longer present. Second, exposure to exogenous antigens, most clearly microbial antigen but perhaps also food antigen, may trigger expression of antibodies that cross-react with brain antigens (Table 1). In general, genetic susceptibility in these diseases either is of less importance or has not yet been characterized. Examples include Sydenham's chorea, a neurological manifestation of rheumatic fever in which antibodies that emerge against Group A β-hemolytic streptococcus (GAS) cross-react with neuronal targets. Finally, individuals with diagnosed or cryptic tumors may exhibit paraneoplastic syndromes in which the tumor expresses an inciting antigen and the resulting antibodies cross-react with an identical or structurally homologous antigen in the brain (Table 2).
Table 1.
Antibody-related disorders of the central nervous system
| Disorder | Defined antigen | Ab in CSF | Ab useful in diagnosis | Clinical responsea | Ab relevant to disease mechanismb | Mechanism | Subcellular site of action | Etiology |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HAM/tropical spastic paraparesis |
hnRNP A1 (244) |
Yes (245) | ND | ND | ND | Inhibits neuronal activity (246) |
Intracellular | Molecular mimicry (246) |
|
| ||||||||
| Neuromyelitis optica |
AQP4(150, 151, 171) |
Yes (171, 247) |
Yes (154) | Yes (248), suppression |
Yes (152, 249) | Receptor-mediated internalization; complement- mediated toxicity |
Extracellular | Autoimmunity |
|
| ||||||||
| Acute disseminated encephalomyelitis |
MOG (138) | Yes (138, 139) |
Yes (250) | Yes (251–253), modulation |
Yes (254) | Complement- mediated demyelination |
Extracellular | Autoimmunity |
|
| ||||||||
| Systemic lupus erythematosus |
NR2A/NR2B | Yes (106–108, 255, 256) |
Yes (103–108, 255, 256) |
Yes (106, 107) |
Yes (2, 100, 257) |
Receptor modulation, apoptosis (50, 100, 101) |
Extracellular | Autoimmunity |
|
| ||||||||
| Neuronal surface P antigen (116) |
Yes (114) | Yes (258) | ND | Yes (116) | Ca2+ influx, apoptosis (116) |
Extracellular | Autoimmunity | |
|
| ||||||||
| Poststreptococcal movement disorders, Sydenham's chorea, and PANDAS |
Lysoganglioside dopamine D2 receptor |
Yes (199, 215, 216) |
Yes | Yes (218) | Yes (213, 214, 217) |
Aberrant cell signaling, neurotransmitter release (216, 259) |
Extracellular |
Molecular mimicry |
| Tubulin (199, 215, 216, 259) |
Intracellular | |||||||
|
| ||||||||
| Celiac disease | Synapsin 1 (260) |
Yes | ND | Yes | Yes (260) | ND | Intracellular | Autoimmunity/ molecular mimicry |
|
| ||||||||
| Transglutaminase | ND | Yes (261) | Yes (262) | ND | ND | Intracellular | Autoimmunity | |
|
| ||||||||
| Autism | ND (238, 239) | ND | ND | ND (263) | ND (239) | ND | ND | ND |
|
| ||||||||
| Limbic encephalitis |
AMPAR (GluR1, GluR2) |
Yes | Yes | Yes | Yes | Altered receptor location (264) |
Extracellular | Autoimmunity |
|
| ||||||||
| NMDAR (265) [NR1/NR2B (224)] |
Yes | Yes | Yes | Yes | Receptor internalization (224) |
Extracellular | Autoimmunity | |
|
| ||||||||
| Lgi1 (24) | Yes (264) | Yes (24) | Yes (24, 266) | ND | ND (24) | Extracellular | Autoimmunity | |
|
| ||||||||
| Rasmussen encephalitis |
GluR3 (267) | Yes | Yes | Yes (268, 269) |
Yes (269) | Complement- mediated toxicity (270) |
Extracellular | Autoimmunity |
|
| ||||||||
| Hashimoto's encephalitis |
Aldehyde reductase (271) |
Yes (271) | ND | ND | ND | ND | Intracellular |
Autoimmunity |
| Thyroglobulin (271, 272) |
Extracellular | |||||||
|
| ||||||||
| Encephalitis lethargica |
ND | Yes (273) | ND | ND | ND | ND | ND | Autoimmunity |
|
| ||||||||
| Stiff-person syndrome |
GAD (274) | Yes (275, 276) |
Yes (233) | Yes (274) | Yes (233) | ND | Intracellular | Autoimmunity |
|
| ||||||||
| Gephryin (275) |
Yes | Yes | Yes | Yes | ND | Intracellular |
Autoimmunity | |
| GABA(B) receptor (277) |
Extracellular | |||||||
|
| ||||||||
| Amphiphysin (233) |
Yes | Yes | Yes | Yes | Synaptic inhibition (233) |
Intracellular | Autoimmunity | |
Abbreviations: Ab, antibody; AMPAR, α-amino-3-hydroxy-5-methyl-4–isoxazolepropionic acid receptor; mGluR, metabotropic glutamate receptor; AQP4, astrocytic aquaporin-4 water channels; CSF, cerebrospinal fluid; GABA, γ-aminobutyric acid; GAD, glutamic acid decarboxylase; HAM, human T-lymphotropic virus type 1–associated myelopathy; hnRNP A1, heterogeneous ribonucleoprotein A1; Lgi1, leucine-rich, glioma-inactivated 1; ND, not determined; NMDAR, N-methyl-d-aspartate receptor; SCLC, small-cell lung cancer; NR1, NR2A, and NR2B, subunits of the NMDAR; MOG; myelin oligodendrocyte glycoprotein; PANDAS, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections.
Is there a clinical response to immune modulation or suppression? Note that the treatment response is not based on randomized controlled trials.
Includes active immunization and passive immunization models and maternal transfer.
Table 2.
Antibody-related paraneoplastic disorders of the central nervous systema
| Associated malignancy | Defined antigen | Auto-Ab in CSF | Ab useful in diagnosis | Ab mechanism | Clinical responseb |
|---|---|---|---|---|---|
|
| |||||
| Intracellular antigens | |||||
|
| |||||
| Breast, SCLC | Amphiphysin/synaptic vesicle | Yes | Yes | Synaptic inhibition (233) | Yes |
|
| |||||
| SCLC, thymoma, non-Hodgkin's lymphoma, renal carcinoma | CV2/CRMP5 | Yes | Yes | ND | Yes |
|
| |||||
| Thymoma, SCLC, non-SCLC, pancreatic carcinoma | GAD (GAD65, GAD67) (278, 279), gephyrin (275) | Yes | Yes | ND (279) | Yes (274) |
|
| |||||
| SCLC, non-SCLC, breast, prostate, ovary, bladder | Hu (ANNA1) | Yes | Yes | ND (280–282) | Yes (283) |
|
| |||||
| Germ cell (testis), SCLC, breast | Ma | ND | Yes | ND | Yes (284) |
|
| |||||
| SCLC | Recoverin | ND | Yes | Increased intracellular Ca2+ (285) | ND |
|
| |||||
| Ovary, breast, SCLC, thymic carcinoma | Ri (ANNA2) | Yes | Yes | ND | ND (286) |
|
| |||||
| Hodgkin's lymphoma | Tr (PCA1) | ND | Yes | ND | Yes (287) |
|
| |||||
| Ovary, breast | Yo (PCA1) | ND | Yes | ND | ND (288) |
|
| |||||
| SCLC, ovary | 2ic4 (PCA1) | Yes (284) | Yes (289) | ND | ND |
|
| |||||
| Membrane antigens | |||||
|
| |||||
| Hodgkin's lymphoma, SCLC, non-SCLC, thymoma, thymic carcinoma, breast | AMPAR, mGluR1, mGluR2, and mGluR5 | Yes | Yes | Decreased AMPAR density (264) | Yes (264) |
|
| |||||
| SCLC | GABA (B1) | Yes (277) | Yes | Yes | Yes (277) |
|
| |||||
| Ovary dermoid, teratoma | NMDAR (290) [NR2A/NR2B (222)] | Yes (290) | Yes | Irreversible loss of NMDAR (224) | Yes (290) |
|
| |||||
| SCLC, thymoma, endometrial carcinoma (rare) | Caspr1 (24) | Yes | Yes (24) | ND (24) | Yes (24) |
Abbreviations: Ab, antibody; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ANNA, neuronal nuclear antibody; Caspr1, contactin-associated protein-like 1 (component of the voltage-gated potassium channel); CRMP5, collapsin response mediator protein 5 [the same antigen as CV2 (291)]; CSF, cerebrospinal fluid; GABA, γ-aminobutyric acid; GAD, glutamate acid decarboxylase; mGluR, metabotropic glutamate receptor; PCA, Purkinje cell cytoplasmic antibody; NMDAR, N-methyl-d-aspartate receptor; SCLC, small-cell lung cancer.
These autoantibodies are found in the serum and often in CSF, and they are critical in early diagnosis when tumors may be difficult to detect. In many situations, especially for autoantibodies to membrane antigens, symptoms are plausibly caused by regional autoantibody binding. Clinical response to immune modulation is often ineffective, especially with movement disorders, and the optimal treatment is tumor removal or eradication.
Is there a response to immune modulation? Note that the treatment response is not based on randomized controlled trials.
In individuals with anti-brain reactivity that results from a more generalized defect in immune tolerance, as occurs with autoimmunity, autoantibody production may be sustained or may have a relapsing and remitting course. Entry of antibody into brain tissue may be intermittent, making the progression of symptomatology intermittent. It might be expected that antibodies that arise in diseases with clear antigenic triggers (e.g., exogenous microbial or dietary antigens or tumor antigens) disappear once the inciting trigger is no longer present; thus, eradication of the exogenous trigger (through antibiotics, dietary change, or a protective immune response) or destruction of a tumor (through surgical, pharmacologic, or radiotherapeutic intervention) would be expected to terminate autoantibody production. This is often observed. Once the source of exogenous or tumor antigen is eliminated, the production of cross-reactive, brain-reactive antibodies ceases, although this response may depend on the thoroughness of the elimination. Whether clinical symptoms remit when autoantibodies cease to be present will depend on the reversibility of the pathologic process initiated by the antibodies. In some cases, there will be full restoration of brain function; in others, neuronal death and/or permanent circuit impairment may preclude full restoration of function.
It should be noted that the activation of B cells to secrete brain-reactive antibody has not been extensively studied, but also that there is no evidence that the pathway of B cell activation is unique in these cases. Indeed, where B cell activation has been characterized, there is evidence of germinal center maturation of B cells, based primarily on molecular characteristics of the antibodies (3, 4).
B CELL SELECTION AND BRAIN REACTIVITY
B cells produce antibody molecules to fulfill two critical functions: (a) to assist in the removal of cellular debris and (b) to neutralize and destroy invading pathogens and their toxins. The first function is mediated by a relatively small number of antibodies. There is no evidence that these antibodies must undergo class-switch recombination from IgM to IgG or that immunologic memory must develop for these antibodies to assist in the clearance of apoptotic debris. To protect against pathogenic microorganisms, in contrast, a large spectrum of antibodies is required. This generation of antibody diversity is possible because antibody genes are formed through combinatorial events that allow a relatively small number of gene segments to assort into a vast number of intact antibody genes. Because the combinatorial process is not informed by any knowledge of the world of self or exogenous antigen, censoring processes that remove autoreactivity from the B cell repertoire must be in place. Once B cells encounter antigen, antigen-specific T cells help them differentiate further to a germinal center response in which immunoglobulin genes undergo class-switch recombination and somatic hypermutation. The latter process again generates autospecificities that need to be eliminated through negative selection mechanisms.
It is reasonable to ask whether failure to censor autoreactive B cells through central and peripheral tolerance mechanisms is more or less likely to occur for brain-reactive B cells. Exchange of soluble molecules and cells between the central nervous system (CNS) and other organs and compartments is highly restricted due to multiple mechanisms including the existence of the BBB (5). Recent evidence suggests that these mechanisms may be relatively well developed by the time the highly diverse B cell repertoire is generated (6, 7); thus, it is likely that many brain-specific antigens are sequestered from immature or transitional B cells that are at a stage of maturation when they can still undergo negative selection and from post–germinal center B cells, which also undergo negative selection. This sequestration may explain why most brain-reactive antibodies appear to target antigens expressed by neurons or astrocytes rather than antigens expressed by microglia (CNS-resident myeloid cells), the latter of which are often also expressed by other myeloid-lineage cells and are therefore available to mediate negative selection of B cells. It is highly likely that neurons and astrocytes express a large number of antigens that are not appreciably expressed in other tissues or that they express a brain-specific isoform or a novel posttranslational modification of a more widely expressed antigen. There is, in fact, little pressure to censor brain-reactive B cells; the presence of CNS-reactive antibodies is, in general, harmless to the host. It is only when BBB integrity is compromised that there is a potential for antibody-mediated pathology.
It is interesting to speculate that just as there may be little need to remove brain-reactive B cells, the removal of all brain-reactive B cells may be deleterious, leaving the host with too restricted a repertoire for optimal protection against infection and toxins. This theoretical construct proposes two critical functions for the BBB and related features of CNS immunity: (a) restricting negative selection against brain-specific antigens to permit an expanded B cell repertoire and (b) shielding the CNS from a potential onslaught of neuropathic antibodies once brain-reactive B cells are activated.
Importantly, these considerations apply only to antibodies directed against CNS antigens. Antibodies to peripheral nerve antigens do not need to penetrate the BBB to mediate pathogenic effects, nor are the antigens of the peripheral nervous system necessarily sequestered from the developing B cell repertoire.
IMMUNE FUNCTION AND THE BRAIN
The CNS has an intricate relationship with the immune system; cytokines maintain homeostasis of neuronal activation, and synaptic and neuronal activities exert powerful regulatory control over many aspects of systemic immunity. CNS immune homeostasis is skewed toward quiescence and tolerance under healthy conditions, and specific anatomical structures, including the BBB, limit interactions between the CNS and the systemic immune system. This phenomenon was originally referred to as immune privilege, although the concept may have subsequently been interpreted more broadly than is accurate (5, 8). In fact, pathways exist for activation of immune cells within the CNS and for interactions with circulating immune cells and soluble factors, including B cells and antibodies. CNS circuits are highly sensitive to these pathways.
It is interesting to consider what evolutionary pressures may have given rise to the anatomical and functional characteristics of CNS immunity. The relative quiescence that characterizes CNS-resident immune cells in healthy individuals, with low neuronal expression of major histocompatibility complex (MHC) antigens, may have evolved as a mea sure to limit inflammatory damage in an organ with little regenerative capacity and extraordinary ongoing metabolic demand. The CNS has a crucial and highly conserved role in orchestrating systemic immune responses (9–11), which contrasts with the restricted immune response that occurs within the brain. It signals primarily through nerves rather than by releasing molecules and cells into the circulation, and it does not generally require a porous vasculature to communicate with other organ systems.
The concept of the immune privilege of the CNS refers strictly to the parenchyma of the CNS. The parenchyma is defined as the CNS tissue that does not immediately contact blood vessels, meninges, or ventricles and that is not localized within a circumventricular organ. This concept was initially elaborated on the basis of classic studies in which a graft implanted within CNS tissue failed to elicit the immune response that was triggered when the graft was implanted in other regions of the body (5). Immunoglobulin concentrations within the CNS parenchyma are extremely low. MHC expression, a requirement for T effector cell function, is low on cells in the CNS under normal conditions (5). Passage of immune cells between the brain and the circulation is very restricted. Microglia are CNS-resident myeloid cells; unlike other myeloid cells, they are not derived from bone marrow precursors but are descended from primitive macrophages that invade the neural tube early in development, and they are replenished by self-replication in the adult (12, 13). Although microglia express many markers of circulating myeloid cells and can be activated by a variety of stimuli to adopt a macrophage-like phenotype, they are characterized in their resting state by a limited capacity for antigen presentation and phagocytosis (13). It is thought that the CNS parenchyma either expresses many antigens that are not found to an appreciable degree in other tissues or expresses a given antigen in a form not present in other tissues. The lack in the CNS parenchyma of a constitutively active antigen-presenting cell capable of migrating to lymphoid organs and the limited passage of soluble antigens into the blood or lymph are likely mechanisms for a lack of negative selection against CNS antigens. In particular, there is no lymphatic drainage in the CNS, only the passage of interstitial fluid into the CSF and then into lymphatic vessels. The unique nature of the CNS immune environment suggests that molecules of the immune system that are present in the brain are involved in different processes in the CNS than in other tissues. For example, early complement components in most tissues are proinflammatory and can initiate a significant recruitment of a myeloid response (14); however, early complement components in the CNS exert important effects on neuronal synapse development (15, 16). Similarly, cytokines, which are proinflammatory in peripheral organs, regulate synaptic activity in the CNS (17–19).
ANTIBODY EFFECTOR MECHANISMS
Antibody effector mechanisms are in general mediated by the fragment crystallizable (Fc) region of Ig. Fc-mediated effector functions of antibody include the activation of complement with the generation of inflammatory mediators such as C5a and the recruitment of the membrane attack complex, which leads to destruction of targeted cells. Thus, complement activation routinely results in both the targeted elimination of pathogens and the generation of a local proinflammatory milieu to further activate distinct mechanisms of immune activity. The Fc region of antibody can also engage Fc receptors, activate Fc receptor–bearing cells, initiate antibody-dependent, cell-mediated cytotoxicity, and trigger the release of additional inflammatory mediators. These processes also lead to the elimination of a targeted cell or pathogen and induce local inflammation.
In principle, all these mechanisms might occur in the CNS, but several would be operative only under inflammatory conditions. Early complement components are important in neural development and in normal homeostasis in the brain. Late complement components that constitute the membrane attack complex are produced by multiple CNS cell types in tissue culture and in vivo (20, 21), but the relevance of complement activation for pathologic in vivo physiology is unclear. Microglial cells are the major phagocytic cell population of the CNS and bear complement receptors, but their phagocytic capacity is limited in the resting state, and it is unclear whether they ingest complement-opsonized cells (15, 21–23). In general, the only Fc receptor that microglia express is FcRIIB, the inhibitory Fc receptor. Under inflammatory conditions, microglia can be induced to express activating Fc receptors, but whether there are neurological conditions characterized by antibody-mediated phagocytosis of CNS cells or cellular elements is not yet determined (22). A similar question exists with respect to antibody-dependent cytotoxicity; it is not clear whether this mechanism of cell loss and initiation of local inflammation occurs in the brain under any pathologic condition.
The fragment antigen-binding (Fab) region of the antibody can mediate pathology through neutralization or alteration of function of targeted molecules. Antibodies to cellular receptors can activate intracellular signaling cascades, thereby modulating neuronal function or triggering apoptotic pathways. Indeed, most brain-reactive antibodies with identified mechanisms of injury seem to operate in this fashion. Moreover, there are now data demonstrating that antibodies can perturb neuronal function through binding to neurotransmitter receptors and mediating receptor internalization. Antibodies to cell surface molecules can also block normal cellular interactions with soluble molecules, other cells, or extracellular matrix components and can thereby change neural function or neural circuitry. Examples from systemic lupus erythematosus (SLE), NMO, Sydenham's chorea, and paraneoplastic disease are described below. Although antibodies in other tissues in the body can also alter cell function, many autoantibodies mediate pathogenicity through complement- or Fc-mediated effector functions. In the brain, these effector functions appear to be much less central to antibody function.
Because Fc receptor engagement and complement activation seem to have limited relevance to antibody pathogenicity in the brain, the importance of defining immunoglobulin class and subclass of brain-reactive antibodies may be more relevant to gaining insight into B cell activation than to identifying particularly pathogenic antibody subsets. Antibody fine specificity is especially relevant to brain-reactive antibodies. For example, patients' antibodies to AQP4, which characterize NMO, do not routinely bind AQP4 present on nonastrocytic cells such as renal collecting duct epithelial cells. Moreover, antibodies to the obligatory NR1 subunit of N-methyl-D-aspartate receptor (NMDAR) that are present in paraneoplastic disease do not bind NR1 on colonic epithelium, platelets, or other NMDAR-expressing cells (see below). It is not yet known whether the selective binding of antibodies to antigen in the brain when the antigen may be expressed in many organs reflects a conformational difference caused by its presence within a macromolecular complex, an isoform difference, posttranslational modifications, or the induction of the antigen on nonbrain tissue only under particular circumstances. Unsurprisingly, however, only brain-specific epitopes that are recognized as epitopes present on other cell types might be expected to mediate negative selection.
Some antibodies implicated in brain disease bind intracellular antigens. Whether these antibodies possess a mechanism to enter the cell or an as yet unrecognized cross-reactivity to an extracellular or membrane antigen is not known. Several such cross-reactivities have been identified. In these cases, pathogenicity appears to reflect cross-reactivity with a membrane antigen as opposed to reactivity with intracellular antigen.
Importantly, both the antibody's mechanism of action and its local concentration determine whether the antibody-mediated symptoms are permanent or reversible. As described below, some antibodies may modulate function when present at one concentration and trigger apoptosis when present at greater concentration. It is also possible that in some situations autoantibodies do not act alone: T cells—possibly specific for the same autoantigen—may also cause tissue damage or secrete cytokines that synergize with autoantibody in causing cell damage.
ASSAYS FOR THE IDENTIFICATION OF BRAIN-REACTIVE ANTIBODIES
Assays to identify brain-reactive antibodies are crucial to the detection of antibody-mediated diseases of the brain. They include enzyme-linked immunosorbent assays (ELISAs) using purified antigen, Western blots of brain or brain cell–specific lysates, and cell-based as-says using an antigen-negative cell line (such as human embryonic kidney cells) transfected with cell surface brain antigen to permit fluorescence detection of antibody binding (24). All these approaches allow a qualitative determination of antibody titer as well as a determination of antigenic specificity. The ELISA assay is high throughput, inexpensive, and rapid. The Western blots often display multiple bands even with normal sera, complicating their interpretation. In principle, Western blots and cell-based assays can be adapted to antigen discovery, but the latter have not yet been used for this purpose. The validity of all these approaches requires that the antigen, either bound to a solid surface or expressed on a cell other than a neuron or astrocyte, preserve the configuration that it has in the CNS. It is therefore possible that antibodies that bind brain antigens will fail to bind antigen in an ELISA, a Western blot, or a cell-based assay. It is also possible to look for brain-reactive specificity using immunohistochemistry on brain tissue. This approach permits an investigation of regional specificity of antibody binding but does not lend itself to the identification of antigenic specificity.
To determine whether brain-reactive antibodies are causal for brain pathology, injection of antibody into brain tissue or into CSF has been used. Alternatively, antibodies have been added to cultured cells to demonstrate their toxicity or ability to alter cell function. In animal models, antibody pathogenicity is confirmed when (a) transfer of serum or purified antibody or (b) active immunization with antigen (to produce serum antibodies with the defined antigenic specificity) leads to functional or structural damage following breach of the BBB.
THE BLOOD-BRAIN BARRIER
It is estimated that the human brain contains up to ten billion capillaries, a number that roughly translates into one vessel for each neuron (25). Thus, the extraordinarily dense neurovascular tree represents a critical interface between the circulation and the CNS. CNS endothelial cells exhibit several unique features that are critical for maintaining CNS homeostasis. The neurovasculature consists of arteries, characterized by the presence of smooth muscle; capillary microvessels, which constitute the great majority of the neurovascular surface; postcapillary venules, which are generally larger and are characterized by distinct perivascular structures (Figure 1); and larger veins such as the sinuses. The capillary surfaces in the CNS form a rigid barrier, preventing molecules from diffusing between the CNS parenchyma and the blood, and join with the glial limitans to be characterized as a two-walled castle moat (26, 27). Barrier function is mediated by tight junctions between the endothelial cells. The sealing action of these junctions creates a size-selective and effective (but not absolute) barrier to diffusion of nonlipid molecules. The tight junction is maintained by proteins from three families (claudins, occludins, and junctional adhesion molecules) and stabilized by associated adhesion and extracellular matrix proteins (28–30). Thus far, the only individual protein shown to be necessary for functional BBB tight junctions is claudin-5 (28, 31). Soluble factors routinely penetrate tissues through a transcellular route by binding to luminal receptors on the endothelial cell and trafficking through the cell in a process known as transcytosis. This process is highly restricted in healthy CNS endothelial cells (32, 33). Of particular note, endothelial cells of the brain microvasculature express the neonatal Fc receptor for immunoglobulin (FcRn), as do endothelial cells throughout the body. In most tissues, these receptors are polarized to the luminal surface of the cell, where they regulate immunoglobulin half-life and permit immunoglobulin to penetrate tissue (34). In contrast, in brain microvasculature, FcRn has abluminal localization and may actually export immunoglobulin from the brain back into the circulation (34–36). Endothelial cells also routinely participate in the recruitment of circulating leukocytes into tissue. In the CNS, the passage of leukocytes across the endothelial wall is highly restricted (37) due to a specific pattern of adhesion molecule expression on brain endothelial cells (38, 39).
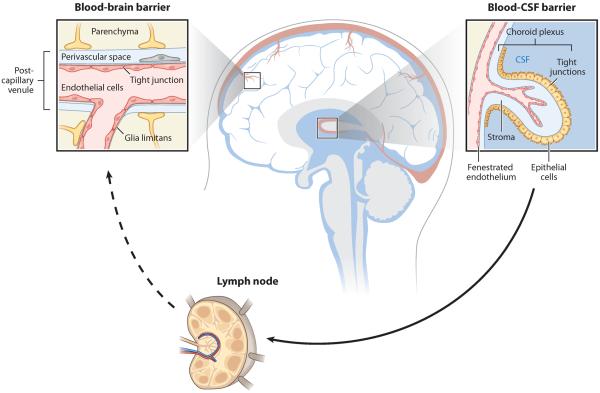
Figure 1.
Anatomical organization of the neurovascular and cerebroventricular systems of the central nervous system (CNS). Most CNS tissue is vascularized by a dense network of blood vessels that exhibit characteristic cellular and physiological features of the blood-brain barrier (BBB). The BBB thus constitutes a critically important interface between the CNS and systemic immunity. Other important interfaces between the CNS and the immune system exist within the ventricular system. Within the circumventricular organs, endothelial cells form a fenestrated capillary network. Within the choroid plexus, specialized epithelial cells filter blood to generate cerebrospinal fluid (CSF), an interstitial fluid that fills the ventricles and the meninges of the CNS. These epithelial cells exhibit barrier properties, including tight junctions, similar to those at the BBB. The CSF communicates with the perivascular space surrounding CNS blood vessels and drains into the lymphatic and venous circulation.
All CNS vessels are enveloped by basement membranes, generated by both endothelial cells and astrocytes (40). Astrocytic foot processes abut the abluminal surface of the endothelial cells and regulate barrier properties (40). Pericytes and other CNS-resident vascular cell types also induce endothelial barrier properties (41). Most of the neurovascular surface is composed of capillary microvessels, where solute exchange occurs in both healthy and pathologic states. Within postcapillary venules, the astrocytic and endothelial basement membranes are distinct, forming a perivascular space containing perivascular macrophages, which unlike microglia are thought to be bone marrow derived, constitutively capable of phagocytosis, and possibly constitutively antigen presenting (8). Soluble factors such as immunoglobulins are thought to enter the perivascular space more easily than the CNS parenchyma; within the perivascular space, these factors may be bound or scavenged by macrophages or may, under certain circumstances, progress into the CNS parenchyma (8). The perivascular space is a preferential site for lymphocytes to infiltrate and accumulate in the CNS (37). To directly alter brain function, antibodies must either traverse the BBB or be synthesized within the brain. In contrast, other immune effector molecules such as cytokines may alter brain function by activating afferent nerves or establishing inflammatory cascades that move from regions lacking a BBB to cells throughout the brain.
In the event of CNS infection, pathogens must be countered and contained despite the risk of inflammatory injury. Although the CNS does not have a conventional lymphatic system, the leptomeningeal and ventricular systems function as a primitive lymphatic organ (5). The cavities of the meninges, which line the outer surface of the CNS, are continuous with the ventricles, which drain into the cervical lymph (Figure 1). These cavities are demarcated from the CNS parenchyma by ependymal membranes and contain CSF elaborated by the choroid plexus. Although antigens of the CNS parenchyma are not actively sampled by antigen-presenting cells, the leptomeningeal and ventricular systems contain immune cells, including dendritic cells and B cells, that are comparable to those found in most tissues (42). Within the choroid plexus, endothelial cells do not present tight junctions. Whereas the passage of soluble factors from the blood to the CSF is restricted by tight junctions between epithelial cells, the passage of soluble factors from the CSF to the brain parenchyma occurs relatively easily (8, 43, 44). Within a few additional specialized CNS regions known as circumventricular organs, the typical tight junction morphology of CNS endothelial cells is replaced by a fenestrated, porous capillary structure that allows passage of soluble factors to and from the blood (45–47). The capillary structure of these regions may be more permissive of autoantibody-mediated pathology, as has been proposed in the case of NMO (48). (For reviews of BBB and CSF structures and functions, see References 8, 43, and 44.)
When the IgG in CSF appears to be polyclonal, it likely originates within secondary lymphoid organs and penetrates the BBB. When the IgG in CSF appears to be oligoclonal, as in MS (see below) and other CNS autoimmune diseases, the antibodies are likely synthesized by B cells that have penetrated within the brain.
Development of the Blood-Brain Barrier and CNS Immunity
Developing neural circuits can be profoundly and irreversibly affected by molecules present in the maternal circulation, including antibodies (49, 50). The developmental time course of CNS barrier formation is thus a critical factor in normal embryonic development. Only recently have some of the molecular pathways in BBB formation been elucidated. The specialized phenotype of CNS endothelial cells is not cell intrinsic but rather is induced by the CNS environment. Astrocytes were initially identified as a cellular source of barrier-inducing factors in the mature CNS (40); furthermore, Sonic hedgehog, likely of astrocytic origin, was shown to induce multiple features of the CNS endothelial phenotype in vivo and in vitro (51). However, CNS endothelial cells appear to exhibit tight junctions even in the developing neural tube, before the development of astrocytes. Pericytes, which are associated with vessels at early time points, have been identified as critical sources of BBB-inducing signals during embryogenesis and also in the aging brain (6, 41, 52). Surprisingly, neural progenitor–derived Wnts also have a role in establishing the CNS endothelial phenotype (53). In humans, the BBB is thought to assume full structural maturity shortly after birth (45), although some regional differences in time of maturation exist, likely because astrocytes develop only postnatally in some brain regions, such as the hippocampus. Although there has been some controversy related to the integrity of the BBB in neonatal rodents, the preponderance of evidence suggests that tight junction expression and some barrier function is evident at early stages of neural tube vascularization (6). Tight junction expression alone, however, is not sufficient for mature BBB function. For example, the MECA-32 antigen is a leukocyte adhesion molecule, highly expressed in peripheral endothelium and lymphatic venules, whose expression is downregulated in CNS endothelial cells; reexpression of the MECA-32 antigen is a proposed marker of BBB breakdown (54). Expression of the MECA-32 antigen is retained in developing CNS vessels even after tight junction proteins are present. Thus, the precise timing of BBB development is incompletely understood. Nevertheless, a convergence of evidence strongly suggests that from an early stage, the BBB does shield the CNS from circulating antibody and that CNS-specific antigens are not present in significant concentrations when and where negative selection of B cells occurs.
Pathologic Disruption of the Blood-Brain Barrier
The BBB is a dynamic system that can be modulated in vivo by physiologically relevant stimuli, such that certain conditions allow for typically nonpermeating molecules such as antibodies to enter brain tissue and alter neural function (55). Paracellular permeability, which affects the size selectivity of tight junctions, and transcytosis are both subject to modulation (26, 56, 57). Although most studies have assumed that large molecules breach the BBB by the paracellular route, recent reports show that compounds including cytokines, chemokines, and antibodies can in some circumstances cross the BBB by the transcellular route using receptor-mediated processes, a phenomenon that may be exploited for drug delivery (Figure 2) (55, 58, 59). Physiological stimuli such as stress, trauma, infection, and inflammation have been proposed to affect BBB function (57, 60–63). These stimuli may differ in the magnitude, timing, compound specificity, and anatomical localization of their effects on the BBB. The most severe form of BBB damage results from rupture or necrosis of vascular tissue following traumatic brain injury or stroke; in addition to the acute physical damage these events cause, long-term BBB disruption may occur as a result of sustained local inflammation (56). Even in the absence of physical injury, inflammatory signals effect disruption of the BBB by activating endothelial cells (32, 64, 65). Endothelial cells can also be activated by vasoactive signaling molecules such as norepinephrine and adenosine. Unsurprisingly, several drugs with brain-related effects (such as nicotine, cocaine, 3,4-methylenedioxymethamphetamine, and tetrahydrocannabinol) also alter the BBB (66). Although most studies have focused on how circulating factors affect BBB integrity, pathologic disruption of the BBB can clearly be initiated by CNS-resident cells. For example, genetic polymorphisms in apolipoprotein E are a major risk factor for Alzheimer's disease and cause BBB disruption in animal models; a recent study pinpointed proinflammatory cytokine secretion by pericytes as the initial insult in this process, which then may be sustained by penetration of proinflammatory circulating factors (67). Many more factors that affect BBB integrity, including other genetic polymorphisms, undoubtedly still await discovery.
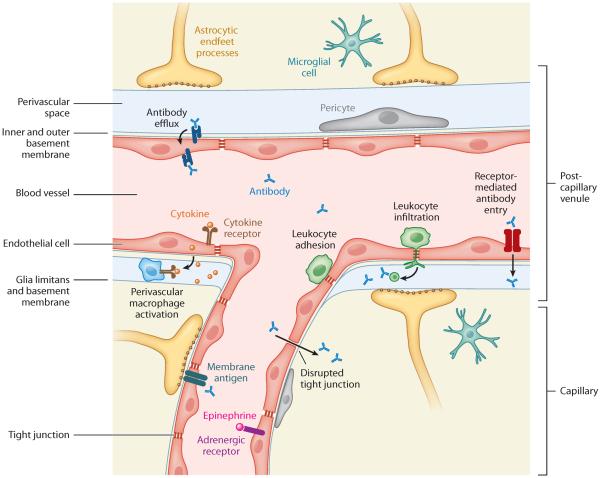
Figure 2.
Diverse mechanisms of antibody entry into the central nervous system (CNS) at the neurovascular interface. Anatomy and cell types: The neurovascular network forms a dense and intricate organ system in which numerous specialized cell types cooperate to form distinct classes of blood vessels with unique functions in regulating antibody access to the CNS parenchyma. The immediate barrier for preventing antibody entry into the CNS is the blood-brain barrier formed by CNS endothelial cells. These cells form tight junctions that restrict paracellular diffusion of soluble factors and exhibit limited transcytosis and a relatively limited propensity for leukocyte adhesion under resting conditions. Astrocytes surround the basal surfaces of vessels with their foot processes and basement membranes. Most of the neurovascular surface area is composed of capillary microvessels, in which exchange of soluble factors occurs and in which astrocyte-derived membranes are apposed to endothelial-derived basement membranes. In larger postcapillary venules, a distinct perivascular space is observed between the astrocytic endfeet and the endothelial wall, where resident cells include pericytes (which induce barrier properties) and perivascular macrophages (which are thought to transduce inflammatory signals). Possible mechanisms of antibody entry: Under normal physiological conditions, entry of immunoglobulins into the CNS is negligible. This restriction may be disrupted or bypassed under certain pathologic circumstances. Activation of CNS endothelial cells can result from activation of luminal receptors, including Toll-like receptors, cytokine receptors, and hormone receptors. Activation of perivascular macrophages might result from endothelial cell activation, which can further enhance disruption of tight junctions, permitting paracellular diffusion, increasing transcellular transport of soluble factors, and upregulating leukocyte adhesion molecules. Leukocyte adhesion to activated endothelial cells is likely to amplify relevant inflammatory signals, even in the absence of overt transmigration into the CNS. These processes are more likely to occur in brain microvascular capillaries. CNS inflammation may also cause inside-out disruption of the BBB. Antibodies with specificity for cell surface molecules expressed by endothelial cells or perhaps by other neurovascular cells may enter the CNS by antigen binding and engaging transcytotic trafficking through receptor-mediated antibody entry. Leukocyte infiltration may result in local antibody production by B lymphocytes and a locally disrupted BBB. Finally, antibody penetration into the CNS may be counteracted by antibody efflux effected by polarized FcRn (neonatal Fc receptor for immunoglobulin) on endothelial cells.
The actual mechanism of barrier breach, as opposed to general conditions that are correlated with barrier breach, is still quite unclear (55). One particularly elegant study recently provided insight into the mechanism of action of adrenergic receptors at the BBB in the context of meningococcal infection. In this instance, the bacteria effectively hijack β-arrestin-dependent trafficking—a central arm of adrenoreceptor signaling—to sequester adhesion molecules away from the tight junction and alter overall endothelial cell morphology (57). It is attractive to hypothesize that similar mechanisms may function in vivo in other conditions as well.
Several mechanisms have been identified that permit blood-borne molecules to enter or signal to CNS parenchyma (Figure 2). Activation of endothelial cells on the luminal surface can lead to abluminal secretion of soluble factors, including prostaglandins (68) and nitric oxide, that then alter neuronal function. In this way, endothelial cell activation may not alter barrier properties but will nonetheless lead to the presence of inflammatory mediators within the brain (65), perhaps affecting BBB function secondarily. Metalloproteinases are secreted by activated endothelial cells and can degrade extracellular matrix, resulting in BBB breakdown (25). Additionally, peripheral nerves could in principle transmit signals to the CNS that would alter endothelial cell barrier function from the inside out. Other mechanisms of barrier breach have also been demonstrated. For example, in HIV infection, nanotubules appear to connect the capillary lumen with brain tissue (69); the mechanism for nanotubule formation is not clear. Microglial and/or astrocytic foot processes are also suggested to penetrate between endothelial cells to reach the capillary lumen and may transmit information directly from the circulation to the brain (70). Activated leukocytes, notably monocytes and autoreactive T cells, can transmigrate into the CNS during autoimmune pathology, as has been well described for EAE (discussed below). In addition, these cells can adhere to the luminal surface of an endothelial cell without overt transmigration (71), and they are likely involved in region-specific impairment of barrier function for soluble factors, including antibodies, through mechanisms that are not well understood but probably involve local release of cytokines in the vessel lumen (72). Immunoglobulins may also access the CNS by first penetrating into the CSF and then progressing through the ventricular circulation into CNS parenchymal regions; this process has been proposed to be particularly important during development, but its relative importance in antibody-mediated pathology has not been extensively addressed (45, 73).
Recent data demonstrate heterogeneity of brain microvasculature, with surface molecules on endothelial cells showing region-specific expression or density. Antibodies may use these molecules to penetrate the brain though receptor-facilitated transport under both quiescent and inflammatory conditions. For example, brain microvascular endothelial cells express receptors for NMDA and dopamine (69, 74–76). It is possible that these receptors facilitate the transport of receptor-binding antibodies into brain parenchyma. There is a growing awareness of inflammatory molecules, such as interleukin-1, tumor necrosis factor, epinephrine, and complement component C5a, that can bind receptors on endothelial cells and impair barrier integrity (55, 61). Cytokines likely play a crucial role in allowing antibodies to cross the BBB and may, due to a nonhomogeneous distribution of their receptors on brain microvasculature, exhibit regional specificity. Type 1 interferon and corticosteroids are the only substances that clearly enhance barrier function, although some data implicate estrogen as well (55, 61, 77).
ANTIBODIES TO THE PERIPHERAL NERVOUS SYSTEM: PROOF OF PRINCIPLE FOR AUTOANTIBODY-TRIGGERED NEUROLOGICAL DISEASE
Antibody-mediated diseases of peripheral nerves are, in principle, similar to antibody-mediated diseases affecting any organ not sequestered behind a specialized barrier. In the peripheral nervous system, antibody has unimpeded access to its target antigen; thus, the conventional approach to identify an antibody-mediated autoimmune disease applies. These diseases, like antibody-mediated diseases of the CNS, may be autoimmune or may be induced by a microbial pathogen or tumor. The metric for relating an antibody to symptomatology is relatively straightforward and includes (a) the presence of antibody in serum; (b) passive transfer models; and (c) immunohistology of the affected nerve. Many antibodies involved in peripheral nerve disease may not cause brain disease even if transported across the BBB, as the targeted antigen may not be present in the brain.
Autoimmune disorders of the peripheral nervous system were, not surprisingly, the first antibody-mediated diseases of the nervous system to be discovered. Antibodies have long been appreciated as the pathogenic mechanism for the autoimmune disease myasthenia gravis. Myasthenia gravis results from anti–acetylcholine receptor antibodies that act at the neuromuscular junction. These antibodies impede the efficiency of neuromuscular transmission and eventually, in conjunction with complement, flatten and destroy the postsynaptic membrane. Transmission of disease from affected mother to newborn and by transferring serum from affected individuals to an experimental animal demonstrated that a serum factor, specifically antibody, was responsible for the symptoms (78, 79). Moreover, immunosuppression (in some situations thymectomy) effectively treated the condition. Myasthenia gravis is particularly instructive in that not all individuals with antibody to the acetylcholine receptor experience symptoms. Elegant studies on the neuromuscular junction have suggested that aspects of the junction itself modulate the pathogenicity of the antibodies (60, 63). This mirrors an observation made with respect to many autoimmune diseases: Target tissue has variable susceptibility to autoantibody-mediated damage. This differential susceptibility may reflect genetic factors or, alternatively, epigenetically determined states of the targeted tissue (66).
Molecular mimicry and antimicrobial antibody cross-reactivity with peripheral nerve antigen can also cause antibody-mediated peripheral nerve disease. Guillain-Barré syndrome is an areflexic progressive paralysis caused by ganglioside-specific autoantibodies that often arise following infection with Campylobacter jejuni or, less frequently, Haemophilus influenza. These antibodies bind a microbial antigen, either a lipooligosaccharide or an unusual disialosyl epitope on the bacterial surface (46). Elegant experiments with truncated bacterial cell surface oligosaccharides demonstrated that these ganglioside mimics are required for the induction of neurotoxic antibodies (80, 81). Once the antibodies are present, complement-dependent mechanisms destroy the perisynaptic Schwann cells through the formation of a membrane attack complex (82). Ex vivo experiments show that the motor nerve terminal also appears to be affected, with complement fixation leading to pore formation and unregulated calcium influx, subsequent acetylcholine release, and neuromuscular paralysis. An animal model has been developed by immunization with bacterial antigen, and a passive transfer model has confirmed the toxicity of the human antibodies (83). The severity of disease is not correlated with antibody concentration, suggesting that clinically critical differences exist in antibody fine specificity or target cell vulnerability to antibody-mediated attack (84). These pathogenic antibodies disappear with time as the microbial antigen is eradicated. Clinical symptomatology may persist, however, due to incomplete neuronal repair.
Peripheral nerve disease can also arise as a paraneoplastic syndrome. Antibodies to the calcium channels on small-cell lung carcinoma cells can cross-react with calcium channels on peripheral nerves. These antibodies block channel function and reduce acetylcholine secretion, leading to clinical symptoms akin to those of myasthenia gravis. Importantly, in all of the above examples, the Fab region of the antibody is believed to identify the target cell; subsequent damage is mediated through Fc-dependent mechanisms.
ANTIBODIES TO BRAIN VASCULAR ELEMENTS
Although it is outside the scope of this review, brain pathology can follow antibody-mediated damage to brain vasculature and disruption of blood flow. For example, antiphospholipid antibodies are present in SLE patients and in individuals with primary antiphospholipid syndrome (85). Antiphospholipid antibodies bind to clotting factors and endothelial cells, directly triggering thrombosis or initiating endothelial cell activation and establishing a thrombogenic nidus within a vessel. The actual autoantigens on endothelial cells bound by these antibodies have not been fully defined. These antibodies cause brain damage indirectly by interfering with normal cerebral blood flow. When these antibodies cause hemorrhagic stroke, a breach of BBB integrity gives serum antibodies access to brain tissue at the site of the hemorrhage. When the antibodies cause a nonhemorrhagic stroke, it is not known whether there is a local loss of barrier integrity.
Systemic Lupus Erythematosus Autoantibodies
SLE is an autoimmune disease primarily affecting women in their childbearing years. The disease is characterized by the production of autoantibodies, primarily to nuclear antigens. These antibodies can trigger inflammation in multiple organs, often through cross-reactivity with tissue-specific antigen. The origin of autoantibodies in SLE is an area of active investigation. Some reports suggest that they arise initially in response to microbial antigen but fail to be regulated appropriately. Other reports suggest that from their first appearance they are a consequence of inadequate clearance of apoptotic debris and that they represent an immune response to self-antigen presented in an immunogenic fashion. Many alleles that predispose to SLE also predispose to other autoimmune diseases, and there is strong evidence that some SLE susceptibility alleles reduce the stringency of B cell negative selection. On the basis of current knowledge, some autoantibodies appear to be markers of disease, whereas others have pathogenic potential.
One common class of autoantibodies in SLE is anti-DNA antibodies, present in over 70% of patients. These autoantibodies clearly contribute to tissue damage in the kidney. An extended search for antigens bound by anti-DNA antibodies in glomeruli has led to the identification of several cross-reactive molecules such as heparan sulfate, laminin, and α-actinin (86–88). Thus, anti-DNA antibodies may bind to chromatin adherent and to glomerular basement membrane or to a cross-reactive, non–nucleic acid renal antigen. Studies of renal pathogenesis have led to the notion that cross-reactivity may be central to the pathogenicity of SLE autoantibodies.
Studies of anti-DNA antibody fine specificity resulted in the identification of cross-reactivity with NMDARs (89, 90). R4A, a glomerulotropic mouse anti-DNA antibody, differs by three amino acids from the 52B3 antibody, which is not glomerulotropic despite having a log greater apparent affinity for DNA (91). R4A was therefore ascertained to have a different fine specificity than 52B3. Using a phage display peptide library as a probe for antibody fine specificity, the consensus peptide sequence D/EWD/EYS/G (DWEYS) (92) was identified as a target of R4A.
The potential relevance of the R4A antibody to brain disease became apparent when a protein database revealed that both the rodent and human NR2A and NR2B subunits of the NMDAR contained the consensus sequence. NMDARs are composed of two NR1 subunits and any two of four NR2 (A–D) subunits and are expressed on neurons throughout the CNS. Together with α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors (AMPARs), they are critical for synaptic transmission (93–97). In particular, NMDARs are required for long-term potentiation (98), the physiological mechanism for memory formation in the brain. Overstimulation of NMDARs due to excessive ligand binding leads to high influx of calcium into the cell, causing neuronal dysfunction and, ultimately, cell death. Timing of receptor modulation, dose of excitatory compound, local density, and receptor subunit composition can all affect downstream receptor activity and cell function or viability.
Anti-DNA, antipeptide autoantibodies are present in 40% of patients with SLE and bind both the NR2A and NR2B subunits and the intact NMDAR. Both murine and human anti-DNA, antipeptide antibodies exhibit dose-dependent neurotoxicity when injected directly into rodent brain and when applied to human fetal brain cells in vitro (99, 100). Antibody has also been eluted from a specimen of human postmortem brain. When this antibody was injected directly into mouse brain, it caused neuronal apoptosis (101). Interestingly, these antibodies bind NMDAR on neurons and can immunoprecipitate neuronal NMDARs. Recently, we have demonstrated that they also bind NMDARs on HEK cells doubly transfected with NR1 and NR2A or NR2B.
Although serum levels of DNA/NMDAR-reactive antibodies do not correlate consistently with cognitive or behavioral disorders in SLE patients (102; but see References 103–105), the presence of these antibodies in CSF of SLE patients correlates with neuropsychiatric lupus, specifically with global nonfocal CNS dysfunction. Antibody titers in CSF correlate with symptom severity and, interestingly, decline during convalescence, whereas cytokine levels do not (106–108). The latter observation suggests that antibodies might be present due to penetration of the BBB at a defined moment, whereas cytokines might be secreted locally by resident inflammatory cells.
Mice immunized with an octameric form of the consensus NMDAR peptide on a branched polylysine backbone (109) develop anti-NMDAR, anti-DNA reactivity in serum. Thus, the peptide is a cross-reactive antigen that is a double-stranded DNA mimetope that can break self-tolerance in BALB/c mice and lead to the presence of potentially neurotoxic antibodies in the circulation. Mice harboring these antibodies in the blood are asymptomatic with respect to brain function. Although there is some antibody deposition around the circumventricular organs, the remainder of the brain is histologically normal. Thus, there needs to be an insult to BBB integrity for these antibodies to penetrate brain parenchyma. Although some data exist that NMDARs are present on endothelial cells, they do not appear to appreciably transport antibody to the brain in this model. Lipopolysaccharide (LPS) and epinephrine—agents that breach BBB integrity—lead to cognitive abnormalities and behavioral impairments, respectively, in antibody-bearing mice. Endothelial cells can express Toll-like receptors, but LPS probably compromises barrier integrity through induction of high levels of systemic cytokines rather than through direct effects on endothelial cells (55, 65, 110). Epinephrine causes a transient increase in blood pressure and cerebral blood flow but also binds to adrenergic receptors on cells of the BBB. When either of these agents is present systemically, the DNA/NMDAR-reactive antibody accesses the brain and causes neuronal injury and loss. Animals harboring antibody and treated with LPS exhibit hippocampal neuronal loss and impaired memory function (2). Similarly, in mice, when intravenous administration of human SLE serum is followed by systemic administration of LPS, the animals exhibit loss of hippocampal neurons and memory impairment. If the serum is first depleted of peptide-reactive antibodies, there is no detectable neurotoxicity, confirming that specific antibody is responsible for the cognitive impairment and that the LPS alone does not cause sustained cognitive impairment. When epinephrine is used as the BBB-breaching agent, animals harboring antibodies display neuronal damage in the amygdala and abnormal fear conditioning, but their memory function remains normal (111). Thus, a single autoantibody specificity can cause distinct manifestations that depend on the brain region exposed to antibody. The region of BBB breach is a function of the breaching agent. This observation is critical, as it demonstrates that the same antibody can give rise to various symptom complexes.
Interestingly, there is no detectable influx of blood-borne inflammatory cells into the brain following exposure to either LPS or epinephrine in this model. Thus, neuronal loss is noninflammatory or only mildly inflammatory. In the human disease, slow accumulation of cognitive and behavioral impairment acquired in this manner would not immediately suggest immune-mediated pathology, as it is not necessarily associated with acute inflammation in the CNS. Indeed, many of the insults to barrier integrity that permit antibodies to penetrate brain tissue are not disease specific, and CNS damage in SLE accrues independent of disease activity, further complicating the association of lupus antibodies with brain symptoms. Therefore, even in a disease such as SLE in which tissue injury throughout the body is clearly initiated by autoantibodies, a relationship between antibody and brain symptomatology has only recently been appreciated.
Using the unique opportunities afforded by the ex vivo hippocampal slice preparation, it was possible to demonstrate that both murine and human DNA/NMDAR-reactive antibodies modulate NMDAR function by preferentially binding the active NMDAR configuration, permitting calcium to enter the cell. Presumably, the antibody increases the time the NMDAR remains in this configuration, thereby enhancing calcium influx. The insult to neurons caused by antibody activity is evident in an assessment of mitochondrial membrane potential. Mitochondria in cells exposed to both antibody and glutamate are characterized by increased inner mitochondrial membrane permeability and swelling. Thus, the antibody is functional only in activated synapses; toxicity therefore occurs as a function of antibody concentration, local glutamate concentration, and prior NMDAR activation (100).
These studies further demonstrated that the antibody alters synaptic activity at a concentration tenfold less than is needed to mediate cell death (100). This finding broadens the potential symptomatology related to DNA/NMDAR-reactive antibodies in that some neuronal dysfunction might be reversible, transiently affecting the amplitude of excitatory postsynaptic potentials, whereas other dysfunction might be permanent, reflecting antibody-mediated neuronal loss (100).
To date, experiments in animals in which the DNA/NMDAR-reactive antibodies were generated through immunization or administered by passive transfer indicate that antibody access to brain is a necessary condition for brain pathology. There is no evidence of complement activation in the brain. Indeed, Fab′ 2 fragments of antibody can also cause neuron death when injected into brain, confirming that antibody toxicity can occur unrelated to complement activation or engagement of Fc receptor–bearing cells, and there is no discernible effect of IgG isotype. Moreover, neuronal death occurs without visible inflammation and does not require synergy with complement or cytokines. Because the damage is insidious and not characterized by intense inflammation, the time between actual damage and its detection may be quite variable.
Recent efforts have identified a cross-reactive neuronal antigen for another classic lupus antibody, anti–ribosomal P autoantibody (112). Early studies suggested an association between serum anti–ribosomal P antibody levels and psychosis, but the data were inconsistent and the notion that an antibody to an intracellular antigen could alter cell function raised the perplexing question of how the antibody might access the antigen. Clinical studies that investigate an association between serum antibody and brain disease must address antibody penetration of brain parenchyma. Multiple studies have demonstrated anti–ribosomal P antibody within the CSF of patients with neuropsychiatric lupus (113–115). A plausible mechanism for the brain symptoms induced by anti–ribosomal P antibodies has recently been proposed (116). Anti–ribosomal P antibodies isolated from patients with SLE bind an integral membrane protein on neurons in rodent cortex, hippocampus, amygdala, and stria terminalis (116). Electrophysiological analyses of neurons in culture demonstrate that anti–ribosomal P antibody alters calcium flux and mediates neuronal apoptosis. These antibodies have been administered to mice through intraventricular injection and lead to a behavioral phenotype (117). No doubt, there will be many more antineuronal autoantibodies present in SLE patients that alter neuronal function, and the potential cross-reactivity of classic autoantibodies to brain antigens needs to be investigated.
ANTIBODIES IN THE CLASSIFICATION OF DEMYELINATING DISEASE
MS is the most frequent neurological disease in young adults and confers a high risk of future disability (118). Patients experience recurrent neurological deficits with complete or partial remission or progressive impairment. Multiple focal brain lesions can be detected in various brain regions of the white and gray matter, and how the immune system attacks the myelin sheaths and finally leads to axonal loss, gliosis, and atrophy remains unresolved (119, 120).
The ongoing search to identify an antigen-specific humoral immune response in patients with MS is mainly based on the hallmark finding in 1948 of persistent oligoclonal immunoglobulin bands (OCBs) in the CSF of more than 90% of patients (121). The search has concerned researchers throughout the past century, most recently leading to a presumptive identification of the potassium channel KIR 4.1 as the antigenic target (122). CSF analysis for OCBs was incorporated in the diagnostic criteria of MS (123); however, the antigenic specificity of the OCBs remains unknown, and OCBs are not unique for MS but can also be observed in other CNS inflammatory diseases (123, 124). MS is widely considered to be a CD4+ T cell–mediated autoimmune disease (125), but the dramatic beneficial effect of B cell depletion in patients with relapsing remitting MS (126, 127) was rather surprising and has resulted in a renewed focus on determining the relevance of B cells and antibodies as either active promoters or secondary phenomena in MS (73, 128, 129). Neuropathological findings suggest antibody-mediated damage of oligodendrocytes in early acute MS plaques of approximately 50% of patients (130, 131). On the basis of clonally expanded B lymphocytes present in chronic MS plaques and OCBs in CSF, an oligoclonal population of B cells is believed to infiltrate the CNS compartment, where the B cells secrete antibody (132–134).
The potential contribution of pathogenic antibodies to MS is supported by studies of the EAE animal model, which shares some pathophysiological similarities with the human disease. EAE is typically induced by immunization with myelin components and adjuvant or by adoptive transfer of encephalitogenic CD4+ T cells. Antibodies directed to the myelin oligodendrocyte glycoprotein (MOG) can augment demyelination (73, 122, 128). Anti-MOG antibodies display pathogenic effects in vitro (135). Few studies have described the presence of such antibodies in active MS brain lesions (136, 137). Interestingly, MOG antibodies can be detected in serum of healthy individuals by ELISA and Western blot but not by cell-based assays. Whether access of antibody to brain tissue or antibody fine specificity distinguishes patients from healthy individuals is not known.
Surprisingly, using MOG-transfected cells for antibody screening revealed MOG as a target antigen in approximately 40% of pediatric patients with acute disseminated encephalomyelitis (ADEM) (138–141). High-titer antibodies to MOG are largely absent in adult MS patients but are detected in some patients with AQP4 IgG–seronegative NMO, pediatric MS, and optic neuritis using a cell-based immunofluorescence assay (138, 140–146). ELISA and Western blot assays largely failed to detect these antibodies. These data illustrate the absence of a uniform assay for all brain-reactive antibodies.
NMO was described clinically at the end of the twentieth century as a severe form of MS with acute bilateral optic neuritis and longitudinal extending myelitis spanning three or more vertebral segments (147–149). In 2004, a specific antibody in the serum of patients was identified using a cell-based indirect immunofluorescence assay (150). The antibody proved to be directed against the extracellular domain of the water channel protein AQP4, which is abundantly expressed on astrocytic perivascular end-foot processes (151, 152). Following the discovery of anti-AQP4 IgG, NMO was no longer regarded as a variant of MS but rather as a separate entity (152). The antibody is absent in MS but can be found in some patients with other autoimmune disorders such as Sjögren's syndrome, myasthenia gravis, neuropsychiatric SLE, and thyroiditis (147).
The unique localization of the targeted antigen in close proximity to the BBB and brain-CSF barrier likely facilitates antibody access to target antigen at sites of BBB disruption. Some data even suggest that glial foot processes penetrate to the vascular lumen; subsequent antibody-mediated attack could itself disrupt BBB integrity. Although NMO was initially thought to spare the brain and to have a monophasic course, NMO is now described as a relapsing disease, and MS-atypical brain lesions are present primarily at sites of high AQP4 expression (153). Selective targeting of the antibody to the optic nerve and spinal cord may relate to the dense expression of AQP4 in these regions; however, AQP4 density is not sufficient to explain a lack of cortical pathology in NMO patients, as AQP4 is abundantly concentrated in the cortex (153–156). It needs to be further investigated whether the absence of cortical pathology can be attributed to (a) regional differences in BBB composition or integrity (156–158); (b) the need for region-specific pathogenic epitope; or (c) a requirement for threshold concentration or duration of exposure to antibody.
AQP4 IgG is present in approximately 80% of NMO patients (159). AQP4 is expressed as either the full-length protein (M1-AQP4) or the shorter isoform (M23-AQP4), a splice variant. These form heterotetramers with differing sensitivity to antibody detection (144, 160). Binding of autoantibodies depends on the formation of AQP4 supramolecular structures, termed orthogonal arrays of particles, which are currently considered to be the targets of AQP4 IgG (144, 161). Differential expression of AQP4 isoforms might even influence the mode of action of the antibody. A recent study suggested that AQP4 IgG binding results in internalization of M1-AQP4; in contrast, antibody binding to M23-AQP4 activates the complement system and inhibits AQP4 water permeability (162). It remains unresolved whether AQP4 autoantibodies directly impair water permeability or do so through a more general impairment of astrocyte function (163).
The most notable histological characteristic of NMO is the extensive demyelination across multiple segments involving gray and white matter, with remarkable loss of AQP4 and acute axonal pathology. Demyelination, oligodendrocyte loss, and cytokine release occur most likely as secondary events of the disease (164, 165). Histopathological staining shows that antibodies and active complement products enclose hyalinized and thick-walled blood vessels in a characteristic rosette-like pattern (166). The localization of disease activity at the vessels undoubtedly relates to antibody access to astrocytic foot processes. Besides AQP4, active lesions lack other astrocytic markers such as glial fibrillary acidic protein (GFAP) and the AQP4-associated glutamate transporter 2 (EAAT2). They also are characterized by an infiltration of macrophages, neutrophils, eosinophils, and some CD3+ and CD8+ T cells (167). Selective targeting of the astrocytes is additionally confirmed by the presence of high concentrations of the astrocyte-specific proteins GFAP and S-100B in CSF (168).
An active involvement of AQP4 antibodies is strongly suggested by clinical observations showing a beneficial effect of antibody- or B cell–targeting therapies (169, 170) as well as a correlation of antibody titer levels with disease activity (171) and length of spinal cord lesions (172, 173). A cytotoxic effect of AQP4 IgG has been shown in several in vitro experiments, demonstrating complement-mediated lysis following opsonization of AQP4 transfected cells and AQP4-positive astrocytes (174–176). An ex vivo spinal cord slice model revealed that NMO-like lesions develop in the presence of patients' antibodies and complement, with astrocytosis and loss of myelin (177). The pathogenic potential of the antibody was further demonstrated in vivo by passive transfer of antibodies from AQP4 IgG–positive NMO patients to rodents with EAE, which appears to be a necessary substrate for antibody-mediated pathology. Compared with animals infused with antibodies from AQP4 IgG–negative NMO patients or IgG from MS patients, the AQP4 IgG–treated animals exhibited exacerbated lesions in the CNS with a distribution similar to that seen in NMO patients (178–180). Preabsorption of patients' antibodies with AQP4-transfected cells, as well as in vivo administration of a monoclonal recombinant antibody generated from a B cell within the CSF of a NMO patient, confirmed an AQP4 IgG–specific pathogenic effect (178, 179). Several studies have examined whether AQP4 antibodies are actively internalized through endothelial transcytosis, enter the CNS through circumventricular organs, or require breach of the BBB by pathogenic T cells. Current data emphasize a contributing role of pathogenic AQP4-specific T cells (181) and further suggest that antibodies to human brain microvascular endothelial cells in the serum of NMO patients may contribute to BBB disruption by stimulating the expression of high levels of vascular endothelial growth factor (129).
Yet antibody access to the target antigen may not be sufficient to explain disease pathogenesis, as one study showed absence of NMO pathology following injection of antibodies from NMO patients into juvenile rats despite apparent extravasation of antibody into the CNS (178, 182). It also remains unclear why AQP4 expressed in peripheral tissues such as kidney, lung, skeletal muscle, stomach, and inner ear is not bound by AQP4 antibodies (183–185). Presumably, AQP4 expressed in these tissues does not routinely express the targeted epitope (48, 186, 187).
INDUCTION OF BRAIN-REACTIVE ANTIBODIES BY MICROBIAL PATHOGENS
The GAS Streptococcus pyogenes is associated with the development of postinfectious autoimmune responses in humans. The best studied of these responses is acute rheumatic fever, a collection of inflammatory disorders in which immune activation by streptococcal antigens appears to initiate responses against the heart (carditis), joints (arthritis), skin (erythema marginatum), and/or brain (Sydenham's chorea) (188).
Sydenham's chorea is a delayed neurological complication of GAS infection that occurs in patients, usually under the age of 18. It usually occurs within a few weeks of a GAS infection but can occur up to six months after the acute infection and may persist (189, 190). The disorder is characterized mainly by chorea and other motor symptoms preceded by neuropsychiatric symptoms such as obsessive-compulsive symptoms, anxiety, tics, hyperactivity, and emotional lability (191). In 1998, researchers identified a group of clinically related disorders named PANDAS (pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection) in which the onset of neuropsychiatric symptoms followed GAS infection (192). These conditions lack the presentation of chorea and hence do not meet the diagnostic criteria of Sydenham's chorea.
There is compelling evidence that GAS infection induces antibodies cross-reactive with neuronal antigens through the process of molecular mimicry (193). The basal ganglia is one of the primary targets of these antibodies (194, 195). Husby et al. (196) were the first to describe antibodies in Sydenham's chorea patients that cross-react with basal ganglia tissue. Subsequent studies using immunofluorescence, ELISA, and Western immunoblotting have confirmed this observation (197–201). Neuroimaging studies suggest that basal ganglia abnormalities associate with the active phase of Sydenham's chorea (202) and that these changes revert to normal on recovery (203–207; but see also References 208–210).
Early studies investigating antibody-mediated behavioral alterations focused on direct infusion of patients' serum into rat striatum. These studies, using serum from PANDAS or Sydenham's chorea patients, failed to demonstrate motor or behavioral abnormalities in the injected rats (211, 212). In studies in which mice were immunized with a GAS homogenate, some of the immunized animals developed motor and behavioral disturbances that were correlated with immunoreactivity to the deep cerebellar nuclei (213, 214). Naive mice given serum from immunized mice also developed some behavioral impairments, although the antibodies in these mice targeted the hippocampus (214) and not the cerebellum as in the donor mice (213). Although these models (213, 214) support a role for anti-brain antibodies in the induction of a behavioral syndrome following GAS exposure, the studies did not precisely replicate neural and immune features reported previously in Sydenham's chorea and PANDAS (196, 199, 215).
Human monoclonal antibodies from a Sydenham's chorea patient that react with N-acetyl-β-d-glucosamine (GlcNAc), the major epitope of GAS carbohydrate, also react with a specific lysoganglioside, GM1, present in the brain and with the intra-cellular cytoskeletal protein tubulin (199). Elevated levels of lysoganglioside GM1- and tubulin-specific IgG were found in acute Sydenham's chorea sera and CSF (199, 215, 216). The Sydenham's chorea monoclonal antibodies were shown to activate calcium-calmodulin kinase II (CaMKII)-dependent signaling and induce dopamine release in the catecholamine-secreting SK-N-SH neuronal cell line. Whether the reactivity to tubulin is critical for antibody-mediated effects in vivo or in vitro remains to be determined.
Recently, an active immunization model in rats has been developed (217) that helps elucidate the antibody-mediated effects on the dopamine pathway. Rodents immunized with GAS antigens displayed impaired fine motor control and gait, excessive grooming, and modest hyperactivity. Behavioral changes were correlated with the presence of antibodies in the striatum, thalamus, and frontal cortex as well as with the activation of CaMKII in neuronal cells. Alterations in dopamine and glutamate levels were also found in the cortex and basal ganglia. Interestingly, this study also suggested a novel brain antigen, the D2 dopamine receptor, as a possible target of anti-GAS antibodies. D2 receptor blockade with a complete antagonist, haloperidol, successfully reversed some of the motor impairments in rats following immunization with GAS antigens and is consistent with either mechanism of antibody activity (217). To further demonstrate the role of the D2 receptor in Sydenham's chorea, Cox and colleagues (C.J. Cox, S.E. Swedo, J.F. Leckman, M.W. Cunningham, personal communication) generated a transgenic mouse expressing an antibody that was derived from a Sydenham's chorea patient and is cross-reactive with GAS and brain. The transgenic autoanti-body induced dose-dependent activation of the D2 receptor ex vivo and targeted dopaminergic neurons in the basal ganglia in vivo. Importantly, sera of acute Sydenham's chorea and PANDAS patients harbor elevated levels of antibodies to the dopamine D2 receptor (193, 217). Whether antibodies to lysoganglioside GM1 routinely cross-react with the dopamine D2 receptor has not yet been established.
Thus, multiple observations suggest the presence of functional autoantibodies in the pathogenesis of Sydenham's chorea: (a) the presence of brain-reactive antibodies in the sera of patients; (b) the correlation of CSF antibody titers with symptoms; (c) the reversibility of symptoms as CSF antibody titers decline; and (d) the effectiveness of plasmapheresis or intravenous immunoglobulin as a treatment (202, 204, 216, 218). How these antibodies penetrate the brain and why there is apparent regional specificity has not been established. Given that the antibodies appear to be polyclonal and that systemic immunomodulatory therapies have beneficial effects in Sydenham's chorea, it would seem reasonable to conclude that antibodies are produced in lymphoid organs and penetrate into the brain. It has been suggested that dopamine receptors on endothelial cells might facilitate the transport of anti–dopamine receptor antibodies into brain parenchyma. These antibodies might then bind neurons and alter dopamine-mediated pathways. Alternatively, the antibodies may not need to penetrate brain tissue to alter neuronal function. Anti–dopamine receptor antibodies might bind dopamine receptors present on endothelial cells, activate endothelial CaMKII, and lead to dopamine release from endothelial cells into the CNS.
It is possible that infection itself facilitates endocytosis or tight junction dysfunction, permitting entry of anti-GAS, anti-brain cross-reactive antibodies into the brain. The presence of antibody deposits on the brain depends upon the inclusion of Bordetella pertussis as an adjuvant in GAS-immunized rats (217). B. pertussis compromises the integrity of cerebral endothelial tight junctions (219) and leads to increased vascular permeability in brain tissue (220).
PARANEOPLASTIC ANTIBODIES
Paraneoplastic syndromes reflect the ability of benign or malignant tumors to provoke an immune response. When the immune response is targeted to a brain antigen, the induced antibodies can alter neural function. In some cases the immune response includes T cell infiltration (CD4+ and CD8+), with activation of microglia, gliosis, and neuron loss (221). Determining the etiology of the neurological dysfunction is clinically extremely important, as the syndromes may develop rapidly and may also precede more local symptoms of the tumor. Current hypotheses regarding pathogenesis of the wide spectrum of neuropsychiatric paraneoplastic disorders propose that tumor cells express antigens that are normally and predominantly expressed in the CNS. The generation of antibodies to tumor cell surface molecules presumably constitutes one aspect of tumor surveillance, whereas antibodies might be induced to either intracellular or cell surface antigens through the immunogenicity of dead tumor cells. Paraneoplastic syndromes involving intracellular antigens are more likely to associate with both B and T cell responses; in contrast, paraneoplastic syndromes provoked by cell surface antigens, often synaptic antigens, provoke mainly B cell responses with rare T cell infiltrates. It is possible, however, that this distinction reflects a more intense effort to identify T cells in conditions characterized by antibodies to intracellular antigens in order to identify pathogenic mechanisms.
CSF analysis in paraneoplastic syndromes depends on the onset and duration of the illness. Abnormal CSF analysis is common (70–90% prevalence) across many studies. The abnormality is often characterized as inflammatory CSF, which includes one or more of the following: pleocytosis, increased immunoglobulins, or increased protein (225). The glucose is normal. Paraneoplastic syndromes may also be associated with OCBs present in CSF (225, 226). Comparing the ratio of Ig to albumin in serum with that in CSF supports intrathecal synthesis (225, 226). This observation is also consistent with a report of pleocytosis in the CSF of 93% of patients, although early in the disease, pleocytosis can occur without increased protein (227). It is also consistent with the observation that OCBs detected in CSF may not be detected in serum from the same patient (227–230).
Paraneoplastic syndromes characterized by neuropsychiatric symptoms such as an altered level of consciousness, amnesia, movement disorder, and seizures occur most often in patients with teratomas that express neural antigens (222), although other tumor types can also elicit antibodies that cause paraneoplastic syndromes (Table 2). The antigens targeted in these conditions include channel receptors, subunits of complex assemblies of neurotransmitter receptors, or proteins critical for synaptic function and cause disorders of the hippocampus, basal ganglia, cerebellum, and brain stem (222, 223). For example, the mechanism of action of anti-NR1 antibody appears to involve either internalization of the receptor, with an ensuing decrease in synaptic density of NMDARs but without a permanent loss or degradation of the synapses, or without permanent destruction of the dendrite or cell body (224). The proposed mechanism is consistent with the reversible symptomatology. In animal studies, the alteration in synapse structure correlates with antibody level. Some women with teratomas develop antibody to the NR2 rather than the NR1 subunit of the NMDAR (222). The symptoms largely overlap those in women with anti-NR1 antibodies. Whether paraneoplastic anti-NR2 antibodies cause receptor internalization is not yet known, but they have an epitope specificity that differs from the anti-NR2 antibodies of SLE; the specific nature of the antibody-receptor interaction is likely an important factor in determining the very different associated neuropsychiatric manifestations.
Remarkable experiments have identified a putative pathogenic antibody to amphiphysin in patients with the paraneoplastic syndrome known as stiff-person syndrome (231), which is characterized by fluctuating but progressive muscle rigidity and spasm. Amphiphysin, an intracellular protein, is crucial to synaptic vesicle endocytosis, and the toxicity of the antibody results from a depletion of the releasable vesicle pool. Some symptoms that are present in patients with the antibodies have been reproduced in a dose-dependent manner by passive transfer of serum from patients to mice (232). Not unexpectedly, however, these passive transfer experiments required breaching of the BBB (with encephalitogenic T helper cells specific for myelin basic protein) for antibodies to penetrate brain tissue. Recent experiments employed direct intraventricular injection of purified antiamphiphysin antibody and led to stiffness, reduced dorsal root potentials, and altered Hoffmann reflex (the electrically elicited monosynaptic deep tendon reflex) in recipient animals. Novel imaging techniques identified the IgG internalized within neurons. Moreover, antibody failed to localize in neurons from amphiphysin-deficient mice; thus, these antibodies appear to access intracellular proteins by an incompletely understood mechanism (233), perhaps related to the well-described role of this antigen in endocytosis. Ex vivo patch-clamp experiments showed that antiamphiphysin antibodies suppress the amplitude of the inhibitory postsynaptic currents (233). Many questions remain; prominent among these are how the antibodies access intracellular proteins and why GABAergic synapses should be preferentially attacked through the amphiphysin binding given that amphiphysin expression is widespread in CNS synapses. Interestingly, both amphiphysin and NR2 are targets of autoantibodies in individuals with no apparent tumor and no classified autoimmune disease. It is not known whether the immunogenicity of these antigens in these patients is due to cryptic tumors, or whether it is due to an unknown mechanism unrelated to the presence of tumor cells.
MATERNAL ANTIBODY TRANSMISSION TO THE FETUS
Antibodies to brain antigens may recognize the immature as well as the mature brain. Thus, they theoretically have the potential to alter brain development. The clinical observation that children of mothers but not fathers with SLE have a greater incidence of learning disabilities (234) stimulated animal experiments to test whether the DNA/NMDAR-reactive antibodies that can alter cognition and behavior in adults might cross both the placenta and the immature BBB during fetal development and alter brain development. Female mice with high titers of DNA/NMDAR-reactive antibodies produce offspring with delayed neonatal reflex acquisition and long-term impairments in some tasks dependent on cortical activity. The developing brain exhibits increased cortical neuronal death, a thinned cortical plate, and ectopic mitotic activity. Injection of a pregnant dam with either murine or human monoclonal DNA/NMDAR-reactive antibody demonstrated a time-linked sensitivity to neurotoxicity. Pregnant dams injected late in gestation have embryos with neural development com parable to controls; earlier injection leads to a thinned and disorganized cortex (50). Interestingly, there is a gender-specific effect of maternal antibody. Whereas the male fetuses exposed to antibody experienced cognitive impairment as adults, the female fetuses exposed to maternal antibody failed to survive. Although the exact mechanism remains unclear, fetal death depends on NR2A expression in the fetus (49).
Complementing the data from the in vivo studies in mice, recent epidemiological data from over 500 women with SLE who had over 700 births demonstrated a trend for a higher male-to-female ratio in children born to mothers with SLE compared with control mothers (234a).
It has been proposed that some cases of autism spectrum disorder might be attributable to in utero exposure to maternal antibodies. Two main observations buttress this hypothesis: First, in a cohort of over 600,000 births in Denmark, the presence of autism spectrum disorder in a child was associated with the presence of either rheumatoid arthritis or celiac disease in the mother (235). This study concluded that the presence of one of these disorders in the mother increased the risk of autism spectrum disorder at least twofold in her child, suggesting that autoimmunity in the mother may predispose to autism spectrum disorder. Second, several investigators have identified the presence of antibodies that bind to the brain in mothers with autism spectrum disorder offspring (236, 237). Indeed, mothers of an autism spectrum disorder child are four times more likely to harbor anti-brain antibodies than unselected women of childbearing age (L. Brimberg, P.K. Gregersen, B. Diamond, unpublished data). In two studies, either (a) serum containing antibodies reactive with neuronal antigens or (b) IgG isolated from pooled sera of mothers with autism spectrum disorder offspring was passively administered to pregnant mice. The offspring were observed to have deficits in social behavior and motor skills as well as cerebellar abnormalities (238). Martin et al. (239) administered IgG purified from a large number of mothers with brain-reactive antibodies and a child with autism spectrum disorder to pregnant cynomolgus monkeys. The resultant offspring were observed to have more social deficits, increased motor activity, and increased stereotypic behaviors compared with offspring born of mothers given control IgG pooled from mothers of a normally developing child (239). These data support the hypothesis that maternal antibody can affect the developing fetal brain. This is clearly an area that requires much more study.
THERAPEUTIC APPROACHES
On the basis of the examples provided above, it seems plausible that many developmental or degenerative diseases of the CNS might be antibody mediated or, at the least, exacerbated by antibody should it penetrate to brain tissue. The importance of aggressively pursuing antibody as the mechanism of both inflammatory and noninflammatory brain disease lies in the relative ease of modulating antibody production and the potential use of decoy antigens to block antibody targeting to brain antigens.
Obviously, B cell-directed therapies, especially those that target plasma cells as well as other B cell subsets, will alleviate progression of disease in many cases. Importantly, as in other tissues, once certain brain-intrinsic degenerative pathways have been triggered, antibody may no longer be required for continued damage; thus, there may be a critical time window for B cell depletion therapy. Because B cell depletion or prevention of antibody secretion has unwanted immunosuppressive toxicities, other therapeutic strategies are needed.
Antibody blockade with decoy antigen has been explored. Because in many antigen-mediated diseases of the brain the offending B cells have not themselves penetrated the BBB, decoy antigen may need to be present only systemically and not within the CNS. The approach of antibody blockade has been most intensively explored for anti-NMDAR antibod ies. In principle, NMDAR antagonists would protect neurons from antibody-mediated toxicity. Indeed, NMDAR antagonists do protect neurons from anti-NMDAR antibodies in SLE models, but the history of attempting to block glutamate receptors in the brain in order to decrease excitotoxic damage is long and disappointing. The use in humans of the irreversible NMDAR inhibitor MK-801 and even the FDA-approved NMDAR antagonist memantine has been limited by toxic effects. Even if a nontoxic receptor antagonist were developed, the strategy of lifelong treatment with these agents would be problematic, as normal NMDAR activity is required for learning and memory and other critical neuronal functions.
An emerging alternative is a strategy of blocking the antibody from binding tissue antigen. The D isoform of the DWEYS pentapep-tide is bound by anti-NMDAR antibody, is resistant to proteolytic cleavage, and exhibits a 6–8 h half-life in blood. Thus, the D peptide is a potential therapeutic agent that might function as a decoy antigen that does not interfere with normal NMDAR physiology. Experiments using the blocking peptide in vivo demonstrate reduced antibody-mediated toxicity in adult animals (240, 241). A more useful therapeutic is FISLE-412, an orally absorbable mimetic of the DWEYS peptide that effectively prevents the toxic effects of murine and human DNA/NMDAR-reactive antibodies in both kidney and brain (241). Thus, an antibody-blocking strategy seems potentially beneficial and should be pursued. An important feature of this approach is that it is not necessary to know the actual tissue antigen bound by the antibody; identifying a surrogate antigen is sufficient to mediate antibody blockade.
CONCLUSIONS
Brain-reactive antibodies are found in humans with autoimmune disease, with exposure to microbes or other exogenous antigens that induce cross-reactivity, and with tumors expressing brain antigens. Importantly, these mechanisms may not be mutually exclusive. For example, celiac disease develops in individuals with an underlying autoimmune predisposition and in the context of dietary exposure to gluten peptides. Thus, both autoimmune predisposition and exposure to cross-reactive antigens are responsible for disease-specific autoantibodies that bind CNS antigens (242, 243). Although these mechanisms likely encompass the great majority of antibody-driven neuropathologies, brain injury such as trauma, seizure, or stroke may also impair the sequestration of brain-specific antigens and lead to an immune response to brain antigen.
In all these situations, reactivity likely develops to brain antigens that do not engage in the routine process of B cell selection. Thus, autoantibody-mediated brain pathology is likely to have the following characteristics:
Reactivity to brain-specific antigen or to brain-specific epitopes of more broadly expressed antigen. Generally, this means that brain-reactive antibodies will target neurons and astrocytes.
Mechanisms of neurotoxicity that do not depend on Fc-associated effector functions, as the immune environment of the brain does not favor complement or Fc receptor activation.
A mechanism for antibody to penetrate the BBB. This may be noninflammatory, such as antigen-mediated endocytosis, or may require an inflammatory breach of barrier integrity. Moreover, the nature of the antibody-mediated neuropsychiatric symptoms may depend on the mechanism of barrier breach, as breaches in barrier integrity appear to be local and nonhomogeneous. This consideration pertains to disease in adults.
In utero exposure to antibody can occur without a specific mechanism of barrier breach. Whether there is regional specificity to the development of barrier function is not known.
The identification of syndromes mediated by brain-reactive antibodies remains challenging, although new neuroimaging modalities hold promise. In humans, these modalities may identify premortem brain pathology—and its progression or cessation of progression—that has, up to now, been unappreciated. In animal models, it will be possible to track antibody penetration into brain tissue and ensuing alterations in brain function. Yet it is important to note that even the detection of brain-reactive antibodies remains problematic, and there is no uniform methodology that will identify all those with known pathogenic potential.
The potential pathogenicity of brain-reactive antibodies is now well appreciated. The number of neuropsychiatric syndromes shown to be mediated, at least in part, by brain-reactive antibodies will undoubtedly increase. Multiple questions need to be addressed as more brain-reactive antibodies are identified: How do antibodies access their CNS targets? Do antibodies access intracellular targets, and if so, how? Are neurons and astrocytes indeed preferentially targeted? Are Fc-mediated mechanisms of antibody toxicity less important in the CNS than in other organs? What maintains BBB function, and what are its key modulators? Is there a systemic, metabolomic, proteomic, or other marker of barrier integrity? Is the developing fetal neuron less vulnerable to the many brain-reactive antibodies that can impair adult neuron function or viability? Why are antigens present in the peripheral tissues not targeted by the autoantibodies?
This group of CNS diseases might seem relatively accessible to therapeutic intervention. The availability of B cell-targeted therapeutics holds promise that many antibody-mediated neuropsychiatric symptoms can be alleviated once pathogenic antibodies are identified. Because targeting B cells impairs protective immunity, the use of decoy antigens warrants further exploration. Perhaps most importantly, learning to modulate BBB function in order to control antibody entry into the brain is a future therapeutic necessity.
ACKNOWLEDGMENTS
The authors thank Peter Gregersen and Richard Ransohoff for helpful comments and Benjamin Obholzer for illustrations.
Glossary
- Blood-brain barrier (BBB)
a physiological characteristic of the CNS vasculature, through which the exchange of compounds between the blood and CNS is highly restricted
- Multiple sclerosis (MS)
autoimmune disease with a heterogeneous clinical presentation; commonly affects young adults and has a high risk of future disability
- Neuromyelitis optica (NMO)
inflammatory demyelinating disease in which patients experience optic neuritis and longitudinal extensive myelitis spanning three or more vertebral segments
- Aquaporin-4 (AQP4)
channel that regulates the passage of water in and out of the CNS; a major self-antigen in NMO
- Sydenham's chorea
neurological complication of GAS that occurs in patients, usually under age 18, with an autoimmune etiology
- GAS
Group A β-hemolytic streptococcus
- Paraneoplastic syndrome
clinical neurologic symptoms caused by an immune response to a tumor antigen that cross-reacts with a neuronal antigen
- Negative selection
censoring of lymphocytes that react with self-components
- Astrocyte
a major CNS cell type belonging to the neuroectodermal lineage; regulates vascular, neuronal, and immune function in the CNS
- Microglia
CNS-resident myeloid cells; quiescent under normal circumstances but may be activated to adopt a macrophage-like phenotype under proinflammatory conditions
- Neuronal synapse
contact between neurons, specialized for the localized release of neurotransmitters; the major mode of signal transduction in the CNS
- Systemic lupus erythematosus (SLE)
autoimmune disease primarily affecting women and characterized by numerous autoantibodies, especially antibodies to nuclear antigens
- N-methyl-d-aspartate receptor (NMDAR)
a glutamate receptor that is important in synaptic plasticity and learning and is an autoimmune target in lupus
- Tight junctions
cell-cell contact structures that tightly restrict the passage of soluble factors; important for the barrier properties of CNS endothelial cells
- Molecular mimicry:
antigen sequence similarities between foreign and self-peptides, such that the T or B cell will recognize them and be activated
- R4A
mouse monoclonal antibody reactive with DNA and NMDAR's NR2A and NR2B subunits; can modulate NMDAR function, altering synaptic function or triggering apoptosis
- OCB
oligoclonal immunoglobulin band
- Acute disseminated encephalomyelitis (ADEM)
CNS demyelinating disease in which a subgroup patients have antibodies against the myelin oligodendrocyte glycoprotein (MOG)
- PANDAS
pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection
- Decoy antigen therapy
the use of soluble antigen to block antibody from binding its target physiological antigen
Footnotes
DISCLOSURE STATEMENT The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Pfeiffer F, Schafer J, Lyck R, Makrides V, Brunner S, et al. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122:601–14. doi: 10.1007/s00401-011-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21:179–88. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–41. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 4.Meinl E, Krumbholz M, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann. Neurol. 2006;59(6):880–92. doi: 10.1002/ana.20890. [DOI] [PubMed] [Google Scholar]
- 5.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–66. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]; These studies demonstrated that CNS blood vessels exhibit BBB properties prenatally and that pericytes are critical for BBB function during embryogenesis and in the adult brain.
- 7.Saunders NR, Habgood MD, Dziegielewska KM. Barrier mechanisms in the brain, II. Immature brain. Clin. Exp. Pharmacol. Physiol. 1999;26:85–91. doi: 10.1046/j.1440-1681.1999.02987.x. [DOI] [PubMed] [Google Scholar]
- 8.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Sun J, Singh V, Kajino-Sakamoto R, Aballay A. Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science. 2011;332:729–32. doi: 10.1126/science.1203411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol. Rev. 2012;248:188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CHY, Jenne CN, Lee W-Y, Léger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–5. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 12.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–45. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 2011;13:1227–35. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 14.Tegla C, Cudrici C, Patel S, Trippe R, Rus V, et al. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol. Res. 2011;51:45–60. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 2012;35:369–89. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 16.Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol. Immunol. 2011;48:1592–603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–59. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 18.Piton A, Michaud JL, Peng H, Aradhya S, Gauthier J, et al. Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Hum. Mol. Genet. 2008;17:3965–74. doi: 10.1093/hmg/ddn300. [DOI] [PubMed] [Google Scholar]
- 19.Pavlowsky A, Gianfelice A, Pallotto M, Zanchi A, Vara H, et al. A postsynaptic signaling pathway that may account for the cognitive defect due to IL1RAPL1 mutation. Curr. Biol. 2010;20:103–15. doi: 10.1016/j.cub.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 20.Lister KJ, Hickey MJ. Immune complexes alter cerebral microvessel permeability: roles of complement and leukocyte adhesion. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H694–704. doi: 10.1152/ajpheart.01271.2005. [DOI] [PubMed] [Google Scholar]
- 21.Teeling J, Carare R, Glennie M, Perry V. Intracerebral immune complex formation induces inflammation in the brain that depends on Fc receptor interaction. Acta Neuropathol. 2012;124:479–90. doi: 10.1007/s00401-012-0995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunnon K, Teeling JL, Tutt AL, Cragg MS, Glennie MJ, Perry VH. Systemic inflammation modulates Fc receptor expression on microglia during chronic neurodegeneration. J. Immunol. 2011;186:7215–24. doi: 10.4049/jimmunol.0903833. [DOI] [PubMed] [Google Scholar]
- 23.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, et al. Microglia sculpt post-natal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133:2734–48. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Bodenheimer TS, Brightman MW. A blood-brain barrier to peroxidase in capillaries surrounded by perivascular spaces. Am. J. Anat. 1968;122:249–67. doi: 10.1002/aja.1001220206. [DOI] [PubMed] [Google Scholar]
- 27.Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS. 2011;8:4–13. doi: 10.1186/2045-8118-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, et al. Size-selective loosening of the blood-brain barrier in claudin-5–deficient mice. J. Cell Biol. 2003;161:653–60. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engelhardt B. β1-Integrin/matrix interactions support blood-brain barrier integrity. J. Cereb. Blood Flow Metab. 2011;31:1969–71. doi: 10.1038/jcbfm.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osada T, Gu Y-H, Kanazawa M, Tsubota Y, Hawkins BT, et al. Interendothelial claudin-5 expression depends on cerebral endothelial cell-matrix adhesion by β1-integrins. J. Cereb. Blood Flow Metab. 2011;31:1972–85. doi: 10.1038/jcbfm.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, et al. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. Histol. Histopathol. 2004;19:535–64. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- 33.Stewart PA. Endothelial vesicles in the blood-brain barrier: Are they related to permeability? Cell. Mol. Neurobiol. 2000;20:149–63. doi: 10.1023/A:1007026504843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007;7:715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J. Neuroimmunol. 2001;114:168–72. doi: 10.1016/s0165-5728(01)00242-9. [DOI] [PubMed] [Google Scholar]
- 36.Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood-brain barrier. J. Neurochem. 2002;81:203–6. doi: 10.1046/j.1471-4159.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- 37.Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: Leucocyte–endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol. Appl. Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 38.Moussion C, Girard J-P. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479:542–46. doi: 10.1038/nature10540. [DOI] [PubMed] [Google Scholar]
- 39.Girard J-P, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol. Today. 1995;16:449–57. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 40.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 41.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–27. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]; These studies demonstrated that CNS blood vessels exhibit BBB properties prenatally and that pericytes are critical for BBB function during embryogenesis and in the adult brain.
- 42.Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J. Exp. Med. 2011;208:1695–705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp. Neurol. 1993;120:245–63. doi: 10.1006/exnr.1993.1059. [DOI] [PubMed] [Google Scholar]
- 44.Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS. 2011;8:3. doi: 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front. Pharmacol. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbott N, Patabendige A, Dolman D, Yusof S, Begley D. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 47.Rodríguez EM, Blázquez JL, Pastor FE, Peláez B, Peña P, et al. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int. Rev. Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 48.Popescu BF, Lennon VA, Parisi JE, Howe CL, Weigand SD, et al. Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications. Neurology. 2011;76:1229–37. doi: 10.1212/WNL.0b013e318214332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Zhou D, Lee J, Niu H, Faust TW, et al. Female mouse fetal loss mediated by maternal autoantibody. J. Exp. Med. 2012;209:1083–89. doi: 10.1084/jem.20111986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat. Med. 2009;15:91–96. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]; Showed that maternal anti-NMDAR antibodies can alter fetal brain development in utero and cause persistent neurological symptoms in the offspring.
- 51.Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–31. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]; Identified Sonic hedgehog signaling as a mechanism for the specific induction of BBB properties in endothelial cells within the CNS.
- 52.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]; These studies demonstrated that CNS blood vessels exhibit BBB properties prenatally and that pericytes are critical for BBB function during embryogenesis and in the adult brain.
- 53.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:641–46. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duijvestijn AM, Schreiber AB, Butcher EC. Interferon-γ regulates an antigen specific for endothelial cells involved in lymphocyte traffic. Proc. Natl. Acad. Sci. USA. 1986;83:9114–18. doi: 10.1073/pnas.83.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr. Pharm. Des. 2005;11:973–84. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- 56.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coureuil M, Lécuyer H, Scott MGH, Boularan C, Enslen H, et al. Meningococcus hijacks a β2-adrenoceptor/β-arrestin pathway to cross brain microvasculature endothelium. Cell. 2010;143:1149–60. doi: 10.1016/j.cell.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 58.Deane R, Sagare A, Hamm K, Parisi M, LaRue B, et al. IgG-assisted age-dependent clearance of Alzheimer's amyloid βpeptide by the blood-brain barrier neonatal Fc receptor. J. Neurosci. 2005;25:11495–503. doi: 10.1523/JNEUROSCI.3697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 60.Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011;585:3770–80. doi: 10.1016/j.febslet.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 61.Flierl M, Rittirsch D, Huber-Lang M, Stahel P. Pathophysiology of septic encephalopathy—an unsolved puzzle. Crit. Care. 2010;14:165. doi: 10.1186/cc9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janigro D. Does leakage of the blood-brain barrier mediate epileptogenesis? Epilepsy Curr. 2007;7:105–7. doi: 10.1111/j.1535-7511.2007.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mildner A, Schlevogt B, Kierdorf K, Böttcher C, Erny D, et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer's disease. J. Neurosci. 2011;31:11159–71. doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dénes Á , Humphreys N, Lane TE, Grencis R, Rothwell N. Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J. Neurosci. 2010;30:10086–95. doi: 10.1523/JNEUROSCI.1227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serrats J, Schiltz JC, García-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dietrich JB. Alteration of blood-brain barrier function by methamphetamine and cocaine. Cell Tissue Res. 2009;336:385–92. doi: 10.1007/s00441-009-0777-y. [DOI] [PubMed] [Google Scholar]
- 67.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–16. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, et al. Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-α in the rat. J. Pharmacol. Exp. Ther. 2007;323:488–98. doi: 10.1124/jpet.107.127035. [DOI] [PubMed] [Google Scholar]
- 69.Hazleton JE, Berman JW, Eugenin EA. Novel mechanisms of central nervous system damage in HIV infection. HIV/AIDS Res. Palliat. Care. 2010;2:39–49. doi: 10.2147/hiv.s9186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 71.Greenwood J, Heasman S, Alvarez J, Prat A, Lyck R, Engelhardt B. Leukocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol. Appl. Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 72.Zhou H, Andonegui G, Wong CHY, Kubes P. Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J. Immunol. 2009;183:5244–50. doi: 10.4049/jimmunol.0901309. [DOI] [PubMed] [Google Scholar]
- 73.Berer K, Wekerle H, Krishnamoorthy G. B cells in spontaneous autoimmune diseases of the central nervous system. Mol. Immunol. 2011;48:1332–37. doi: 10.1016/j.molimm.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 74.Scott GS, Bowman SR, Smith T, Flower RJ, Bolton C. Glutamate-stimulated peroxynitrite production in a brain-derived endothelial cell line is dependent on N-methyl-D-aspartate (NMDA) receptor activation. Biochem. Pharmacol. 2007;73:228–36. doi: 10.1016/j.bcp.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neuhaus W, Freidl M, Szkokan P, Berger M, Wirth M, et al. Effects of NMDA receptor modulators on a blood-brain barrier in vitro model. Brain Res. 2011;1394:49–61. doi: 10.1016/j.brainres.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Barker C, Billingham R. Immunologically privileged sites. Adv. Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- 77.Jacob A, Hack B, Chiang E, Garcia JGN, Quigg RJ, Alexander JJ. C5a alters blood-brain barrier integrity in experimental lupus. FASEB J. 2010;24:1682–88. doi: 10.1096/fj.09-138834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Medawar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 79.Engelhardt B, Ransohoff R. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–95. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 80.Pardridge W. Blood-brain barrier delivery. Drug Discov. Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 81.Betz L, Goldstein G, Katzman R. Blood-brain-cerebrospinal fluid barriers. In: Siegel GJ, editor. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 4th ed. Raven; New York: 1989. pp. 591–606. [Google Scholar]
- 82.Hafer-Macko CE, Sheikh KA, Li CY, Ho TW, Cornblath DR, et al. Immune attack on the Schwann cell surface in acute inflammatory demyelinating polyneuropathy. Ann. Neurol. 1996;39:625–35. doi: 10.1002/ana.410390512. [DOI] [PubMed] [Google Scholar]
- 83.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin. Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 84.Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- 85.Bernard I, Fournie GJ, Saoudi A. Genomics studies of immune-mediated diseases using the BNLEW rat model. Methods Mol. Biol. 2010;597:389–402. doi: 10.1007/978-1-60327-389-3_26. [DOI] [PubMed] [Google Scholar]
- 86.Lindahl P, Johansson B, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–45. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 87.Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin L. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 2001;153:933–46. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J. Neuropathol. Exp. Neurol. 2008;67:1113–21. doi: 10.1097/NEN.0b013e31818f9ca8. [DOI] [PubMed] [Google Scholar]
- 89.Hickey W, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–92. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 90.Greter M, Heppner F, Lemos M, Odermatt B, Goebels N, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 2005;11:328–34. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 91.Katz JB, Limpanasithikul W, Diamond B. Mutational analysis of an autoantibody: differential binding and pathogenicity. J. Exp. Med. 1994;180:925–32. doi: 10.1084/jem.180.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaynor B, Putterman C, Valadon P, Spatz L, Scharff MD, Diamond B. Peptide inhibition of glomerular deposition of an anti-DNA antibody. Proc. Natl. Acad. Sci. USA. 1997;94:1955–60. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–38. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 94.Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog. Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- 95.Blahos J, Wenthold RJ. Relationship between N-Methyl-D-aspartate receptor NR1 splice variants and NR2 subunits. J. Biol. Chem. 1996;271:15669–74. doi: 10.1074/jbc.271.26.15669. [DOI] [PubMed] [Google Scholar]
- 96.Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J. Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huntley GW, Vickers JC, Morrison JH. Cellular and synaptic localization of NMDA and non-NMDA receptor subunits in neocortex: organizational features related to cortical circuitry, function and disease. Trends Neurosci. 1994;17:536–43. doi: 10.1016/0166-2236(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 98.Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 2001;7:1189–93. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]; Showed that anti-DNA antibodies can cross-react with NMDARs and modulate receptor activity.
- 100.Faust TW, Chang EH, Kowal C, Berlin R, Gazaryan IG, et al. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc. Natl. Acad. Sci. USA. 2010;107:18569–74. doi: 10.1073/pnas.1006980107. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrated dose-dependent cellular alterations for the subset of anti-DNA/NMDAR-reactive antibodies.
- 101.Kowal C, DeGiorgio LA, Lee JY, Edgar MA, Huerta PT, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl. Acad. Sci. USA. 2006;103:19854–59. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harrison MJ, Ravdin LD, Lockshin MD. Relationship between serum NR2a antibodies and cognitive dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2515–22. doi: 10.1002/art.22030. [DOI] [PubMed] [Google Scholar]
- 103.Omdal R, Brokstad K, Waterloo K, Koldingsnes W, Jonsson R, Mellgren SI. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur. J. Neurol. 2005;12:392–98. doi: 10.1111/j.1468-1331.2004.00976.x. [DOI] [PubMed] [Google Scholar]
- 104.Lapteva L, Nowak M, Yarboro CH, Takada K, Roebuck-Spencer T, et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2505–14. doi: 10.1002/art.22031. [DOI] [PubMed] [Google Scholar]
- 105.Gono T, Kawaguchi Y, Kaneko H, Nishimura K, Hanaoka M, et al. Anti-NR2A antibody as a predictor for neuropsychiatric systemic lupus erythematosus. Rheumatology. 2011;50:1578–85. doi: 10.1093/rheumatology/keq408. [DOI] [PubMed] [Google Scholar]
- 106.Husebye ES, Sthoeger ZM, Dayan M, Zinger H, Elbirt D, et al. Autoantibodies to a NR2A peptide of the glutamate/NMDA receptor in sera of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2005;64:1210–13. doi: 10.1136/ard.2004.029280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoshio T, Onda K, Nara H, Minota S. Association of IgG anti-NR2 glutamate receptor antibodies in cerebrospinal fluid with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2006;54:675–78. doi: 10.1002/art.21547. [DOI] [PubMed] [Google Scholar]
- 108.Fragoso-Loyo H, Cabiedes J, Orozco-Narváez A, Dávila-Maldonado L, Atisha-Fregoso Y, et al. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PLoS ONE. 2008;3:e3347. doi: 10.1371/journal.pone.0003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J. Exp. Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gosselin D, Rivest S. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol. Psychiatry. 2008;13:480–97. doi: 10.1038/sj.mp.4002122. [DOI] [PubMed] [Google Scholar]
- 111.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: Antibodies alter emotion. Proc. Natl. Acad. Sci. USA. 2006;103:678–83. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bonfa E, Elkon KB. Clinical and serological associations of the antirobosomal P protein antibody. Arthritis Rheum. 1986;29:981–85. doi: 10.1002/art.1780290806. [DOI] [PubMed] [Google Scholar]
- 113.Bonfa E, Golombek SJ, Kaufman LD, Skelly S, Weissbach H, et al. Association between lupus psychosis and antiribosomal P protein antibodies. N. Engl. J. Med. 1987;317:265–71. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- 114.Golombek SJ, Graus F, Elkon KB. Autoantibodies in the cerebrospinal fluid of patients with systemic lupus erythematosus. Arthritis Rheum. 1986;29:1090–97. doi: 10.1002/art.1780290906. [DOI] [PubMed] [Google Scholar]
- 115.Yoshio T, Hirata D, Onda K, Nara H, Minota S. Antiribosomal P protein antibodies in cerebrospinal fluid are associated with neuropsychiatric systemic lupus erythematosus. J. Rheumatol. 2005;32:34–39. [PubMed] [Google Scholar]
- 116.Matus S, Burgos PV, Bravo-Zehnder M, Kraft R, Porras OH, et al. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. J. Exp. Med. 2007;204:3221–34. doi: 10.1084/jem.20071285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Katzav A, Ben-Ziv T, Chapman J, Blank M, Reichlin M, Shoenfeld Y. Anti-P ribosomal antibodies induce defect in smell capability in a model of CNS -SLE (depression) J. Autoimmun. 2008;31:393–98. doi: 10.1016/j.jaut.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 118.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 119.Bruck W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J. Neurol. 2005;252(Suppl. 5):v3–9. doi: 10.1007/s00415-005-5002-7. [DOI] [PubMed] [Google Scholar]
- 120.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N. Engl. J. Med. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 121.Kabat EA, Glusman M, Knaub V. Quantitative estimation of the albumin and gamma globulin in normal and pathologic cerebrospinal fluid by immunochemical methods. Am. J. Med. 1948;4:653–62. doi: 10.1016/s0002-9343(48)90389-1. [DOI] [PubMed] [Google Scholar]
- 122.Srivastava R, Aslam M, Kalluri SR, Schirmer L, Buck D, et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N. Engl. J. Med. 2012;367:115–23. doi: 10.1056/NEJMoa1110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Freedman MS, Thompson EJ, Deisenhammer F, Giovannoni G, Grimsley G, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch. Neurol. 2005;62:865–70. doi: 10.1001/archneur.62.6.865. [DOI] [PubMed] [Google Scholar]
- 124.Link H, Huang YM. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. J. Neuroimmunol. 2006;180:17–28. doi: 10.1016/j.jneuroim.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 125.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 126.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann. Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 127.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 2008;358:676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 128.Pöllinger B, Krishnamoorthy G, Berer K, Lassmann H, Bösl MR, et al. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J. Exp. Med. 2009;206:1303–16. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–41. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 130.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 2000;47:707–17. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 131.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–18. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Colombo M, Dono M, Gazzola P, Roncella S, Valetto A, et al. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J. Immunol. 2000;164:2782–89. doi: 10.4049/jimmunol.164.5.2782. [DOI] [PubMed] [Google Scholar]
- 133.Owens GP, Ritchie AM, Burgoon MP, Williamson RA, Corboy JR, Gilden DH. Single-cell repertoire analysis demonstrates that clonal expansion is a prominent feature of the B cell response in multiple sclerosis cerebrospinal fluid. J. Immunol. 2003;171:2725–33. doi: 10.4049/jimmunol.171.5.2725. [DOI] [PubMed] [Google Scholar]
- 134.Qin Y, Duquette P, Zhang Y, Talbot P, Poole R, Antel J. Clonal expansion and somatic hy-permutation of V(H) genes of B cells from cerebrospinal fluid in multiple sclerosis. J. Clin. Investig. 1998;102:1045–50. doi: 10.1172/JCI3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kerlero de Rosbo N, Honegger P, Lassmann H, Matthieu JM. Demyelination induced in aggregating brain cell cultures by a monoclonal antibody against myelin/oligodendrocyte glycoprotein. J. Neurochem. 1990;55:583–87. doi: 10.1111/j.1471-4159.1990.tb04173.x. [DOI] [PubMed] [Google Scholar]
- 136.Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat. Med. 1999;5:170–75. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- 137.O'Connor KC, Appel H, Bregoli L, Call ME, Catz I, et al. Antibodies from inflamed central nervous system tissue recognize myelin oligodendrocyte glycoprotein. J. Immunol. 2005;175:1974–82. doi: 10.4049/jimmunol.175.3.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O'Connor KC, McLaughlin KA, De Jager PL, Chitnis T, Bettelli E, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat. Med. 2007;13:211–17. doi: 10.1038/nm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovery of conformation-dependent autoantibodies in a subset of patients with ADEM but not in patients with adult-onset MS.
- 139.Di Pauli F, Mader S, Rostasy K, Schanda K, Bajer-Kornek B, et al. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin. Immunol. 2011;138:247–54. doi: 10.1016/j.clim.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 140.Brilot F, Dale RC, Selter RC, Grummel V, Kalluri SR, et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann. Neurol. 2009;66:833–42. doi: 10.1002/ana.21916. [DOI] [PubMed] [Google Scholar]
- 141.Pröbstel AK, Dornmair K, Bittner R, Sperl P, Jenne D, et al. Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology. 2011;77:580–88. doi: 10.1212/WNL.0b013e318228c0b1. [DOI] [PubMed] [Google Scholar]
- 142.Rostasy K, Mader S, Schanda K, Huppke P, Gärtner J, et al. Anti-myelin oligodendrocyte glyco-protein antibodies in pediatric patients with optic neuritis. Arch. Neurol. 2012;69:752–56. doi: 10.1001/archneurol.2011.2956. [DOI] [PubMed] [Google Scholar]
- 143.McLaughlin KA, Chitnis T, Newcombe J, Franz B, Kennedy J, et al. Age-dependent B cell autoimmunity to a myelin surface antigen in pediatric multiple sclerosis. J. Immunol. 2009;183:4067–76. doi: 10.4049/jimmunol.0801888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mader S, Lutterotti A, Di Pauli F, Kuenz B, Schanda K, et al. Patterns of antibody binding to aquaporin-4 isoforms in neuromyelitis optica. PLoS ONE. 2010;5:e10455. doi: 10.1371/journal.pone.0010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mader S, Gredler V, Schanda K, Rostasy K, Dujmovic I, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J. Neuroinflamm. 2011;8:184. doi: 10.1186/1742-2094-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79:1273–77. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 147.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–15. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 148.Cree B. Neuromyelitis optica: diagnosis, pathogenesis, and treatment. Curr. Neurol. Neurosci. Rep. 2008;8:427–33. doi: 10.1007/s11910-008-0066-2. [DOI] [PubMed] [Google Scholar]
- 149.Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53:1107–14. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 150.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–12. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]; Pioneering discovery in NMO patients of highly specific autoantibodies that enable early treatment; these antibodies have helped produce further insights into the disease pathogenesis.
- 151.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005;202:473–77. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hinson SR, McKeon A, Lennon VA. Neurological autoimmunity targeting aquaporin-4. Neuro-science. 2010;168:1009–18. doi: 10.1016/j.neuroscience.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 153.Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch. Neurol. 2006;63:964–68. doi: 10.1001/archneur.63.7.964. [DOI] [PubMed] [Google Scholar]
- 154.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–89. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 155.Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch. Neurol. 2006;63:390–96. doi: 10.1001/archneur.63.3.390. [DOI] [PubMed] [Google Scholar]
- 156.Popescu BF, Parisi JE, Cabrera-Gomez JA, Newell K, Mandler RN, et al. Absence of cortical demyelination in neuromyelitis optica. Neurology. 2010;75:2103–9. doi: 10.1212/WNL.0b013e318200d80c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Suzuki M, Obara K, Sasaki Y, Matoh K, Kitabatake A, et al. Comparison of perivascular astrocytic structure between white matter and gray matter of rats. Brain Res. 2003;992:294–97. doi: 10.1016/j.brainres.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 158.Hirano A, Kawanami T, Llena JF. Electron microscopy of the blood-brain barrier in disease. Microsc. Res. Tech. 1994;27:543–56. doi: 10.1002/jemt.1070270609. [DOI] [PubMed] [Google Scholar]
- 159.Waters P, Jarius S, Littleton E, Leite MI, Jacob S, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch. Neurol. 2008;65:913–19. doi: 10.1001/archneur.65.7.913. [DOI] [PubMed] [Google Scholar]
- 160.Neely JD, Christensen BM, Nielsen S, Agre P. Heterotetrameric composition of aquaporin-4 water channels. Biochemistry. 1999;38:11156–63. doi: 10.1021/bi990941s. [DOI] [PubMed] [Google Scholar]
- 161.Crane JM, Tajima M, Verkman AS. Live-cell imaging of aquaporin-4 diffusion and interactions in orthogonal arrays of particles. Neuroscience. 2010;168:892–902. doi: 10.1016/j.neuroscience.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hinson SR, Romero MF, Popescu BF, Lucchinetti CF, Fryer JP, et al. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc. Natl. Acad. Sci. USA. 2012;109:1245–50. doi: 10.1073/pnas.1109980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nicchia GP, Mastrototaro M, Rossi A, Pisani F, Tortorella C, et al. Aquaporin-4 orthogonal arrays of particles are the target for neuromyelitis optica autoantibodies. Glia. 2009;57:1363–73. doi: 10.1002/glia.20855. [DOI] [PubMed] [Google Scholar]
- 164.Misu T, Fujihara K, Itoyama Y. Neuromyelitis optica and anti-aquaporin 4 antibody—an overview. Brain Nerve. 2008;60:527–37. In Japanese. [PubMed] [Google Scholar]
- 165.Popescu BF, Lucchinetti F. Pathology of demyelinating diseases. Annu. Rev. Pathol. Mech. Dis. 2012;7:185–217. doi: 10.1146/annurev-pathol-011811-132443. [DOI] [PubMed] [Google Scholar]
- 166.Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 167.Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain. 2002;125:1450–61. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Misu T, Takano R, Fujihara K, Takahashi T, Sato S, Itoyama Y. Marked increase in cerebrospinal fluid glial fibrillar acidic protein in neuromyelitis optica: an astrocytic damage marker. J. Neurol. Neurosurg. Psychiatry. 2009;80:575–77. doi: 10.1136/jnnp.2008.150698. [DOI] [PubMed] [Google Scholar]
- 169.Watanabe S, Nakashima I, Misu T, Miyazawa I, Shiga Y, et al. Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult. Scler. 2007;13:128–32. doi: 10.1177/1352458506071174. [DOI] [PubMed] [Google Scholar]
- 170.Cree BA, Lamb S, Morgan K, Chen A, Waubant E, Genain C. An open label study of the effects of rituximab in neuromyelitis optica. Neurology. 2005;64:1270–72. doi: 10.1212/01.WNL.0000159399.81861.D5. [DOI] [PubMed] [Google Scholar]
- 171.Jarius S, Aboul-Enein F, Waters P, Kuenz B, Hauser A, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072–80. doi: 10.1093/brain/awn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–43. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- 173.Dujmovic I, Mader S, Schanda K, Deisenhammer F, Stojsavljevic N, et al. Temporal dynamics of cerebrospinal fluid anti-aquaporin-4 antibodies in patients with neuromyelitis optica spectrum disorders. J. Neuroimmunol. 2011;234:124–30. doi: 10.1016/j.jneuroim.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 174.Kinoshita M, Nakatsuji Y, Moriya M, Okuno T, Kumanogoh A, et al. Astrocytic necrosis is induced by anti-aquaporin-4 antibody-positive serum. Neuroreport. 2009;20:508–12. doi: 10.1097/wnr.0b013e32832776f4. [DOI] [PubMed] [Google Scholar]
- 175.Hinson SR, McKeon A, Fryer JP, Apiwattanakul M, Lennon VA, Pittock SJ. Prediction of neuromyelitis optica attack severity by quantitation of complement-mediated injury to aquaporin-4-expressing cells. Arch. Neurol. 2009;66:1164–67. doi: 10.1001/archneurol.2009.188. [DOI] [PubMed] [Google Scholar]
- 176.Sabater L, Giralt A, Boronat A, Hankiewicz K, Blanco Y, et al. Cytotoxic effect of neuromyelitis optica antibody (NMO-IgG) to astrocytes: an in vitro study. J. Neuroimmunol. 2009;215:31–35. doi: 10.1016/j.jneuroim.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 177.Zhang H, Bennett JL, Verkman AS. Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann. Neurol. 2011;70:943–54. doi: 10.1002/ana.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann. Neurol. 2009;66:630–43. doi: 10.1002/ana.21837. [DOI] [PubMed] [Google Scholar]
- 179.Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann. Neurol. 2009;66:617–29. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kinoshita M, Nakatsuji Y, Kimura T, Moriya M, Takata K, et al. Neuromyelitis optica: passive transfer to rats by human immunoglobulin. Biochem. Biophys. Res. Commun. 2009;386:623–27. doi: 10.1016/j.bbrc.2009.06.085. [DOI] [PubMed] [Google Scholar]
- 181.Shimizu F, Sano Y, Takahashi T, Haruki H, Saito K, et al. Sera from neuromyelitis optica patients disrupt the blood-brain barrier. J. Neurol. Neurosurg. Psychiatry. 2012;83:288–97. doi: 10.1136/jnnp-2011-300434. [DOI] [PubMed] [Google Scholar]
- 182.Pohl M, Fischer MT, Mader S, Schanda K, Kitic M, et al. Pathogenic T cell responses against aquaporin 4. Acta Neuropathol. 2011;122:21–34. doi: 10.1007/s00401-011-0824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J. Cell Sci. 1995;108:2993–3002. doi: 10.1242/jcs.108.9.2993. [DOI] [PubMed] [Google Scholar]
- 184.Takumi Y, Nagelhus EA, Eidet J, Matsubara A, Usami S, et al. Select types of supporting cell in the inner ear express aquaporin-4 water channel protein. Eur. J. Neurosci. 1998;10:3584–95. doi: 10.1046/j.1460-9568.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- 185.Frigeri A, Gropper MA, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc. Natl. Acad. Sci. USA. 1995;92:4328–31. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Apiwattanakul M, Popescu BF, Matiello M, Weinshenker BG, Lucchinetti CF, et al. Intractable vomiting as the initial presentation of neuromyelitis optica. Ann. Neurol. 2010;68:757–61. doi: 10.1002/ana.22121. [DOI] [PubMed] [Google Scholar]
- 187.Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y. Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology. 2005;65:1479–82. doi: 10.1212/01.wnl.0000183151.19351.82. [DOI] [PubMed] [Google Scholar]
- 188.Stollerman GH. Rheumatic fever in the 21st century. Clin. Infect. Dis. 2001;33:806–14. doi: 10.1086/322665. [DOI] [PubMed] [Google Scholar]
- 189.Cardoso F, Eduardo C, Silva AP, Mota CC. Chorea in fifty consecutive patients with rheumatic fever. Mov. Disord. 1997;12:701–3. doi: 10.1002/mds.870120512. [DOI] [PubMed] [Google Scholar]
- 190.Cardoso F, Vargas AP, Oliveira LD, Guerra AA, Amaral SV. Persistent Sydenham's chorea. Mov. Disord. 1999;14:805–7. doi: 10.1002/1531-8257(199909)14:5<805::aid-mds1013>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 191.Gordon N. Sydenham's chorea, and its complications affecting the nervous system. Brain Dev. 2009;31:11–14. doi: 10.1016/j.braindev.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 192.Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am. J. Psychiatry. 1998;155:264–71. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- 193.Cunningham MW. Streptococcus and rheumatic fever. Curr. Opin. Rheumatol. 2012;24:408–16. doi: 10.1097/BOR.0b013e32835461d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dale RC. Autoimmunity and the basal ganglia: new insights into old diseases. QJM. 2003;96:183–91. doi: 10.1093/qjmed/hcg026. [DOI] [PubMed] [Google Scholar]
- 195.Dale RC. Post-streptococcal autoimmune disorders of the central nervous system. Dev. Med. Child Neurol. 2005;47:785–91. doi: 10.1017/S0012162205001647. [DOI] [PubMed] [Google Scholar]
- 196.Husby G, van de Rijn I, Zabriskie JB, Abdin ZH, Williams RC., Jr Antibodies reacting with cytoplasm of subthalamic and caudate nuclei neurons in chorea and acute rheumatic fever. J. Exp. Med. 1976;144:1094–110. doi: 10.1084/jem.144.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bronze MS, Dale JB. Epitopes of streptococcal M proteins that evoke antibodies that cross-react with human brain. J. Immunol. 1993;151:2820–28. [PubMed] [Google Scholar]
- 198.Church AJ, Cardoso F, Dale RC, Lees AJ, Thompson EJ, Giovannoni G. Anti-basal ganglia antibodies in acute and persistent Sydenham's chorea. Neurology. 2002;59:227–31. doi: 10.1212/wnl.59.2.227. [DOI] [PubMed] [Google Scholar]; First study to demonstrate the functional role of monoclonal antibody isolated from a Sydenham's chorea patient in neuronal cell signaling.
- 199.Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat. Med. 2003;9:914–20. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- 200.Kotby AA, El Badawy N, El Sokkary S, Moawad H, El Shawarby M. Antineuronal antibodies in rheumatic chorea. Clin. Diagn. Lab. Immunol. 1998;5:836–39. doi: 10.1128/cdli.5.6.836-839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Morshed SA, Parveen S, Leckman JF, Mercadante MT, Bittencourt Kiss MH, et al. Antibodies against neural, nuclear, cytoskeletal, and streptococcal epitopes in children and adults with Tourette's syndrome, Sydenham's chorea, and autoimmune disorders. Biol. Psychiatry. 2001;50:566–77. doi: 10.1016/s0006-3223(01)01096-4. [DOI] [PubMed] [Google Scholar]
- 202.Giedd JN, Rapoport JL, Kruesi MJ, Parker C, Schapiro MB, et al. Sydenham's chorea: magnetic resonance imaging of the basal ganglia. Neurology. 1995;45:2199–202. doi: 10.1212/wnl.45.12.2199. [DOI] [PubMed] [Google Scholar]
- 203.Lee PH, Nam HS, Lee KY, Lee BI, Lee JD. Serial brain SPECT images in a case of Sydenham chorea. Arch. Neurol. 1999;56:237–40. doi: 10.1001/archneur.56.2.237. [DOI] [PubMed] [Google Scholar]
- 204.Aron AM. Sydenham's chorea: positron emission tomographic (PET) scan studies. J. Child Neurol. 2005;20:832–33. doi: 10.1177/08830738050200101101. [DOI] [PubMed] [Google Scholar]
- 205.Weindl A, Kuwert T, Leenders KL, Poremba M, Gräfin von Einsiedel H, et al. Increased striatal glucose consumption in Sydenham's chorea. Mov. Disord. 1993;8:437–44. doi: 10.1002/mds.870080404. [DOI] [PubMed] [Google Scholar]
- 206.Goldman S, Amrom D, Szliwowski HB, Detemmerman D, Bidaut LM, et al. Reversible striatal hypermetabolism in a case of Sydenham's chorea. Mov. Disord. 1993;8:355–58. doi: 10.1002/mds.870080318. [DOI] [PubMed] [Google Scholar]
- 207.Citak EC, Gucuyener K, Karabacak NI, Serdaroglu A, Okuyaz C, Aydin K. Functional brain imaging in Sydenham's chorea and streptococcal tic disorders. J. Child Neurol. 2004;19:387–90. doi: 10.1177/088307380401900513. [DOI] [PubMed] [Google Scholar]
- 208.Hill A, Herkes GK, Roche P. SPECT and MRI findings in Sydenham's chorea. J. Neurol. Neurosurg. Psychiatry. 1994;57:763. doi: 10.1136/jnnp.57.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Heye N, Jergas M, Hotzinger H, Farahati J, Pohlau D, Przuntek H. Sydenham chorea: clinical, EEG, MRI and SPECT findings in the early stage of the disease. J. Neurol. 1993;240:121–23. doi: 10.1007/BF00858729. [DOI] [PubMed] [Google Scholar]
- 210.Demiroren K, Yavuz H, Cam L, Oran B, Karaaslan S, Demiroren S. Sydenham's chorea: a clinical follow-up of 65 patients. J. Child Neurol. 2007;22:550–54. doi: 10.1177/0883073807302614. [DOI] [PubMed] [Google Scholar]
- 211.Loiselle CR, Lee O, Moran TH, Singer HS. Striatal microinfusion of Tourette syndrome and PANDAS sera: failure to induce behavioral changes. Mov. Disord. 2004;19:390–96. doi: 10.1002/mds.10522. [DOI] [PubMed] [Google Scholar]
- 212.Ben-Pazi H, Sadan O, Offen D. Striatal microinjection of Sydenham chorea antibodies: using a rat model to examine the dopamine hypothesis. J. Mol. Neurosci. 2012;46:162–66. doi: 10.1007/s12031-011-9559-6. [DOI] [PubMed] [Google Scholar]
- 213.Hoffman KL, Hornig M, Yaddanapudi K, Jabado O, Lipkin WI. A murine model for neuropsychiatric disorders associated with group A β-hemolytic streptococcal infection. J. Neurosci. 2004;24:1780–91. doi: 10.1523/JNEUROSCI.0887-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yaddanapudi K, Hornig M, Serge R, De Miranda J, Baghban A, et al. Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol. Psychiatry. 2010;15:712–26. doi: 10.1038/mp.2009.77. [DOI] [PubMed] [Google Scholar]
- 215.Kirvan CA, Swedo SE, Kurahara D, Cunningham MW. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham's chorea. Autoimmunity. 2006;39:21–29. doi: 10.1080/08916930500484757. [DOI] [PubMed] [Google Scholar]
- 216.Kirvan CA, Cox CJ, Swedo SE, Cunningham MW. Tubulin is a neuronal target of autoantibodies in Sydenham's chorea. J. Immunol. 2007;178:7412–21. doi: 10.4049/jimmunol.178.11.7412. [DOI] [PubMed] [Google Scholar]
- 217.Brimberg L, Benhar I, Mascaro-Blanco A, Alvarez K, Lotan D, et al. Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology. 2012;37:2076–87. doi: 10.1038/npp.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Garvey MA, Snider LA, Leitman SF, Werden R, Swedo SE. Treatment of Sydenham's chorea with intravenous immunoglobulin, plasma exchange, or prednisone. J. Child Neurol. 2005;20:424–29. doi: 10.1177/08830738050200050601. [DOI] [PubMed] [Google Scholar]
- 219.Brückener KE, el Bayâ A, Galla HJ, Schmidt MA. Permeabilization in a cerebral endothelial barrier model by pertussis toxin involves the PKC effector pathway and is abolished by elevated levels of cAMP. J. Cell Sci. 2003;116:1837–46. doi: 10.1242/jcs.00378. [DOI] [PubMed] [Google Scholar]
- 220.Linthicum DS, Munoz JJ, Blaskett A. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell Immunol. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- 221.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N. Engl. J. Med. 2003;349:1543–54. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 222.Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349–61. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J. Neurosci. 2010;30:5866–75. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–94. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 226.Deisenhammer F, Bartos A, Egg R, Gilhus NE, Giovannoni G, et al. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur. J. Neurol. 2006;13:913–22. doi: 10.1111/j.1468-1331.2006.01493.x. [DOI] [PubMed] [Google Scholar]
- 227.Psimaras D, Carpentier AF, Rossi C. Cerebrospinal fluid study in paraneoplastic syndromes. J. Neurol. Neurosurg. Psychiatry. 2010;81:42–45. doi: 10.1136/jnnp.2008.159483. [DOI] [PubMed] [Google Scholar]
- 228.Stich O, Rauer S. Antigen-specific oligoclonal bands in cerebrospinal fluid and serum from patients with anti-amphiphysin- and anti-CV2/CRMP5 associated paraneoplastic neurological syndromes. Eur. J. Neurol. 2007;14:650–53. doi: 10.1111/j.1468-1331.2007.01802.x. [DOI] [PubMed] [Google Scholar]
- 229.Stich O, Graus F, Rasiah C, Rauer S. Qualitative evidence of anti-Yo-specific intrathecal antibody synthesis in patients with paraneoplastic cerebellar degeneration. J. Neuroimmunol. 2003;141:165–69. doi: 10.1016/s0165-5728(03)00257-1. [DOI] [PubMed] [Google Scholar]
- 230.Storstein A, Monstad SE, Honnorat J, Vedeler CA. Paraneoplastic antibodies detected by isoelectric focusing of cerebrospinal fluid and serum. J. Neuroimmunol. 2004;155:150–54. doi: 10.1016/j.jneuroim.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 231.Folli F, Solimena M, Cofiell R, Austoni M, Tallini G, et al. Autoantibodies to a 128-kd synaptic protein in three women with the stiff-man syndrome and breast cancer. N. Engl. J. Med. 1993;328:546–51. doi: 10.1056/NEJM199302253280805. [DOI] [PubMed] [Google Scholar]
- 232.Sommer C, Weishaupt A, Brinkhoff J, Biko L, Wessig C, et al. Paraneoplastic stiff-person syndrome: passive transfer to rats by means of IgG antibodies to amphiphysin. Lancet. 2005;365:1406–11. doi: 10.1016/S0140-6736(05)66376-3. [DOI] [PubMed] [Google Scholar]
- 233.Geis C, Weishaupt A, Hallermann S, Grünewald B, Wessig C, et al. Stiff person syndrome-associated autoantibodies to amphiphysin mediate reduced GABAergic inhibition. Brain. 2010;133:3166–80. doi: 10.1093/brain/awq253. [DOI] [PubMed] [Google Scholar]
- 234.Lahita RG. Systemic lupus erythematosus: learning disability in the male offspring of female patients and relationship to laterality. Psychoneuroendocrinology. 1988;13:385–96. doi: 10.1016/0306-4530(88)90045-5. [DOI] [PubMed] [Google Scholar]
- 234a.Vinet E, Bernatsky S, Pineau CA, Clarke AE, Platt R, et al. Increased male-to-female ratio in children born to women with systemic lupus erythematosus. Arthritis Rheum. 2013 doi: 10.1002/art.37852. In press. [DOI] [PubMed] [Google Scholar]
- 235.Atladóttir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124:687–94. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]; A significant association between maternal history of celiac disease or rheumatoid arthritis and autism spectrum disorder was observed for the first time
- 236.Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, et al. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol. Psychiatry. 2008;64:583–88. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J. Neuroimmunol. 2008;194:165–72. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 238.Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, et al. Maternal neuronal antibodies associated with autism and a language disorder. Ann. Neurol. 2003;53:533–37. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- 239.Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J. Amaral DG. 2008. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav. Immun. 22:806–16. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Diamond B, Bloom O, Al Abed Y, Kowal C, Huerta PT. Volpe BT. 2011. Moving towards a cure: blocking pathogenic antibodies in systemic lupus erythematosus. J. Intern. Med. 269:36–44. doi: 10.1111/j.1365-2796.2010.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bloom O, Cheng KF, He M, Papatheodorou A, Volpe BT, et al. Generation of a unique small molecule peptidomimetic that neutralizes lupus autoantibody activity. Proc. Natl. Acad. Sci. USA. 2011;108:10255–59. doi: 10.1073/pnas.1103555108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sollid LM, Jabri B. Celiac disease and transglutaminase 2: a model for posttranslational modification of antigens and HLA association in the pathogenesis of autoimmune disorders. Curr. Opin. Immunol. 2011;23:732–38. doi: 10.1016/j.coi.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hadjivassiliou M, Sanders DS, Grünewald RA, Woodroofe N, Boscolo S, Aeschlimann D. Gluten sensitivity: from gut to brain. Lancet Neurol. 2010;9:318–30. doi: 10.1016/S1474-4422(09)70290-X. [DOI] [PubMed] [Google Scholar]
- 244.Lee SM, Dunnavant FD, Jang H, Zunt J, Levin MC. Autoantibodies that recognize functional domains of hnRNPA1 implicate molecular mimicry in the pathogenesis of neurological disease. Neurosci. Lett. 2006;401:188–93. doi: 10.1016/j.neulet.2006.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jernigan M, Morcos Y, Lee SM, Dohan FC, Jr, Raine C, Levin MC. IgG in brain correlates with clinicopathological damage in HTLV-1 associated neurologic disease. Neurology. 2003;60:1320–27. doi: 10.1212/01.wnl.0000059866.03880.ba. [DOI] [PubMed] [Google Scholar]
- 246.Kalume F, Lee SM, Morcos Y, Callaway JC, Levin MC. Molecular mimicry: cross-reactive antibodies from patients with immune-mediated neurologic disease inhibit neuronal firing. J. Neurosci. Res. 2004;77:82–89. doi: 10.1002/jnr.20137. [DOI] [PubMed] [Google Scholar]
- 247.Jarius S, Franciotta D, Paul F, Ruprecht K, Bergamaschi R, et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: frequency, origin, and diagnostic relevance. J. Neuroinflammation. 2010;7:52. doi: 10.1186/1742-2094-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur. J. Neurol. 2010;17:1019–32. doi: 10.1111/j.1468-1331.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- 249.Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–31. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- 250.Olsson T. White matter disease: roles of anti-MOG antibodies in demyelinating diseases. Nat. Rev. Neurol. 2011;7:248–49. doi: 10.1038/nrneurol.2011.45. [DOI] [PubMed] [Google Scholar]
- 251.Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58:143–46. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- 252.Nishikawa M, Ichiyama T, Hayashi T, Ouchi K, Furukawa S. Intravenous immunoglobulin therapy in acute disseminated encephalomyelitis. Pediatr. Neurol. 1999;21:583–86. doi: 10.1016/s0887-8994(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 253.Shahar E, Andraus J, Savitzki D, Pilar G, Zelnik N. Outcome of severe encephalomyelitis in children: effect of high-dose methylprednisolone and immunoglobulins. J. Child Neurol. 2002;17:810–14. doi: 10.1177/08830738020170111001. [DOI] [PubMed] [Google Scholar]
- 254.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am. J. Pathol. 1988;130:443–54. [PMC free article] [PubMed] [Google Scholar]
- 255.Arinuma Y, Yanagida T, Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2008;58:1130–35. doi: 10.1002/art.23399. [DOI] [PubMed] [Google Scholar]
- 256.Yoshio T, Okamoto H, Minota S. Antibodies to bovine serum albumin do not affect the results of enzyme-linked immunosorbent assays for IgG anti-NR2 glutamate receptor antibodies: reply to the letter by Hirohata et al. Arthritis Rheum. 2007;56:2813–14. doi: 10.1002/art.22827. [DOI] [PubMed] [Google Scholar]
- 257.Diamond B, Kowal C, Huerta PT, Aranow C, Mackay M, et al. Immunity and acquired alterations in cognition and emotion: lessons from SLE. Adv. Immunol. 2006;89:289–320. doi: 10.1016/S0065-2776(05)89007-8. [DOI] [PubMed] [Google Scholar]
- 258.Karassa FB, Afeltra A, Ambrozic A, Chang D-M, De Keyser F, et al. Accuracy of anti–ribosomal P protein antibody testing for the diagnosis of neuropsychiatric systemic lupus erythematosus: an international meta-analysis. Arthritis Rheum. 2006;54:312–24. doi: 10.1002/art.21539. [DOI] [PubMed] [Google Scholar]
- 259.Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J. Neuroimmunol. 2006;179:173–79. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 260.Alaedini A, Okamoto H, Briani C, Wollenberg K, Shill HA, et al. Immune cross-reactivity in celiac disease: Anti-gliadin antibodies bind to neuronal synapsin I. J. Immunol. 2007;178:6590–95. doi: 10.4049/jimmunol.178.10.6590. [DOI] [PubMed] [Google Scholar]
- 261.Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D. Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann. Neurol. 2008;64:332–43. doi: 10.1002/ana.21450. [DOI] [PubMed] [Google Scholar]
- 262.Sander HW, Magda P, Chin RL, Wu A, Brannagan TH, 3rd, et al. Cerebellar ataxia and coeliac disease. Lancet. 2003;362:1548. doi: 10.1016/S0140-6736(03)14743-5. [DOI] [PubMed] [Google Scholar]
- 263.Chez MG, Guido-Estrada N. Immune therapy in autism: historical experience and future directions with immunomodulatory therapy. Neurotherapeutics. 2010;7:293–301. doi: 10.1016/j.nurt.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann. Neurol. 2009;65:424–34. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pleasure D. Diagnostic and pathogenic significance of glutamate receptor autoantibodies. Arch. Neurol. 2008;65:589–92. doi: 10.1001/archneur.65.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–12. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 267.Cohen-Kashi Malina K, Ganor Y, Levite M, Teichberg VI. Autoantibodies against an extracellular peptide of the GluR3 subtype of AMPA receptors activate both homomeric and heteromeric AMPA receptor channels. Neurochem. Res. 2006;31:1181–90. doi: 10.1007/s11064-006-9143-6. [DOI] [PubMed] [Google Scholar]
- 268.Andrews PI, Dichter MA, Berkovic SF, Newton MR, McNamara JO. Plasmapheresis in Rasmussen's encephalitis. Neurology. 2001;57:S37–41. 1996. [PubMed] [Google Scholar]
- 269.Rogers SW, Andrews PI, Gahring LC, Whisenand T, Cauley K, et al. Autoantibodies to glutamate receptor GluR3 in Rasmussen's encephalitis. Science. 1994;265:648–51. doi: 10.1126/science.8036512. [DOI] [PubMed] [Google Scholar]
- 270.Whitney KD, McNamara JO. GluR3 autoantibodies destroy neural cells in a complement-dependent manner modulated by complement regulatory proteins. J. Neurosci. 2000;20:7307–16. doi: 10.1523/JNEUROSCI.20-19-07307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gini B, Lovato L, Cianti R, Cecotti L, Marconi S, et al. Novel autoantigens recognized by CSF IgG from Hashimoto's encephalitis revealed by a proteomic approach. J. Neuroimmunol. 2008;196:153–58. doi: 10.1016/j.jneuroim.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 272.Schiess N, Pardo CA. Hashimoto's encephalopathy. Ann. N. Y. Acad. Sci. 2008;1142:254–65. doi: 10.1196/annals.1444.018. [DOI] [PubMed] [Google Scholar]
- 273.Dale RC, Church AJ, Surtees RA, Lees AJ, Adcock JE, et al. Encephalitis lethargica syndrome: 20 new cases and evidence of basal ganglia autoimmunity. Brain. 2004;127:21–33. doi: 10.1093/brain/awh008. [DOI] [PubMed] [Google Scholar]
- 274.Dalakas MC, Fujii M, Li M, Lutfi B, Kyhos J, McElroy B. High-dose intravenous immune globulin for stiff-person syndrome. N. Engl. J. Med. 2001;345:1870–76. doi: 10.1056/NEJMoa01167. [DOI] [PubMed] [Google Scholar]
- 275.Butler MH, Hayashi A, Ohkoshi N, Villmann C, Becker CM, et al. Autoimmunity to gephyrin in Stiff-Man syndrome. Neuron. 2000;26:307–12. doi: 10.1016/s0896-6273(00)81165-4. [DOI] [PubMed] [Google Scholar]
- 276.Solimena M, Folli F, Denis-Donini S, Comi GC, Pozza G, et al. Autoantibodies to glutamic acid decarboxylase in a patient with stiff-man syndrome, epilepsy, and type I diabetes mellitus. N. Engl. J. Med. 1988;318:1012–20. doi: 10.1056/NEJM198804213181602. [DOI] [PubMed] [Google Scholar]
- 277.Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hernández-Echebarría L, Saiz A, Arés A, Tejada J, García-Tuñón L, et al. Paraneoplastic encephalomyelitis associated with pancreatic tumor and anti-GAD antibodies. Neurology. 2006;66:450–51. doi: 10.1212/01.wnl.0000196488.87746.7b. [DOI] [PubMed] [Google Scholar]
- 279.Saiz A, Blanco Y, Sabater L, González F, Bataller L, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131:2553–63. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 280.Bien CG, Vincent A, Barnett MH, Becker AJ, Blumcke I, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–38. doi: 10.1093/brain/aws082. [DOI] [PubMed] [Google Scholar]
- 281.Graus F, Keime-Guibert F, Reñe R, Benyahia B, Ribalta T, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124:1138–48. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- 282.Orange D, Frank M, Tian S, Dousmanis A, Marmur R, et al. Cellular immune suppression in paraneoplastic neurologic syndromes targeting intracellular antigens. Arch. Neurol. 2012;69:1132–40. doi: 10.1001/archneurol.2012.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Vernino S. Paraneoplastic cerebellar degeneration. Handb. Clin. Neurol. 2012;103:215–23. doi: 10.1016/B978-0-444-51892-7.00013-9. [DOI] [PubMed] [Google Scholar]
- 284.Bataller L, Wade DF, Graus F, Stacey HD, Rosenfeld MR, Dalmau J. Antibodies to Zic4 in paraneoplastic neurologic disorders and small-cell lung cancer. Neurology. 2004;62:778–82. doi: 10.1212/01.wnl.0000113749.77217.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Adamus G, Webb S, Shiraga S, Duvoisin RM. Anti-recoverin antibodies induce an increase in intracellular calcium, leading to apoptosis in retinal cells. J. Autoimmun. 2006;26:146–53. doi: 10.1016/j.jaut.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 286.Kastrup O, Meyring S, Diener HC. Atypical paraneoplastic brainstem encephalitis associated with anti-ri-antibodies due to thymic carcinoma with possible clinical response to immunoglobulins. Eur. Neurol. 2001;45:285–87. doi: 10.1159/000052145. [DOI] [PubMed] [Google Scholar]
- 287.Shams'ili S, Grefkens J, de Leeuw B, van den Bent M, Hooijkaas H, et al. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain. 2003;126:1409–18. doi: 10.1093/brain/awg133. [DOI] [PubMed] [Google Scholar]
- 288.Rojas-Marcos I, Rousseau A, Keime-Guibert F, Reñé R, Cartalat-Carel S, et al. Spectrum of paraneoplastic neurologic disorders in women with breast and gynecologic cancer. Medicine. 2003;82:216–23. doi: 10.1097/01.md.0000076004.64510.ce. [DOI] [PubMed] [Google Scholar]
- 289.Kerasnoudis A, Rockhoff M, Federlein J, Gold R, Krogias C. Isolated ZIC4 antibodies in paraneoplastic cerebellar syndrome with an underlying ovarian tumor. Arch. Neurol. 2011;68:1073. doi: 10.1001/archneurol.2011.176. [DOI] [PubMed] [Google Scholar]
- 290.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Vernino S, Tuite P, Adler CH, Meschia JF, Boeve BF, et al. Paraneoplastic chorea associated with CRMP-5 neuronal antibody and lung carcinoma. Ann. Neurol. 51:625–30. doi: 10.1002/ana.10178. [DOI] [PubMed] [Google Scholar]