Abstract
Granulation, the process of particle enlargement by agglomeration technique, is one of the most significant unit operations in the production of pharmaceutical dosage forms, mostly tablets and capsules. Granulation process transforms fine powders into free-flowing, dust-free granules that are easy to compress. Nevertheless, granulation poses numerous challenges due to high quality requirement of the formed granules in terms of content uniformity and physicochemical properties such as granule size, bulk density, porosity, hardness, moisture, compressibility, etc. together with physical and chemical stability of the drug. Granulation process can be divided into two types: wet granulation that utilize a liquid in the process and dry granulation that requires no liquid. The type of process selection requires thorough knowledge of physicochemical properties of the drug, excipients, required flow and release properties, to name a few. Among currently available technologies, spray drying, roller compaction, high shear mixing, and fluid bed granulation are worth of note. Like any other scientific field, pharmaceutical granulation technology also continues to change, and arrival of novel and innovative technologies are inevitable. This review focuses on the recent progress in the granulation techniques and technologies such as pneumatic dry granulation, reverse wet granulation, steam granulation, moisture-activated dry granulation, thermal adhesion granulation, freeze granulation, and foamed binder or foam granulation. This review gives an overview of these with a short description about each development along with its significance and limitations.
Keywords: Granulation technique and technology, Pneumatic dry granulation, Reverse wet granulation, Steam granulation, Moisture-activated dry granulation, Thermal adhesion granulation
Introduction
Granulation, a technique of particle enlargement by agglomeration, is one of the most significant unit operations in the production of pharmaceutical dosage forms, mostly tablets and capsules.1 During the granulation process, small fine or coarse particles are converted into large agglomerates called granules. Generally, granulation commences after initial dry mixing of the necessary powder ingredients along with the active pharmaceutical ingredient (API), so that a uniform distribution of each ingredient throughout the powder mixture is achieved. Although granules used in the pharmaceutical industry have particle size in the range of 0.2-4.0 mm, they are primarily produced as an intermediary with a size range of 0.2-0.5 mm to be either packed as a dosage form or be mixed with other excipients before tablet compaction or capsule filling.1,2
Granules are produced to enhance the uniformity of the API in the final product, to increase the density of the blend so that it occupies less volume per unit weight for better storage and shipment, to facilitate metering or volumetric dispensing, to reduce dust during granulation process to reduce toxic exposure and process-related hazards, and to improve the appearance of the product.2 Consequently, the ideal characteristics of granules include spherical shape for improved flow, narrow particle size distribution for content uniformity and volumetric dispensing, sufficient fines to fill void spaces between granules for better compaction and compression characteristics, and adequate moisture and hardness to prevent breaking and dust formation during process.
Granulation is an exemplary of particle design and the properties of the particles acquired after granulation depend on particle size of the drug and excipients, the type, concentration, and volume of binder and/or solvents, granulation time, type of granulator, drying rate (temperature and time), etc. The primary methods by which the agglomerated granules are formed include solid bridges, sintering, chemical reaction, crystallization and deposition of colloidal particles.1,3 Besides, binding can also be accomplished through adhesive and cohesive forces by utilizing high viscous binders. The series of mechanisms by which granules are formed from the powder particles encompass wetting and nucleation, coalescence or growth, consolidation, and attrition or breakage.3-5
Blend of powders containing pharmaceutical excipients and API can be compressed into tablets either by direct compression or after making granules by agglomeration or granulation techniques ( Fig. 1). The granulation technique may be widely categorized in to two types, dry granulation and wet granulation, based on the type of method used to facilitate the agglomeration of powder particles ( Fig. 1). Dry granulation uses mechanical compression (slugs) or compaction (roller compaction) to facilitate the agglomeration of dry powder particles, while the wet granulation uses granulation liquid (binder/solvent) to facilitate the agglomeration by formation of wet mass by adhesion. Among these two techniques, wet granulation is the most widespread granulation technique used despite the fact that it involves multiple unit processes such as wet massing, drying and screening, which are complex, time consuming, and expensive requiring large space and multiple equipment.1,2,5
Fig. 1 .
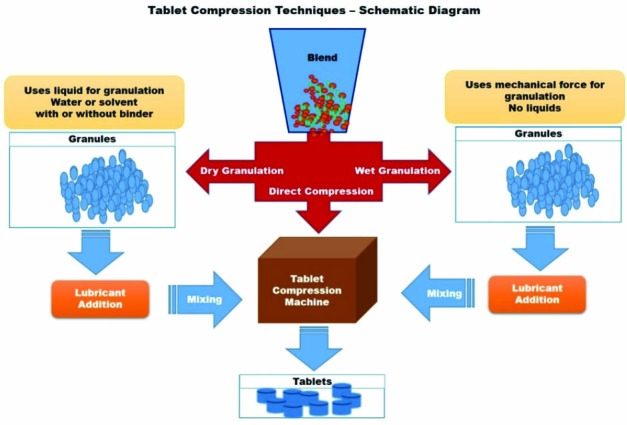
Schematic diagram of tablet compression techniques
The type of process selection requires thorough knowledge of physicochemical properties of the drug, excipients, required flow and release properties, etc. Granulation technologies like roller compaction, spray drying, supercritical fluid, low/high shear mixing, fluid bed granulation, extrusion/spheronization, etc. have been successful for many decades in the preparation of various pharmaceutical dosage forms. Pharmaceutical granulation technology continues to change, and various improved, modified, and novel techniques and technologies have been made available along the course. The aim of this review is to give the reader a glimpse of the latest techniques and technologies with regard to pharmaceutical granulation. Subsequently, this review gives a short description about each development along with its significance and limitations, which are summarized in Table 1.
Table 1 . Summary of recent progresses in granulation techniques and technologies .
|
Techniques/
technologies |
Description | Granule Characteristics | Merits | Limitations | Equipment |
|
Pneumatic dry
granulation |
• Dry granulation • Mild Compaction and pneumatic classification |
√ Porous, highly compressible √ Taste masking √ Fast disintegration √ Release time modification |
↑ Drug loading √ Thermolabile and moisture sensitive drugs ↑ Product stability ↓ Cost and waste |
X Recycled granule quality X Segregation potential X Friability |
√ Roller compaction with air stream or vacuum |
| Reverse wet granulation |
• Wet granulation • Water or solvent is granulating liquid |
√ Uniform wetting √ Uniform erosion |
↓ Particle size √ Spherical shape √ Poorly water soluble drugs |
X Larger particle size1 X Lower porosity X Many problems similar to conventional wet granulation |
√ High speed mixer |
| Steam granulation |
• Wet granulation • Steam is granulating liquid |
↑ Diffusionrate ↑ Uniform distribution ↑ Surface area √ Spherical shape |
√ Eco-friendly √ Sterility Process time √ No solvent use √ No health hazards |
X Local over heating/wetting X High energy inputs X Thermolabile drugs X Limited binders |
√ High speed mixer with steam generator/regulator |
| Moisture-Activated Dry Granulation |
• Wet granulation 1-4% water is granulating liquid and moisture-absorbing material |
√ Uniform size ↑ Flowability ↑ Compressibility |
√ Less energy input √ No drying process √ Wide applicability √ Continuous processing ↓ Shorter process time Process variables |
X Moisture sensitive drugs X Impossible high drug loading X Limited absorbents |
√ High-shear mixer coupled with a sprayer |
| Thermal adhesion granulation |
• Wet granulation • Low water/solvent is granulating liquid and heating at 30-130 °C |
√ Flowability √ Friability Tensile strength |
↑ Drug loading √ No drying process Dust |
X High energy inputs X Thermolabile and moisture sensitive drugs X Limited binders |
√ Tumble blender or similar equipment coupled with heating system |
| Melt granulation |
• Wet granulation • Meltable binder as granulating liquid, heating at 50–90 ◦C |
√ Possible modified release ↑ Dissolution |
√ No water or solvent √ No drying process ↓ Energy input ↓ Cost and process time √ Water sensitive drugs |
X Thermolabile drugs X Limited binders |
√ High shear mixer √ Fluidized bed |
| Freeze granulation |
• Wet granulation • Spray freezing and subsequent freeze drying for slurry or suspensions |
√ Uniform size √ Flowability √ Spherical shape |
↑ Granule homogeneity √ Thermolabile drugs √ Granule density control ↓ Material waste |
X Limited solvent medium X Only suitable for conversion of liquid slurry or suspension to granules |
√ Spray freezer coupled with freeze dryer |
| Foam granulation |
• Wet granulation • Foam as granulating liquid |
√ Uniform binder distribution √ No over wetting ↑ Surface area |
↓ Water requirement No spray nozzle use √ Low water required ↓ Cost and process time √ Water sensitive drugs |
X Moisture sensitive drugs X Limited binders |
√ High shear mixer or fluidized bed granulator coupled with foam generator/regulator |
↓ reduced or decreased; ↑ increased or high; √ possibility or suitability or availability; X Unsuitable or not applicable. 1 at lower binder concentration.
Recent progress in dry granulation
Dry granulation could be achieved either by roller compaction or by slugging. The two different types are illustrated in the schematic diagram Fig. 2. There has not been much progress in the dry granulation technique and technology in comparison to wet granulation, except for one important innovation known as pneumatic dry granulation technology developed by Atacama LabsOy (Helsinki, Finland), which is described below.6 The description of its significance and limitations are summarized in Table 1.
Fig. 2 .
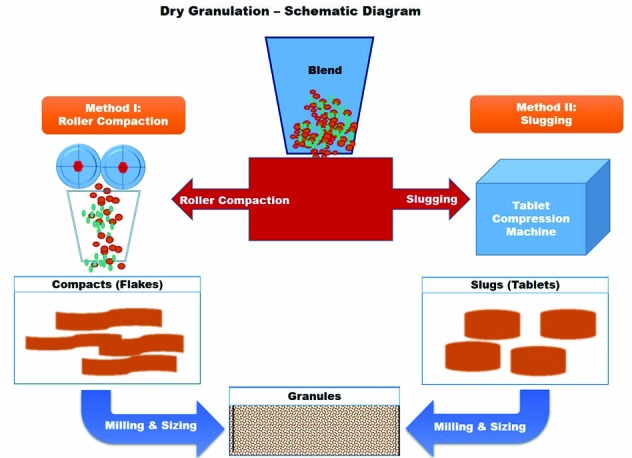
Schematic diagram of dry granulation and two different techniques. Method I is roller compaction and Method II is slugging.
Pneumatic Dry Granulation (PDG)
Pneumatic dry granulation (PDG), an innovative dry granulation technology, utilizes roller compaction together with a proprietary air classification method to produce granules with extraordinary combination of flowability and compressibility.6,7 In this method, granules are produced from powder particles by initially applying mild compaction force by roller compactor to produce a compacted mass comprising a mixture of fine particles and granules. The fine particles and/or smaller granules are separated from the intended size granules in a fractioning chamber by entraining in a gas stream (pneumatic system), whereas the intended size granules pass through the fractioning chamber to be compressed into tablets. The entrained fine particles and/or small granules are then transferred to a device such as a cyclone and are either returned to the roller compactor for immediate re-processing (recycling or recirculation process) or placed in a container for reprocessing later to achieve the granules of desired size.7,8 The schematic diagram of this process is represented as Fig. 3.
Fig. 3 .
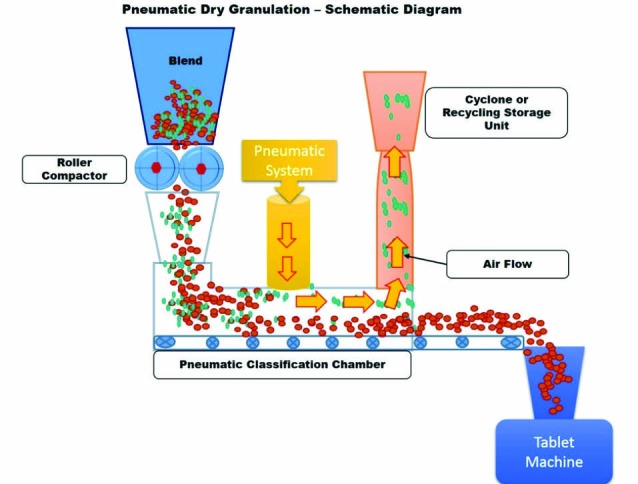
Schematic diagram of pneumatic dry granulation
PDG technology could successfully be used to produce good flowing granules for any formulations that produce compacts with a tensile strength of ~ 0.5 MPa. Also, this technology enables the use of high drug loads of up to 70-100%, because sufficient flowability could be achieved even at lower roll compaction forces (lower solid fractions) compared to usual roller compaction.9 In addition to these, this technology avails various other benefits such as faster processing speed, low cost, little or no material wastage, low dust exposure due to the closed nature of this unit, etc. However, the influence of recycling on the granule quality, suitability with low dose formulations, friability, etc. remains a major issues regarding this technology. The description of its significance and limitations are summarized in Table 1.
Recent progress in wet granulation
Wet granulation is the widely used technique and the granules are produced by wet massing of the excipients and API with granulation liquid with or without binder. The steps involved in conventional wet granulation technique could be seen in Fig. 4. Wet granulation has witnessed various technical and technological innovations such as steam granulation, moisture-activated dry granulation or moist granulation, thermal adhesion granulation, melt granulation, freeze granulation, foamed binder or foam granulation, and reverse wet granulation. The significance and limitations of the recent wet granulation techniques and technologies are summarized in Table 1.
Fig. 4 .
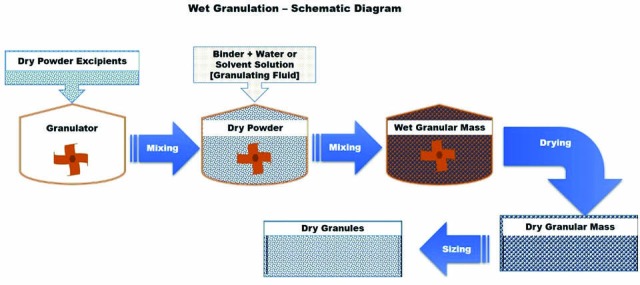
Schematic diagram of conventional wet granulation
Reverse wet granulation
Reverse wet granulation or reverse-phase wet granulation is a new development in the wet granulation technique that involves the immersion of the dry powder formulation into the binder liquid followed by controlled breakage to form granules.10 According to this invention, the binder solution was prepared initially and the dry powder excipients were added to the binder solution under mixing in granulator. Alternatively, the drug was mixed with a solution of hydrophilic polymer and/or binder to form a drug-polymer/binder slurry as a granulating fluid. Granules were then formed by immersing a mixture of other dry excipients into the drug-polymer/binder slurry. The resulted wet granules were milled after drying. The granules produced by this process were found to have good flow and handling characteristics like those produced with wet granulation process. In addition, tablets formed from these granules eroded more uniformly during dissolution testing as compared to usual wet granulation technique. The schematic diagram of this process is presented in Fig. 5.
Fig. 5 .
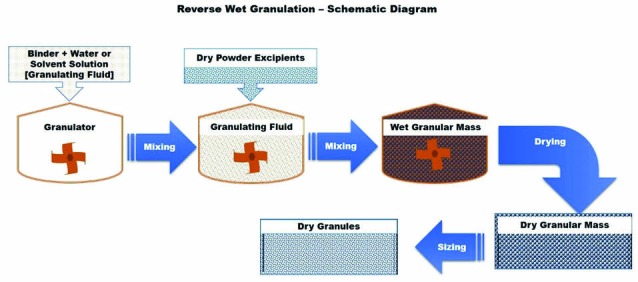
Schematic diagram of reverse wet granulation
Controlled breakage was proposed to be the predominant granule formation mechanisms in reverse wet granulation technique.11,12 It is purported that this technique improves the dissolution characteristics of the poorly water-soluble drugs by allowing uniform distribution of the binder that acts as a wetting agent and enable adequate wetting of the drug substance during granulation. It also increases the chances of adequate and uniform contact between the drug and hydrophilic polymer for better dissolution. These improved granule characteristics result in even erosion of tablets during dissolution.11,12
The advantages of this technique over conventional wet granulation include small and spherical-shaped granules with improved flow properties, uniform wetting and erosion of the granules. This technique could be suitable for poorly water-soluble drugs because of the intimate association between a drug and the polymer. Usability of currently available equipment such as high speed mixer is another merit of this technique. However, this technique produced granules with a greater mass mean diameter and lower intragranular porosity when compared to the conventional wet granulation at lower binder concentrations.11,12
Steam Granulation
In steam granulation as a new wet granulation technique, water steam is used as binder instead of traditional liquid water as granulation liquid.13 Fig. 6 shows the schematic diagram of steam granulation. Steam, at its pure form is transparent gas, and provides a higher diffusion rate into the powder and a more favorable thermal balance during the drying step. After condensation of the steam, water forms a hot thin film on the powder particles, requiring only a small amount of extra energy for its elimination, and evaporates more easily.13,14
Fig. 6 .
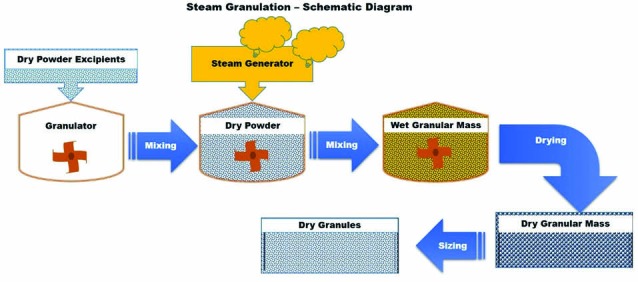
Schematic diagram of steam granulation
The advantages of this process include the higher ability of the steam to distribute uniformly and diffuse into the powder particles, production of spherical granules with larger surface area, and shorter processing time ecofriendly (no involvement of organic solvents). An equipment such as high-shear mixer coupled with a steam generator would be enough for this technique. However, this method requires high energy inputs for steam generation. Besides, this process is not suitable for all binders and is sensitive to thermolabile drugs. The granules produced by this process have higher dissolution rate due to increased surface area of the granules compared to conventional wet granulation process.13-17
Moisture-Activated Dry Granulation (MADG)
This technique is a variation of conventional wet granulation technique. It uses very little water to activate a binder and initiate agglomeration.18 This technique involves two steps, 1) wet agglomeration of the powder particles, and 2) moisture absorption or distribution. Agglomeration is facilitated by adding a small amount of water, usually less than 5% (1-4% preferably), to the mixture of drug, binder and other excipients. The two steps of this MADG are presented in Fig. 7. Agglomeration takes place when the granulating fluid (water) activates the binder. Once the agglomeration is achieved, moisture-absorbing material such as microcrystalline cellulose, silicon dioxide, etc. is added to facilitate the absorption of excess moisture. The moisture absorbents absorb the moisture from the agglomerates, resulting in moisture redistribution within the powder mixture, leading to relatively dry granule mixture. During this moisture redistribution process, some of the agglomerates remain intact in size without change, while some larger agglomerates may break leading to more uniform particle size distribution. It does not require an expensive drying step.19-21
Fig. 7 .
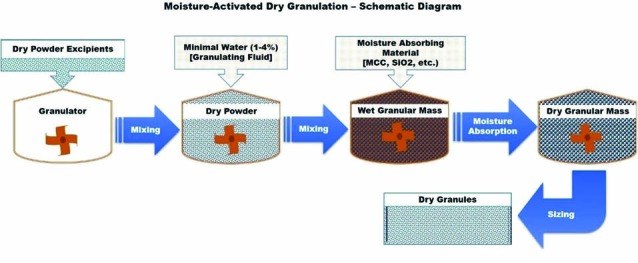
Schematic diagram of moisture-activated dry granulation
The process does not lead to larger lumps formation since the amount of water used is very small compared to usual wet granulation. The particle size of the agglomerates is mainly accounted to be in the range of 150-500 µm. This technique is also known as “moist granulation technique” leading to confusions with the use of appropriate terminology. Some authors believe that dry granulation involves the use of a roller compaction or a slugging step followed by milling to obtain granules.19 However, this technique did not use either of those steps. Besides, given that this technique utilizes a small amount of water, the use of the term “dry granulation” would be inappropriate. Therefore, the authors believe that “moist granulation” would be an appropriate terminology for this technique. In either case, the technique is the same and this review uses the terminology “Moisture-Activated Dry Granulation (MADG)” coined by the inventors of this technique in 1987.18
The application of MADG to an immediate-release and controlled-release dosage forms showed the advantages of wet granulation such as increased particle size, better flow and compressibility.20,21 Additional advantages of this technique include wide applicability, time efficiency and less energy input, and involvement of few process variables with suitability of continuous process. However, this technique could not be used for the preparation of granules that require high drug load and for moisture sensitive drugs and hygroscopic drugs due to stability and processing problems associated with these types of drugs. A high-shear mixer coupled with a sprayer would be a suitable equipment for the MADG process. An ideal machine should be equipped with efficient impellers, blades, and choppers to allow good mass movement and proper mixing of the granulation mass.18-25
Thermal Adhesion Granulation (TAG)
Wei-Ming Pharmaceutical Company (Taipei, Taiwan) has developed this technique, and the thermal adhesion granulation, analogous to moist granulation, utilizes addition of a small amount of granulation liquid and heat for agglomeration.26 This is clearly presented in Fig. 8 as a schematic diagram. Unlike moisture activated dry granulation which uses water alone as granulation liquid, this process uses both water and solvent as granulation liquid. In addition to this, heat is used to facilitate the granulation process. In this process, the drug and excipient mixture is heated to a temperature range of 30–130 °C in a closed system under tumble rotation to facilitate the agglomeration of the powder particles. This technique eliminates the drying process due to the addition of low amount of granulation liquid, which is mostly consumed by the powder particles during agglomeration. Granules of the required particle size can be obtained after cooling and sieving.26,27
Fig. 8 .
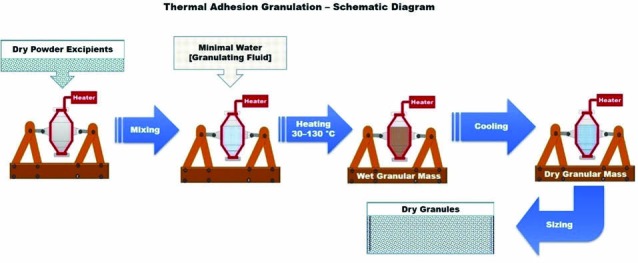
Schematic diagram of thermal adhesion granulation
This technique is quite simple and convenient with low moisture and binder contents in a closed system for preparing highly compressible materials or for modifying the poor characteristics of excipients. Besides, this technique provides granules with better particle size, good flow properties and high tensile strength that could be directly compressed into tablets with adequate hardness and low friability. The limitations of this technique are requirement of considerably high energy inputs and special equipment for heat generation and regulation. This technique is not suitable for all binders and is sensitive to thermolabile drugs.26-29
Melt granulation
Melt granulation or thermoplastic granulation is a technique that facilitates the agglomeration of powder particles using meltable binders, which melts or softens at relatively low temperature (50–90 °C).30 Fig. 9 represents the schematic diagram of melt granulation. Cooling of the agglomerated powder and the consequent solidification of the molten or soften binder complete the granulation process.31,32 Low melting binders can be added to the granulation process either in the form of solid particles that melt during the process (melt-in procedure or in situ melt granulation) or in the form of molten liquid, optionally containing the dispersed drug (spray-on or pump-on procedure), which displays a variety of options to design final granular properties. More specifically, the melt-in procedure of melt granulation process includes heating a mixture of drug, binder and other excipients to a temperature within or above the melting range of the binder. On the contrary, the spray-on procedure encompasses spraying of a molten binder, optionally containing the drug, onto the heated powders.33-35
Fig. 9 .
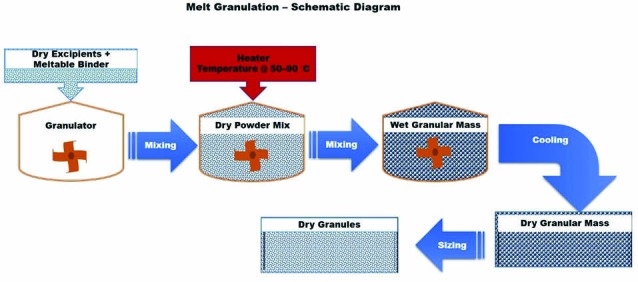
Schematic diagram of melt granulation
Melt granulation is an appropriate alternative to other wet granulation techniques which are used for water sensitive materials.36 Moreover, in comparison with the conventional wet granulation process, it proposes several advantages.31,32,34,37,38 Generally, organic or aqueous solvents are not demanded for the melt granulation process, hence the environmental requirements of organic solvent capture and recycling are eliminated, while the absence of water excludes the wetting and drying phases, making the entire process less energy- and time-consuming. Melt granulation method could be efficiently applied in order to enhance the stability of moisture sensitive drug and further to improve the poor physical properties of the drug substance.36,39 The major drawback of this process is the need of high temperature during the process, which can cause degradation and/or oxidative instability of the ingredients, especially of the thermolabile drugs.
The binders used for this process could be either hydrophilic or hydrophobic. The selection of a meltable binder with a hydrophilic/hydrophobic feature is critical factor for the dissolution behavior of the drugs. The equipments that could be used for melt granulation are high-shear mixer and fluidized bed granulator.33,38,40-42 Interest in melt granulation has increased in recent years, owing to the numerous advantages of this technique over conventional wet granulation process.
Freeze granulation
Freeze granulation technology, spray freezing and subsequent freeze drying, involves spraying droplets of a liquid slurry or suspension into liquid nitrogen followed by freeze-drying of the frozen droplets.43 By spraying a powder suspension into liquid nitrogen, the drops are instantly frozen into granules, and in the subsequent freeze drying process, the granules are dried by sublimation of ice without any segregation effects. The above-mentioned steps are depicted as schematic diagram in Fig. 10. This process yields spherical free-flowing granules that could be formed by using both water based and solvent based slurries. The significance of this technology is that the structure and homogeneity of the particles in the slurry or suspension are retained in the granules. Although various kinds of material in dispersed form can be granulated using this technology, it is suitable for the preparation of fine powder mixes with proper additives for subsequent processing. 43-45
Fig. 10 .
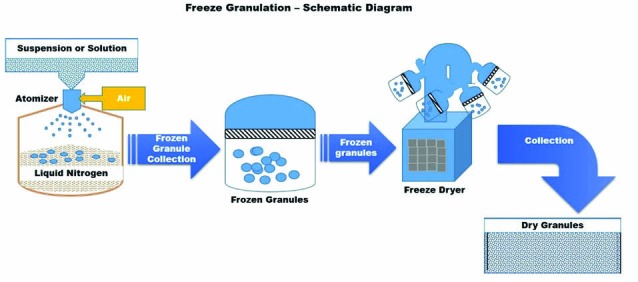
Schematic diagram of freeze granulation
This technology could be useful for the preparation of granules that needs to be prepared from suspensions whose particle size and homogeneity need to be preserved. Eventually, re-dispersible parenteral formulations, nanomaterials, solid self-emulsifying drug delivery systems, etc. could benefit from this technology given its ability to maintain size and homogeneity. The suspension quality always determines and reflects the granule quality in terms of homogeneity. In pharmaceutical industry, the low-temperature and soft freeze drying has vital advantage to minimize damage of organic compounds and improve stability and/or solubility. According to PowderPro AB, compared with spray drying, freeze granulation obviously produces protein particles with light and porous characteristics, and making powders with superior aerosol performance due to favorable aerodynamic properties.43-45
The major advantages of this process include ability to control the granule density through the solid content of the suspension, preparation of granules with no cavities, a high degree of granule homogeneity due to the absence of migration of small particles and/or binder molecules, use of heat sensitive compounds due to mild drying procedure, high product yield due to low waste of material, and possibility of recycling organic solvents. Although organic solvents with suitable freezing point (-25 to +10 °C) can be used, water as medium is preferred in this process, which could be a limiting criteria given the poor solubility of various drugs and processing excipients. Originally, this process was developed by Swedish Ceramic Institute in the late 1980.43-45 Currently, PowderPro AB, the spinoff company (year 2000) from Swedish Ceramic Institute, develops, manufactures, markets, and sells equipment for freeze granulation.
Foam granulation
Foam granulation or foamed binder granulation technology, analogous to spray agglomeration, involves the addition of liquid/aqueous binder as foam instead of spraying or pouring liquid onto the powder particles. Fig. 11 shows the schematic diagram of this technology. This foam binder technology was first introduced by Dow Chemical Company (Midland, MI) in 2003 for delivering aqueous binder systems in high shear and fluid bed wet granulation applications.46 A foam generator can be installed in the binder solution tank with high-shear granulator or fluid bed granulator to introduce the binder as foam rather than spraying or pouring in binder onto the moving powder particles. Adding the binder solution as foam rather than a spray eliminates the problems of inconsistent and unpredictable binder distribution that can affect tablet hardness and drug release.
Fig. 11 .
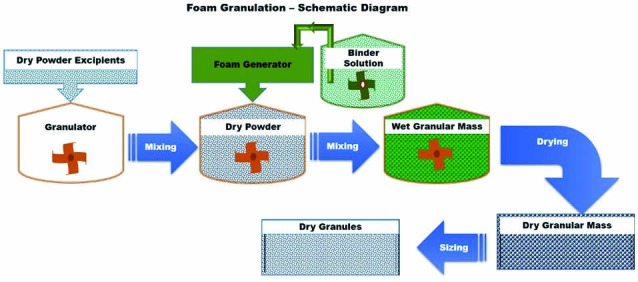
Schematic diagram of foam granulation
The surface area and volume of the foamed binder/water are phenomenally high compared to the sprayed water. This technology exploits the characteristics of the foamed binder to successfully improve the distribution of binder onto the powder particles, even at a binder amount lower than that required in the conventional spray granulation method. Besides, the sprayed liquid droplets have a low spread-to-soak ratio, which means they tend to soak into powders and cause overwetting rather than spreading on the surface of the particles, requiring high levels of water and binder, and eventually drying to remove excess water. On the contrary, foamed binders have a high spread-to-soak ratio, and because of this the binders are coated onto the particles rather than soaked, leading to less amount of binder and more consistent binder distribution. These factors improve the reproducibility and shorten the processing time. Most importantly, this technology eliminates the spray nozzles and its related processing variables and clogging problems.47
In addition to the above-mentioned advantages, this technology would prove useful for high potent/low dose drug formulations due to its ability to distribute drugs evenly. Due to the involvement of low amount of water and short process time, water sensitive formulations could also be prepared using this technology in addition to immediate release and controlled release formulations. Standard equipment such as high/low shear mixer, fluid bed granulator, etc. could be used for this technology in association with a foam generator. Although this technology merits in myriad ways, further understanding of foam quality, process parameters, equipment, flow patterns, mixing behavior, etc. needs to be explored. Besides, the regulatory approval would be a huge hurdle that needs to be overcome.46-50
Concluding Remarks
Technical and technological innovations that improve and ease existing processes could contribute to improved processability and quality of the product formulations in addition to a substantial impact on the product development, time and economy. Obviously, the pharmaceutical granulation techniques and technologies have improved over the years. Nevertheless, efficient and cost-effective manufacturing methods have always been the keen interest of the pharmaceutical industries, which catapults the research and development of new and improved technologies by the interdisciplinary scientists of pharmaceutical companies globally. During the formulation development, each drug substance poses a unique challenge that must be taken into consideration at the process selection stage by the formulation development scientists. Each technique has its own merits and limitations, and the type of technique and technology selection requires thorough knowledge of physicochemical properties of the drug, excipients, required flow and release properties, etc. in addition to the granulation techniques and technologies itself. This review discussed the recent developments in granulation technology for conventional release dosage formulations only. Nonetheless, when it comes to orally disintegrating tablets (ODTs), new technologies like Orasolv®, Durasolv®, Wowtab®, Flashtab®, Zydis®, Flashdose®, Oraquick®, Lyoc®, Advatab®, Frosta®, Quick-Disc® and Nanomelt® have been introduced by various pharmaceutical companies for the production of ODTs, which is beyond the scope of this review. In the pharmaceutical industry, although various technologies have been introduced from time to time, only few have emerged as successful for real time utilization due to different kinds of hurdles such as manufacturing efficiency, economy, regulatory issues, etc. The author’s opinion is that the new techniques and technologies discussed in this review would need enhancements in terms of equipment, process, etc. before being industrialized successfully. Nevertheless, these could provide a platform for further technological innovation.
Acknowledgements
I greatly appreciate Dinesh Babu Nithyanandha Babu for his remarkable help in providing resource and proof reading of this review. I also appreciate Saravanakumar Selvaraj for his support in providing resources for this review.
Ethical issues
Author declares no ethical issues.
Competing interests
There is no known conflict of interest.
Research Highlights
√ Granulation is a technique of particle enlargement by agglomeration in the production of tablets and capsules.
√ During the granulation process, small fine or coarse particles are converted into larger agglomerates called granules.
√ Granules enhance the uniformity of the API, increase the density of the blend, facilitate metering or volumetric dispensing, reduce dust, and improve the appearance of product.
√ The granules are formed by the following methods: solid bridges, sintering, chemical reaction, crystallization, and deposition of colloidal particles.
√ Granules are formed from the powder particles by wetting and nucleation, coalescence or growth, consolidation, and attrition or breakage.
√ Granulation technique is broadly classified into two types, dry granulation and wet granulation, with wet granulation being the most widely used granulation technique.
√ Currently available granulation technologies include roller compaction for dry granulation, and spray drying, supercritical fluid, low/high shear mixing, fluid bed granulation, and extrusion/spheronization for wet granulation.
√ Recent progress includes pneumatic dry granulation technology for dry granulation, and reverse wet granulation, steam granulation, moisture-activated dry granulation or moist granulation, thermal adhesion granulation, melt granulation, freeze granulation, foamed binder or foam granulation for wet granulation.
References
- 1. Anonymous. Handbook of Pharmaceutical Granulation Technology. 3rd ed. Parikh DM, editor. Marcel Dekker, INC.; 2009.
- 2. Anonymous. Pharmaceutics: The Science of Dosage Form Design. 2nd ed. Churchill Livingstone; 2001.
- 3. BJ E, J L. Size enlargement and size reduction. In: Green Don W, PR H, editors. Perry’s Chemical Engineers’ Handbook. 7th ed. New York: McGraw-Hill; 1994.
- 4. BJ E. Solids-solids processing. In: D G, P R, editors. Perry’s Chemical Engineers’ Handbook. 8th ed. New York: McGraw Hill; 2005.
- 5.Iveson SM, Litster JD, Hapgood K, Ennis BJ. Nucleation, growth and breakage phenomena in agitated wet granulation processes: a review. Powder Technology. 2001;117:3–39. doi: 10.1016/S0032-5910(01)00313-8. [DOI] [Google Scholar]
- 6. Politi G, Heilakka E. Granules, tablets and granulation. Google Patents; 2008.
- 7. Politi G, Heilakka E. Method and apparatus for dry granulation. Google Patents; 2009.
- 8. Heilakka E, Rahja P, Lammens R, Sandler N, editors. Pneumatic Dry Granulation (PDG) in solid dosage form manufacture. AAPS Annual Meeting and Exposition 2010 November 14–18; New Orleans.
- 9.Sandler N, Lammens RF. Pneumatic dry granulation: potential to improve roller compaction technology in drug manufacture. Expert Opin Drug Deliv. 2011;8:225–36. doi: 10.1517/17425247.2011.548382. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Reynolds TD. Granulates, process for preparing them and pharmaceutical products containing them. Google Patents. 2010 [Google Scholar]
- 11.Wade JB, Martin GP, Long DF. Feasibility assessment for a novel reverse-phase wet granulation process: The effect of liquid saturation and binder liquid viscosity. Int J Pharm. 2014;475:450–61. doi: 10.1016/j.ijpharm.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Wade JB, Martin GP, Long DF. Controlling granule size through breakage in a novel reverse-phase wet granulation process; the effect of impeller speed and binder liquid viscosity. Int J Pharm. 2014 doi: 10.1016/j.ijpharm.2014.11.067. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez L, Cavallari C, Passerini N, Albertini B, Gonzalez-Rodriguez M, Fini A. Preparation and characterization by morphological analysis of diclofenac/PEG 4000 granules obtained using three different techniques. Int J Pharm. 2002;242:285–9. doi: 10.1016/s0378-5173(02)00189-8. [DOI] [PubMed] [Google Scholar]
- 14.Cavallari C, Albertini B, Gonzalez-Rodriguez ML, Rodriguez L. Improved dissolution behaviour of steam-granulated piroxicam. Eur J Pharm Biopharm. 2002;54:65–73. doi: 10.1016/s0939-6411(02)00021-8. [DOI] [PubMed] [Google Scholar]
- 15.Albertini B, Cavallari C, Passerini N, Gonzalez-Rodriguez ML, Rodriguez L. Evaluation of beta-lactose, PVP K12 and PVP K90 as excipients to prepare piroxicam granules using two wet granulation techniques. Eur J Pharm Biopharm. 2003;56:479–87. doi: 10.1016/s0939-6411(03)00114-0. [DOI] [PubMed] [Google Scholar]
- 16.Vialpando M, Albertini B, Passerini N, Bergers D, Rombaut P, Martens JA. et al. Agglomeration of mesoporous silica by melt and steam granulation Part I: a comparison between disordered and ordered mesoporous silica. J Pharm Sci. 2013;102:3966–77. doi: 10.1002/jps.23700. [DOI] [PubMed] [Google Scholar]
- 17.Vialpando M, Albertini B, Passerini N, Vander Heyden Y, Rombaut P, Martens JA. et al. Agglomeration of mesoporous silica by melt and steam granulation part II: screening of steam granulation process variables using a factorial design. J Pharm Sci. 2013;102:3978–86. doi: 10.1002/jps.23699. [DOI] [PubMed] [Google Scholar]
- 18.Ullah I, Corrao R, Wiley G, Lipper R. Moisture activated dry granulation: A general process. Pharm Technol. 1987;11:48–54. [Google Scholar]
- 19.Railkar AM, Schwartz JB. Evaluation and comparison of a moist granulation technique to conventional methods. Drug Dev Ind Pharm. 2000;26:885–9. doi: 10.1081/ddc-100101313. [DOI] [PubMed] [Google Scholar]
- 20.Railkar AM, Schwartz JB. The effects of formulation factors on the moist granulation technique for controlled-release tablets. Drug Dev Ind Pharm. 2001;27:893–8. doi: 10.1081/DDC-100107669. [DOI] [PubMed] [Google Scholar]
- 21.Railkar AM, Schwartz JB. Use of a moist granulation technique (MGT) to develop controlled-release dosage forms of acetaminophen. Drug Dev Ind Pharm. 2001;27:337–43. doi: 10.1081/DDC-100103733. [DOI] [PubMed] [Google Scholar]
- 22.Gazikalovic E, Obrenovic D, Nidzovic Z, Colic O. Manufacture of tetracaine hydrochloride tablets using direct compression and moist granulation. Vojnosanit Pregl. 2002;59:621–4. [PubMed] [Google Scholar]
- 23.Takasaki H, Yonemochi E, Messerschmid R, Ito M, Wada K, Terada K. Importance of excipient wettability on tablet characteristics prepared by moisture activated dry granulation (MADG) Int J Pharm. 2013;456:58–64. doi: 10.1016/j.ijpharm.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Ullah I, Wang J, Chang S-Y, Guo H, Kiang S, Jain NB. Moisture-activated dry granulation part II: the effects of formulation ingredients and manufacturing-process variables on granulation quality attributes. Pharmaceutical Technology. 2009;33:42–51. [Google Scholar]
- 25.Ullah I, Wang J, Chang S-Y, Wiley GJ, Jain NB, Kiang S. Moisture-Activated Dry Granulation—Part I: A Guide to Excipient and Equipment Selection and Formulation Development. Pharm Technol. 2009;33:62–70. [Google Scholar]
- 26.Yeh TS, Yeh DH. Subjecting mixture of diluent excipients and pharmaceutically active ingredient, binder excipient, optionally with disintegrant excipient, to heating under condition of low moisture and tumble rotation to form tablets. Google Patents. 2004 [Google Scholar]
- 27. Yeh Ta-Shuong YDH, inventor Wei Ming Pharmaceutical Mfg. Co., Ltd. (Taipei, TW) assignee. Process for the preparation of direct tabletting formulation and aids. USA patent 6,761,905. 2004 July 13.
- 28.Chen YC, Ho HO, Chiou JD, Sheu MT. Physical and dissolution characterization of cilostazol solid dispersions prepared by hot melt granulation (HMG) and thermal adhesion granulation (TAG) methods. Int J Pharm. 2014;473:458–68. doi: 10.1016/j.ijpharm.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 29.Lin HL, Ho HO, Chen CC, Yeh TS, Sheu MT. Process and formulation characterizations of the thermal adhesion granulation (TAG) process for improving granular properties. Int J Pharm. 2008;357:206–12. doi: 10.1016/j.ijpharm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Haramiishi Y, Kitazawa Y, Sakai M, Kataoka K. [Study on fluidized melt-granulation I Examination of the factors on the granulation] Yakugaku Zasshi. 1991;111:515–23. doi: 10.1248/yakushi1947.111.9_515. [DOI] [PubMed] [Google Scholar]
- 31.Maejima T, Kubo M, Osawa T, Nakajima K, Kobayashi M. Application of tumbling melt granulation (TMG) method to prepare controlled-release fine granules. Chem Pharm Bull (Tokyo) 1998;46:534–6. doi: 10.1248/cpb.46.534. [DOI] [PubMed] [Google Scholar]
- 32.Maejima T, Osawa T, Nakajima K, Kobayashi M. Application of tumbling melt granulation method to prepare controlled-release beads by coating with mixture of functional non-meltable and meltable materials. Chem Pharm Bull (Tokyo) 1998;46:531–3. doi: 10.1248/cpb.46.531. [DOI] [PubMed] [Google Scholar]
- 33.Abberger T. Influence of binder properties, method of addition, powder type and operating conditions on fluid-bed melt granulation and resulting tablet properties. Pharmazie. 2001;56:949–52. [PubMed] [Google Scholar]
- 34.Passerini N, Calogera G, Albertini B, Rodriguez L. Melt granulation of pharmaceutical powders: a comparison of high-shear mixer and fluidised bed processes. Int J Pharm. 2010;391:177–86. doi: 10.1016/j.ijpharm.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Aleksic I, Duris J, Ilic I, Ibric S, Parojcic J, Srcic S. In silico modeling of in situ fluidized bed melt granulation. Int J Pharm. 2014;466:21–30. doi: 10.1016/j.ijpharm.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 36.Kowalski J, Kalb O, Joshi YM, Serajuddin AT. Application of melt granulation technology to enhance stability of a moisture sensitive immediate-release drug product. Int J Pharm. 2009;381:56–61. doi: 10.1016/j.ijpharm.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 37.Lakshman JP, Kowalski J, Vasanthavada M, Tong WQ, Joshi YM, Serajuddin AT. Application of melt granulation technology to enhance tabletting properties of poorly compactible high-dose drugs. J Pharm Sci. 2011;100:1553–65. doi: 10.1002/jps.22369. [DOI] [PubMed] [Google Scholar]
- 38.Panda RR, Tiwary AK. Hot melt granulation: a facile approach for monolithic osmotic release tablets. Drug Dev Ind Pharm. 2012;38:447–61. doi: 10.3109/03639045.2011.609562. [DOI] [PubMed] [Google Scholar]
- 39.Shah S, Maddineni S, Lu J, Repka MA. Melt extrusion with poorly soluble drugs. Int J Pharm. 2013;453:233–52. doi: 10.1016/j.ijpharm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Abberger T, Henck JO. [Granule formation mechanisms in fluid-bed melt granulation and their effects on tablet properties] Pharmazie. 2000;55:521–6. [PubMed] [Google Scholar]
- 41.Aoki H, Iwao Y, Uchimoto T, Noguchi S, Kajihara R, Takahashi K, et al. Fine granules showing sustained drug release prepared by high-shear melt granulation using triglycerin full behenate and milled microcrystalline cellulose. Int J Pharm. 2014 doi: 10.1016/j.ijpharm.2014.11.058. [DOI] [PubMed] [Google Scholar]
- 42.Van Melkebeke B, Vermeulen B, Vervaet C, Remon JP. Melt granulation using a twin-screw extruder: a case study. Int J Pharm. 2006;326:89–93. doi: 10.1016/j.ijpharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Nyberg B, Carlstrom E, Carlsson R. Granulation of Ceramic Powders for Pressing by Spray-Freezing and Freeze-Drying. Euro-Ceramics II. 1993;1:447–51. [Google Scholar]
- 44.Nyberg B, Carlstrom E, Carlsson R. Freeze-Granulation of Liquid Phase Sintered Silicon Carbide. Ceramic Transactions. 1994;42:107–13. [Google Scholar]
- 45.Rundgren K, Lyckfeldt O, Sjostedt M. Improving Powders with Freeze Granulation. Ceramic Industry. 2003;153:40–4. [Google Scholar]
- 46.Keary CM, Sheskey PJ. Preliminary report of the discovery of a new pharmaceutical granulation process using foamed aqueous binders. Drug Dev Ind Pharm. 2004;30:831–45. doi: 10.1081/ddc-200030504. [DOI] [PubMed] [Google Scholar]
- 47.Tan MX, Nguyen TH, Hapgood KP. Drug distribution in wet granulation: foam versus spray. Drug Dev Ind Pharm. 2013;39:1389–400. doi: 10.3109/03639045.2012.719233. [DOI] [PubMed] [Google Scholar]
- 48.Koo OM, Ji J, Li J. Effect of powder substrate on foaml drainage and collapse: implications to foam granulation. J Pharm Sci. 2012;101:1385–90. doi: 10.1002/jps.23053. [DOI] [PubMed] [Google Scholar]
- 49.Rocca KE, Weatherley S, Sheskey PJ, Thompson MR. Influence of filler selection on twin screw foam granulation. Drug Dev Ind Pharm. 2013 doi: 10.3109/03639045.2013.845839. [DOI] [PubMed] [Google Scholar]
- 50.Thompson MR, Weatherley S, Pukadyil RN, Sheskey PJ. Foam granulation: new developments in pharmaceutical solid oral dosage forms using twin screw extrusion machinery. Drug Dev Ind Pharm. 2012;38:771–84. doi: 10.3109/03639045.2011.633265. [DOI] [PubMed] [Google Scholar]


