Abstract
The goal of the present protocol is to describe the forced swim test (FST), which is one of the most commonly used assays for the study of depressive-like behavior in rodents. The FST is based on the assumption that when placing an animal in a container filled with water, it will first make efforts to escape but eventually will exhibit immobility that may be considered to reflect a measure of behavioral despair. This test has been extensively used because it involves the exposure of the animals to stress, which was shown to have a role in the tendency for major depression. Additionally, the FST has been shown to share some of the factors that are influenced or altered by depression in humans, including changes in food consumption, sleep abnormalities and drug-withdrawal-induced anhedonia. The main advantages of this procedure are that it is relatively easy to perform and that its results are easily and quickly analyzed. Moreover, its sensitivity to a broad range of antidepressant drugs that makes it a suitable screening test is one of the most important features leading to its high predictive validity. Despite its appeal, this model has a number of disadvantages. First, the issue of chronic augmentation is problematic in this test because in real life patients need to be treated for at least several weeks before they experience any relief from their symptoms. Last, due to the aversiveness of the FST, it is important to take into account possible influences it might have on brain structure/function if brain analyses are to be carried out following this procedure.
Keywords: Behavior, Issue 97, Depression, forced swim test, FST, mouse, rat, animal model, behavioral neuroscience, antidepressants, SSRI
Introduction
Depression is a life-threatening psychiatric disorder and a major public health concern worldwide with an incidence of 5% and a lifetime prevalence of 15-20%. Moreover, it is estimated that by 2020 depression will be in the top three contributors to the burden of disease1,2. Depression is associated with disability, decreased quality of life, increased health-related costs and is considered a main risk factor for many diseases, including cardiovascular, metabolic and neuropsychiatric disorders3,4.Current pharmaco-therapeutic treatments have limited efficacy and are associated with many deleterious side effects5,6. Therefore, a better understanding of the pathophysiology of this disorder alongside with the development of innovative and improved treatments remains crucial. Hence, animal models are essential for advancing research in this field.
There are many models used for the study of this disorder (e.g. sucrose preference test, tail suspension test) with the forced swimming test (FST, also known as Porsolt’s test after the developer of this model 7,8) being one of the most commonly used assays 7,9-12.
During the FST an animal is placed in a container filled with water from which it cannot escape. The animal will first try to escape but eventually will exhibit immobility (i.e. floating with the absence of any movement except for those necessary for keeping the nose above water). The FST is a very popular model in animal research for a number of reasons. First, it involves the exposure of the animals to stress, which was shown to have a role in the tendency for major depression 12-14. Moreover depression is often viewed as a lack of ability to handle with stress 15-17. Second, pharmacological treatment with antidepressants prior to the test has been shown to reduce immobility in the FST 18-23. Therefore, it is often used as a screening assay for novel compounds with potential antidepressant properties 15-17,24. Additionally, the FST has been shown to share some of the factors that are influenced or altered by depression in humans, such as changes in food consumption, sleep abnormalities and drug-withdrawal-induced anhedonia 15-17,24. This is also the reason why this test is sometimes used to evaluate depressive-like behavior in mutant mice, with increase or decrease in basal immobility (compared to ‘wild-type’ mice) 25,26.
Protocol
NOTE: All experimental protocols were approved by the International Committee for Animal Care and Use in Israel. All efforts were made to minimize the number of animals used and their suffering.
1. Preparation for the Forced Swim Test
Use two adjacent rooms. Use one room as a “waiting room” for holding the animals prior to behavioral testing, and the other for carrying out the procedure.
Prepare transparent cylindrical glass containers (the only restriction for the number of containers is the space available) measuring 50 cm in height and a diameter of 20 cm. NOTE: It is possible to run several animals simultaneously in the same room. If so, separate the containers from one another using a dark screen so that the animals will not see each other during the procedure.
Prepare video camera(s) in front of the containers so that every camera perceives one or more containers in a way that will allow the clear observation of the animals’ behavior later on while viewing the footage.
Prepare clean drying cages, heat lamps and heat pads for the animals that have finished the procedure to avoid hypothermia. Make sure the bottom of the cage has blotting paper and change it when it gets wet. Do not place the cages in the testing room. NOTE: Make sure not to mix animals from different home cages while they are staying in the transient drying cages.
2. Animal Handling Prior to Testing
House animals in a room with a 12 hr light/dark cycle.
During the days of the experimental procedure give the animals free access to food and water, except for the specific time spent in the procedure room.
When using rats, handle the animals for about 2 min daily, 5 days prior to the beginning of the experimental procedure.
3. Training Procedure
The procedure is carried out differently for rats and mice.
NOTE: For both mice and rats, watch the animals while they are in the water at all times. In case an animal appears in serious distress (e.g. very tired, cannot stay up float) remove the animal from the water and exclude it from the experiment.
- For mice — There is one session 6 min long, divided into pretest (the first 2 min) and test (the last 4 min).
- In order for the mice to get acclimated to the testing environment, transport the animals in their home cages at least 30 min prior to behavioral testing to the waiting room.
- Fill the cylinders with tap water at 25 °C and adjust the water depth according to the mouse’ size, so that it cannot touch the bottom of the container with its hind legs.
- Mark the cylinder with the animal number for the purpose of the identification of the animal later on while viewing the footage.
- Turn on the video camera/s and then place each mouse in the water filled cylinder container for 6 min.
- After 6 min have elapsed, turn the camera/s off, remove the mouse from the container and place it in the transient drying cage with the heat lamp above it and the heat pad under it. The mice should be closely and continuously monitored while recovering in this cage. For this purpose it is possible to place a thermometer at the level of the mice to make sure the temperature in the cage does not exceed 37 °C. Also, place the cage such that not all of it falls under the lamp or over the pad; this allows the mouse to move to a cooler area if desired.
- Change the water after every session to avoid any influence on the next mouse.
- For rats — There are 2 sessions, 24 hr apart. The first session is the pre-test stage (15 min) and the second session is the test stage (5 min).
- In order for the rats to get acclimated to the testing environment, transport the animals in their home cages at least 30 min prior to behavioral testing to the waiting room.
- Fill the cylinders with tap water at 23 ± 1 °C and adjust the water depth according to the rat’s size, so that it cannot touch the bottom of the container with its hind legs.
- Mark the cylinder with the animal number for the purpose of the identification of the animal later on while viewing the footage.
- Place each rat in the water filled cylinder container for 15 min.
- After 15 min have elapsed remove the rat from the container and place it in the transient drying cage with the heat lamp above it and the heat pad under it. The rat should be closely and continuously monitored while recovering in this cage. For this purpose it is possible to place a thermometer at the level of the rat to make sure the temperature in the cage does not exceed 37 °C. Also, place the cage such that not all of it falls under the lamp or over the pad; this allows the rat to move to a cooler area if desired.
- Change the water after every session to avoid any influence on the next rat. NOTE: This is the end of the pretest stage.
- Twenty-four hours later, in order for the rats to get acclimated to the testing environment, transport the animals in their home cages at least 30 min prior to behavioral testing to the waiting room.
- Fill the cylinders with tap water at 23 ± 1 °C and adjust the water depth according to the rat’s size, so that it cannot touch the bottom of the container with its hind legs.
- Mark the cylinder with the animal number for the purpose of the identification of the animal later on while viewing the footage.
- Turn on the video camera/s and then place the rat in the water filled cylinder container for 5 min. Make sure that each rat is tested in the same container and position in the room as the previous day.
- After 5 min have elapsed turn the camera/s off, remove the rat from the container and place it in the transient drying cage with the heat lamp above it and the heat pad under it. The rat should be closely and continuously monitored while recovering in this cage. For this purpose it is possible to place a thermometer at the level of the rat to make sure the temperature in the cage does not exceed 37 °C. Also, place the cage such that not all of it falls under the lamp or over the pad; this allows the rat to move to a cooler area if desired.
- Change the water after every session to avoid any influence on the next rat. NOTE: This is the end of the test stage.
4. The Behavioral Coding
For mice, code the last 4 min defined as the test stage. For rats, code the 5 min of the test stage.
Code the duration of time spent as “Immobile” if the mouse is floating with the absence of any movement except for those necessary for keeping the nose above water.
Code the duration of time spent as “Struggling/climbing” if quick movements of the forelimbs are observed such that the front paws break the surface of the water.
Code the duration of time spent as “Swimming” if movement of forelimbs or hind limbs in a paddling fashion is observed. NOTE: It is possible to use an alternative way to code these behaviors, this includes a time-sampling method. Rate the swimming, struggling or immobility as the frequency of episodes at 5 sec intervals throughout the test session.
Representative Results
The following results are based on unpublished data from our lab. In this experiment, adult ICR female mice were tested after 3 weeks of treatment with the selective serotonin reuptake Inhibitor (SSRI) escitalopram or novel herbal anti-depressive and anti-anxiety treatment (NHT) (for additional information regarding the herbal treatment, see 12,27,28). One-way ANOVA revealed that the treatment reduced depressive-like behavior in the FST [F(2,58) = 4.88, p <0.05]. One-sided Dunnet analysis revealed that treatment with either escitalopram or the NHT reduced depressive-like behavior in the FST (see Fig. 1A for post hoc comparisons). The treatment also increased struggling behavior in the FST [F(2,58) = 4.36, p <0.05]. One-sided Dunnet analysis revealed that treatment with escitalopram increased struggling behavior in the FST (see Figure 1B for post hoc comparisons). The treatment had no effect on swimming behavior [F(2,58) = 2.89, p >0.05, Figure 1C].
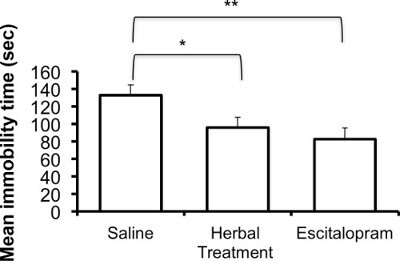 Figure 1A:Representative results of the effects of the NHT and escitalopram treatment (3 weeks) on immobility time in the FST (N: NHT = 19, escitalopram = 19, control = 21). *p <0.05, **p <0.005.
Figure 1A:Representative results of the effects of the NHT and escitalopram treatment (3 weeks) on immobility time in the FST (N: NHT = 19, escitalopram = 19, control = 21). *p <0.05, **p <0.005.
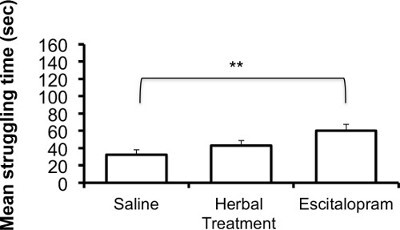 Figure 1B:Representative results of the effects of the NHT and escitalopram treatment (3 weeks) on struggling time in the FST (N: NHT = 19, escitalopram = 19, control = 21). *p <0.05, **p <0.005.
Figure 1B:Representative results of the effects of the NHT and escitalopram treatment (3 weeks) on struggling time in the FST (N: NHT = 19, escitalopram = 19, control = 21). *p <0.05, **p <0.005.
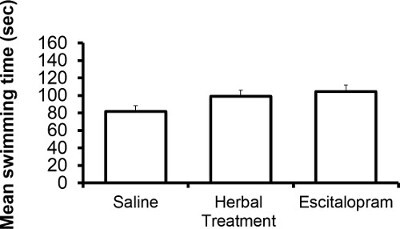 Figure 1C: Representative results of the effects of the NHT and escitalopram treatment (3 weeks) on swimming time in the FST (N: NHT = 19, escitalopram = 19, control = 21).
Figure 1C: Representative results of the effects of the NHT and escitalopram treatment (3 weeks) on swimming time in the FST (N: NHT = 19, escitalopram = 19, control = 21).
Discussion
The FST is used to monitor depressive-like behavior and is based on the assumption that immobility reflects a measure of behavioral despair 3. The main advantages of this procedure lie in its relatively easy operation and fast results. Moreover, its sensitivity to a broad range of antidepressant drugs that makes it a suitable screening test is one of the most important features leading to its high predictive validity 29. Importantly, this test can also differentiate between drugs that are not aimed for the treatment of depression such as benzodiazepines, which have been shown to possess anti-anxiety effects 3,30. Another example of the value of the FST in the study of depression, which also contributes to its face validity, is the fact that models of predisposition to depression were found to influence the immobility behavior. For example, its use as a marker of depressive-like behavior in genetic research. Genetic animal models of depression have been successfully breeding rodents on the basis of their immobility in the FST 32, suggesting that immobility is determined by a heritable trait, although some strains have shown innately high immobility in the FST 33,34. Additionally, it is important to mention that genetic models that are not based on the performance in the FST were also found to be successful in providing valid models for studying depressive-like behavior 35,36. In addition to the genetic research, the immobility behavior in the FST has been shown to be influenced in several animal models that are linked with predisposition to depression, such as early exposure to stress 37,38, clinical diabetes 39,40 and acute drug withdrawal41.
Despite its appeal, reservations regarding its construct validity that cast doubt on it being a model for depression have also been raised. For example, the issue of chronic augmentation is problematic in this test because in real life patients need to be treated for at least several weeks before they experience any relief from their symptoms 42-44. That raises the question of whether immobility in the FST and depression share the same long-term adaptive changes in neuronal circuitry that underlie the effects of antidepressants in humans. With that said, it is important to mention that several recent studies have shown effects of antidepressants in this test following chronic treatment at much lower doses than those needed to induce effects after acute augmentation 24,45. Another much-discussed issue is the precise meaning of the immobility behavior as a measure that reflects the symptoms of depression 18,25. It is important to note that the immobility in these tests seems to be the result of an inability or reluctance to maintain effort rather than a generalized hypoactivity. This is of special importance due to the fact that patients suffering from depression show psychomotor impairments, particularly in those tests requiring the duration of effort 46. The active behaviors in this model (i.e. struggling and swimming) could potentially lead to escape and as a result decrease stress, whereas the passive behavior (i.e. immobility) may preserve energy while waiting for a possible escape. The animal’s choice of behavior varies and depends on numerous factors (e.g. pre-exposure, energy status, treatment, etc.). SSRIs have shown to postpone the transition from active to passive coping strategies, whereas factors that were found associated with depression accelerate this transition 18. Moreover, few questions have been raised regarding immobility as a learned process, meaning that the animal might learn that the best solution would be to be passive and wait to be removed from the water, what has been described as learned immobility 47-49. However, one might claim that this view leads to an anthropomorphic perspective of this model. In addition, negative correlation between longer immobility duration and stress hormones was not found 50,51. Furthermore, SSRIs were found to reduce immobility in a single test session following chronic administration in rats 52 or even following acute administration in mice 29 suggesting that where SSRIs are concerned learned immobility does not seem to play a role.
Another important notion is the role of the active behaviors during the FST. Although immobility is the behavior that is usually presented in articles, the other two measures have also been shown to be significant. Specifically, antidepressants that increase serotonergic neurotransmission led to longer swimming durations whereas those that were found to increase catacholaminergic neurotransmission led to longer struggling durations 18. This may help us to differentiate the neurochemical mechanisms underlying this behavior in different experiments. This was observed mainly in rats 53-56 and may be the cause that in our results exposure to the SSRI escitalopram resulted in the increase of the struggling but not the swimming behavior.
In addition, due to the fact that some antidepressants are known for reducing locomotor activity 18 and also that drugs such as psychomotor stimulants were found to reduce immobility in the FST 18,20,31 but are not effective for treating depression, it is recommended to preform locomotor activity tests in addition to the FST to rule out that the basal activity level is not the determining factor in this model. Moreover, due the aversiveness of the FST, it is important to take into account possible influences it might have on brain structure/function if brain analyses are to be carried out following this procedure. Also when preforming a number of behavioral tests, if the other paradigms are not considered stressful, it is recommended that the FST will be the last assay that is carried out.
Last, although in the past the scoring in this procedure was submitted to the possibility of bias by the experimenter, it is becoming more and more common to use designated software that eliminates this disadvantage 12,57,58.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This research was supported by the Israel Science Foundation (grant No. 738/11), by the National Institute for Psychobiology in Israel (NIPI-7-2011-12), and by the Open University Foundation
References
- Levinson DF. The genetics of depression: a review. Biological psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nature reviews. Drug discovery. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Thase ME. Managing depressive and anxiety disorders with escitalopram. Expert opinion on pharmacotherapy. 2006;7:429–440. doi: 10.1517/14656566.7.4.429. [DOI] [PubMed] [Google Scholar]
- Lam RW, Kennedy SH. Evidence-based strategies for achieving and sustaining full remission in depression: focus on metaanalyses. Canadian journal of psychiatry. Revue canadienne de psychiatrie. 2004;49:17S–26S. [PubMed] [Google Scholar]
- Dording CM, et al. The pharmacologic management of SSRI-induced side effects: a survey of psychiatrists. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2002;14:143–147. doi: 10.1023/a:1021137118956. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Archives internationales de pharmacodynamie et de therapie. 1977;229:327–336. [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends in Pharmacological Sciences. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, et al. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. European Journal of Pharmacology. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Doron R, et al. A novel herbal treatment reduces depressive-like behaviors and increases BDNF levels in the brain of stressed mice. Life sciences. 2014;94:151–157. doi: 10.1016/j.lfs.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Caspi A, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Kaufman J, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zacharko RM. Multiple neurochemical and behavioral consequences of stressors: Implications for depression. Pharmacology and Therapeutics. 1990;46:119–136. doi: 10.1016/0163-7258(90)90039-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annual Review of Psychology. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience and biobehavioral reviews. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: The effects of water depth. Behavioural Brain Research. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Hemby SE, et al. Potential antidepressant effects of novel tropane compounds, selective for serotonin or dopamine transporters. Journal of Pharmacology and Experimental Therapeutics. 1997;282:727–733. [PubMed] [Google Scholar]
- Bouvard M, Stinus L. In the rat forced swimming test, chronic but not subacute administration of dual 5-HT/NA antidepressant treatments may produce greater effects than selective drugs. Behavioural Brain Research. 2002;136:521–532. doi: 10.1016/s0166-4328(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology. 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neuroscience and biobehavioral reviews. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Molecular Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Sang KP, et al. Par-4 links dopamine signaling and depression. Cell. 2005;122:275–287. doi: 10.1016/j.cell.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Doron R, et al. Anxiolytic effects of a novel herbal treatment in mice models of anxiety. Life sciences. 2012;90:995–1000. doi: 10.1016/j.lfs.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Doron R, et al. Escitalopram or novel herbal mixture treatments during or following exposure to stress reduce anxiety-like behavior through corticosterone and BDNF modifications. PloS one. 2014;9:e91455. doi: 10.1371/journal.pone.0091455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity. Psychopharmacology. 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Reinhold JA, Mandos LA, Rickels K, Lohoff FW. Pharmacological treatment of generalized anxiety disorder. Expert opinion on pharmacotherapy. 2011;12:2457–2467. doi: 10.1517/14656566.2011.618496. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Interaction between estrogens and antidepressants in the forced swimming test in rats. Psychopharmacology. 2004;173:139–145. doi: 10.1007/s00213-003-1707-4. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Kilts CD. Animal models of depression and schizophrenia. Textbook of Psychopharmacology. 1998. pp. 89–131.
- Armario A, Gavaldà A, Martí J. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology. 1995;20:879–890. doi: 10.1016/0306-4530(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Paré WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiology and Behavior. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neuroscience and biobehavioral reviews. 2005;29:739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Piras G, Piludu MA, Giorgi O, Corda MG. Effects of chronic antidepressant treatments in a putative genetic model of vulnerability (Roman low-avoidance rats) and resistance (Roman high-avoidance rats) to stress-induced depression. Psychopharmacology. 2014;231:43–53. doi: 10.1007/s00213-013-3205-7. [DOI] [PubMed] [Google Scholar]
- Bielajew C, et al. Strain and Gender Specific Effects in the Forced Swim Test. Effects of Previous Stress Exposure. Stress. 2003;6:269–280. doi: 10.1080/10253890310001602829. [DOI] [PubMed] [Google Scholar]
- Fujisaki C, et al. An immnosuppressive drug, cyclosporine-A acts like anti-depressant for rats under unpredictable chronic stress. Journal of Medical and Dental Sciences. 2003;50:93–100. [PubMed] [Google Scholar]
- Gomez R, Vargas CR, Wajner M, Barros HMT. Lower in vivo brain extracellular GABA concentration in diabetic rats during forced swimming. Brain research. 2003;968:281–284. doi: 10.1016/s0006-8993(03)02340-0. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke LA, Wozniak KM, Durcan MJ, Linnoila M. Behavior of streptozotocin-diabetic mice in tests of exploration, locomotion, anxiety, depression and aggression. Physiology and Behavior. 1990;48:429–433. doi: 10.1016/0031-9384(90)90339-6. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biological psychiatry. 2003;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Portella MJ, et al. Can we really accelerate and enhance the selective serotonin reuptake inhibitor antidepressant effect? A randomized clinical trial and a meta-analysis of pindolol in nonresistant depression. The Journal of clinical psychiatry. 2011;72:962–969. doi: 10.4088/JCP.09m05827blu. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA. Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. The Journal of clinical psychiatry. 2008;69:946–958. doi: 10.4088/jcp.v69n0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Thomas P, Dupuis B. Effect of pindolol on onset of action of paroxetine in the treatment of major depression: intermediate analysis of a double-blind, placebo-controlled trial. Reseau de Recherche et d'Experimentation Psychopharmacologique. The American journal of psychiatry. 1998;155:1346–1351. doi: 10.1176/ajp.155.10.1346. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Willner P. Animal models of depression: An overview. Pharmacology and Therapeutics. 1990;45:425–455. doi: 10.1016/0163-7258(90)90076-e. [DOI] [PubMed] [Google Scholar]
- Jefferys D, Funder J. The effect of water temperature on immobility in the forced swimming test in rats. European Journal of Pharmacology. 1994;253:91–94. doi: 10.1016/0014-2999(94)90761-7. [DOI] [PubMed] [Google Scholar]
- West AP. Neurobehavioral studies of forced swimming: The role of learning and memory in the forced swim test. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1990;14:863–877. doi: 10.1016/0278-5846(90)90073-p. [DOI] [PubMed] [Google Scholar]
- De Pablo JM, Parra A, Segovia S, Guillamon A. Learned immobility explains the behavior of rats in the forced swimming test. Physiology and Behavior. 1989;46:229–237. doi: 10.1016/0031-9384(89)90261-8. [DOI] [PubMed] [Google Scholar]
- Dal-Zotto S, Martí O, Armario A. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behavioural Brain Research. 2000;114:175–181. doi: 10.1016/s0166-4328(00)00220-5. [DOI] [PubMed] [Google Scholar]
- Rittenhouse PA, López-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–318. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Keeney A, Hogg S. Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. European Journal of Pharmacology. 2004;492:195–201. doi: 10.1016/j.ejphar.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Chaki S, et al. MGS0039: A potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004;46:457–467. doi: 10.1016/j.neuropharm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Mague SD, et al. Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. Journal of Pharmacology and Experimental Therapeutics. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Molina-Hernández M, Téllez-Alcántara NP. Antidepressant-like actions of pregnancy, and progesterone in Wistar rats forced to swim. Psychoneuroendocrinology. 2001;26:479–491. doi: 10.1016/s0306-4530(01)00007-5. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernández-Guasti A, López-Rubalcava C. Antidepressant-like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2003;28:830–838. doi: 10.1038/sj.npp.1300097. [DOI] [PubMed] [Google Scholar]
- Gersner R, Gordon-Kiwkowitz M, Zangen A. Automated behavioral analysis of limbs' activity in the forced swim test. Journal of neuroscience. 2009;180:82–86. doi: 10.1016/j.jneumeth.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Einat H. Partial effects of the protein kinase C inhibitor chelerythrine in a battery of tests for manic-like behavior in black Swiss mice. Pharmacological reports : PR. 2014;66:722–725. doi: 10.1016/j.pharep.2014.03.013. [DOI] [PubMed] [Google Scholar]


