Abstract
Background
The neutrophil-to-lymphocyte ratio (NLR) is a useful predictive factor in several cancers. However, the prognostic value of NLR in patients with esophageal cancer (EC) is still controversial. Therefore, it was necessary for us to perform a meta-analysis to evaluate the prognostic value of NLR in patients with EC.
Methods
A systematic literature search was performed by using Web of Science, PubMed Central, and Medline to evaluate the prognostic value of NLR in patients with EC. The deadline of our primary search was July 10, 2014. This meta-analysis was conducted in accordance with PRISMA guidelines. Pooled hazard ratio (HR) with 95% confidence interval (CI) was used to assess the association of NLR and overall survival (OS) and disease-free survival (DFS).
Results
Six studies involving 1,633 patients were included in our meta-analysis. Our pooled results demonstrated that high NLR was associated with poor OS (HR: 1.54, 95% CI: 1.32–1.80, I 2=25.3%, P=0.254) and DFS (HR: 1.74, 95% CI: 1.25–2.43, I 2=63.9%, P=0.096). Subgroup analysis between NLR and OS was performed in a further investigation. When the patients were segregated according to country, sample size, and pathological type, high NLR was also significantly correlated with OS.
Conclusion
High NLR is associated with poor prognosis in patients with EC. NLR may be a significant predictive biomarker in patients with EC.
Keywords: esophageal cancer, neutrophil-to-lymphocyte ratio, prognosis, meta-analysis, overall survival, disease-free survival
Introduction
Esophageal cancer (EC) is one of the most common cancers worldwide.1 Although there is a significant improvement in the treatment of patients with EC, the prognosis is still poor due to late diagnosis, rapid progression, and high rate of recurrence.2,3 Therefore, it is important for us to identify better predictive factors, especially serum predictive biomarkers, for prognosis in patients with EC.
In recent years, systemic inflammatory response has been shown to correlate with prognosis in various cancers.4,5 Various inflammatory biomarkers, such as cytokine, C-reactive protein (CRP), and Glasgow prognostic score, have been investigated in a variety of cancers.6–9 Recent studies have demonstrated that neutrophil-to-lymphocyte ratio (NLR) is associated with prognosis in various cancers.10–12 However, due to the inconsistent results, the prognostic value of NLR in EC remains controversial.13–20 Therefore, it was necessary for us to perform a meta-analysis to evaluate the prognostic value of NLR in patients with EC.
In this study, we conducted a meta-analysis to evaluate the prognostic value of NLR for survival in patients with EC. To the best of our knowledge, this is the first meta-analysis to investigate the prognostic role of NLR in patients with EC.
Materials and methods
Literature search
A systematic literature search was performed by using Web of Science, PubMed, and Medline to evaluate the prognostic value of NLR in patients with EC. The search strategy was based on combinations of the following search terms: (“neutrophil–lymphocyte ratio” or “neutrophil-to-lymphocyte ratio” or “neutrophil lymphocyte ratio” or “NLR”) and (“esophageal cancer” or “esophageal carcinoma” or “EC”). The deadline of our primary search was July 10, 2014. Only human research was included in our meta-analysis. In addition, the reference lists of identified studies were also checked for further relevant studies.
Inclusion/exclusion criteria
Inclusion criteria were as follows: 1) patients were diagnosed as having EC by pathology; 2) the NLR was measured before treatment; 3) the NLR was measured by serum-based methods; 4) the relationship between NLR and prognosis (overall survival [OS] and disease-free survival [DFS]) was evaluated; 5) full-text studies without language limits. The exclusion criteria were 1) review, letter, case report, or nonhuman research; 2) insufficient data to extract the hazard ratios (HRs) and the 95% confidence intervals (CIs).
Data extraction
Data from each study were extracted independently by two authors (Yang and Huang). If the results reported had possible overlap, only the most recent or the most complete study was included in this study. The following data were collected for each study: author, year, sample, country, treatment, pathological type, cutoff level, TNM stage, follow-up, and HRs and 95% CIs for the correlation between NLR and prognosis.
Statistical analysis
Pooled HRs and 95% CIs were used to analyze the relationship between NLR and prognosis (OS and DFS). The heterogeneity of combined HRs was initially evaluated by χ-square test and expressed by inconsistency index I2. A significant heterogeneity was defined as P<0.10 or I2>50%.21 A combined HR >1 indicated a worse OS, and it was considered statistically significant if the 95% CI for the HR did not overlap. Publication bias was assessed using Begg’s funnel plot and Egger’s linear regression test. All statistical analyses were conducted with Stata version 12.0 (Stata Corporation, College Station, TX, USA).
Results
According to the search strategies, a total of six eligible studies, including 1,633 patients with EC, were included in this meta-analysis.13–18 Sato et al19 demonstrated the correlation between NLR and the response to neoadjuvant chemotherapy. Thus, we excluded it from this study. Rashid et al20 reported the opposite conclusion. However, we could not extract the data from the manuscript because of insufficient data. Therefore, we excluded Rashid’s study as well. The main characteristics of the six included studies are listed in Table 1.
Table 1.
Characteristics of included studies
| Author | Country | Sample size (M/F) | Treatment | Survival analysis | Pathological type | HR (95% CI) | Cutoff level | Follow-up (M)* | TNM stage |
|---|---|---|---|---|---|---|---|---|---|
| Sharaiha et al13 | USA | 295 (85/27) | C/R + S | OS, DFS | SCC + AC | 2.32 (1.53–3.50) 2.26 (1.44–3.56) |
5.0 | 31 (13–61) | I–IV |
| Miyata et al14 | Japan | 152 (132/20) | C + S | OS | SCC | 1.30 (0.76–2.22) | 4.0 | 60.2 (20.1–120.8) | II–IV |
| Feng et al15 | People’s Republic of China | 483 (411/72) | S | OS | SCC | 1.339 (1.015–1.768) | 3.5 | 45 (3–84) | I–III |
| Yoo et al17 | South Korea | 138 (132/6) | C/R | OS | SCC + AC | 2.115 (1.193–3.749) | 2.0 | 39.5 (1.1–93.4) | II–III |
| Wang et al18 | People’s Republic of China | 90 (72/18) | S | OS, DFS | SCC | 1.316 (0.783–2.214) 1.279 (0.780–2.097) |
1.0 | NA | I–III |
| Chen and He16 | People’s Republic of China | 475 (382/93) | S + C/R | OS | SCC | 1.50 (1.12–2.02) | 2.5 | 44.9 (2–84.4) | I–III |
Notes:
Data shown as mean (minimum-maximum).
Abbreviations: M/F, male/female; S, surgery; C/R, chemotherapy/radiotherapy; OS, overall survival; DFS, disease-free survival; SCC, squamous cell carcinoma; AC, adenocarcinoma; NA, not available; M, months.
All these six studies presented data on NLR and OS,13–18 but only two studies reported data on NLR and DFS.13,17 Our pooled results showed that high NLR was associated with poor OS (HR: 1.54, 95% CI: 1.32–1.80, I 2=25.3%, P=0.245) (Figure 1) and DFS (HR: 1.74, 95% CI: 1.25–2.43, I2=63.9%, P=0.096) (Figure 2).
Figure 1.
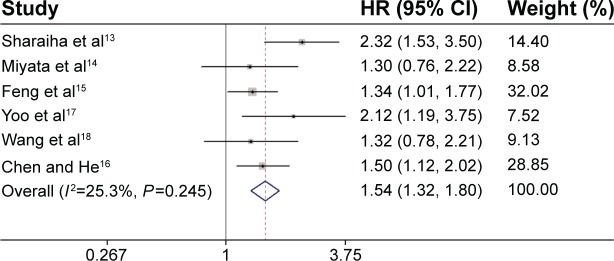
Forest plot of the association between NLR and OS in EC.
Abbreviations: HR, hazard ratio; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; EC, esophageal cancer.
Figure 2.
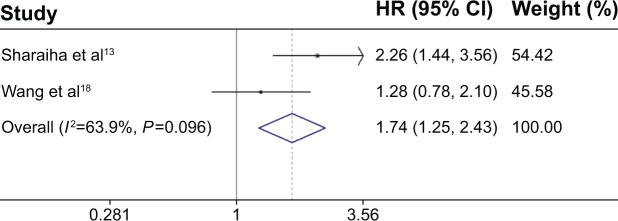
Forest plot of the association between NLR and DFS in EC.
Abbreviations: HR, hazard ratio; CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; DFS, disease-free survival; EC, esophageal cancer.
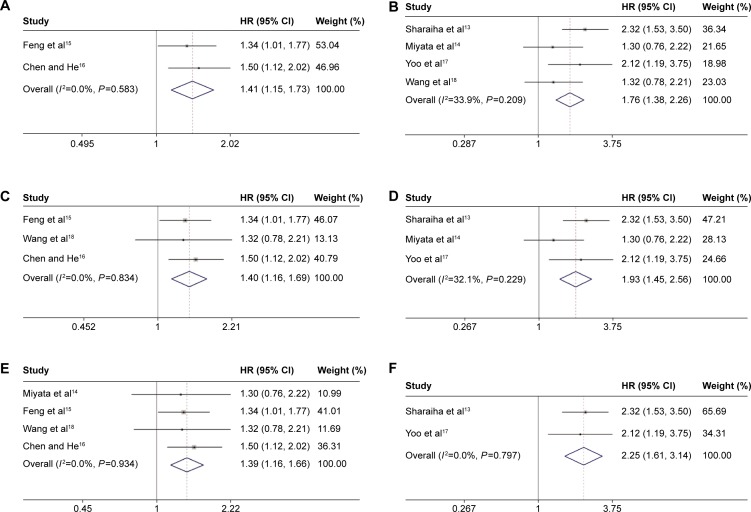
In a further investigation, subgroup analyses were performed (Figure 3). When stratified by “sample size,” the “>300” group yielded a HR of 1.41, and the 95% CI was 1.15–1.73 (Figure 3A). The “≤300” group yielded a HR of 1.76, and the 95% CI was 1.38–2.26 (Figure 3B). In the subgroup analysis by “country,” we found that no matter the patients were Chinese (Figure 3C) or non-Chinese (Figure 3D), high NLR was still a poor predictor for OS. The subgroup analysis by “pathological type” showed that high NLR still yielded a worse OS (Figure 3E and F).
Figure 3.
Forest plot of the association between serum CRP and OS in EC by subgroup analyses.
Notes: Subgroup analysis by sample size (A: >300, B: ≤300), country (C: People’s Republic of China, D: not People’s Republic of China), and pathological type (E: SCC, F: SCC + AC).
Abbreviations: HR, hazard ratio; CI, confidence interval; CRP, C-reactive protein; OS, overall survival; EC, esophageal cancer; SCC, squamous cell carcinoma; AC, adenocarcinoma.
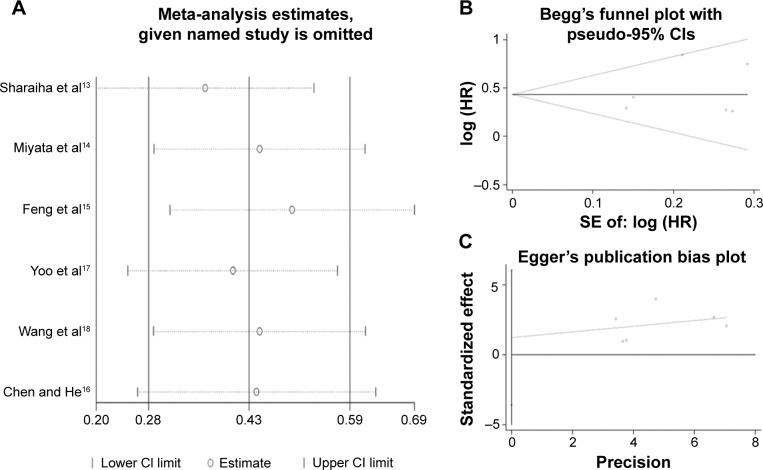
Sensitivity analysis showed that no single study could affect the pooled HRs in the present meta-analysis (Figure 4A). The Begg’s funnel plot and Egger’s linear regression test were performed to analyze the publication bias. There was no significant publication bias in our study (Begg’s test: P=0.707, Egger’s test: P=0.521) (Figure 4B and C).
Figure 4.
Sensitivity analysis (A) and funnel plot of publication biases (B and C) on the relationship between NLR and OS in EC.
Abbreviations: CI, confidence interval; HR, hazard ratio; SE, standard error; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; EC, esophageal cancer.
Discussion
To the best of our knowledge, this is the first meta-analysis to investigate the prognostic role of NLR in patients with EC. In this meta-analysis, our pooled results demonstrated that high NLR was associated with poor OS (HR: 1.54, 95% CI: 1.32–1.80, I2=25.3%, P=0.245) and DFS (HR: 1.74, 95% CI: 1.25–2.43, I2=63.9%, P=0.096) in patients with EC. Subgroup analyses between NLR and OS were performed in a further investigation. When the patients were segregated according to country, sample size, and pathological type, high NLR was also significantly correlated with OS.
Several meta-analyses demonstrated that NLR can be used as an independent predictive factor and can predict the prognosis of various cancers, including urinary cancer,22 hepatocellular carcinoma,23 and colorectal cancer.24 The results of our study are consistent with those of published reports. Furthermore, the sensitivity analysis showed that no single study could affect the pooled HRs in our meta-analysis. There was no significant publication bias in our study. This finding suggests that our meta-analysis was stable and reliable.
The mechanism of the prognostic value of NLR in patients with EC remains unclear. Several reports showed that NLR was associated with tumor-associated macrophages, which promote interleukin-6.25 Furthermore, cancer has been shown to produce various cytokines, such as granulocyte colony-stimulating factor, tumor necrosis factor-α, transforming growth factor-β, interleukin-1, and interleukin-6, which may influence tumor-related neutrophilia.26,27
There were several limitations in our study. First, the number of included studies was limited. Second, the pooled HRs in our study between NLR and OS/DFS were not strong. Empirically, a HR >2 is considered a strongly predictive factor. Therefore, the results should be regarded with caution when using NLR to predict OS/DFS in EC. Moreover, because of the lack of a relevant prospective study, all of the included studies were retrospective. In addition, Rashid’s study was important for our study. However, we excluded it due to insufficient data, which may have influenced our analysis. Therefore, more prospective studies are needed to confirm whether NLR is a prognostic factor in patients with EC.
In conclusion, our meta-analysis demonstrated that high NLR was significantly associated with poor OS and DFS in patients with EC. We conclude that NLR might serve as a useful biomarker for EC. However, further large prospective studies should be carried out to confirm whether NLR has a prognostic value in patients with EC.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Feng JF, Huang Y, Zhao Q. Tumor length in elderly patients with esophageal squamous cell carcinoma: is it a prognostic factor? Ups J Med Sci. 2013;118(3):145–152. doi: 10.3109/03009734.2013.792887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng JF, Chen QX. Significance of the prognostic nutritional index in patients with esophageal squamous cell carcinoma. Ther Clin Risk Manag. 2014;10:1–7. doi: 10.2147/TCRM.S56159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 6.Nakanishi H, Araki N, Kudawara I, et al. Clinical implications of serum C-reactive protein levels in malignant fibrous histiocytoma. Int J Cancer. 2002;99(2):167–170. doi: 10.1002/ijc.10343. [DOI] [PubMed] [Google Scholar]
- 7.Platt JJ, Ramanathan ML, Crosbie RA, et al. C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol. 2012;19(13):4168–4177. doi: 10.1245/s10434-012-2498-9. [DOI] [PubMed] [Google Scholar]
- 8.Vashist YK, Loos J, Dedow J, et al. Glasgow prognostic score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol. 2011;18(4):1130–1138. doi: 10.1245/s10434-010-1383-7. [DOI] [PubMed] [Google Scholar]
- 9.Feng JF, Zhao HG, Liu JS, et al. Significance of preoperative C-reactive protein as a parameter in patients with small cell carcinoma of the esophagus. Onco Targets Ther. 2013;6:1147–1151. doi: 10.2147/OTT.S50039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 11.Halazun KJ, Aldoori A, Malik HZ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34(1):55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Gomez D, Farid S, Malik HZ, et al. Pre-operative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32(8):1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 13.Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18(12):3362–3369. doi: 10.1245/s10434-011-1754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyata H, Yamasaki M, Kurokawa Y, et al. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Exp Ther Med. 2011;2(5):879–885. doi: 10.3892/etm.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. doi: 10.1186/1477-7819-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, He J. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after radical resection of esophageal squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi. 2014;36(4):294–297. Chinese. [PubMed] [Google Scholar]
- 17.Yoo EJ, Park JC, Kim EH, et al. Prognostic value of neutrophil- to-lymphocyte ratio in patients treated with concurrent chemoradiotherapy for locally advanced oesophageal cancer. Dig Liver Dis. 2014;46(9):846–853. doi: 10.1016/j.dld.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Jia Y, Wang N, et al. The clinical significance of tumor infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med. 2014;12:7. doi: 10.1186/1479-5876-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg. 2012;36(3):617–622. doi: 10.1007/s00268-011-1411-1. [DOI] [PubMed] [Google Scholar]
- 20.Rashid F, Waraich N, Bhatti I, et al. A pre-operative elevated neutrophil:lymphocyte ratio does not predict survival from oesophageal cancer resection. World J Surg Oncol. 2010;8:1. doi: 10.1186/1477-7819-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei Y, Jiang YZ, Qian WH. Prognostic role of NLR in urinary cancers: a meta-analysis. PLoS One. 2014;9:e92079. doi: 10.1371/journal.pone.0092079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao WK, Chen D, Li SQ, et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:117. doi: 10.1186/1471-2407-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li MX, Liu XM, Zhang XF, et al. Prognostic role of neutrophil- to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134(10):2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 25.Canna K, McArdle PA, McMillan DC, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92(4):651–654. doi: 10.1038/sj.bjc.6602419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kusumanto YH, Dam WA, Hospers GA, et al. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6(4):283–287. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 27.Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22(9):913–922. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]