Abstract
The identification of mesenchymal stem cells (MSCs) in adult human tissues and the disclosure of their self-renew-al and multi-lineage differentiation capabilities have provided exciting prospects for cell-based regeneration and tis-sue engineering. Although a considerable amount of data is available describing MSCs, there is still lack of information regarding the molecular mechanisms that govern their adhesion and migration. In this work, we will review the current state of knowledge on integrins and other adhesion molecules found to be expressed on MSCs. The dis-cussed topics include the characteristics of MSCs and their clinical applications, integrins and their central role in cell-matrix attachment and migration, and comments on mobilization, differentiation and contribution to tumour development. Finally, by understanding the complex and fundamental pathways by which MSCs attach and migrate, it might be possible to fine-tune the strategies for effective and safe use of MSCs in regenerative therapies.
Keywords: mesenchymal stem cells, differentiation, tissue engineering, integrins, focal adhesions, tumorigenesis
Mesenchymal stem cells and their clinical relevance
Alternative names and criteria to define MSCs
Mesenchymal stem cells (MSCs) is the designation commonly applied to the plastic-adherent cells isolated from bone marrow (BM) and other tissues, which possess multi-potent differentiation capacities in vitro[1]. Excellent reviews on MSCs discussing various topics have already been published. In this review, we will focus on surface characteristics and the integrin system in human MCSs (hMSCs).
The pioneering work of Friedenstein [2], which demonstrated that BM-derived cells were capable of osteogenesis, led to the accelerating interest in identifying, isolating and characterizing cells that reside in a diverse host of tissues, possessing the ability to regenerate cell types specific for these tissues. These advances have furthered our understanding of MSC biology but have also created differences in terminology and readout measurements. Marrow stromal cells, colony-forming unit fibroblasts (CFU-Fs), bone marrow stromal (stem) cells (BMSSCs), stromal precursor cells (SPCs), skeletal stem cells (SSCs), multi-potent adult progenitor cells (MAPCs) are examples among others of terms given to mesenchymal stem cells [3, 4]. Although none of these terms can accurately account for both the developmental origin and differentiation capacity of these cells, the initialisation MSC is currently the most often employed. Nonetheless, the International Society for Cellular Therapy (ISCT) has encouraged the scientific community to adopt this initialisation in their reports only when the investigators have provided a set of standards identifying MSCs. The ISCT has proposed three criteria to define MSCs [5]. First, MSCs must be adherent to plastic when maintained in culture. Second, MSC populations must be positive for several antigens such as CD105, CD73 and CD90. Additionally, these cells must lack the expression of haematopoietic antigens like CD45, CD34 and markers for monocytes, macrophages and B cells. Third, the cells must be able to differentiate at least to osteoblasts, adipocytes and chodroblasts under standard in vitro differentiating conditions.The differentiation can then be demonstrated by well-accepted staining protocols.
We present the above-discussed criteria in Fig. 1. Shown are the phenotypes of typical cultures of hMSCs (derived from BM and purchased from Cambrex, USA) in a subconfluent monolayer, stained with the anti-CD105 antibody and when grown in control, osteogenic, adipogenic and chondrogenic media.
1.
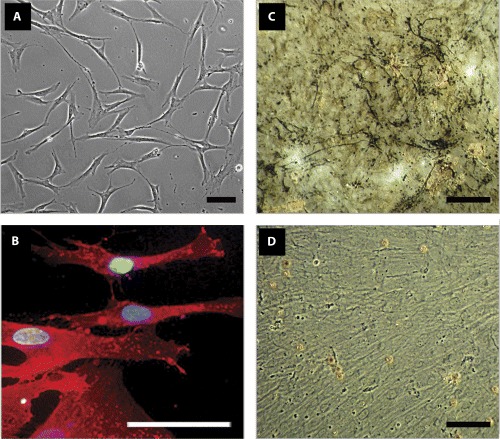
An example of some accepted criteria that define mesenchymal stem cells. (A) Phase-contrast photomicrograph of human MSCs (supplier: Cambrex, USA) showing their fibroblastoid adherent phenotype. (B) Detection of CD105 antigen in hMSCs. Primary anti-CD105 antibody (Cat. no. P3D1, DSHB, USA), secondary antibody conjugated to Texas Red (in red), (Cat. no. 715-075-151, Dianova, Germany) and DAPI nuclear stain (in blue), (Cat. no. D1306, Molecular Probes, Germany) were used. (C and D) hMSCs were induced to differentiate into osteoblasts and the deposition of a mineralized matrix visualized by von Kossa stain is shown in (C). The non-induced control is shown in (D). (E and F) Under adipogenic conditions, hMSCs accumulated lipid vacuoles, which are positively stained by Oil Red O assay (E), whereas in control media no such vacuoles were observed (F). (G and H) Chondrogenesis was indicated by collagen type II staining. A strong signal was detected in stimulated hMSC pellet-cultures (G). Control hMSC pellets clearly
However, it should be noted that this set of criteria introduced by the ISCT displays several deficiencies. For instance, a substantiation of MSC self-renewal ability and functional differentiation in vivo has to be mandatory to the enduring definition of stem cells. Granted, these are strict criteria, but they need to be adopted by the field. The best studied adult stem cells are the haematopoietic stem cells (HSCs) which undergo in vivo self-renewing cell divisions, differentiate at the single cell level into all mature blood elements and functionally repopulate the haematopoietic system of myeloablated animal or human being [6]. With regard to MSCs, such a consensus is still to be achieved mostly due to lack of a single definitive marker, heterogeneity of the cultures, differences in read-out measures and shortage of in vivo confirmations for their plasticity. MSCs do not possess a unique marker that can be reliably used for isolation. Although the ability of MSCs to adhere to plastic surfaces is accepted to define these cells, it should be kept in mind that pre-B-cell progenitors and granulocytic/monocytic precursors also show plastic adherence [7]. Moreover, not all cells within a given population are stem cells. Several similar works strongly suggest that MSCs and isolated clones are heterogeneous not only with respect to their self-renewal capacity but also multi-potentiality (reviewed in Bianco et al.[8]). The heterogeneity of adult MSCs, demonstrated in both in vitro and in vivo studies, can be explained by the notion that one does not establish a ‘stem cell’ culture, but rather a culture in which a subset of cells are stem cells. Furthermore, it is perceived that true plasticity can only be demonstrated by a series of experiments where a single (clonogenic) cell is able to form a progeny of multiple phenotypes in vivo and this phenomenon should reoccur at high frequency and be persistent (reviewed in Lakshmipathy and Verfaillie [9]). Despite the considerable amounts of data available describing the putative MSCs, there is still an insufficiency of unequivocal evidence indicating that MSCs indeed exist in vivo. In this respect, convincing results were presented by Jiang et al.[10] who have reported that cells, termed multi-potent adult progenitor cells (MAPCs), differentiate at the single cell level not only into connective tissue, but also into blood vessels. Moreover, MAPCs proliferated without obvious senescence or loss of differentiation potential and when injected into an early blastocyst, single MAPCs contributed to most somatic cell types. Even though the status of MAPCs and their relation to both MSCs and HSCs is still debatable, this subset of cells fully gratifies the stem cell definition. A proof of similar complexity is still awaited for other postnatal stem cells derived from various tissue sources.
Tissue sources
Despite the fact that BM is considered a well-accepted source of MSCs, MSCs have been isolated from other tissue sources including adipose tissue, periostium, synovial membrane, skeletal muscle, dermis, pericytes, blood (reviewed in Tuan et al.[11]), trabecular bone [12], human umbilical cord [13], lung [14], dental pulp and periodontal ligament [15], suggesting that MSCs are diversely distributed in vivo. It is now of great importance that MSCs derived from different sources are distinguished from one another in order not to pick a ‘one size fits all’ label. To date, there have been very few direct comparisons of MSCs isolated from different tissues. A recent one was delivered from Kern et al.[16] and presented a juxtaposing of MSCs isolated from three sources and expanded under identical culture conditions. Irrespectively of the origin, the MSCs demonstrated no significant difference concerning the morphology and the immune phenotype. Differences, though, could be observed concerning the success rate of isolation, proliferation and differentiation capacity.
While the most accessible, enriched and studied source of MSCs is the BM and because human MSCs are the most relevant for clinical applications, this review will primarily focus on the integrin system of these cells.
Some ‘good’ and ‘bad’ sides of MSCs
It is suggested that recruitment of MSCs occurs during the growing period of an organism as well as in adult life during tissue repair (reviewed in Minguell et al.[17]). Discussed is the concept, that BM stroma-resident MSCs feed progenitors and contribute to tissue regeneration via entering into long- or short-distance trafficking. Recent data support the existence of a pool of circulating MSCs, capable of migrating in and out of the BM and of colonizing sites of organ damage (reviewed in Roufosse et al.[18] and He et al.[19]). In the murine model, MSCs circulate in the blood and can migrate into various tissues [20]. Furthermore, Kuznetsov et al.[21] reported the isolation of adherent, clonogenic fibroblast-like cells with osteogenic and adipogenic potential from the blood of human beings and three other species, but such MSCs have not been detected in other studies [22, 23]. Although there is evidence for the existence of circulating MSCs, a word of caution is warranted because of the controversy underlying this subject and the unpersuasive fulfilment of the above stem cell criteria, i.e. proof of plasticity in vivo.
Based on their commitment to natural processes of tissue repair, MSCs are of great interest when it comes to the development of therapies against a variety of degenerative and age-related diseases. Additionally, MSCs are accepted as most ideal due to their easy isolation, versatile growth and differentiation potential. It is envisaged that MSCs can be used in systemic transplantation procedures for generalized diseases, in local implantation for tissue defects, as a vehicle in gene therapy protocols, or to generate transplantable tissues and organs in tissue engineering protocols (reviewed in Kassem et al.[24]). A few examples on each of the mentioned areas of clinical use will follow.
Sequel to pre-clinical studies in mice [25], the systemic transplantation of allogenic normal MSCs has been shown in children with severe osteogenesis imperfecta [26]. Homing of MSCs to the bone and the production of normal collagen by the transplanted cells were demonstrated in the above study. However, several concerns for an overall clinical approval still remain at present. One concern is particularly pertinent to the experiments with murine MSCs, which are notoriously known to be highly contaminated with macrophages and other haematopoietic cells [27]. These experiments [25] did not verify that the engrafted cells were not of macrophage origin or that they had indeed extravagated the vasculature into the bone lacunae. Along the same lines, in the study of Horwitz et al.[26] no evidence was provided that donor osteoblasts were present and it remains to be determined whether clinical improvement was because of replacement of host osteoblasts with the administrated donor cells. Although many critical points are to be further clarified, continuous efforts proving or disproving the in vivo plasticity of MSCs will have far-reaching consequences for secure clinical applications.
Furthermore, several animal and clinical case reports (reviewed in Kassem et al.[24]) have concluded the successful treatment of bone and cartilage defects, vascular ischemia and coronary artery disease, and of chronic skin wounds upon local administration of MSCs to sites of injury. The injected cells were well tolerated and some spectacular healing results were obtained. Finally, there are few studies that report the differentiation of MSCs into other cell types, such as muscle cells, nerve cells and cardiomyocytes (reviewed in Barry and Murphy [28] and Zipori [29]). Of note, a rigorous assessment of functionality on a cellular level is still to be conducted.
In the above view, there are several current interpretations for MSC plasticity; interpretations which should be further cautioned, debated and strictly evaluated. Thus, apart from the direct conversion of MSCs to other cell types, cell-to-cell fusion and trophic effects, such as MSCs secreting growth factors and cytokines that encourage regeneration by endogenous stem/progenitor cells, perhaps contribute to the positive influence of these cells (reviewed in Grove et al.[30]).
Promising approaches have been examined and used to introduce exogenous DNA into MSCs (reviewed in Damme et al.[31]). Thus, MSCs can be obliged to secrete a therapeutic factor that, once infused into the recipient, can assist in tissue repair. Another scenario for gene therapy is the use of genetically modified MSCs to deliver a fatal signal thereby repressing tumour growth. Recent studies have indicated the tropism of MSCs for chronically inflamed gastric tissue [32] and MSC engraftment into glioma tumours [33]. Additionally, few studies working with hMSCs, immortalized with human telomerase reverse transcriptase (hTERT) have shown their involvement in tumorigenesis [34, 35]. Importantly, these studies also provide new models to explore the stem cell hypothesis in cancer and to develop anti-cancer therapeutics. The link among tissue repair, stem cell renewal and cancerogenesis has been widely discussed (reviewed in Beachy et al.[36] and Dittmar et al.[37]). The present idea is that MSCs can localize to neoplastic tissues under physiological conditions as a default response to the building of a new stroma. Hence, they can conduce to the acceleration of certain tumours. Because of this intrinsic ability, MSCs can be used as a tumour suicide mechanism. Although the anti-tumorigenic use of MSCs is an exciting prospect, several difficulties must be overcome. Such difficulties include the inadequate efficiency of DNA integration, spontaneous genetic changes, cellular toxicity associated with the viral vectors and long-term monitoring of the in vivo function of gene-transduced cells [38].
Another emerging field of regenerative medicine is tissue engineering. Tissue engineering, in a classical sense, implies the use of organ-specific cells for seeding a scaffold ex vivo[39, 40]. Such scaffolds can then be introduced into the wound site to support tissue regeneration. For example, MSC-based constructs have already shown the success of this approach for the treatment of large bone defects in animal models ([41] and reviewed in Otto and Rao [42]).
In short, these stem cell-founded methods for disease therapy and tissue reconstruction have already opened great applicative and market opportunities. Despite the tremendous progress, many fundamental questions remain to be answered; questions regarding MSC phenotype and differentiation as well as the functions of MSCs in vivo and their long-term effectiveness and safety. Furthermore, the elaboration of an expedient approach as well as the comprehension of its underlying molecular mechanisms are dependent on knowledge about integrins and other adhesional molecules, which are expressed and function in MSCs. Bearing on the subject, our understanding of processes like the mobilization and homing of MSCs, adhesion to scaffolds, survival signals in the sites of implantation but also migration towards tumours can exceptionally benefit from the above knowledge.
Integrins and their importance
The integrin receptors
The integrins are a large family of receptors, which mediate cell-matrix and cell-cell adhesion. An individual integrin receptor consists of two non-covalently bound subunits –α and β. Therefore, they are categorized as heterodimeric receptors. Each subunit is a type I transmembrane glycoprotein that has a relatively large extracellular domain and short cytoplasmic tail. Mammals contain 18 α and 8 β subunits that combine to produce at least 24 different heterodimers, each of which can bind to a specific repertoire of cell-surface-, extracellular matrix- (ECM) or soluble protein-ligands [43]. The combinations of integrin sub-units and most of their protein ligands are summarized in Table 1, adapted from Reddy and Mangale [44].
1.
Integrin receptors and their protein ligands †
| Subunits | Ligands | |||
|---|---|---|---|---|
| β | α1 | Collagens, laminins ‡ | ||
| α2 | Collagens, laminins | |||
| α3 | Collagens, laminins, fibronectin, entactin | |||
| α4 | Fibronectin, VCAM-1 ‡ | |||
| α5 | Fibronectin | |||
| α6 | Laminins | |||
| α7 | Laminins ‡ | |||
| α8 | Vitronectin, fibronectin, tenascin | |||
| α9 | Vitronectin, fibronectin, tenascin | |||
| α10 | Collagens ‡ | |||
| α11 | Collagens ‡ | |||
| αV | Fibronectin, vitronectin | |||
| β2 | αL | ICAM-1, ICAM-2, ICAM-3 | ||
| αM | iC3b, fibrinogen, ICAM-1, coagulation factor X | |||
| αX | Fibrinogen, iC3b | |||
| αD | ICAM-3 | |||
| β3 | αIIβ | Fibrinogen, fibronectin, von Willebrand factor, vitronectin, thrombospondin, tenascin | ||
| αV | Fibrinogen, fibronectin, von Willebrand factor, vitronectin, thrombospondin, osteopontin, collagens | |||
| β4 | α6 | Laminins | ||
| β5 | αV | Vitronectin, fibronectin | ||
| β6 | αV | Fibronectin, tenascin | ||
| β7 | α4 | Fibronectin, VCAM-1, MAdCAM-1 ‡ | ||
| αE | E-cadherin | |||
| β8 | αV | Vitronectin | ||
Integrins are versatile receptors, transmitting inside-out and outside-in signals, which are crucial for the establishment of appropriate interactions between the exterior and interior of the cell. Many processes like cell morphology, motility, proliferation, differentiation and death are contingent on this incessant dialog.This bi-directional signalling requires, on one side, the integrin receptor to bind to ligands outside of the cell, and on the other to subcellular components. Many integrins bind beyond the cell to ECM proteins and thereby mediate cell–ECM interactions. Among ECM ligands for integrins are fibronectin, laminin, various collagens, tenascin, vitronectin and members of the SIBLINGs family (Small Integrin Binding LIgand, N-linked Glycoproteins), such as osteopontin, bone sialoprotein and dentin matrix protein 1 [45]. Other integrins bind to cell membrane receptors, mediating cell—cell adhesion. Such counter receptors are VCAM-1 and ICAM-1/2. In the third mode of interaction, for example, the αIIbβ3 integrin promotes the binding of platelets to one another through soluble, multivalent mediator molecules. Fibrinogen and von Willebrand factor function as the primary ligands for the platelet receptor {46}. Next, if we look at the inner face of the cell membrane, there are a lot of associated proteins, which can interact with the integrin transmembrane or cytoplasmic domains and their number is growing constantly. Once integrins are bound to their ligands, they move laterally in the plain of the membrane to form specialized clusters called focal adhesion sites. These specialized ECM attachment organelles and signalling centres assure substrate adhesion as well as targeted location of actin filaments and signalling components and hence they are essential for establishing cell polarity, directed cell migration, and maintaining cell growth and survival {47}. Some of these structural and functional features of focal adhesion are schematized in Fig. 2. In addition, we show staining for the focal adhesion component – paxillin in hMSCs.
2.
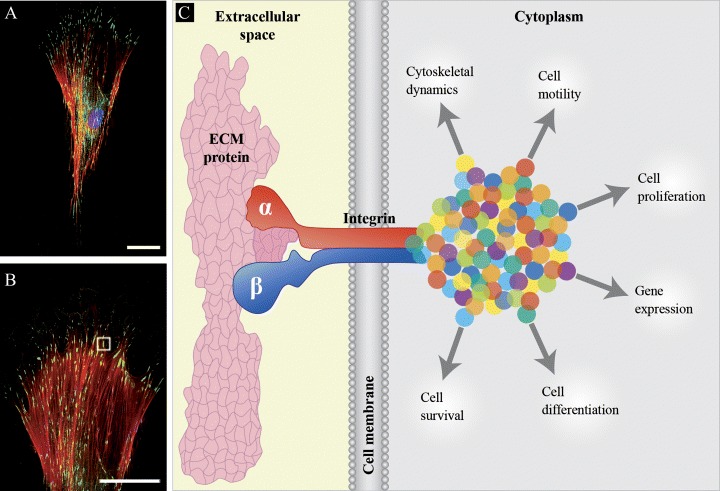
A schematic model depicting the structure of focal adhesion and paxillin staining. (A and B) Fluorescent photomicrograph of human MSC showing the immuno-histological detection of paxillin, a multi-domain adaptor that recruits both structural and signalling molecules to focal adhesions (see gray outline in B). Primary anti-paxillin antibody (Cat. no. 1500-1, Epitomics, USA) was combined with secondary Alexa Fluor 488 antibody (in green), (Cat. no. A11034, Molecular Probes, Germany). Actin fibres and nucleus were stained respectively with Phalloidin (in red), (Cat. no. H-22284, Molecular Probes, Germany) and DAPI (in blue), (Cat. no. D1306, Molecular Probes, Germany). Bars: 50 μm (C) Focal adhesions are sites, where the heterodimeric (and) integrin receptors (depicted by the red and blue larger forms) engage with an extracellular matrix (ECM) protein (the shape in pink) and a cascade of integrin-, membrane-, actin- and signalling-associated molecules (represented as multi-colour dots). Besides having central roles in cell motility and cytoskeletal dynamics, focal adhesions convey information across the cell membrane to regulate cell proliferation, differentiation, gene expression and survival.
Focal adhesions and their molecular composition
Focal adhesions are complex in their molecular composition. Studies have revealed presence of cytoskeletal proteins (tensin, vinculin, paxillin, α-actinin, parvin/actopaxin and talin), tyrosine kinases (Src, FAK, PYK2, Csk and Abl), serine/threonine kinases (ILK, PKC and PAK), modulators of small GTPases (ASAP1, Graf and PSGAP), tyrosine phosphatases (SHP-2 and LAR PTP) and other enzymes (PI 3-kinase and the protease calpain II). Some of these proteins can directly bind actin filaments (vinculin, tensin, -actinin, VASP, parvin/actopaxin and ERM-proteins) and/or directly bind to the cytoplasmic tails of integrins (talin, tensin, FAK, ILK and α-actinin). Moreover, many of these components and their variants are expressed in a cell-type restricted fashion, introducing yet another level of complexity [48].
Integrins affect cell morphology, migration, proliferation, differentiation and survival
Multiple protein-to-protein interactions defined at the site of focal adhesions allow the cell to construct various signalling complexes leading to diverse behaviours. For example, differences in the focal adhesion and actin cytoskeleton properties determine the variety of cell morphology. The actin cytoskeleton provides a structural framework around which cell shape and polarity are defined. Its dynamic properties provide the driving force for cells to move and divide. For instance, when integrin-linked kinase (ILK), a crucial binding partner of an integrin cytoplasmic domain, is ablated in fibroblasts, cell shape spreading, F-actin aggregation, focal adhesion formation and proliferative rates are impaired [49]. Further downstream of integrins, members of the Rho family of small guanosine triphosphatases (GTPases) have emerged as key regulators of the actin cytoskeleton, and further-more, through their interaction with multiple target proteins, these Rho GTPases ensure the co-ordinated control of other cellular activities such as gene transcription and adhesion (reviewed in Hall [50]).
It has been suggested that the strength of focal adhesions influences cell motility [51]. Cell migration is diminished in cells exhibiting strong adhesion, as characterized by abundant actin stress fibers and numerous focal contacts, therefore preventing the cells from releasing its cytoskeleton-ECM linkages. Intermediate state of adhesion facilitates cell migration, whereas weak adhesion does not generate the contractile force necessary for directed cell movement (reviewed in Murphy-Ullrich [52]). Integrin signalling promotes cell migration by inducing changes in the cytoskeletal organization and by increased cellular contractility. Cell motility is additionally facilitated by a partial destruction of the surrounding ECM. Such degradation is catalyzed by matrix metalloproteinases (MMPs). Various publications have indicated that integrin-induced signalling is involved in the control of MMP expression (reviewed in Brakebusch et al.[53]).
The initiation of integrin-mediated cell adhesion has also an impact on proliferation. Integrins can regulate, in a cooperative manner some members of the Cyclin family and thereby progression through the cell cycle. Establishment of specific integrin-ECM stimuli can as well lead to the augmentation of gene expression related to differentiation. On the contrary, loss of adhesion causes endothelial and epithelial cells to undergo apoptosis, a process referred to as anoikis (reviewed in Danen and Sonnenberg [54]).
Consequences of integrins dysregulation
Emerging from these cellular events are the effects on physiological processes like the homing and mobilization of HSCs [55], matrix remodeling (reviewed in Larsen et al.[56]), morphogenesis (reviewed in De Arcangelis and Georges-Labouesse [57]) and development (reviewed in Reddy and Mangale [44]). Gene targeting technology has made it possible to generate mice that lack specific integrins and to gain knowledge about their function in vivo (reviewed in Bouvard et al.[58]). For instance, mice deficient in β 3 integrin produce altered osteoclasts, leading to osteosclerosis characterized by increased bone mass [59]. Moreover, dysregulation of integrin function has been repeatedly linked to pathological invasion and tumorigenesis. As cancer cells become metastatic, they develop altered affinity and avidity for their ECM. This is pursued with dysregulated protease activity and changed integrin expression. The consequences are loss of contact inhibition, anchorage independence and increased agility. In fact, inhibitors of the interaction between MMP-2 and αVβ3 integrin potently suppress the growth of melanomas and gliomas [60]. For a more detailed description of the link between integrins and cancer, a reference to some excellent reviews can be recommended (Hood and Cheresh [61], Guo and Giancotti [62]).
Different focal adhesions in 2D versus 3D culture
Finally, given the high tissue and cell-type specifici ty of integrin signalling and its far-reaching consequences for cell fate, it is important to emphasize that many of the integrinactin interactions and regulatory mechanisms cannot claim to be universally valid. Much of the available data was obtained from experiments using cell lines cultured on a rigid two-dimensional (2D) matrix [63]. Intriguingly, several studies showed that cells do form three-dimen sional (3D) matrix adhesions but are not quite the -same as their 2D counterparts (reviewed in Wozniak et al.[64]). For example, Cukierman et al.[65] have shown with embryonic mouse mesenchymal cells that 5 integrin and paxillin colocalize in focal adhesions on a 3D cell-derived matrix, but not in a 2D fibronectin matrix. Additionally, the phosphorylation of FAK kinase gets lost in in vivo-like matrix adhesions, suggesting that different signalling events might occur in comparison to what is known so far from 2D research systems. The role of integrins therefore yearns to be elucidated on a wider spectrum of cells and in 3D systems that mimic cell-matrix interactions in living organisms. Gaining knowledge on these points can be very much of appliance to hMSCs and their accurate use.
Cell surface molecules on MSCs
HMSCs express a variety of different cell surface proteins, including numerous integrins (see Table 2), growth factor receptors (bFGFR, PDGFR, EGFR, TGFβIR/IIR), chemokine receptors (some inter-leukins, CC and CXC receptors) and cell adhesion molecules (VCAM-1, ICAM-1/2, ALCAM-1, L-selectin, CD105, CD44) [17, 66]. Moreover, hMSCs produce a vast array of matrix molecules including fibronectin, collagen type I, III, IV, laminin, hyaluronan and proteoglycans. These cells should therefore be highly responsive to signals from a diverse nature. However, before we concentrate on the integrin system of hMSCs, we will briefly discuss some recent studies regarding alternative pathways for the stimulation of adhesion and migration in hMSCs.
2.
Integrin subunits detected on human mesenchymal stem cells
| Term | Identified integrins | Method(s) | Reference(s) |
|---|---|---|---|
| Bone marrow stromal cells (BMSC) | αVβ3 | FACS | Karadag and Fisher [83]† |
| BMSC | α2, α4 and β1 | FACS | Walsh et al.[79]† |
| BMSC | α2, α5, α6, αL, β1 and β2 | FACS | Majumdar et al.[99] |
| Colony-forming unit fibroblasts (CFU-Fs) | α1β1, α2β1, α4β1, α5β1, α6β1, αVβ3, αVβ5, β1 and β3 | FACS | Gronthos et al.[77]† |
| Human mesenchymal progenitor cells (hMPC) | α1, α2, α3 and β1 | Real-time PCR | Heckmann et al.[90]† |
| Human mesenchymal stem cell (hMSC) | α1, α2, α3, αV, α6, β1 and β4 | Immuno-histochemistry | Klees et al.[81]† |
| hMSC | β1 | FACS | Aslan et al.[75], Lisignoli et al.[73], Wagner et al.[76] |
| hMSC | α2, α3, αV and β1 | FACS | Chang et al.[91]† |
| hMSC | α1, α3, α4 and αV | FACS | Neuss et al.[100] |
| hMSC | α2, α5, αV, β1 and β3 | FACS | Grayson et al.[92]† |
| hMSC | α1, α2, α3, α5, α6, αV, β1, β3 and β4 | FACS | Majumdar et al.[71] |
| hMSC | α2b, α3, α5, α10, αV, β1, β3 and β5 | Microarray assay | Goessler et al.[85]† |
| hMSC | α2, α3, α4, α5, α6, β1, β2, β3, β5 and β6 | PCR | Chastain et al.[93]† |
| hMSC | β1 | Western blot, PCR | Lee et al.[94]† |
| hMSC | α2 and β1 | Western blot | Meyers et al.[80]† |
| hMSC - hTERT | α2, α4, α5, α6, α11, αV, β1 and β5 | Proteomics | Foster et al.[78]† |
| Multipotent adult progenitor cell (MAPC) | α2 and αV β5 | FACS / Immuno-histochemistry | Reyes et al.[101] |
Marked articles demonstrate a functional role for integrins in hMSC.
Several groups have in parallel investigated the expression and role of chemokine receptors in BM-derived cells [66–69]. Chemokines are small polypeptides that navigate not only haematopoietic cell trafficking and homing, but also cell activation, differentiation and survival, and thus contribute to the formation of specific BM microenvironments. Honczarenko et al.[66] have found that hMSCs express a unique set of chemokine receptors: three CC receptors (CCR1, CCR7 and CCR9) and three CXC receptors (CXCR4, CXCR5 and CXCR6). HMSCs also secrete several of the following ligands: CCL2, CCL4, CCL5, CCL20, CXCL12 and CXCL8. Upon ligand binding, phosphorylation of MAPK and FAK kinases occurs. Additionally, the activation of signal transducer and activator of transcription (STAT) factors and the polymerization of cytoskeletal Factin happen. Interestingly, the long-term culture of hMSCs caused a marked decrease in chemokine receptor expression.
Altogether, these findings suggested that several chemokine axes are important in the biology of hMSCs. A particular chemokine axis, such as SDF-1/CXCR4, has been already investigated in great depth. Stromal deriver factor-1 (SDF-1) is assumed to be involved in the stimulation of haematopoiesis by undifferentiated hMSCs. Indeed, blocking of SDF-1 or its receptor during co-culture of hMSCs and haematopoietic stem/progenitor cells (HSPC) led to reduction of HSPC proliferation and cycling [68]. SDF-1 and hepatocyte growth factor (HGF) become up-regulated during tissue/organ damage and are implicated as chemo-attractants in cell migration. Son et al.[69] have determined the expression of their respective receptors – CXCR4 and c-met (transmembrane tyrosin kinase encoded by MET proto-oncogene) in hMSCs and have observed the positive effect of SDF-1 and HGF on hMSC migration in a matrigel invasion assay. Moreover, the authors found that hMSCs secrete MMP-2 and membrane type 1 (MT1)-MMP and when the MT1-MMP inhibitor was used the chemo-invasion was significantly diminished. These in vitro results suggest that SDF1/CXCR4 and HGF/c-met axes, along with MMPs, may be involved in the recruitment of expanded hMCS to damaged tissue. Intriguing is the fact that cancer stem cells also express CXCR4 on their surface and thereby can be directed towards metastasis in organs that highly express SDF-1 (reviewed in Kucia et al.[70]).
Surface molecules of the immunoglobulin super-family participate in cell-to-cell interactions within the BM compartment. Flow cytometry analysis on hMSCs determined that they express surface molecules whose ligands are present on mature cells of the haematopoietic lineage, including ICAM-1 and 2 and both VCAM-1 and ALCAM-1. Majumdar et al.[71] then showed the augmentation of hMSCs ICAM-1 expression in exposure to IL-1α, TNF-α or IFN-γ. Furthermore, they looked into the role of VCAM-1/4 integrin binding in hMSCs/T lymphocytes interaction. For this purpose, the authors performed a whole-cell binding assay and the pre-incubation of T lympho-cytes, with blocking antibodies against α4, resulted in tremendous inhibition in T-cell binding to hMSCs. This report also provided evidence that binding between the two cell types under the appropriate conditions can result in antigen presentation and cytokine production, suggesting an in vivo role for hMSCs influencing both haematopoietic and immune functions.
Another surface molecule, which is strongly expressed in hMSCs and has recently received much attention, is the hyaluronan receptor CD44 [72]. Hyaluronan, in BM stroma, is the major non-protein glycosaminoglycan component of the ECM and is involved in cell positioning, proliferation and differentiation as well as in receptor-mediated gene expression. Lisignoli et al.[73] have made an interesting finding, showing that the CD44 and ICAM-1 receptors are directly involved in the modulation of some CXC chemokines in a study where hMSCs were grown on a hyaluronan-based scaffold. They also found that due to the contact with the scaffold material, the expression of some MMPs was modified in hMSCs. Their data demonstrated that hyaloronan-CD44 or CD54 interactions affect the hMSC inflammatory and degradative properties, which have to be considered in tissue engineering applications with hyaluronan-based scaffolds. A recent article on the CD44 receptor in rat mesenchymal progenitor cells has emphasized its role in cell migration in response to PDGF stimulation. First, the adhesion of the cells to hyaluronan was strongly suppressed by anti-CD44 antibody and by CD44 small interfering RNA. Thereafter, the migration was significantly inhibited. Such a migratory mechanism has to be questioned for the recruitment of MSCs into wound sites for the preposition of tissue regeneration as well as for migration of fibroblast progenitors to allografts in the development of graft fibrosis [74].
Integrin system of MSCs
In the remaining part of the review, we will return to the primary receptors in consideration here – the integrins. Plenty are the articles, describing integrin subunits detected on hMSCs. Examples are included in Table 2.We have ordered the publications in alphabetical order to the original terms used for naming hMSCs. Moreover, we have mainly favoured recent publications, where the functional importance of integrins in hMSC behaviour has been reported (see table footnotes).
The fluorescence-activated cell sorting (FACS) method has been mostly used for the identification of integrin subunits presented on the cell surface of hMSCs. Expression of integrin subunits like α1, α2, α3, α5, α6, αV, β1, β3, β4 [71] among others have been independently reported. Nevertheless, contradictory results exist and it is still unclear if all subunits are indeed expressed. Few studies have relied on RNA- and protein-based approaches. The detection of the ubiquitous integrin β1 in hMSCs has frequently accompanied several other antigens to immuno-phenotype the cells in vitro[73, 75, 76].
Some articles not only provide evidence of integrin expression but interestingly also that of integrin engagement in the biology of hMSCs. It is these that will be diligently discussed in the following chapter. For the sake of clarity, our survey will maintain the term hMSCs.
Engagement of integrins in hMSC differentiation, attachment to coating materials, survival on scaffolds and neoplastic transformation
Osteogenic differentiation
We will start out discussion with a significant study from Gronthos et al.[77]. They examined the mechanisms mediating the growth of hMSCs on different ECM components, typically found in the loose meshwork of marrow reticular fibers (collagen type III), the endothelial basal laminae of sinusoids (collagen type IV and laminin) and the surrounding, calcified matrix of bone (collagen type I). HMSCs demonstrated a higher colony-forming efficiency when seeded onto collagen type IV, fibronectin, vitronectin and laminin in comparison with collagen type I and III. The differential growth patterns may be a function of their original location in the BM, stage of commitment and unique integrin expression. Therefore, they further analysed the integrin expression on the hMSCs surface and the functional importance of the receptors was determined, using a panel of blocking antibodies against a range of integrin heterodimers. The integrin heterodimers found were α1β1, α2β1, α5β1, α6β1, αVβ3 and αVβ5, using duo-colour analysis. Subsequent adhesion studies using blocking anti-bodies showed that hMSC propagation on collagens, laminin and fibronectin was mediated by the α1 integrins. In contrast, the cloning efficiency in the presence of vitronectin was mediated by αVβ3. Finally, they investigated the role of β1 integrin during cell differentiation in osteoinductive conditions in vitro. The ability of hMSCs to form a mineralized matrix was significantly diminished in the presence of 1 blocking antibody. The results of this study, therefore, indicate the 1 integrin subfamily to be predominant in hMSCs and utilized for adhesion and proliferation on matrix proteins found in the BM. Furthermore, β1 integrin seems to be important for the differentiation of hMSCs into osteoblasts in vitro.
Using a more global approach, quantitative mass spectrometry-based proteomics, Foster et al.[78] profiled the differential expression of membrane proteins in the hMSC-hTERT line undergoing osteogenic differentiation. Twenty-nine integrins and cell adhesion molecules, 20 receptors and 18 small GTPases were identified. CD105, CD73, CD90, integrin subunits β5, α6, αV, β1, fibronectin and collagen type IV among many other proteins were detected in the cellular membrane fraction. Interestingly, during osteogenic differentiation, several integrins and adhesion-associated molecules were up-regulated such as integrin α11, α2 (collagen receptors) and ILK. The increase of integrin β1 has also been confirmed. Most strikingly, versican, a chondroitin sulphate proteoglycan, and two binding partners, CD44 and tenascin, were increased. Tenascin likely functions to stop cell migration and hence the authors have hypothesized that cell motility is reduced in order to proceed for osteogenic differentiation.
Two other studies have looked from a more clinical angle on the role of α2 and β1 integrins during commitment of hMSCs towards osteoblasts. Walsh et al.[79] have tested the effects of different concentrations of dexamethasone, a glucocorticoid which can cause osteoporosis in clinical settings but which also is used as a component of the osteoinductive media on hMSCs in vitro. At a physiological concentration, dexamethasone had no influence on cell adhesion and expectedly did promote osteogenic differentiation. Moreover, the expression of integrin α2β1 was increased which is in line with previous reports. This heterodimer binds collagen type I and may therefore play a pivotal role in the regulation of osteogenic differentiation. Meyers et al.[80] investigated the collagen type I/α2β1integrin signalling from the perspective of reduced osteoblastogenesis in weight-bearing bones due to spaceflights. As a model, they have used national aeronautics and space administration (NASA)-developed rotary cell culture system to simulate microgravity in vitro. Surprisingly, they found that osteogenic-stimulated hMSCs, cultured in microgravity conditions, increased their expression of α2β1 integrin while the downstream activation of MAPK and FAK kinases was reduced.
Another interesting observation regarding β1 integrin and hMSCs differentiation towards osteoblasts came from Klees et al.[81]. They stained bone specimens for laminin 5, which is usually found in tissues with ectodermal and endodermal origin.This ECM protein is suggested to promote cellular growth, morphogenesis and wound healing processes. Laminin 5 gave a positive signal in the preiosteum where it is believed that osteoblasts colocalize with MSCs. Because of relatively low expression levels, the authors have used enzyme-labelled fluorescence and ultraviolet irradiation for reducing tissue autofluorescence in order to uncover its presence. However, it remains unclear exactly which cell type expresses this protein in vivo. More convincingly, when they plated hMSCs on laminin 5-coated dishes, the levels of integrin α3β1 were up-regulated and the phosphorylation of osteogenic transcription factor Runx2 followed. Another, more recent study from the same group further showed FAK as an important signalling mediator and that its phosphorylation is indispensable for the up-regulation of osteogenic genes in MSCs grown on laminin 5 [82].
The last article, selected to discuses hMSCs and their osteoblast progeny implicated the integrin αVβ3/MMP-2 complex as an important migratory mechanism [83]. Briefly, the cellular migration was enhanced by bone sialoprotein, a protein produced by osteoblasts and it was accompanied with an increase in integrin αVβ3 expression. MMP-2 involvement was proved by the use of a specific inhibitor causing the suppression of migration.
Chondrogenic differentiation
A promising therapeutic application of hMSCs has been delineated due to their ability to differentiate into chondrocytes, especially because isolated chondrocytes dedifferentiate in vitro. HMSCs have already been grown on composite materials to mimic cartilage tissue geometry and some auspicious results have been obtained (reviewed in Tuli et al.[84]). As the interactions between the ECM and the cellular compartment can alter cell behaviour, Goessler et al.[85] investigated the expression of integrins using microarray analysis during chondrogenic differentiation of hMSCs in comparison with dedifferentiating human chondrocytes. The appreciable outcome showed that fibronectin, α5β1 and αIIbβ3 integrins, and ILK were all down-regulated, whereas the vitronectin receptor – V3 integrin was not changed during stimulation of hMSCs towards the chondrocyte lineage. On the contrary, dedifferentiating chondrocytes exhibited higher expression of fibronectin, α5β1 integrin and ILK suggesting their involvement in a signal transmission during the dedifferentiating program.
Attachment to scaffold materials
One of the most attractive directions for use of hMSCs is in the means of tissue engineering and regenerative medicine. Mostly, this approach is represented as the cultivation of reparative cells on an appropriate scaffold, which is then introduced into the site of defect tissue [40]. Scaffolds can be prepared from a wide variety of materials and their ingredients can be influential to cell attachment in vitro and the in vivo tissue response. Likewise, cell survival can be affected. Some of these aspects have been investigated in the testing of bone replacement materials with the use of osteosarcoma cell lines like MG63, Saos-2 and U-2 OS ([86] and reviewed in Siebers et al.[87]). For example, MG63 human osteosarcoma cells, when cultured on titanium (a substrate used in the replacement of teeth, knee joints and deteriorated hips) with increasing roughness, showed the augmented expression of α2, α3, α5, β1 and β3 integrins [88]. The use of hydroxylapatite surfaces instead of titanium is characterized by the increase of osteoblast adhesion [89]. On that account, biomaterials and integrins have become of interest in tissue engineering. Another advantage of the scaffolds is that they provide the bases for analysing the properties of 3D-formed adhesions. Several works, using hMSCs, have already reported on the above topics. Heckmann et al.[90] have documented the growth of hMSCs on collagen type I gels and concluded that the interaction is dependent on α1, α2 and β1 integrins. Besides, when β1 integrin function was blocked with an antibody, the cells rounded up and MMP-1 expression was elevated irrespective of the blocking. In an alternative study, where hMSCs were cultivated on collagen type II 3D fibres, a spontaneous chondrogenic differentiation was observed and the blocking of α2 or β1 integrins significantly impeded the collagen fibre remodelling by the cells [91]. An earlier investigation by Grayson et al.[92] showed another noteworthy finding. HMSCs seeded on 3D poly(ethylene terephthalate) (PET) scaffolds secreted and embedded themselves in an extensive ECM network composed of collagen type I and IV, fibronectin and laminin. Integrin 21 was increased whereas α5β1 was slightly decreased in 3D compared to 2D cultures. Paxillin expression and localization patterns were changed in 3D cultures and integrin αVβ3 was present only in 2D.
Taken together, these results provide clues for the designing of 3D scaffolds, which can later be used as tissue engineering constructs. In the same direction, a recent article, showed that the osteogenic potential of hMSCs differed according to the applied polymer scaffold [93]. In this study, Poly(lactide-co-glycolide) (PLGA) and poly(caprolactone) (PCL), two widely used polymeric biomaterials were implicated. Adhesion-blocking studies revealed that hMSCs adhere to PLGA primarily via collagen type I, while vitronectin mediates their attachment to PCL. Most interesting was the gene expression profiles of the β3 integrin gene, which can mediate the binding of osteoblasts to fibronectin, vitronectin and osteopontin. In this case, 3 integrin RNA levels were quite high in hMSCs seeded on PLGA scaffolds after only 3 days in culture and nearly undetectable in cells on PCL scaffolds. Over the 5-week time course, the expression levels essentially reversed. Although these profiles are somewhat difficult to interpret, it was obvious that demand for the β3 integrin protein, and thus the level of gene expression, was changing over time in a fashion depending on the type of scaf-fold. Lee et al.[94] have implicated β1 integrin in the adhesion of hMSCs on similar, poly(lactic acid) PLA scaffolds. Cell attachment to the polymers was lowered to only 2–8% when the β1 integrin receptor was blocked by a specific antibody. Overall, these results allude how applicable such knowledge can be for scaffold development.
Neoplastic transformation
One critical point as mentioned before is that hMSCs can also be a target for neoplastic transformation. These cells, as the supportive stroma of the BM, can also be embroiled into the interaction with altered blood cells. Several research groups have already explored this field and have shared the evidence that α4β1 and α5β1 integrins dominate the traffic of leukemic cells in human BM stroma. For example, K562 cells, an erythroleukemia control cell line, lack the α4 and α5 receptors and show the absence of binding to the BM [95]. The same group also proposes fibronectin as a matrix for tumour cell adhesion in the BM stroma. In vitro confocal laser microscope analysis showed that hMSCs produce high amounts of fibronectin, especially at contact points between cells. By using α5β1 integrin- and fibronectin-blocking anti-bodies, it was clarified that the tested tumour cell lines were not able to adhere on hMSCs [96]. Last to be discussed is an article where a mechanism for the metastasis of primary prostatic epithelial cells has been investigated. First, it was shown that collagen type I and fibronectin increased the tumour cell adhesion whereas vitronectin and laminin did not. Inhibition studies demonstrated the involvement of α2β1 in the adhesion of the prostatic epithelial cells to the hMSCs [97]. Altogether, further efforts in understanding the role of integrins and hMSCs in the origination and propagation of tumours will be highly awarded not only because of their contribution to basic biological comprehension but also to the development of low-risk hMSC-based therapeutic strategies.
Conclusions
HMSCs, as a source for exciting interdisciplinary applications with the endmost goal to functionally restore damaged tissues, have been extensively studied on different levels of complexity. Voluminous amounts of data have already been provided on the question of which integrins hMSC express and utilize. Surely, there are many points to be secured and further explored. For example, a frequently used approach is the blocking of receptor signalling via antibodies, which can now be performed in a neater way with small interference RNAs. Furthermore, 3D systems in vitro are mimicking the in vivo situation more closely than monolayer cultures, allowing better interpretation of the composition and functioning of focal adhesions.
In summary, we have delivered examples of integrin participation in the eccentric life of hMSCs as we believe there is still a lot to wonder and discover about the essence of their mutual relations.
Acknowledgments
We acknowledge the financial support of the Bavarian Research Foundation (grant DPA-51/05) and the AO Foundation (grant 05-D83). We also thank Khrystyna Ern and Florian Haasters for providing microscopic material included in Fig. 1, Antje von Hoyer for preparing the cartoon part of Fig. 2 and Edward Fellows for carefully reading the manuscript.
References
- 1.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–5. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ. Osteogenetic activity of transplanted transitional epithelium. Acta Anat (Basel) 1961;45:31–59. doi: 10.1159/000141739. [DOI] [PubMed] [Google Scholar]
- 3.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–16. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robey PG, Bianco P. The use of adult stem cells in rebuilding the human face. J Am Dent Assoc. 2006;137:961–72. doi: 10.14219/jada.archive.2006.0317. [DOI] [PubMed] [Google Scholar]
- 5.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 6.Verfaillie CM. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 2002;12:502–8. doi: 10.1016/s0962-8924(02)02386-3. [DOI] [PubMed] [Google Scholar]
- 7.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–85. [PubMed] [Google Scholar]
- 8.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 9.Lakshmipathy U, Verfaillie C. Stem cell plasticity. Blood Rev. 2005;19:29–38. doi: 10.1016/j.blre.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 11.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060–9. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 13.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–10. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 14.Sabatini F, Petecchia L, Tavian M, Jodon DV, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest. 2005;85:962–71. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 15.Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191–9. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 16.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 17.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med. 2001;226:507–20. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 18.Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36:585–97. doi: 10.1016/j.biocel.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 19.He Q, Wan C, Li G. Multi-potent mesenchymal stromal cells in blood. Stem Cells. 2006;Sep 14 doi: 10.1634/stemcells.2006-0335. [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Piersma AH, Ploemacher RE, Brockbank KG. Transplantation of bone marrow fibroblastoid stromal cells in mice via the intravenous route. Br J Haematol. 1983;54:285–90. doi: 10.1111/j.1365-2141.1983.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–40. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazarus HM, Haynesworth SE, Gerson SL, Caplan AI. Human bone marrow-derived mesenchymal (stromal) progenitor cells (MPCs) cannot be recovered from peripheral blood progenitor cell collections. J Hematother. 1997;6:447–55. doi: 10.1089/scd.1.1997.6.447. [DOI] [PubMed] [Google Scholar]
- 23.Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–74. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 24.Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol. 2004;95:209–14. doi: 10.1111/j.1742-7843.2004.pto950502.x. [DOI] [PubMed] [Google Scholar]
- 25.Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1998;95:1142–7. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark BR, Keating A. Biology of bone marrow stroma. Ann N Y Acad Sci. 1995;770:70–8. doi: 10.1111/j.1749-6632.1995.tb31044.x. [DOI] [PubMed] [Google Scholar]
- 28.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Zipori D. Mesenchymal stem cells: harnessing cell plasticity to tissue and organ repair. Blood Cells Mol Dis. 2004;33:211–5. doi: 10.1016/j.bcmd.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22:487–500. doi: 10.1634/stemcells.22-4-487. [DOI] [PubMed] [Google Scholar]
- 31.Van Damme A, Vanden Driessche T, Collen D, Chuah MK. Bone marrow stromal cells as targets for gene therapy. Curr Gene Ther. 2002;2:195–209. doi: 10.2174/1566523024605645. [DOI] [PubMed] [Google Scholar]
- 32.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–71. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 33.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 34.Burns JS, Abdallah BM, Guldberg P, Rygaard J, Schroder HD, Kassem M. Tumorigenic heterogeneity in cancer stem cells evolved from long-term cultures of telomerase-immortalized human mesenchymal stem cells. Cancer Res. 2005;65:3126–35. doi: 10.1158/0008-5472.CAN-04-2218. [DOI] [PubMed] [Google Scholar]
- 35.Serakinci N, Guldberg P, Burns JS, Abdallah B, Schrodder H, Jensen T, Kassem M. Adult human mesenchymal stem cell as a target for neoplastic transformation. Oncogene. 2004;23:5095–8. doi: 10.1038/sj.onc.1207651. [DOI] [PubMed] [Google Scholar]
- 36.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–31. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 37.Dittmar T, Seidel J, Zaenker KS, Niggemann B. Carcinogenesis driven by bone marrow-derived stem cells. Contrib Microbiol. 2006;13:156–69. doi: 10.1159/000092971. [DOI] [PubMed] [Google Scholar]
- 38.Rubio D, Garcia-Castro J, Martin MC, De La FR, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 39.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–21. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 40.Schieker M, Seitz H, Drosse I, Seitz S, Mutschler W. Biomaterials as scaffold for bone tissue engineering. Eur J Trauma. 2006;32:114–24. [Google Scholar]
- 41.Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M, Giardino R, Cancedda R, Quarto R. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49:328–37. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 42.Otto WR, Rao J. Tomorrow's skeleton staff: mesenchymal stem cells and the repair of bone and cartilage. Cell Prolif. 2004;37:97–110. doi: 10.1111/j.1365-2184.2004.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calderwood DA. Integrin activation. J Cell Sci. 2004;117:657–66. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- 44.Reddy KV, Mangale SS. Integrin receptors: the dynamic modulators of endometrial function. Tissue Cell. 2003;35:260–73. doi: 10.1016/s0040-8166(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 45.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–5. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- 46.Ivaska J, Heino J. Adhesion receptors and cell invasion: mechanisms of integrin-guided degradation of extracellular matrix. Cell Mol Life Sci. 2000;57:16–24. doi: 10.1007/s000180050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brakebusch C, Fassler R. The integrin-actin connection, an eternal love affair. EMBO J. 2003;22:2324–33. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–90. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 49.Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fassler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–40. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 51.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–40. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 52.Murphy-Ullrich JE. The de-adhesive activity of matri-cellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–90. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R. Integrins in invasive growth. J Clin Invest. 2002;109:999–1006. doi: 10.1172/JCI15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;200:471–80. doi: 10.1002/path.1416. [DOI] [PubMed] [Google Scholar]
- 55.Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 56.Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–71. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 57.De Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16:389–95. doi: 10.1016/s0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- 58.Bouvard D, Brakebusch C, Gustafsson E, Aszodi A, Bengtsson T, Berna A, Fassler R. Functional consequences of integrin gene mutations in mice. Circ Res. 2001;89:211–23. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- 59.McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–40. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silletti S, Kessler T, Goldberg J, Boger DL, Cheresh DA. Disruption of matrix metalloproteinase 2 binding to integrin alpha vbeta 3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci USA. 2001;98:119–24. doi: 10.1073/pnas.011343298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 62.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–26. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 63.Wiesner S, Legate KR, Fassler R. Integrin-actin interactions. Cell Mol Life Sci. 2005;62:1081–99. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–19. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 66.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–41. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 67.Honczarenko M, Glodek AM, Swierkowski M, Na IK, Silberstein LE. Developmental stage-specific shift in responsiveness to chemokines during human B-cell development. Exp Hematol. 2006;34:1093–100. doi: 10.1016/j.exphem.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 68.Overstraeten-Schlogel N, Beguin Y, Gothot A. Role of stromal-derived factor-1 in the hematopoietic-sup-porting activity of human mesenchymal stem cells. Eur J Haematol. 2006;76:488–93. doi: 10.1111/j.1600-0609.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- 69.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–64. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 70.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–94. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 71.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–41. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 72.Schieker M, Pautke C, Reitz K, Hemraj I, Neth P, Mutschler W, Milz S. The use of four-colour immuno-fluorescence techniques to identify mesenchymal stem cells. J Anat. 2004;204:133–9. doi: 10.1111/j.1469-7580.2004.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lisignoli G, Cristino S, Piacentini A, Cavallo C, Caplan AI, Facchini A. Hyaluronan-based polymer scaffold modulates the expression of inflammatory and degradative factors in mesenchymal stem cells: involvement of Cd44 and Cd54. J Cell Physiol. 2006;207:364–73. doi: 10.1002/jcp.20572. [DOI] [PubMed] [Google Scholar]
- 74.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–35. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 75.Aslan H, Zilberman Y, Kandel L, Liebergall M, Oskouian RJ, Gazit D, Gazit Z. Osteogenic differentiation of noncultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells. 2006;24:1728–37. doi: 10.1634/stemcells.2005-0546. [DOI] [PubMed] [Google Scholar]
- 76.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–16. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001;28:174–81. doi: 10.1016/s8756-3282(00)00424-5. [DOI] [PubMed] [Google Scholar]
- 78.Foster LJ, Zeemann PA, Li C, Mann M, Jensen ON, Kassem M. Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells. 2005;23:1367–77. doi: 10.1634/stemcells.2004-0372. [DOI] [PubMed] [Google Scholar]
- 79.Walsh S, Jordan GR, Jefferiss C, Stewart K, Beresford JN. High concentrations of dexamethasone suppress the proliferation but not the differentiation or further maturation of human osteoblast precursors in vitro: relevance to glucocorticoid-induced osteoporosis. Rheumatology. 2001;40:74–83. doi: 10.1093/rheumatology/40.1.74. [DOI] [PubMed] [Google Scholar]
- 80.Meyers VE, Zayzafoon M, Gonda SR, Gathings WE, McDonald JM. Modeled microgravity disrupts collagen I(integrin signaling during osteoblastic differentiation of human mesenchymal stem cells. J Cell Biochem. 2004;93:697–707. doi: 10.1002/jcb.20229. [DOI] [PubMed] [Google Scholar]
- 81.Klees RF, Salasznyk RM, Kingsley K, Williams WA, Boskey A, Plopper GE. Laminin-5 induces osteogenic gene expression in human mesenchymal stem cells through an ERK-dependent pathway. Mol Biol Cell. 2005;16:881–90. doi: 10.1091/mbc.E04-08-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salasznyk RM, Klees RF, Boskey A, Plopper GE. Activation of FAK is necessary for the osteogenic differentiation of human mesenchymal stem cells on laminin-5. J Cell Biochem. 2006;Aug 22 doi: 10.1002/jcb.21074. [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 83.Karadag A, Fisher LW. Bone sialoprotein enhances migration of bone marrow stromal cells through matrices by bridging MMP-2 to alpha(v)beta3-integrin. J Bone Miner Res. 2006;21:1627–36. doi: 10.1359/jbmr.060710. [DOI] [PubMed] [Google Scholar]
- 84.Tuli R, Li WJ, Tuan RS. Current state of cartilage tissue engineering. Arthritis Res Ther. 2003;5:235–8. doi: 10.1186/ar991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goessler UR, Bieback K, Bugert P, Heller T, Sadick H, Hormann K, Riedel F. In vitro analysis of integrin expression during chondrogenic differentiation of mesenchymal stem cells and chondrocytes upon dedifferentiation in cell culture. Int J Mol Med. 2006;17:301–7. [PubMed] [Google Scholar]
- 86.Pautke C, Schieker M, Tischer T, Kolk A, Neth P, Mutschler W, Milz S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004;24:3743–8. [PubMed] [Google Scholar]
- 87.Siebers MC, Ter Brugge PJ, Walboomers XF, Jansen JA. Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review. Biomaterials. 2005;26:137–46. doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 88.Lange R, Luthen F, Beck U, Rychly J, Baumann A, Nebe B. Cell-extracellular matrix interaction and physico-chemical characteristics of titanium surfaces depend on the roughness of the material. Biomol Eng. 2002;19:255–61. doi: 10.1016/s1389-0344(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 89.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J Biomed Mater Res. 2000;51:475–83. doi: 10.1002/1097-4636(20000905)51:3<475::aid-jbm23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 90.Heckmann L, Fiedler J, Mattes T, Brenner RE. Mesenchymal progenitor cells communicate via alpha and beta integrins with a three-dimensional collagen type I matrix. Cells Tissues Organs. 2006;182:143–54. doi: 10.1159/000093964. [DOI] [PubMed] [Google Scholar]
- 91.Chang CF, Lee MW, Kuo PY, Wang YJ, Tu YH, Hung SC. Three-dimensional collagen fiber remodeling by mesenchymal stem cells requires the integrin-matrix interaction. J Biomed Mater Res A. 2006;Sep 29 doi: 10.1002/jbm.a.30963. [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 92.Grayson WL, Ma T, Bunnell B. Human mesenchymal stem cells tissue development in 3D PET matrices. Biotechnol Prog. 2004;20:905–12. doi: 10.1021/bp034296z. [DOI] [PubMed] [Google Scholar]
- 93.Chastain SR, Kundu AK, Dhar S, Calvert JW, Putnam AJ. Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. J Biomed Mater Res A. 2006;78:73–85. doi: 10.1002/jbm.a.30686. [DOI] [PubMed] [Google Scholar]
- 94.Lee JW, Kim YH, Park KD, Jee KS, Shin JW, Hahn SB. Importance of integrin beta1-mediated cell adhesion on biodegradable polymers under serum depletion in mesenchymal stem cells and chondrocytes. Biomaterials. 2004;25:1901–9. doi: 10.1016/j.biomaterials.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 95.Van der Velde-Zimmermann D, Smits AJ, Verdaasdonk MA, Rademakers LH, Werner N, Spierings DC, De Weger RA, Van den Tweel JG, Joling P. beta1-Integrins dominate cell traffic of leukemic cells in human bone marrow stroma. Int J Cancer. 1996;66:225–33. doi: 10.1002/(SICI)1097-0215(19960410)66:2<225::AID-IJC15>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 96.Van der Velde-Zimmermann D, Verdaasdonk MA, Rademakers LH, De Weger RA, Van den Tweel JG, Joling P. Fibronectin distribution in human bone marrow stroma: matrix assembly and tumor cell adhesion via alpha5 beta1 integrin. Exp Cell Res. 1997;230:111–20. doi: 10.1006/excr.1996.3405. [DOI] [PubMed] [Google Scholar]
- 97.Lang SH, Clarke NW, George NJ, Testa NG. Primary prostatic epithelial cell binding to human bone marrow stroma and the role of alpha2beta1 integrin. Clin Exp Metastasis. 1997;15:218–27. doi: 10.1023/a:1018465213641. [DOI] [PubMed] [Google Scholar]
- 98.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 99.Majumdar MK, Banks V, Peluso DP, Morris EA. Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J Cell Physiol. 2000;185:98–106. doi: 10.1002/1097-4652(200010)185:1<98::AID-JCP9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 100.Neuss S, Becher E, Woltje M, Tietze L, Jahnen-Dechent W. Functional expression of HGF and HGF receptor(c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405–14. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- 101.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–46. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]


