Abstract
Intracellular antibodies (intrabodies) constitute a potent tool to neutralize the function of target proteins inside specific cell compartments (cytosol, nucleus, mitochondria and ER). The intrabody technology is an attractive alternative to the generation of gene-targeted knockout animals and complements or replaces knockdown techniques such as antisense-RNA, RNAi and RNA aptamers. This article focuses on intrabodies targeted to the ER. Intracellular anti-bodies expressed and retained inside the ER (ER intrabodies) are shown to be highly efficient in blocking the translocation of secreted and cell surface molecules from the ER to the cell surface.The advantage of ER intrabodies over cytoplasmic intrabodies is that they are correctly folded and easier to select. A particular advantage of the intrabody technology over existing ones is the possibility of inhibiting selectively post-translational modifications of proteins.The main applications of ER intrabodies so far have been (i) inactivation of oncogenic receptors and (ii) functional inhibition of virus envelope proteins and virus-receptor molecules on the surface of host cells.In cancer research, the number of in vivo mouse models for evaluation of the therapeutic potential of intrabodies is increasing.In the future, endosomal localized receptors involved in bacterial and viral infections, intracellular oncogenic receptors and enzymes involved in glycosylation of tumour antigens might be new targets for ER intrabodies.
Keywords: cell surface molecules, ER intrabodies, secretory pathway
Introduction
Various approaches are used to study specific protein function: gene-targeted knockout animals and knockdown techniques such as anti-sense RNA, RNAi and RNA aptamers [1–3]. Alternatively, intracellular expressed antibodies (intrabodies) can be applied to inhibit protein function [4]. Gene-targeted knockout mice are a powerful experimental system to examine the role of specific genes or protein iso-forms [1]. Nevertheless, the generation of knockout mice is very time consuming and in addition the technique is not applicable in human beings compared to the RNAi, RNA aptamers and intrabody technology which might be applicable in human diseases [3, 5, 6].
Intrabodies can be targeted to different subcellular compartments (cytosol, nucleus, ER, mitochondria) by the use of specific signal peptides [4]. Inside the targeted cell compartment, they can exert their function very effectively and specifically. They have the capacity to block or promote protein–protein or protein–DNA interactions [7–9], influence the function of enzymes [9, 10] and inhibit as ER-intrabodies the translocation of cell surface molecules from the ER to the cell surface [4].
This review is focused on the applications of intra-bodies targeted to the ER for inhibiting the transport of cell surface molecules to the plasma membrane. In the paper, the intrabody technology is explained and the in vitro and in vivo applications are reviewed and compared with the gene-silencing technique RNAi and RNA aptamers. Intracellularly applied aptamers are also called ‘intramers’[11]. The RNAi-mediated gene silencing at the transcriptional and post-transcriptional level is an emerging technology platform, which has become the method of choice for targeted knockdown of gene expression in mammalian cells. RNA interference is mediated by small interfering RNAs (siRNAs), which are intracellularly generated from long endogenous double-stranded RNA molecules (dsRNAs) through the cleavage activity of a ribonuclease III-type protein [5]. Alternatively, short hairpin RNA (shRNA) are expressed resulting in knockdown of the target message too [5]. Furthermore, the potential and limitations of ER intrabodies as therapeutic reagents are discussed.
The advantages of the intrabody technology are (i) the excellent specifity [12], (ii) very stable expression in mammalian cells (compared to small siRNAs and shRNAs [13] and RNA intramers [14]) and (iii) the possibility to inactivate and study the function of a specific protein domain (Table 1). Specific protein–protein interactions and post-translational modifications of proteins can be targeted that are not possible with gene-targeted knockout animals and knockdown techniques such as RNAi and anti-sense RNA. The RNAi approach is much less technically challenging than the intrabody-mediated knockout of protein function and different vector systems have been developed to allow stable promoter-driven intracellular expression of siRNAs and shRNAs [15, 16]. Furthermore, alleles differing by a single nucleotide polymorphism can be targeted by siRNAs [17, 18], but its major limitation is non-specifity [19, 20]. In addition, transfection of RNAi may result in up-regulation of IFN-stimulated genes [21] depending on the sequence and size of the siRNAs (Table 1).
1.
Intracellular antibodies versus RNA interference and intramers
| Intrabodies | RNAi | Intramers |
|---|---|---|
| Prerequisite is a specific antibody | Prerequisite is the sequence of the mRNA or promoter of the target | Prerequisite is a single-stranded library of oligonucleotides |
| Time consuming technology | Much less technical challenge | Much less technical challenge |
| Very high specificity to the target | Non-specific effects | Very high specifity to the target |
| Long active half-life | Relatively short active half-life | Relatively short active half-life |
| Targeting of specific protein domains | Loss of multiple functions of the target | Targeting of specific protein domains |
| Inhibition of post-translational modifications | Not possible | Maybe possible |
| No activation of the IFN system known | Sequence and size-dependent activation of the IFN system | Not investigated |
The advantages of the RNA aptamer(intramer technology is the high specifity, the possibility to select RNAs with a high affinity to the target by an in vitro selection (Selex: systematic evolution of ligands by exponential enrichment [3]) and the possibility to target specific protein domains.The main limitation of the intrabody technology is that an antibody against the specific target must exist.
ER intrabodies will give insights into the function of newly detected cell surface molecules and, furthermore, some will have potential application as therapeutic antibodies. A large number of different molecules with specific biological functions are expressed on the cell surface and are involved in cell growth, apoptosis, differentiation, adhesion, bacterial and viral infection and antigen presentation. ER intrabodies are transported to the lumen of the ER and bind to their specific secretory molecule (Fig. 1A). After transport via COPII-coated vesicles, the intrabody-target protein complex binds via the C-terminal retention sequence (KDEL) inside the cis Golgi network to the human ER receptor hERD2 [22]. Proteins retained inside the ER share a common carboxy-terminal tetrapeptide: KDEL [23]. Initially it was shown that fusion of the sequence SEKDEL from the resident luminal ER protein grp78 to lysozyme led to 100% retention [23]. In addition, fusion of the tetrapeptide KDEL to the secreted protein human proneuropeptide Y (pro-NPY) led to retention, too [24]. For the retention of ER intrabodies, the sequence SEKDEL has been used in almost all studies [4].
1a.
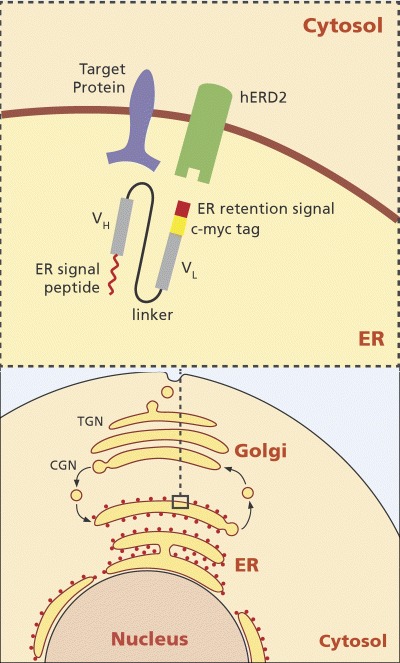
ScFv intrabody targeted to the ER. Shown is the ER intrabody as scFv fragment. VH= variable domain of the heavy chain, VL= variable domain of the light chain. The VH and VL domains are fused by a 15 amino acid flexible linker shown as a black line. The red line at the N-terminus of the VH domain represents the ER signal peptide. The red rectangle at the C-terminus of the VL domain represents the ER retention sequence and the yellow rectangle the c-myc tag. In addition is shown the target protein (cell surface molecule) and the hERD2 receptor that binds to the ER retention sequence of the scFv fragment. The complex consisting of the scFv fragment and the target protein binds to the hERD2 receptor inside the cis-Golgi and is transported through the Golgi apparatus back to the ER where the scFv-target protein complex is released. (CGN: cis-Golgi Network, TGN: trans-Golgi Network).
The KDEL signal induces oligomerization of hERD2, recruitment of ArfGAP (involved in COPI coat assembly) and formation of COPI-coated budding complexes [25]. The protein complex is then recycled back via COPI-coated vesicles to the ER (Fig. 1A). This results in a very efficient down-regulation of the expression of the specific cell surface target molecule on the cell surface. After retrieval to the ER, the proteins dissociate from the receptor and are degraded directly inside the ER [26] or by the cytoplasmic proteosome [27]. Recently, it was shown that the degradation of the intrabody target ß-amyloid precursor protein (APP) was blocked by an inhibitor of the cytosolic proteasome [28].
Mouse intrabodies will induce a major histocompatibility (MHC) class I-restricted cytotoxic T-cell immune response in human beings. After degradation of the intrabody molecule, the peptides are complexed with MHC I molecules inside the ER and transported to the cell surface. The MHC class I-peptide complex will then be recognized by cytotoxic T cells that have been primed before by antigen-presenting cells.
In the cancer field, ER intrabodies could block the cell surface expression of oncogenically activated proteins [Table 2, 29–46]. They down-regulate viral coat proteins and co-receptors of HIV-1 [Table 2, 47–54] and decrease the immunogenicity of allogeneic cell transplants by inhibiting the transport of MHC I from the ER to the cell surface [Table 2, 55–57]. Intrabodies neutralizing integrins are suitable tools for studying the significance of specific integrins for cell phenotype and differentiation [Table 2, 58–60]. Furthermore, ER intrabodies were successfully targeted to a transmembrane protein involved in the pathogenesis of Alzheimers's disease [28] and to cellular prion protein (PrPc) [61, 62]. Recently we could show that the function of toll-like receptors (TLRs) could be inhibited by ER intrabodies and have developed an anti-TLR2 intrabody that inhibits very effectively TLR2 signalling (Böldicke et al., manuscript in preparation). Interestingly, some of the cellular proteins targeted by the intrabody approach have been successfully silenced with similar efficiency performed with siRNAs or shRNAs: (ErbB-2, [63], EGFR [64], VEGFR-2 [65], HIV gp120 [66], CCR5 [67], CXCR4 [68], V integrin [69] and prion-like protein PrP [70]).
2.
Cellular targets of ER intrabodies
| ER Intrabodies inhibiting oncogenic proteins | ||||
|---|---|---|---|---|
| Target | Target function | Cellular targets | Performance | References |
| Human IL-2 receptor | Signal transduction | Jurkat T cells | In vitro studies with intrabody plasmid | [29] |
| Human IL-2 receptor | Signal transduction | T-cell leukaemia cell line, HTLV-1 transformed cell lines and primary human T cells | In vitro studies with tetracycline responsible intrabody plasmid and HIV-1-based lentivirus vector | [30, 31] |
| ErbB-2 | Signal transduction | NIH/3T3 cells expressing human erbB-2 and mammary carcinoma cells | In vitro studies with murine leukaemia virus vector | [32, 33] |
| ErbB-2 | Signal transduction | Human breast cancer and ovarian carcinoma cell lines | In vitro studies with recombinant adenovirus and intrabody plasmid | [34, 35] |
| ErbB-2 | Signal transduction | Human ovarian carcinoma cells transplanted in SCID mice | In vivo studies with recombinant adenovirus | [36] |
| ErbB-2 | Signal transduction | Human ovarian cancer cells | Phase I study with ovarian cancer patients performed with recombinant adenovirus | [37] |
| ErbB-2 | Signal transduction | Human erbB-2-positive tumour cell lines | In vitro study with intrabody plasmid containing a high affinity anti-erbB-2 scFv fragment | [38] |
| ErbB-2/androgen receptor | Signal transduction | Human prostate cancer tumour cell line | In vitro studies with murine leukaemia virus vector | [39] |
| EGFR | Signal transduction | Human tumour cell lines overexpressing EGFR | In vitro studies with murine leukaemia virus vector | [40] |
| VEGFR-2/KDR | Signal transduction | Porcine aortic endothelial cells overexpressing humanVEGFR-2 | In vitro studies with intrabody plasmid | [41] |
| VEGFR-2/KDR | Signal transduction | HUVECs | In vitro studies with recombinant adenovirus | [42] |
| Tie-2 | Signal transduction | Mouse model with Kaposi's sarcoma and human colon carcinoma xenografts | In vivostudies with adenovirus-delivered anti-Tie-2 intrabody | [43] |
| VEGFR-2/KDR-Tie-2 | Signal transduction | Mouse model with human melanoma xenografts | In vivo studies with adenovirus-delivered anti-VEGFR-2-Tie-2 intradiabody | [44, 45] |
| Human α folate receptor | Signal transduction | Human ovarian carcinoma cell lines | In vitro studies with intrabody plasmid | [46] |
| Cathepsin L | Endopeptidase | Human melanoma cell lines | In vitro studies with intrabody plasmid | [81] |
| Metalloproteinase MMP-2 and MMP-9 | Degradation of collagen IV | Human lung carcinoma cell line | In vitro studies with intrabody plasmid | [82] |
| ER intrabodies targeting HIV-1 gp160/gp120 and HIV-1 co-receptors | ||||
| Target | Target function | Cellular targets | Performance | References |
| HIV-1 gp120 | Viral coat protein | Human lymphocytes and CD4+ T cells from HIV-positive patients | In vitro studies with recombinant adeno-associated virus and murine leukaemia virus vector | [47, 48] |
| HIV-1 gp160 | Viral coat protein | CD4+ human T cells (Jurkat) | In vitro studies with intrabody plasmid | [49] |
| CCR5 | HIV-1 co-receptor | CCR5+/CD4+ human lymphocyte cell line | In vitro studies with murine leukaemia virus vector | [50] |
| CCR5 | HIV-1 co-receptor | CD34+ fetal liver stem cells | In vitro studies with HIV-1-derived self-inactivating lentivirus vector and in vivo studies with NOD/SCID mice transduced with the intrabody expressing CD34+ fetal liver stem cells | [51] |
| CCR5 | HIV-1 co-receptor | Human monocyte-derived macrophages and microglia cells from human fetal brain tissue | In vitro studies with Tag-deleted SV40-derived vector and hammerhead CCR5 specific ribozyme | [52] |
| CXCR4 | HIV-1 co-receptor | CD4+Human T lymphocytic cell line and HeLa-CD4/ βgal-CCR5 cells | In vitro studies with murine leukemia virus and SV40-based virus vector | [53] |
| CXCR4 | HIV-1 co-receptor | Primary human brain microvascular endothelial cells and post-mitotic differentiated human neurons | In vitro studies with HIV-1-based lentivirus vector | [54] |
| ER intrabodies targeting other viruses as HIV-1 | ||||
| Target | Target function | Cellular targets | Performance | References |
| Hepatitis C virus core protein | Viral core protein | Human hepatocellular carcinoma cell line and HEK 293 cells | In vitro studies with intrabody plasmid | [83] |
| Maedi-visna virus gp46 protein | Envelope glycoprotein | Sheep choroid plexus cell line | In vitro studies with the soluble scFv fragment, intracellular ER expression of the antibody is planned | [84] |
| Root-knot nematode Meloidogyne incognita | Nematoide in fection | Tobacco leaf protoplasts | In vitro studies with intrabody plasmid | [85] |
| ER intrabodies abrogating antigen presenting molecules | ||||
| Target | Target function | Cellular targets | Performance | References |
| MHC I | Antigen presentation | Primary rat keratinocytes | In vitro studies with intrabody plasmids | [55] |
| MHC I | Antigen presentation | Human CD4+ Jurkat T-cell line, human primary keratinocytes and several cell lines of divergent tissue sources | In vitro studies with intrabody plasmid and recombinant adenovirus | [56] |
| MHC I | Antigen presentation | Human umbilical vein endothelial cells | In vitro studies with recombinant adenovirus | [57] |
| α1,3-Galactosyl-transferase | Glycosylation | Pig epithelial kidney cells | In vitro studies with intrabody plasmid | [87] |
| ER intrabodies targeting integrins | ||||
| Target | Target function | Cellular target | Performance | References |
| αV integrin | Mediation of cell-cell and cellmatrix interactions | Saos-2 human osteosarcoma cell line and WM-266-4 melanoma cell line | In vitro studies with intrabody plasmid | [58, 59] |
| αV integrin | Mediation of cell-cell and cell-matrix interactions | Human metastatic melanoma cell lines and xenograft melanoma SCID mouse model | In vitro and in vivo studies with recombinant adenovirus | [60] |
| ER Intrabodies targeting proteins involved in Alzheimer's and Prion disease | ||||
| Target | Target function | Cellular target | Performance | References |
| Human β-amy-loid precursor protein (APP) | Transmembrane protein involved in the pathogenesis of Alzheimer's disease | HEK 293 cells transient transfected with cDNA of human APP | In vitro studies with intrabody plasmid | [28] |
| Cellular prion protein | Pathogenesis of Prion diseases | Nerve growth factor-differentiated PC12 cells infected with a suspension of mouse brains from scrapie-affected mice and scrapie mouse model | In vitro and in vivo studies with PC 12 cells stably expressing the intrabody | [61, 62] |
Intrabodies expressed in the ER are correctly folded through interaction with molecular chaperones such as BiP and GRP94 and the oxidizing environment of the ER favours intradomain disulfide bond formation [71]. On the contrary, cytoplasmic intrabodies often have folding and stability problems, resulting in low expression levels and limited halflife of antibody domains due to the reduced environment [72].To bypass these problems, the intracellular antibody capture (IAC) technology based on a yeast antibodyantigen two hybrid system was used to select scFv-fragments that tolerate the absence of the intra-chain disulfide bond in the reducing environment of the cytoplasm [73]. Additionally, a stable anti-GCN4 scFv intrabody could be expressed inside the cytoplasm of yeast by grafting the CDR loops to a highly stable single-chain framework [8].
Generation of ER-targeted intrabodies
The main format of an intrabody is the single-chain variable fragment (scFv) of an antibody, which consists of the H- and L-chain variable antibody domain (VH and VL) held together by a short, flexible linker sequence (Fig.1A and B).The source of the intrabody genes are existing hybridomas. To construct the intrabody, gene VH and VL can be amplified by an immunoglobulin-specific consensus primer, the scFv fragment assembled by overlapping PCR introducing the linker (Gly4Ser)3 and cloned into an ER-targeting vector (Fig. 1B). The ER-targeting vector contains a secretory leader, an ER retention signal and a peptide tag (for detection of the antibody, for example c-myc tag) that are both located at the end of the anti-body gene (Fig. 1B). The ER retention signal (SEKDEL, [23]) retains the antibody inside the lumen of the ER, where its binds the corresponding secretory target molecule and prevents further transport of the antigen to the cell surface (Fig. 1A). In general, expression of the intrabody is performed via the CMV promoter. The other intrabody format used is the Fab fragment. Alternatively, scFv fragments can be selected performed with in vitro display systems, such as phage, bacterial, yeast cell surface or ribosome display. In addition to the most commonly used intrabody formats, the scFv and Fab fragments, other very effective antibody formats have now been constructed: a bispecific intradiabody [44, 45] and single-domain intrabodies [74]. The bispecific intradiabody simultaneously silences two independent signalling pathways and shows high intracellular stability compared to scFv intrabodies [44, 45]. Single-domain antibodies are a new generation of small size, stable antibodies that can be isolated from existing phage display libraries.
1b.
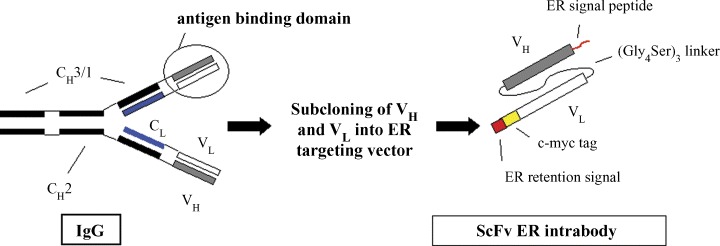
Construction of an scFv ER intrabody. The scheme shows the construction of an scFv ER intrabody starting from a complete hybridoma antibody. Shown are the heavy chain constant domains CH3/1 and CH2, the constant region of the light chain CL, and VH and VL constituting the antigen binding domain.The red square represents the ER retention signal and the yellow rectangle the c-myc tag for detection of the scFv ER intrabody. The 15 amino acid linker assembles the VH and VL domains of the scFv fragment.
However, it seems to be not possible to predict if an antibody is fully active as an intrabody. Nevertheless, we generated two scFv intrabodies recognizing VEGFR-2 from two soluble scFv fragments isolated by phage display [41, 75] and one scFv-intrabody against TLR2 from a complete hybridoma antibody [Böldicke et al., unpublished results]. In all three cases, the intrabodies could inhibit the translocation of the intracellular antigen from the ER to the cell surface to near 100%.Two factors will influence the activity of intrabodies inside the cell: (i) the amount of expressed intrabody and (ii) the stability and structure of the protein. The amount of the expressed intrabody can be controlled by the promoter used. Performed with a stronger promoter as the CMV promoter generally used (for example EF-BOS) could lead to high expression of the intrabody, but could also lead to ‘donut-like’ aggregates of intra-bodies targeted to the cytoplasm or ER compartment [76]. The stability of ER intrabodies (that are correctly folded in contrast to cytosolic intrabodies [72] can be enhanced performed with bivalent fomats [44, 45]. In this context, it would be very interesting to compare the stability of an scFv and Fab intrabody fragment. A recent published paper shows that the change of an scFv fragment to a Fab format improves the in vivo stability significantly [77].
Blocking translocation of growth factor receptors
Numerous key molecules, proto-oncogenes, hormones, peptides, growth factors and their corresponding receptors expressed by cancer cells, are involved in the proliferation of these cells [78]. Growth factor receptors, often carrying tyrosine kinase activities in their cytoplasmic domains, are overexpressed in many cancers and have been successfully targeted by intra-bodies. Cell growth and differentiation is induced via intracellular signalling after binding of the specific growth factor to the extracellular domain of the receptor [79]. Performed with ER-intrabodies, the function of oncogenic receptors and the corresponding tumour growth was inhibited (for example: IL2 receptor [29–31], ErbB-2 [32–39], EGFR [40], VEGFR-2 [41, 42], Tie-2 [43], simultaneously knockout of VEGFR-2/Tie-2 [44, 45] and human-folate receptor [46]).
Overexpression of ErbB-2 is observed in many tumours including breast and ovary. Intraperitoneal delivery of adenovirus encoding an anti-erbB-2 intra-body enhances survival and reduces tumour growth in a xenograft model of human ovarian carcinoma in SCID mice [36]. A phase I trial with ovarian cancer patients shows the limitations of an in vivo approach mainly due to insufficient adenoviral gene transfer [37]. Furthermore, performed with the ER intrabody approach, the crosstalk between ErbB-2 and the androgen receptor (AR) was experimentally verified. Expression of an ER-targeted anti-erbB2 intrabody in LNCaP prostate cancer tumour cells inhibited androgren-stimulated recruitment of AR to the androgen responsive enhancer of the prostate specific antigen (PSA) gene and histone acetylation of the enhancer, endogenous (PSA) expression and androgen-stimulated growth [39]. It showed that ErbB-2 signalling is required for optimal transcriptional activity of AR in prostate cancer cells.
The human vascular endothelial growth factor receptor-2 (VEGFR-2/KDR) and the Tie-2 receptor and their corresponding ligands vascular endothelial growth factor (VEGF-A) and angiopoietin-1/angiopoietin-2, respectively, play an important role in tumour angiogenesis and in haematological diseases [80]. Anti-VEGFR-2 intrabodies were described to block VEGFR-2 expression on the surface of porcine aortic endothelial cells and human umbilical vein endothelial cells (HUVECs) [41, 42]. The intrabodies inhibited in vitro angiogenesis. Interestingly, tumour angiogenesis was inhibited by intrabodies in vivo performed with human tumour xenograft mouse models. This was shown with an adenovirus-delivered anti-Tie-2 intra-body given to mice with well-established human Kaposi's sarcoma (SLK) or human colon carcinoma (SW1222) [43]. Furthermore, a new bispecific, tetravalent intradiabody was able to simultaneously knockout both cell surface receptors KDR and Tie-2 [44, 45]. Performed with a human melanoma xenograft mouse model, the intradiabody inhibited tumour growth by 92.2%[45]. In summary, it could be concluded that ER-targeted anti-VEGFR-2/KDR or anti-Tie-2 intrabodies might be useful for therapeutic application against solid tumours.
Another application of antioncogenic receptor ER intrabodies was shown with the human folate receptor [46].The α-folate receptor (FR) is selectively overexpressed in 90% of non-mucinous ovarian carcinomas. Folate uptake induces signal transduction associated with dysregulated cell proliferation. Expression of anti-FR intrabodies in ovarian cancer cells led to a dramatic decrease in cell proliferation rate and increased adhesion to various extracellular matrix components accompanied by reversion towards normal phenotype [46].
Last but not least, the ER intrabody technology was used to neutralize proteins involved in tumour development that do not belong to the growth factor receptor family. Intrabodies were generated that block the secretion of proteinases. This was shown for (i) procathepsin L that strongly increases the tumourigenicity and metastatic potential of human melanoma cells [81] and (ii) for the metalloproteinase MMP-2 and MMP-9 which both degrade collagenase IV in the basement membrane and play an important role in tumour invasion and formation of metastasis [82].
ER intrabodies against infectious diseases
ER intrabodies against HIV
To prevent the entry of HIV-1 into CD4+ T cells, the HIV-1 glycoprotein gp120 and the corresponding co-receptors can be targeted by neutralizing antibodies or small molecule antagonists. To inhibit different stages of HIV-1 life cycles, anti-HIV intrabodies have been targeted to structural, regulatory and enzymatic proteins of the virus inside the different compartments of the host cell [47]. The targets of anti-HIV ER intra-bodies are the HIV-1 env glycoprotein gp120 [47, 48], the precursor gp160 [49] and the binding counterpart: the CD4 co-receptor CCR5 or CXCR4 [50–54].
Anti-HIV-1 gp120(gp160 intrabodies
An ER intrabody against the envelope protein gp120 led to inhibition of proteolytic processing of the env precursor gp160, decrease of envelope-mediated syncytium formation and reduced infectivity of HIV-1 particles released by intrabody expressing cells [47, 48]. Studies are evaluating the efficiency of the anti-gp120 intrabody in asymptomatic HIV-1-infected patients, by reinfusing autologous CD4+ T cells that have been transduced ex vivo with a murine leukaemia virus vector encoding this intrabody [47]. Furthermore, an scFv with an ER or trans-Golgi network (TGN) retention signal recognizing the envelope precursor gp160 inhibited HIV-1-induced syncytial formation too [49].
Anti-HIV-1 co-receptor intrabodies
HIV requires a co-receptor, in addition to CD4, for entry into target cells. Macrophage (M)-tropic viruses require the chemokine receptor CCR5 for entry, while T-cell-line (TCL)-tropic viruses use CXCR4 for entry. Intrabodies against CCR5 and CXCR4 led to protection against HIV-1 infection [50–54]. Interestingly, the anti-CCR5 intrabody [50] reacts with CCR5 from nonhuman primates and the strategy to establish an HIV-1 resistant cell pool in infected patients can be tested in SIV and chimeric simian-human immunod-eficiency virus (SHIV) models of human AIDS. Recently the intrabody was transduced into CD34+ foetal liver stem cells and this cells gave rise to CD4+ and CD8+ thymocytes in non-obese diabetic (NOD)/severely combined-immunodeficient (SCID)-human thymus/liver (hu thy/liv) mice [51].
In another approach, the CCR5 surface expression was down-regulated in monocyte-derived macrophages after co-infection of Tag-deleted SV40-derived vector encoding an anti-CCR5 scFv fragment and a hammerhead CCR5-specific ribozyme. The combination of both transgenes prevented the cells from HIV-1 infection after challenge with high dosis of HIV-1 [52]. In addition, recently a new anti-CXCR4 intrabody was described that inhibits infectious entry of HIV-1 in primary isolated human brain microvascular endothelial cells and post-mitotic differentiated human neurons [54]. This example opens the possibility to develop strategies to apply such intrabodies against HIV-1-related neurodegenerative disorders and neuroinvasion. In summary, the anti-envelope intrabodies might be used to generate cells that produce virions that are markedly less infectious, whereas the anti-CCR5/CXCR4 intrabodies might be used in clinical gene therapy strategies to generate a cell pool in infected patients that is protected from HIV-1 infection.
ER intrabodies to other viruses
A recombinant ER-targeted Fab fragment against the Hepatitis C virus core protein was described, and co-localization and intracellular binding was shown [83]. Two soluble scFv fragments against the transmembrane envelope glycoprotein gp46 of maedi-visna virus (a retrovirus which causes pneumonitis, encephalomyelitis and arthritis in sheep) recognized maedivisna virus in the cytoplasma of fixed virus-infected sheep choroids plexus (SCP) cells [84]. These antibodies targeted to the ER as intrabodies could prevent the maturation process of the gp150 precursor envelope glycoprotein into gp135 and gp46 in virus-infected cells resulting in less infectious virus particles. Last, but not least, an ER intrabody was described that recognizes secretory salivary proteins of the root-knot nematode Meloidogyne incognita [85].
Abrogation of MHC I molecules
Presentation of intracellularly synthesized peptides in a complex with MHC class I molecules on the cell surface of donor cells is the central reason for rejection of allogeneic cell and tissue transplants. The generation of transplantable in vitro generated skin sheets for replacement of skin defects has become a standard procedure. Nevertheless, after transplantation the allogeneic MHC class I expressing keratoni-cytes are able to induce an alloimmune response via CD8+ T cells and infiltrating recipient APCs. Methods to down-regulate MHC I cell surface expression such as deletion of the MHC I associated protein ß2 microglobulin (ß2m) and deletion of the intracellular TAP (transporter associated with antigen processing) led to cells that still exhibit small amounts of MHC I molecules on the cell surface [86]. The intrabody technology represents an efficient promising approach to suppress allorecognition against MHC I. It could be shown that intrabody expression in primary rat and human keratinocytes prevented the cells from CTL-mediated lysis to a great extend [55, 56].
In another approach, surface MHC I molecules were declined in human umbilical vein endothelial cells (HUVECs) [57] and also in this case the intra-bodies protect allogeneic HUVECs from CTL-mediated lysis. This might be important in the field of vascular surgery where transplantation of small-calibre vascular prothesis seeded with autologous endothelial cells is performed. Following the studies with the keratinocytes and HUVECs, the ER intrabody strategy is a promising tool to avoid the allograft rejection induced by MHC I molecules of the host.
In addition, ER intrabodies against α1,3-galacto-syltransferase have been described [87]. The carbohydrate structure Gal α1,3 Gal expressed on pig cells is the major antigen recognized by xenoreactive natural antibodies in higher primates. The expression of the anti-α1,3-galactosyltransferase intrabodies in pig epithelial kidney cells inhibits almost completely complement dependent cytotoxicity mediated by purified anti-Gal antibodies. Nevertheless, it only partially reduced the cytotoxicity of whole serum due to non-αGal xenoantigens recognized by human xenoreactive natural antibodies. The combination of the expression of such intrabodies with the expression of human regulators of complement activity and expression of enzymes that compete with α1,3-galactosyltransferase for acceptor molecules might lead to a more efficient inhibition of xenogenic cytotoxicity.
ER intrabodies targeting integrins
The integrins are a large family of transmembrane glycoproteins that mediate cell–cell or cell–matrix interactions. In mammals, 24 integrins have been identified to date, resulting from different pairings among the 18 α- and 8 β-subunits. They play an important role in the regulation of cell survival, differentiation, proliferation, haemostasis and host defence. They bind to various extracellular matrix proteins such as fibronectin, vitronectin and osteo-pontin. In addition αV integrins play a role in the progression of cancer. Performed with an anti-αV integrin intrabody, new insights into the function of integrins during differentiation of osteosarcoma cells, in the regulation of cell surface expression of αvβ1 integrin and in the involvement of integrins during survival of metastatic cancer cells were obtained [58–60].
After transfection of an anti-αV integrin intrabody into osteosarcoma cells, the expression of the osteoblast differentiation marker genes, alkaline phosphatase and osteopontin, was induced whereas the expression of matrix metalloproteinase-2 was decreased [58]. These data indicated that αV integrins are important regulators of osteosarcoma cell phenotypes. The same group has also analysed whether the expression of integrin αVβ1 is selectively regulated by the hierarchical formation of other αV-containing heterodimers. They showed that transfection of the same intrabody into melanoma cells resulted in the reduction of cell surface expression of only integrin αvβ1 [59]. This leads to the conclusion that the expression level of αvβ1 on the surface of melanoma cells is dependent on the number of v subunits available after the formation of other αv-containing heterodimers. Furthermore, the authors constructed a recombinant adenovirus with the intrabody gene and used this adenovirus to express the intra-body in three metastatic melanoma cell lines. The adenovirus effectively inhibited the cell surface expression of αV integrins and prevented tumour growth of one metastatic melanoma cell line in SCID mice [60].
ER intrabodies targeting proteins involved in Alzheimer's and Prion disease
Misfolded and accumulated intracellular proteins characterize a wide range of neurodegenerative disorders. Proteins involved in the Huntington's and Parkinson's diseases were successfully targeted by cytoplasmic and nucleus-targeted intrabodies [88] whereas ER intrabodies inhibited the cleavage or translocation of cell surface proteins from the ER to the cell surface that play an important role in the bio-genesis of Alzheimer's and Prion diseases: β-APP [28] and PrPc[61, 62].
Endoproteolysis of the β-APP by β- and γ-secretases generates the toxic amyloid β-peptide (Aβ), which accumulates in the brain of Alzheimer's disease (AD) patients. Generation of the toxic amyloid β-peptide was prevented by ER expression of an intrabody recognizing an epitope adjacent to the β-secretase cleavage site of the human β-APP [28]. In the case of Prion disease, SEKDEL-tagged anti-prion intrabodies were able to prevent abnormal scrapie isoform accumulation and inhibit prion infectivity in mice [61, 62]. These ER intrabodies are useful tools to study in more detail the cellular traffic and degradation of this important protein.
Intrabody gene delivery
The transfer of intrabody genes into living cells can be performed with viral or nonviral transfer systems.
Viral vectors
ER intrabody gene transfer was performed with aden-ovirus [34–37, 42–45, 56, 57, 60], murine leukaemia virus [32, 33, 39, 40, 47, 48, 50, 53], HIV-1-based lentivirus [30, 31, 51, 54], SV40-derived vector [52, 53, 89] and adeno-associated virus (AAV) [47]. Recombinant adenoviruses are the preferred vectors for gene therapy.The advantage of this DNA virus is the extremely efficient transduction of most tissues and dividing as well as quiescent cells and a high titre that can be produced. The expression of the transgene is transient and the viral genome does not normally integrate into the host genome with no risk for insertional mutagenesis. A signifcant problem associated with adenoviral vectors is elicitation of an immune response against the vector and systemic delivery and is accompanied by rapid hepatic uptake of virus. Some improvements to extend plasma circulation of recombinant ade-novirus have been reported [90]. Nevertheless, for in vitro transduction of ER intrabodies into cell lines [34, 35, 56, 60] and primary cells [42, 56, 57] and in mouse tumour models, recombinant adenovirus has been successfully employed [36, 43, 45, 60].
The advantage of lentiviral vectors is their capacity of integrating into non-deviding cells that constitute the targets of HIV-1 infection such as resting T cells, dendritic cells and macrophages and have been widely used in preclinical studies [91].They can also infect proliferating cells and lentiviruses transfer genes to haematopoietic stem cells with a superior gene transfer efficiency. In addition, cells of the central nervous system have been successfully transduced.
Retroviral vectors are not able to transduce non-deviding post-mitotic cells. Further disadvantages are low vector titre and low transfection efficiency and particle instability. The retroviral vectors are suitable for ex vivo gene therapy, and despite the disadvantages, retroviral gene delivery systems have been used already in a number of clinical trials [92]. However, integration of retrovirus including lentivirus genomes into the host genome might induce oncogenesis in some clinical applications. The AAV, which is currently being tested in several human gene therapy trials, has several unique features that distinguish it from other gene therapy vectors: sustained transgene gene expression with low risk of insertional mutagenesis and the fact that host cellular immune responses have not yet been observed [93].
A major goal of gene delivery systems is the development of vectors that are able to target specific cell types. New approaches are transcriptional and transductional targeting [94–96]. Transcriptional targeting is generally achieved by placing the transgene under the control of a cell-type-specific promoter. Transductional targeting can be performed by pseudotyping (substitution of a part or all of the virusreceptor with those from other virus strains or serotypes). Pseudotyping has been well established for lentiviral vectors. In this case, pseudo-types bearing glycoproteins of lyssavirus, lympho-cytic choriomeningitis, alphavirus, filovirus, gammaretrovirus and baculovirus have been described [97]. Genetic fibre pseudotyping is performed to generate chimeric adenovirus with enhanced infectivity of ovarian cells without increasing gene delivery to murine livers [98]. Transductional targeting can also be carried out with bispecific molecular adaptors (usually bispecific antibodies) that simultaneously block native receptor binding and redirect the vector capsid to new cellular receptors or by genetically altering receptor-binding proteins in the virus capsid so that they bind to alternative receptors [94–96]. Performed with this approach, the normal receptor binding is abolished and/or a small peptide ligand for alternative receptor binding is incorporated into the capsid structure.
Adenovirus targeting was successfully performed in several cases [95]. In contrast to aden-oviral vectors, envelope alterations of retroviral vectors including lentivirus affect in most cases viron assembly and lead to low fusion or defective fusion activity [96, 99]. With regard to the retargeting of lentivirus, two new reports performed with Sindbis pseudotyped lentiviral vectors were described displaying an anti-CCR5 scFv fragment [100] and an anti-P-glycoprotein antibody [101]. The modified lentivirus expressing the anti-P-glycoprotein antibody on the viron surface were successfully retargeted to P-glycoprotein on metastatic melanoma through intravenous injection of mice [101]. These two studies can be considered as a powerful approach for specific viral gene delivery mediated by Sindbis envelope that displays single-chain antibody fragments recognizing specific cellular surface proteins.
Non-viral transfer systems
Liposomes and other non-viral delivery systems (naked DNA, DNA-coated polymer, cationic peptide-DNA complexes, metal-coated DNA) are under investigation for use in cancer gene therapy [102]. At present, the main disadvantage is the low transduction efficiency and transient transgene expression. Nevertheless, the delivery of genes by cationic liposomes is rather effective and used in worldwide human clinical trials of gene therapy. Recent studies combining structural and biological techniques are now unravelling the relationship between the distinctly structured cationic liposomes-DNA complexes and a new generation of liposomes is now in the process of evaluation [103].
Recent advances indicate that efficient, longterm gene expression can be maintained as small episomal plasmids or artificial chromosomes and integration of DNA can be targeted to specific genomic sites [102]. In addition, inclusion of ligands for receptor-mediated endocytosis, peptide sequences that enhance DNA compaction and endosomal disruption sequences enhances non-viral delivery to cells [102].
The use of cationic protein transduction domains (PTDs) that cargo peptides, proteins and nucleic acids into cells by a receptor-independent, fluid phase macropinocytosis was reported in different mouse models of cancer, ischaemia and inflammation [104]. These reports of successful in vivo transduction are exciting and encouraging. Nevertheless, a human scFv fragment recognizing the ED-B domain of fibronectin (a marker of the modified extra-cellular matrix associated with the tumour enovasculature) fused to the TAT peptide showed severely reduced tumour-targeting performance compared to the unconjugated antibody [105]. The properties of PTD-linked intrabody genes need to be examined in detail. In another promising approach, specific targeting of the therapeutic herpes simplex virus thymidine kinase gene was achieved with a so-called immunoporter consisting of a Fab fragment against EGF receptor conjugated with a cationic polylysine chain [106]. It was shown that the Fab immunore-porter carrying the herpes TK gene was effective in preventing the growth of EGF receptor-overexpressing tumour cells.
Conclusions
The intrabody technology is a promising method to inactivate and to study specific protein functions inside different compartments of a cell. It complements the RNAi and intramer technology and replaces the RNAi technology in cases in which the RNAi approaches fail due to unspecific effects. The advantage of ER intrabodies is that they are correctly folded in contrast to cytoplasmic expressed intra-bodies. In vitro, they inhibit very efficiently the translocation of growth factor receptors from the ER to the cell surface, prevent HIV-1 virus infection and decrease the immunogenicity of allogenic tissue and transplants and target successfully proteins involved in the genesis of neurological disorders. However, until now they have not been applied for clinical applications in spite of some very promising results obtained from in vivo mouse models. A prerequisite for clinical approaches is the improvement of efficient cell- and tissue-specific intrabody gene transfer. Nevertheless, in the future ER intrabodies will be potent reagents to inactivate endosomally localized receptors involved in bacterial and viral infection diseases, intracellular oncogenic receptors and enzymes involved in glycosylation of tumour markers.
References
- 1.Bućcan M, Abel T. The mouse: genetics meets behaviour. Nature Rev Genet. 2002;3:114–23. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- 2.Brantl S. Antisense-RNA regulation and RNA interference. Biochim Biophys Acta. 2002;1575:15–25. doi: 10.1016/s0167-4781(02)00280-4. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich H. RNA aptamers: from basic science towards therapy. Handb Exp Pharmacol. 2006;173:305–26. doi: 10.1007/3-540-27262-3_15. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler YY, Chen S-Y, Sane DC. Intrabody and intrakine strategies for molecular therapy. Mol Ther. 2003;8:355–66. doi: 10.1016/s1525-0016(03)00183-7. [DOI] [PubMed] [Google Scholar]
- 5.Barik S. Silence of the transcripts: RNA interference in medicine. J Mol Med. 2005;83:764–73. doi: 10.1007/s00109-005-0690-0. [DOI] [PubMed] [Google Scholar]
- 6.Lobato MN, Rabbits TH. Intracellular antibodies and challenges facing their use as therapeutic agents. Trends Mol Med. 2003;9:390–6. doi: 10.1016/s1471-4914(03)00163-1. [DOI] [PubMed] [Google Scholar]
- 7.De Fromentel CC, Gruel N, Venot C, Debussche L, Conseiller E, Dureuil C, Teillaud J-L, Tocque B, Bracco L. Restoration of transcriptional activity of p53 mutants in human tumour cells by intracellular expression of anti-p53 single chain Fv fragments. Oncogene. 1999;18:551–7. doi: 10.1038/sj.onc.1202338. [DOI] [PubMed] [Google Scholar]
- 8.Wörn A, Auf der Maur A, Escher D, Honegger A, Barberis A, Plückthun A. Correlation between in vitro stability and in vivo performance of anti-GCN4 intra-bodies as cytoplasmic inhibitors. J Biol Chem. 2000;275:2795–803. doi: 10.1074/jbc.275.4.2795. [DOI] [PubMed] [Google Scholar]
- 9.Tse E, Rabbitts TH. Intracellular antibody-caspase-mediated cell killing: an approach for application in cancer therapy. Proc Natl Acad Sci USA. 2000;97:12266–71. doi: 10.1073/pnas.97.22.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herschhorn A, Admon A, Hizi A. Recombinant human antibodies against the reverse transcriptase of human immunodefiency virus type-1. Biochim Biophys Acta. 2003;1648:154–63. doi: 10.1016/s1570-9639(03)00118-3. [DOI] [PubMed] [Google Scholar]
- 11.Famulok M, Blind M, Mayer G. Intramers as promising new tools in functional proteomics. Chem Biol. 2001;8:931–9. doi: 10.1016/s1074-5521(01)00070-9. [DOI] [PubMed] [Google Scholar]
- 12.Cao T, Heng BC. Intracellular antibodies (intrabodies) versus RNA interference for therapeutic applications. Ann Clin Lab Sci. 2005;35:227–9. [PubMed] [Google Scholar]
- 13.Fish RJ, Kruithof EKO. Shortterm cytotoxic effects and longterm instability of RNAi delivered using lentiviral vectors. BMC Mol Biol. 2004;5:1–15. doi: 10.1186/1471-2199-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Famulok M, Mayer G, Blind M. Nucleic acid aptamers-from selection in vitro to application in vivo. Acc Chem Res. 2000;33:591–9. doi: 10.1021/ar960167q. [DOI] [PubMed] [Google Scholar]
- 15.Tran N, Cairns MJ, Dawes IW, Arndt GM. Expressing functional siRNAs in mammalian cells using convergent transcription. BMC Biotechnol. 2003;3:1–9. doi: 10.1186/1472-6750-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snove O, Jr, Rossi JJ. Expressing short hairpin RNAs in vivo. Nature Meth. 2006;3:689–95. doi: 10.1038/nmeth927. [DOI] [PubMed] [Google Scholar]
- 17.Dykxhoorn DM, Schlehuber LD, London IM, Lieberman J. Determinants of specific RNA interference-mediated silencing of human β-globin alleles differing by a single nucleotide polymorphism. Proc Natl Acad Sci USA. 2006;103:5953–8. doi: 10.1073/pnas.0601309103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato M, Vaughan MB, Girard L, Peyton M, Lee W, Shames DS, Ramirez RD, Sunaga N, Gazdar AF, Shay JW, Minna JD. Multiple oncogenic changes (K-RASV12p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 2006;66:2116–28. doi: 10.1158/0008-5472.CAN-05-2521. [DOI] [PubMed] [Google Scholar]
- 19.Bilanges B, Stokoe D. Direct comparison of the specificity of gene silencing using antisense oligonu-cleotides and RNAi. Biochem J. 2005;388:573–83. doi: 10.1042/BJ20041956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu S, Adema CM, Lane T. A computational study of off-target effects of RNA interference. Nucleic Acids Res. 2005;33:1834–47. doi: 10.1093/nar/gki324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marques JT, Williams BRG. Activation of the mammalian immune system by siRNAs. Nature Biotechnol. 2005;23:1399–405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- 22.Lewis MJ, Pelhalm HRB. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell. 1992;68:353–64. doi: 10.1016/0092-8674(92)90476-s. [DOI] [PubMed] [Google Scholar]
- 23.Munro S, Pelham HRB. C-terminal signal prevents secretion of luminal ER proteins. Cell. 48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 24.Andres DA, Dickerson IM, Dixon JE. Variants of the carboxyl-terminal KDEL sequence direct intracellular retention. J Biol Chem. 265:5952–5. [PubMed] [Google Scholar]
- 25.Stornaiuolo M, Lotti LV, Borgese N, Torrisi M-R, Mottola G, Martire G, Bonatti S. KDEL and KKXX retrieval signals appended to the same reporter protein determine different trafficking between endoplasmic reticulum, intermediate compartment, and Golgi complex. Mol Biol Cell. 2003;14:889–902. doi: 10.1091/mbc.E02-08-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donoso G, Herzog V, Schmitz A. Misfolded BIP is degraded by a proteasome-independent endoplasmic-reticulum-associated degradation pathway. Biochem J. 2005;387:897–903. doi: 10.1042/BJ20041312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nature Cell Biol. 2005;7:766–72. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 28.Paganetti P, Calanca V, Galli C, Stefani M, Molinari M. β-site specific intrabodies to decrease and prevent generation of Alzheimer´s Aβ peptide. J Cell Biol. 2005;168:863–8. doi: 10.1083/jcb.200410047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson JH, Sodroski JG, Waldmann TA, Marasco WA. Phenotypic knockout of the high-affinity human interleukin 2 receptor by intracellular single-chain antibodies against the subunit of the receptor. Proc Natl Acad Sci USA. 1995;92:3137–41. doi: 10.1073/pnas.92.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson JH, Waldmann TA, Sodroski JG, Marasco WA. Inducible knockout of the interleukin-2 receptor chain: expression of the high-affinty IL-2 receptor is not required for the in vitro growth of HTLV-I-transformed cell lines. Virology. 1997;237:209–16. doi: 10.1006/viro.1997.8779. [DOI] [PubMed] [Google Scholar]
- 31.Richardson JH, Hofmann W, Sodroski JG, Marasco WA. Intrabody-mediated knockout of the high-affinity IL-2 receptor in primary human T cells using a bicistronic lentivirus vector. Gene Ther. 1998;5:635–44. doi: 10.1038/sj.gt.3300644. [DOI] [PubMed] [Google Scholar]
- 32.Beerli RR, Wels W, Hynes NE. Intracellular expression of single chain antibodies reverts ErbB-2 transformation. J Biol Chem. 1994;39:23931–6. [PubMed] [Google Scholar]
- 33.Graus-Porta D, Beerli RR, Hynes NE. Single-chain antibody-mediated intracellular retention of ErbB-2 impairs neu differentiation factor and epidermal growth factor signaling. Mol Cell Biol. 1995;15:1182–91. doi: 10.1128/mcb.15.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright M, Grim J, Deshane J, Kim M, Strong TV, Siegal GP, Curiel DT. An intracellular anti-erb-B-2 single-chain antibody is specifically cytotoxic to human breast carcinoma cells overexpressing erb-B-2. Gene Ther. 1997;4:317–22. doi: 10.1038/sj.gt.3300372. [DOI] [PubMed] [Google Scholar]
- 35.Grim J, Deshane J, Siegal GP, Alvarez RD, DiFiore P, Curiel DT. The level of erbB2 expression predicts sensitivity to the cytotoxic effects of an intracellular anti-erbB2 scFv. J Mol Med. 1998;76:451–8. doi: 10.1007/s001090050237. [DOI] [PubMed] [Google Scholar]
- 36.Deshane J, Siegal GP, Wang M, Wright M, Bucy RP, Alvarez RD, Curiel DT. Transductional efficacy and safety of an intraperitoneally delivered adenovirus encoding an anti-erbB-2 intracellular single-chain anti-body for ovarian cancer gene therapy. Gynecol Oncol. 1997;64:378–85. doi: 10.1006/gyno.1996.4566. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez RD, Barnes MN, Gomez-Navarro J, Wang M, Strong TV, Arafat W, Arani RB, Johnson MR, Roberts BL, Siegal GP, Curiel DT. A cancer gene therapy approach utilizing an anti-erbB-2 single-chain antibody-encoding adenovirus (AD21): a phase I trial. Clin Cancer Res. 2000;6:3081–7. [PubMed] [Google Scholar]
- 38.Arafat W, Gómez-Navarro J, Xiang J, Siegal GP, Alvarez RD, Curiel DT. Antineoplastic effect of anti-erbB-2 intrabody is not correlated with scFv affinity for its target. Cancer Gene Ther. 2000;7:1250–6. doi: 10.1038/sj.cgt.7700228. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Majumder S, McCall W, Sartor CI, Mohler JL, Gregory CW, Earp HS, Whang YE. Inhibition of HER-2/neu kinase impairs androgen receptor recruitment to the androgen responsive enhancer. Cancer Res. 2005;65:3404–9. doi: 10.1158/0008-5472.CAN-04-4292. [DOI] [PubMed] [Google Scholar]
- 40.Jannot CB, Beerli RR, Mason S, Gullick WJ, Hynes NE. Intracellular expression of a single-chain antibody directed to the EGFR leads to growth inhibition of tumor cells. Oncogene. 1996;13:275–82. [PubMed] [Google Scholar]
- 41.Böldicke T, Weber H, Mueller PP, Barleon B, Bernal M. Novel highly efficient intrabody mediates complete inhibition of cell surface expression of the human vascular endothelial growth factor receptor-2 (VEGFR-2/KDR) J Immunol Meth. 2005;300:146–59. doi: 10.1016/j.jim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Wheeler YY, Kute TE, Willingham MC, Chen Si-Yi, Sane DC. Intrabody-based strategies for inhibition of vascular endothelial growth factor receptor-2: effects on apoptosis, cell growth, and angiogenesis. FASEB J. 2003;17:1733–5. doi: 10.1096/fj.02-0942fje. [DOI] [PubMed] [Google Scholar]
- 43.Popkov M, Jendreyko N, McGavern DB, Rader C, Barbas IIICF. Targeting Tumour Angiogenesis with Adenovirus-delivered anti-Tie-2 intrabody. Cancer Res. 2005;65:972–81. [PubMed] [Google Scholar]
- 44.Jendreyko N, Popkov M, Beerli RR, Chung J, McGavern DB, Rader C, Barbas IIICF. Intradiabodies, bispecific, tetravalent antibodies for the simultaneous functional knockout of two cell surface receptors. J Biol Chem. 2003;278:47812–9. doi: 10.1074/jbc.M307002200. [DOI] [PubMed] [Google Scholar]
- 45.Jendreyko N, Popkov M, Rader C, Barbas IIICF. Phenotypic knockout of VEGF-R2 and Tie-2 with an intradiabody reduces tumor growth and angiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:8293–8. doi: 10.1073/pnas.0503168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Figini M, Ferri R, Mezzanzanica D, Bagnoli M, Luison E, Miotti S, Canevari S. Reversion of transformed phenotype in ovarian cancer cells by intracelllular expression of antifolate receptor antibodies. Gene Ther. 2003;10:1018–25. doi: 10.1038/sj.gt.3301962. [DOI] [PubMed] [Google Scholar]
- 47.Rondon IJ, Marasco WA. Intracellular antibodies (intrabodies) for gene therapy of infectious diseases. Annu Rev Microbiol. 1997;51:257–83. doi: 10.1146/annurev.micro.51.1.257. [DOI] [PubMed] [Google Scholar]
- 48.Poznansky MC, Foxall R, Mhashilkar A, Coker R, Jones S, Ramstedt U, Marasco W. Inhibition of human immunodefiency virus replication and growth advantage of CD4+ T cells from HIV-infected individuals that express intracellular antibodies against HIV-1 gp120 or Tat. Hum Gene Ther. 1998;9:487–96. doi: 10.1089/hum.1998.9.4-487. [DOI] [PubMed] [Google Scholar]
- 49.Zhou P, Goldstein S, Devadas K, Tewari D, Notkins AL. Cells transfected with a non-neutralizing antibody gene are resistant to HIV infection: targeting the endoplasmic reticulum and trans-Golgi network. J Immunol. 1998;160:1489–96. [PubMed] [Google Scholar]
- 50.Steinberger P, Andris-Widhopf J, Bühler B, Torbett BE, Barbas IIICF. Functional deletion of the CCR5 receptor by intracellular immunization produces cells that are refractory to CCR5-dependent HIV-1 infection and cell fusion. Proc Natl Acad Sci USA. 2000;97:805–10. doi: 10.1073/pnas.97.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swan CH, Bühler B, Tschan MP, Barbas IIICF, Torbett BE. T-cell protection and enrichment through lentiviral CCR5 intrabody gene delivery. Gene Ther. 2006;13:1480–92. doi: 10.1038/sj.gt.3302801. [DOI] [PubMed] [Google Scholar]
- 52.Cordelier P, Kulkowsky JW, Ko C, Matskevitch AA, McKee HJ, Rossi JJ, Bouhamdan M, Pomerantz RJ, Kari G, Strayer DS. Protecting from R5-tropic HIV: individual and combined effectiveness of a hammer-head ribozyme and a single-chain Fv antibody that targets CCR5. Gene Ther. 2004;11:1627–37. doi: 10.1038/sj.gt.3302329. [DOI] [PubMed] [Google Scholar]
- 53.BouHamdan M, Strayer DS, Wei D, Mukhtar M, Duan L-X, Hoxie J, Pomerantz RJ. Inhibition of HIV-1 infection by down-regulation of the CXCR4 co-receptor using an intracellular single chain variable fragment against CXCR4. Gene Ther. 2001;8:408–18. doi: 10.1038/sj.gt.3301411. [DOI] [PubMed] [Google Scholar]
- 54.Mukhtar M, Acheampong E, Khan MA, BouHamdan M, Pomerantz RJ. Down-modulation of the CXCR4 co-receptor by intracellular expression of a single chain variable fragment (SFv) inhibits HIV-1 entry into primary human brain microvascular endothelial cells and postmitotic neurons. Mol. Brain Res. 2005;135:48–57. doi: 10.1016/j.molbrainres.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 55.Busch A, Marasco WA, Doebis C, Volk H-D, Seifert M. MHC class I manipulation on cell surfaces by gene transfer of anti-MHC class I intrabodies-a tool for decreased immunogenicity of allogeneic tissue and cell transplants. Methods. 2004;34:240–9. doi: 10.1016/j.ymeth.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Mhashilkar AM, Doebis C, Seifert M, Busch A, Zani C, Soo Hoo J, Nagy M, Ritter T, Volk H-D, Marasco WA. Intrabody-mediated phenotypic knockout of major histocompatibility complex class I expression in human and monkey cell lines and in primary human ker-atinocytes. Gene Ther. 2002;9:307–19. doi: 10.1038/sj.gt.3301656. [DOI] [PubMed] [Google Scholar]
- 57.Beyer F, Doebis C, Busch A, Ritter T, Mhashilkar A, Marasco WM, Laube H, Volk H-D, Seifert M. Decline of surface MHC I by adenoviral gene transfer of anti-MHC I intrabodies in human endothelial cells - new perspectives for the generation of universal donor cells for tissue transplantation. J Gene Med. 2004;6:616–23. doi: 10.1002/jgm.548. [DOI] [PubMed] [Google Scholar]
- 58.Koistinen P, Pulli T, Uitto V-J, Nissinen L, Hyypiä T, Heino J. Depletion of αV integrins from osteosarcoma cells by intracellular antibody expression induces bone differentiation marker genes and suppresses gelati-nase (MMP-2) synthesis. Matrix Biol. 1999;18:239–51. doi: 10.1016/s0945-053x(99)00022-0. [DOI] [PubMed] [Google Scholar]
- 59.Koistinen P, Heino J. The selective regulation of αvβ1 integrin expression is based on the hierarchical formation of αv-containing heterodimers. J Biol Chem. 2002;27:24835–41. doi: 10.1074/jbc.M203149200. [DOI] [PubMed] [Google Scholar]
- 60.Koistinen P, Ahonen M, Kähäri V-M, Heino J. V integrin promotes n vitro and in vivo survival of cells in metastatic melanoma. Int J Cancer. 2004;112:61–70. doi: 10.1002/ijc.20377. [DOI] [PubMed] [Google Scholar]
- 61.Cardinale A, Filesi I, Vetrugno V, Pocchiari M, Sy M-S, Biocca S. Trapping prion protein in the endoplasmic reticulum impairs PrPc maturation and prevents PrPSc Accumulation. J Biol Chem. 2005;280:685–94. doi: 10.1074/jbc.M407360200. [DOI] [PubMed] [Google Scholar]
- 62.Vetrugno V, Cardinale A, Filesi I, Mattei S, Sy M-S, Pocchiari M, Biocca S. KDEL-tagged anti-prion intra-bodies impair PrP lysosomal degradation and inhibit scrapie infectivity. Bioch Biophys Res Comm. 2005;338:1791–7. doi: 10.1016/j.bbrc.2005.10.146. [DOI] [PubMed] [Google Scholar]
- 63.Hu X, Su F, Qin L, Jia W, Gong C, Yu F, Guo J, Song E. Stable RNA interference of ErbB-2 gene synergistic with epirubicin suppresses breast cancer growth in vitro and in vivo. Biochem Biophys Res Comm. 2006;346:778–85. doi: 10.1016/j.bbrc.2006.05.206. [DOI] [PubMed] [Google Scholar]
- 64.Kang C-S, Zhang Z-Y, Jia Z-F, Wang G-X, Qiu M-Z, Zhou H-X, Yu S-Z, Chang J, Jiang H, Pu P-Y. Suppression of EGFR expression by antisense or small interference RNA inhibits U251 glioma cell growth in vitro and in vivo. Cancer Gene Ther. 2006;13:530–8. doi: 10.1038/sj.cgt.7700932. [DOI] [PubMed] [Google Scholar]
- 65.Kou R, SenBanerjee S, Jain MK, Michel T. Differential regulation of vascular endothelial growth factor receptors (VEGFR) revealed by RNA interference: interactions of VEGFR-1 and VEGFR-2 in endothelial cell signalling. Biochemistry. 2005;44:15064–73. doi: 10.1021/bi0509898. [DOI] [PubMed] [Google Scholar]
- 66.Park W-S, Hayafune M, Miyano-Kurosaki N, Takaku H. Specific HIV-1 env gene silencing by small interfering RNAs in human peripheral blood mononuclear cells. Gene Ther. 2003;10:2046–50. doi: 10.1038/sj.gt.3302099. [DOI] [PubMed] [Google Scholar]
- 67.An DS, Qin FX-F, Auyeung VC, Mao SH, Kung SKP, Baltimore D, Chen ISY. Optimization and functional effects of stable short hairpin RNA expression in primary human lymphocytes via lentiviral vectors. Mol Ther. 2006;14:494–504. doi: 10.1016/j.ymthe.2006.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y-L, Yu J-M, Song X-R, Wang X-W, Xing Li-G, Gao B-B. Regulation of the chemokine receptor CXCR4 and metastasis by hypoxia-inducible factor in non-small cell lung cancer cell lines. Cancer Biol Ther. 2006;5:1320–6. doi: 10.4161/cbt.5.10.3162. [DOI] [PubMed] [Google Scholar]
- 69.Graef T, Steidl U, Nedbal W, Rohr U, Fenk R, Haas R, Kronenwett R. Use of RNA interference to inhibit integrin subunit αV-mediated angiogenesis. Angiogenesis. 2005;8:361–72. doi: 10.1007/s10456-005-9026-5. [DOI] [PubMed] [Google Scholar]
- 70.Qin K, Zhao L, Tang Y, Bhatta S, Simard JM, Zhao RY. Doppel-induced apoptosis and counteraction by cellular prion protein in neuroblastoma and astrocytes. Neurosciences. 2006;141:1375–88. doi: 10.1016/j.neuroscience.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 71.Anken E, Braakman I, Craig E. Versatility of the endoplasmic reticulum protein-folding factory. Crit Rev Biochem Mol Biol. 2005;40:191–228. doi: 10.1080/10409230591008161. [DOI] [PubMed] [Google Scholar]
- 72.Wörn A, Plückthun A. Stability engineering of anti-body single-chain Fv fragments. J Biol Chem. 2001;305:989–1010. doi: 10.1006/jmbi.2000.4265. [DOI] [PubMed] [Google Scholar]
- 73.Visintin M, Quondam M, Cattaneo A. The intracellular antibody capture technology: towards the high-throughput selection of functional intracellular antibodies for target validation. Methods. 2004;34:200–14. doi: 10.1016/j.ymeth.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 74.Verheesen P, De Kluijver A, Van Koningsbruggen S, De Brij M, De Haard HJ, Van Ommen G-JB, Van Der Maarel SM, Verrips CT. Prevention of oculopharyngeal muscular dystrophyassociated aggregation of nuclear poly (A)-binding protein with a single-domain intracellular antibody. Hum Mol Genet. 2006;15:105–11. doi: 10.1093/hmg/ddi432. [DOI] [PubMed] [Google Scholar]
- 75.Böldicke T, Tesar M, Griesel C, Rohde M, Gröne H-J, Waltenberger J, Kollet O, Lapidot T, Yayon A, Weich H. Anti-VEGFR-2 scFvs for cell isolation. Single-chain antibodies recognizing the human vascular endothelial growth factor receptor-2 (VEGFR-2/flk-1) on the surface of primary endothelial cells and pre-selected CD34+ cells from cord blood. Stem Cells. 2001;19:24–36. doi: 10.1634/stemcells.19-1-24. [DOI] [PubMed] [Google Scholar]
- 76.Persic L, Righi M, Roberts A, Hoogenboom HR, Cattaneo A, Bradbury A. Targeting vectors for intracellular immunisation. Gene. 1997;187:1–8. doi: 10.1016/s0378-1119(96)00627-0. [DOI] [PubMed] [Google Scholar]
- 77.Quintero-Hernández V, Juárez-González VR, Ortíz-León M, Sánchez R, Possani LD, Becerril B. The change of the scFv into the Fab format improves the stability and in vivo toxin neutralization capacity of recombinant antibodies. Mol Immunol. 2007;44:1307–15. doi: 10.1016/j.molimm.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 78.Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Hum Reprod Update. 2006. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts; pp. 1–21. [DOI] [PubMed] [Google Scholar]
- 79.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nature Rev Cancer. 2004;4:361–70. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 80.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 81.Guillaume-Rousselet N, Jean D, Frade R. Cloning and characterization of anti-cathepsin L single chain variable fragment whose expression inhibits procathepsin L secretion in human melanoma cells. Biochem J. 2002;367:219–27. doi: 10.1042/BJ20020350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang W, Zhou J, Xu L, Zhen Y. Antineoplastic effect of intracellular expression of a single-chain antibody directed agianst type IV collagenese. J Environ Pathol Toxicol Oncol. 2000;19:61–8. [PubMed] [Google Scholar]
- 83.Heintges T, Zu Putlitz J, Wands JR. Characterization and binding of intracellular antibody fragments to the Hepatitis C Virus core protein. Bioch Biophys Res Comm. 1999;263:410–8. doi: 10.1006/bbrc.1999.1350. [DOI] [PubMed] [Google Scholar]
- 84.Blazek D, Celer V, Navrátilová I, Skládal P. Generation and characterization of single-chain anti-body fragments specific against transmembrane envelope glycoprotein gp46 of maedivisna virus. J Virol Methods. 2004;115:83–92. doi: 10.1016/j.jviromet.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 85.Rosso M-N, Schouten A, Roosien J, Borst-Vrenssen T, Hussey RS, Gommers FJ, Bakker J, Schots A, Abad P. Expression and functional characterization of a single chain FV antibody directed against secretions involved in plant nematode infection process. Biochem Biophys Res Comm. 1996;220:255–63. doi: 10.1006/bbrc.1996.0428. [DOI] [PubMed] [Google Scholar]
- 86.Freland S, Chambers BJ, Andersson M, Kaer LV, Ljunggren H-G. Rejection of allogeneic and syngeneic but not MHC class I-deficient tumor grafts by MHC class I-deficient mice. J Immunol. 1998;160:572–79. [PubMed] [Google Scholar]
- 87.Vanhove B, Charreau B, Cassard A, Pourcel C, Soulillou J-P. Intracellular expression in pig cells of anti-α1,3galactosyltransferase single-chain FV anti-bodies reduces galα1,3 gal expression and inhibits cytotoxicity mediated by anti-gal xenoantibodies. Transplantation. 1998;66:1477–85. doi: 10.1097/00007890-199812150-00011. [DOI] [PubMed] [Google Scholar]
- 88.Miller TW, Messer A. Intrabody applications in neurological disorders: progress and future prospects. Mol Ther. 2005;12:394–401. doi: 10.1016/j.ymthe.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 89.Vera M, Fortes P. Simian virus-40 as a gene therapy vector. DNA Cell Biol. 2004;23(5):271–82. doi: 10.1089/104454904323090903. [DOI] [PubMed] [Google Scholar]
- 90.Green NK, Herbert CW, Hale SJ, Hale AB, Mautner V, Harkins R, Hermiston T, Ulbrich K, Fisher KD, Seymour LW. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11:1256–63. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
- 91.Romano G. Current development of lentiviral-mediated gene transfer. Drug news Perspect. 2005;18:128–34. doi: 10.1358/dnp.2005.18.2.886481. [DOI] [PubMed] [Google Scholar]
- 92.Rainov NG, Ren H. Clinical trials with retrovirus mediated gene therapy-what we have learned? J Neurooncol. 2003;65:227–36. doi: 10.1023/b:neon.0000003652.71665.f2. [DOI] [PubMed] [Google Scholar]
- 93.Goncalves MAFV. Adeno-associated virus: from defective virus to effective vector. Virology J. 2005;2(43):1–17. doi: 10.1186/1743-422X-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Naure Rev Genet. 2003;4:346–58. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 95.Glasgow JN, Bauerschmitz GJ, Curiel DT, Hemminki A. Transductional and transcriptional targeting of adenovirus for clinical applications. Curr Gene Ther. 2004;4:1–14. doi: 10.2174/1566523044577997. [DOI] [PubMed] [Google Scholar]
- 96.Lavillette D, Russell SJ, Cosset F-L. Retargeting gene delivery using surface-engineered retroviral vector particles. Curr Opin Biotechnol. 2001;12:461–6. doi: 10.1016/s0958-1669(00)00246-9. [DOI] [PubMed] [Google Scholar]
- 97.Cronin J, Zhang X-Y, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5:387–98. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rein DT, Breidenbach M, Kirby TO, Han T, Siegal GP, Bauerschmitz GJ, Wang M, Nettelbeck DM, Tsuruta Y, Yamamoto M, Dall P, Hemminki A, Curiel DT. A fiber-modified, secretory leukoprotease inhibitor promoter-based conditionally replicating adenovirus for treatment of ovarian cancer. Clin Cancer Res. 2005;11:1327–35. [PubMed] [Google Scholar]
- 99.Zhao Yi, Zhu L, Lee S, Li L, Chang E, Soong N-W, Douer D, Anderson WF. Identification of the block in targeted retroviral-mediated gene transfer. Proc Natl Acad Sci USA. 1999;96:4005–10. doi: 10.1073/pnas.96.7.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Da Silva FA, Costa MJL, Corte-Real S, Goncalves J. Cell type-specific targeting with Sindbis pseudotyped lentiviral vectors displaying anti-CCR5 single-chain antibodies. Hum Gene Ther. 2005;16:223–34. doi: 10.1089/hum.2005.16.223. [DOI] [PubMed] [Google Scholar]
- 101.Morizono K, Xie Y, Ringpis G-E, Johnson M, Nassanian H, Lee B, Wu L, Chen ISY. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–52. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 102.Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nature Rev Genet. 2005;6:299–310. doi: 10.1038/nrg1577. [DOI] [PubMed] [Google Scholar]
- 103.Ewert K, Ahmad A, Evans HM, Safinya CR. Cationic lipid-DNA complexes for non-viral gene therapy: relating supramolecular structures to cellular pathways. Exert Opin Biol Ther. 2005;5:33–53. doi: 10.1517/14712598.5.1.33. [DOI] [PubMed] [Google Scholar]
- 104.Dowdy SF, Snyder EL. Recent advances in the use of protein transduction domains for the delivery of peptides, proteins and nucleic acids in vivo. Expert Opin Drug Deliv. 2005;2:1–9. doi: 10.1517/17425247.2.1.43. [DOI] [PubMed] [Google Scholar]
- 105.Niesner U, Halin C, Lozzi L, Günthert M, Neri P, Wunderli-Allenspach H, Zardi L, Neri D. Quantitation of the tumor-targeting properties of antibody fragments conjugated to cell-permeating HIV-1 TAT peptides. Bioconjug Chem. 2002;13:729–36. doi: 10.1021/bc025517+. [DOI] [PubMed] [Google Scholar]
- 106.Suzuki M, Takayanagi A, Shimizu N. Targeted gene delivery using humanized single-chain antibody with negatively charged oligopeptide tail. Cancer Sci. 2004;95:424–9. doi: 10.1111/j.1349-7006.2004.tb03226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


