Abstract
Toll-like receptor 4 (TLR4) is key regulators of both innate and adaptive immune responses. TLR4 recognizes pathogen-associated molecular patterns (PAMPs) and activates the inflammatory cells. The function of TLR4 in atherosclerosis has been investigated in mouse knockout studies and epidemiological studies of human TLR4 polymorphisms. These studies have shown that TLR4 function affects the initiation and progression of atherosclerosis. This article reviews the biological functions and clinical implications of TLR4 in atherosclerosis.
Keywords: Toll-like receptor 4, atherosclerosis, endothelial cells, vascular smooth muscle cells, adventitial fibroblasts, dendritic cells, macrophages, arterial remodelling
Introduction
It has become evident that atherosclerosis is an inflammatory disease with immune responses during initiation and progression of this disease [1]. The presence of inflammatory cells can be observed in all stages of atherosclerosis [2]. Activated inflammatory cells have been suggested to induce atherosclerotic plaque formation and destabilization. Moreover, these cells play an important role in defense against infectious agents. In general, the immune system responds effectively to harmful agents by two closely related pathways: the innate and the adaptive immune recognition systems. The adaptive immune system involves dynamic adaptation to unique antigenic epitopes present on pathogens in the environment. The innate immune response is the first line of defense and is limited to recognize highly conserved pathogen motifs, entitled pathogen-associated molecular patterns (PAMPs) [3]. In the first line of defense, the receptors capable of recognizing these PAMPs are toll-like receptors (TLRs) and scavenger receptors. In addition to their crucial role in innate immunity, TLRs have recently been associated with atherosclerosis. This review will focus on the role of TLR4 in the initiation and progression of atherosclerotic disease.
Toll-like receptor 4 structure and its functions
The Toll was originally described as a type I transmembrane receptor that controls the embryonic dorsal-ventral pattern of the Drosophila[4]. Recent studies have identified mammalian homologues of the toll receptor proteins now referred to as TLR proteins [5, 6]. TLRs are type I transmembrane proteins with extracellular amino terminus and a carboxy terminal intracellular domain. The extracellualr domain contains a varying number of leucine-rich repeat (LRR), which are presumably involved in ligand binding. The intracellular domain of TLR proteins is similar to the intracellular domain of the interleukin-1 receptor, and the conserved region is called toll/interleukin 1 receptor domain [6]. To date, 10 members of the TLR family have been identified in human [7]. Different members of this receptor family recognize different PAMPs, such as peptidoglycan for TLR2 [8], lipopolysaccharide (LPS) for TLR4 [9], flagellin for TLR5 [10], and CpG-DNA-repeats for TLR9 [11]. In short, TLRs play an importat role in recognizing microbial components.
Toll-like receptor 4 was identified as the first human homologue of the Drosophila Toll [5]. The extracellular domain of TLR4 that contain over 600 amino acids is highly polymorphic compared with the transmembrane and intracellular domain of the protein [12]. This TLR4 polymorphism contributes to species-specific differences in recognition of LPS, the prototypic TLR4 ligand [13]. The intracellular TIR domain, which is composed of three highly conserved regions, contains 150 amino acids [14]. The TIR domain modulates protein–protein interactions between the TLRs and signal transduction elements. TLR4 was shown to be involved in the recognition of LPS, a major cell wall component of Gram-negative bacteria. In addition to LPS, TLR4 recognizes several other ligands, such as lipoteichoicacid, heat-shock proteins (HSP), and EDA in fibronectin.
Activation of TLR4 was shown to elicit the production of cytokines and chemokines [15]. Therefore, it was suggested that the TLR4 might be involved in immune responses, especially in the activation of innate immunity. Activated TLR4 triggers not only innate immunity but also adaptive immunity. TLR4 activation on dendritic cells induces the expression of co-stimulatory molecules and production of inflammatory cytokines [16]. Then, activated dendritic cells present microorganism-derived peptide antigens expressed on the cell surface with MHC class II antigen to naive T cells, thereby initiating an antigen-specific adaptive immune response [17]. Now, evidence is accumulating that TLR4 could affect atherosclerosis in multiple ways (Fig. 1).
1.
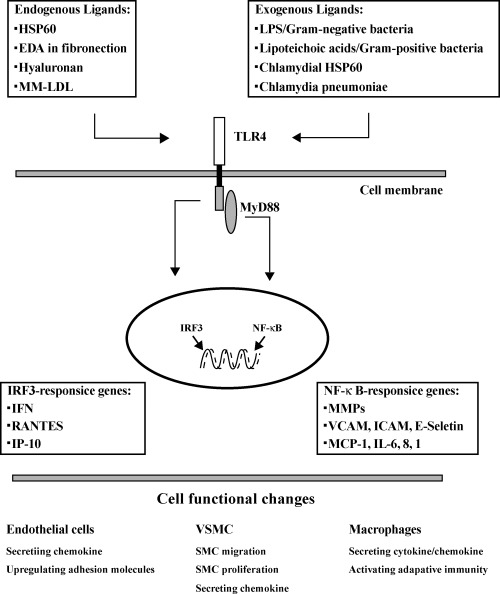
TLR4 signaling pathway and its relation with atherosclerosis. Both endogenous and exogenous ligands can activate TLR4 on cells, such as endothelial cells, vascular smooth muscle cells, adventitial fibroblasts, dendritic cells and macrophages. Activated TLR4 lead to activation of the transcription factor nuclear factor κ B-(NF-κB) or IRF3-responsive genes. Activated transcription factors mediate secretion of pro-inflammatory cytokines and chemokines and also induce expression of adhesion molecules. Ultimately, these processes might initiate or promote atherosclerotic lesions.
TLR4 expression in atherosclerotic lesions
Consistent with its role in pathogen recognition, TLR4 is expressed by cells involved in the first line of host defense, including neutrophils, macrophages, and dendritic cells [18]. In addition to innate immune cells, TLR4 is also expressed in several other types of cells that contribute to the more complex adaptive immunity such as B and T cells. Recently, accumulating interest has focused on TLR4 expression in atherosclerotic lesions.
Endothelial cells
The endothelium plays a central role in maintaining vascular health by virtue of its vital anti-inflammatory and anti-coagulant properties. Endothelial dysfunction is the earliest detectable manifestation of atherothrombosis [19]. Endothelial cells respond to pharmacologic or hemodynamic stimuli by modulating the induction and/or repression of several genes. Edfeldt et al. recently found that TLR4 is markedly augmented in endothelial cells of human atherosclerotic lesions. However, TLR4 was expressed at low levels by endothelial cells in normal arteries [20]. Furthermore, cultured human vascular endothelial cells express little TLR4 under baseline conditions, and they express high levels of TLR4 on stimulation with pro-inflammatory cytokines [21]. Although these data suggested that TLR4 in endothelial cells was associated with initiation of atherosclerosis, it remain unclear how TLR4 in endothelial cells play roles in atherosclerosis.
Vascular smooth muscle cells (VSMCs)
VSMCs reside mostly in the media of healthy adult arteries, where their role is to regulate vascular tone. Under cytokines influences, VSMC migrate to the intima and undergo a phenotypic change characterized by a reduction in content of contractile proteins and a large increase in the number of synthetic organelles. These changes were thought be a crucial step in the development of atherosclerosis. It was shown that TLR4 expression is up-regulated in the medial smooth muscle of human atherosclerotic vessel [22]. In contrast, few α-smooth muscle actin–positive smooth muscle cells express TLR4 in normal arteries. Moreover, it is shown that Chlamydia pneumoniae may signal through TLR4 to induce the proliferation of human vascular smooth muscle cell. These activated vascular smooth muscle cells express high levels of TLR4 [23].
Adventitial fibroblasts
The tunica media of all arteries is contained within a connective tissue layer that contains blood vessels and nerves and that is known as the tunica adventitia. In the adventitia, fibroblasts are the most prominent cells and the involvement of adventitial fibroblasts in the formation of intimal lesions has been demonstrated in animal models [24]. It was found that adventitial fibroblasts express a functional TLR4 in the human atherosclerotic arteries [25]. Furthermore, Vink et al. found that TLR4 in adventitial fibroblast is associated with intimal lesion formation in a mouse femoral cuff mode [25].
Dendritic cells (DCs)
Bobryshev and Lord first described the identification of DCs in arteries [26]. Morphological studies show that vascular dendritic cells are rare in the normal artery but accumulate in the atherosclerotic lesions [27]. They demonstrated that vascular DCs have cell–cell contact with monocytes/macrophages and leukocytic cells [28]. Furthermore, these DCs colocalized with T cells [29]. These data implicate a possible role for DCs in the immune mediation of atherosclerosis. Recent data show that endogenous HSP70 and Chlamydia pneumoniae HSP60 were demonstrated in DCs plaques [30]. It was suggested that TLR4 in DCs involved in atherogenesis.
Macrophages
A cytokine or growth factor produced in the inflamed intima induces monocytes entering the plaque to differentiate into macrophages. Such cytokines also stimulate the expression of TLRs that allow macrophages to ingest oxidized lipids and to develop into macrophage foam cells.This step is critical for the development of atherosclerosis [31]. Xiaoou et al. have found that TLR4 is expressed in macrophage-infiltrated atherosclerotic lesions of mice and humans during this step. Moreover, TLR-4 mRNA in cultured macrophages is up-regulated by ox-LDL [20, 32].
Role of TLR4 in atherosclerosis
Expression of TLR4 in atherosclerotic lesions has led to the hypothesis that TLR4 is involved in the development and progression of atherosclerotic disease.
TLR4 in early atherosclerotic lesion
Early atherosclerotic lesions are precursors of advanced lesions and categorized into three types as initial, fatty streak and intermediate lesions. These lesions are characterized by an accumulation of lipid-laden cells beneath the endothelium [2]. Most of these cells are macrophages. Early study revealed that C3H/HeJ mice are atherosclerosis resistant on a high cholesterol diet compared with C57BL/6 mice [33]. C3H/HeJ mice carry a point mutation in the intra-cytoplasmic region of TLR4 resulting in a non-functional Tlr4. Furthermore, endothelial cells derived from C3H/HeJ mice presented a lack of an inflammatory response toward MM-LDL (minimally modified low density lipoprotein), supporting the hypothesis that MM-LDL indeed initiates atherosclerosis via TLR4 [34]. Transfer of bone marrow derived from an atheroscle-rosis-prone mouse strain into C3H/HeJ mice failed to reverse the phenotype of C3H/HeJ mice, supporting an important role of TLR4 on endothelial cells during initiation of atherosclerosis [35].
Studies in animals and humans have shown that arterial endothelial cells are activated in the early-stage of atherogenesis. Endothelial cell activation is characterized by the expression of cell adhesive molecules, which cause circulating monocytes rolling along the vascular surface to adhere at the site of activation [36]. It was shown that recognition of MM-LDL by TLR4 on endothelial cells results in the secretion of the chemokine IL-8 [37]. LPS is considered a potent activator in endothelial dysfunction and expression of pro-inflammatory cytokines. It is now well established that LPS up-regulates the expression of intracellular adhesion molecule-1, vascular adhesion molecule and MCP-1 by TLR4 of human coronary artery endothelial cells [38]. These proteins will stimulate the expression of TLR4 on macrophages that allow macrophages to ingest oxidized lipids and to develop into macrophage foam cells, contributing to atherogenesis [31]. The activated TLR4 on macrophage can initiate a signal cascade that induces expression of inflammatory cytokines, proteases, and cytotoxic oxygen and nitrogen radical molecules [39]. These proteins further aggravate the development of atherosclerosis. Interestingly, TLR4 can directly interfere with cholesterol metabolism in macrophages [40], suggesting an additional mechanism by which TLR4 may affect atherogenesis. However, the potential role of TLR4 in early atherosclerotic lesion is still largely unexplored.
TLR4 in advanced atherosclerotic lesion
Atherosclerotic lesions are considered advanced by histological criteria when accumulations of lipid, cells, and matrix components, including minerals, are associated with structural disorganization, repair, and thickening of the intima, as well as deformity of the arterial wall. Lesions consist mainly of thick layers of fibrous connective tissue and lipid-laden SMC [41]. The most predominant change in advanced ath-erosclerosis lesions is functional modulations and numerical increases in intimal smooth muscle cells.
In atherosclerosis, LPS induces expression of genes encoding the elastin-degrading enzyme, cathepsin S via TLR4 in human cervical smooth muscle cells [42]. These proteinases allow SMCs to migrate to the site of inflammation or injury in response to chemokines. In addition, on the site of inflammation or injury, microorganism may signal through TLR4 to induce the proliferation of human vascular smooth muscle cell [23]. These results provide evidence for a link between TLR4 on SMCs and intimal hyperplasia in atherosclerosis. In other study, Vink et al. found that stimulation of TLR4 on adventitial fibroblasts augmented neointima formation, an effect that was reduced in TLR4-defective mice by using a mouse femoral cuff model [25]. It was suggested that TLR4 on adventitial fibroblasts was involved in neointima formation in atherosclerosis.
Apart from lipid-laden SMCs, there are numerous macrophages without lipid droplet inclusions or with lipid droplet inclusions (named as macrophage foam cells) in advanced atherosclerotic lesion. Several descriptive studies show that TLR4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques [20, 32]. The activated TLR4 on macrophage can initiate a signal cascade that induce expression of inflammatory cytokines and proteases, which may play a role in formation and modeling of advanced lesions [39].
In a word, these results suggest that TLR4 play a pivotal role in progression of atherosclerotic disease.
TLR4 in plaque rupture
Plaque rupture is dangerous because it exposes pro-thrombotic material from the core of the plaque to the blood. It was demonstrated that genetic deficiency of TLR4 reduces aortic atherosclerosis in apoE-deficient mice. Furthermore, TLR4 deficiency was associated with alterations in plaque composition, including reduction in lipid and macrophage content, and markedly decreased expression of the pro-inflammatory factors [43, 50]. These changes suggest greater structural stability.Therefore, it is has been suggested that TLR4 signaling might be important in plaque destabilization.
Several studies have shown that activated macrophage cells within the plaque are capable of degrading extracellular matrix by secretion of matrix metalloproteinases (MMP) and proteolytic enzymes, which lead to plaque rupture [44]. It was demonstrated that LPS mediates express MMP-9 by TLR4 in human macrophages and MMP-9 degrades collagen of fibrous cap [45]. Other researches also show that LPS induced proteolytic enzymes by via TLR4 in macrophages [42]. These proteolytic enzymes may weaken the fibrous cap and predispose plaque to rupture by degrading the components of extracellular matrix [46]. Recently, apoptosis is considered as an important event in plaque rupture. VSMCs apoptosis in the fibrous cap may result in plaque rupture [47]. It has been reported that activated TLR4 signaling pathway induce expression of apoptotic molecules of the Fas death pathway [48].
In short, these results suggested a potential role for TLR4 in plaque destabilization. However, the exact mechanism role of TLR4 in plaque stabilization remains obscure.
TLR4 in arterial remodelling
In atherosclerosis, arterial remodelling is defined as a structural change in total arterial circumference. It ranges from expanse to contraction and therefore can compensate for or aggravated the effect of plaque or hyperplasia load on the ultimately useful lumen. Several studies have shown that intimal hyperplasia and adventitial changes relate to remodelling of the artery in atherosclerosis. In a mouse model of cuff, it was seen that neointiam formation was accompanied with femoral artery remodelling. However, this phenomenon could not be detected in TLR4-deficient mice [49]. Moreover, adventitial stimulation of TLR4 induced arterial remodelling, an effect that was reduced in TLR4-defective mice [25]. These results provide evidence for a link between TLR4 and arterial remodelling.
It is likely that matrix breakdown and synthesis play an important role in arterial remodelling. Changes in collagen, elastin and glycosaminoglycan contribute most to the arterial remodelling. Several lines of evidence suggest that MMP system play a role in degrading elastin and collagen. Recent in vitro studies revealed that LPS induce secretion of MMP-9 by TLR4 in activated macrophages that are present in adventitial layer of human coronary arteries [45]. In other studies, it was shown that LPS induces expression of genes encoding the elastin-degrading enzyme, cathepsin S via TLR4 in human cervical smooth muscle cells [42]. Therefore, TLR4 activation could play a role in arterial remodelling by production of MMP and proteolytic enzymes. Other potential mechanisms of TLR4 in arterial remodelling should be explored further.
TLR4 polymorphisms in atherosclerosis
The characterization of the human TLR4 polymorphisms Asp299Gly and Thr399Ile is a single nuleotide polymorphism in the TLR4 gene, which impairs the efficacy of LPS signaling and the capacity to elicit inflammation [51]. Recently, several clinical studies have investigated the role of TLR4 polymorphisms in the development of atherosclerotic disease.
The Asp299Gly TLR4 polymorphism was first described as associated to lower risk of carotid artery atherosclerosis [52]. Other studies also reported that this polymorphism imparts protection from carotid artery atherosclerosis and acute coronary syndrome [51, 53]. However, studies in a large cohort of 1400 participants failed to show an association between Asp299Gly TLR4 polymorphism and coronary atherosclerosis [54]. Furthermore, development of carotid atherosclerosis was not affected by this polymorphism in familiar hypercholesterolaemic patients [55]. Recently, a report from the Stockholm Heart Epidemiology Program found that the co-segregating 299Gly/399Ile variant was demonstrated to confer a higher risk of myocardial infarction [56]. For most of clinical studies involved relatively few patients, larger clinical studies will be required to reconcile these divergent results.
Conclusion
Toll-like receptor 4 was identified as the first human homologue of the Drosophila Toll. Recently, accumulating interest has focused on TLR4 expression in atherosclerotic lesions. Different cell types in atherosclerotic vessel wall, such as endothelial cells, vascular smooth muscle cells, adventitial fibroblasts, dendritic cells and macrophages, express TLR4. Expression of TLR4 in atherosclerotic lesions is involved in the development and progression of atherosclerotic disease. With a better understanding of TLR4 involvement in atherosclerosis, we can begin to develop effective therapies to this life-threatening disease in the future.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis. American Heart Association. Circulation. 1994;89:2462–78. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto C, Hudson KL, Anderson KV. The toll gene of Drosophila required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–79. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 6.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila toll. Proc Natl Acad Sci USA. 1998;95:588–93. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Means TK, Golenbock DT, Fenton MJ. The biology of toll-like receptors. Cyt Growth Fact Rev. 2000;11:219–32. doi: 10.1016/s1359-6101(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge. recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 9.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in the tlr4 gene. J Exp Med. 1999;189:615–25. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlet DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 11.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 12.Smirnova I, Poltorak A, Chan EK, McBride C, Beutler B. Phylogenetic variation and polymorphism at the toll-like receptor 4 locus (TLR4) Genome Biol. 2000;1:1–10. doi: 10.1186/gb-2000-1-1-research002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002;3:354–9. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill LA, Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today. 2000;21:206–9. doi: 10.1016/s0167-5699(00)01611-x. [DOI] [PubMed] [Google Scholar]
- 15.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 16.Akira S, Takeda K, Kaisho T. Toll like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 17.Reis e Sousa C. Dendritic cells as sensors of infection. Immunity. 2001;14:495–8. doi: 10.1016/s1074-7613(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 18.Imler JL, Hoffmann JA. Toll receptors in innate immunity. Trends Cell Biol. 2001;11:304–11. doi: 10.1016/s0962-8924(01)02004-9. [DOI] [PubMed] [Google Scholar]
- 19.Bonetti PO, Lerman LO, Lerman A. Endothelial dys-function: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 20.Edfeldt K, Swedenborg J, Hansson GK, Yan Z-Q. Expression of Toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–61. [PubMed] [Google Scholar]
- 21.Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-gamma induce toll-like receptor 2 and toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J Immunol. 2001;166:2018–24. doi: 10.4049/jimmunol.166.3.2018. [DOI] [PubMed] [Google Scholar]
- 22.Stoll LL, Denning GM, Li WG, Rice JB, Harrelson AL, Romig SA, Gunnlaugsson ST, Miller FJ, Jr, Weintraub NL. Regulation of endotoxin-induced proinflammatory activation in human coronary artery cells: expression of functional membrane-bound CD14 by human coronary artery smooth muscle cells. J Immunol. 2004;173:1336–43. doi: 10.4049/jimmunol.173.2.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasu S, LaVerda D, Qureshi N, Golenbock DT, Beasley D. Chlamydia pneumoniae and Chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ Res. 2001;89:244–50. doi: 10.1161/hh1501.094184. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–5. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 25.Vink A, Schoneveld AH, Van Der Meer JJ, Van Middelaar BJ, Sluijter JP, Smeets MB, Quax PH, Lim SK, Borst C, Pasterkamp G, De Kleijn DP. In vivo evidence for a role of Toll-like receptor 4 in the development of intimal lesions. Circulation. 2002;106:1985–90. doi: 10.1161/01.cir.0000032146.75113.ee. [DOI] [PubMed] [Google Scholar]
- 26.Bobryshev YV, Lord RS. S-100 positive cells in human arterial intima and in atherosclerotic lesions. Cardiovasc Res. 1995;29:689–96. [PubMed] [Google Scholar]
- 27.Bobryshev YV, Ikezawa T, Watanabe T. Formation of Birbeck granule-like structures in vascular dendritic cells in human atherosclerotic aorta. Lag-antibody to epidermal Langerhans cells recognizes cells in the aortic wall. Atherosclerosis. 1997;133:193–202. doi: 10.1016/s0021-9150(97)00129-9. [DOI] [PubMed] [Google Scholar]
- 28.Bobryshev YV, Lord RS. Structural heterogeneity and contacting interactions of vascular dendritic cells in early atherosclerotic lesions of the human aorta. J Submicrosc Cytol Pathol. 1996;28:49–60. [PubMed] [Google Scholar]
- 29.Bobryshev YV, Lord RS. Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immuneinflammatory reactions. Cardiovasc Res. 1998;37:799–810. doi: 10.1016/s0008-6363(97)00229-0. [DOI] [PubMed] [Google Scholar]
- 30.Bobryshev YV, Cao W, Phoon MC, Tran D, Chow VT, Lord RS, Lu J. Detection of Chlamydophila pneumoniae in dendritic cells in atherosclerotic lesions. Atherosclerosis. 2004;173:185–95. doi: 10.1016/j.atherosclerosis.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 31.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92:8264–8. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–8. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 33.Ishida BY, Blanche PJ, Nichols AV, Yashar M, Paigen B. Effects of atherogenic diet consumption on lipoproteins in mouse strains C57BL/6 and C3H. J Lipid Res. 1991;32:559–68. [PubMed] [Google Scholar]
- 34.Shi W, Haberland ME, Jien ML, Shih DM, Lusis AJ. Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation. 2000;102:75–81. doi: 10.1161/01.cir.102.1.75. [DOI] [PubMed] [Google Scholar]
- 35.Shi W, Wang X, Tangchitpiyanond K, Wong J, Shi Y, Lusis AJ. Atherosclerosis in C3H/HeJ mice reconstituted with apolipoprotein E-null bone marrow. Arterioscler Thromb Vasc Biol. 2002;22:650–5. doi: 10.1161/01.atv.0000013388.03553.31. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson EE, Xie X, Werr J, Thoren P, Lindbom L. Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J Exp Med. 2001;194:205–18. doi: 10.1084/jem.194.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walton KA, Hsieh X, Gharavi N, Wang S, Wang G, Yeh M, Cole AL, Berliner JA. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoylsn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8: a role for Toll like receptor 4 and a glycosylphos-phatidylinositol-anchored protein. J Biol Chem. 2003;278:29661–6. doi: 10.1074/jbc.M300738200. [DOI] [PubMed] [Google Scholar]
- 38.Zeuke S, Ulmer AJ, Kusumoto S, Katus HA, Heine H. TLR4-mediated inflammatory activation of human coronary artery endothelial cells by LPS. Cardiovas Res. 2002;56:126–34. doi: 10.1016/s0008-6363(02)00512-6. [DOI] [PubMed] [Google Scholar]
- 39.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–94. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 40.Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and Toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805–16. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 41.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis a report from the committee on vascular lesions of the council on arte-riosclerosis, American Heart Association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 42.Watari M, Watari H, Nachamkin I, Strauss JF. Lipopolysaccharide induces expression of genes encoding pro-inflammatory cytokines and the elastindegrading enzyme, cathepsin S, in human cervical smooth-muscle cells. J Soc Gynecol Invest. 2000;7:190–8. doi: 10.1016/s1071-5576(00)00054-x. [DOI] [PubMed] [Google Scholar]
- 43.Michelsen KS, Wong MH, Shah PK. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. PNAS. 2004;101:10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90:775–8. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 45.Grenier D, Grignon L. Response of human macrophage-like cells to stimulation by Fusobacterium nucleatum ssp. nucleatum lipopolysaccharide. Oral Microbiol Immunol. 2006;21:190–6. doi: 10.1111/j.1399-302X.2006.00278.x. [DOI] [PubMed] [Google Scholar]
- 46.Shah PK, Galis ZS. Matrix metalloproteinase hypothesis of plaque rupture: players keep piling up but questions remain. Circulation. 2001;104:1878–80. [PubMed] [Google Scholar]
- 47.Geng YJ, Libby P. Progression of atheroma: a struggle between death and procreation. Arterioscler Thromb Vasc Biol. 2002;22:1370–80. doi: 10.1161/01.atv.0000031341.84618.a4. [DOI] [PubMed] [Google Scholar]
- 48.Fukui M, Imamura R, Umemura M, Kawabe T, Suda T. Pathogen-associated molecular patterns sensitize macrophages to Fas ligand-induced apoptosis and IL-1 beta release. J Immunol. 2003;171:1868–74. doi: 10.4049/jimmunol.171.4.1868. [DOI] [PubMed] [Google Scholar]
- 49.Hollestelle SC, De Vries MR, Van Keulen JK, Schoneveld AH, Vink A, Strijder CF, Van Middelaar BJ, Pasterkamp G, Quax PH, De Kleijn DP. Toll-like receptor 4 is involved in outward arterial remodeling. Circulation. 2004;109:393–8. doi: 10.1161/01.CIR.0000109140.51366.72. [DOI] [PubMed] [Google Scholar]
- 50.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced athero-sclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–21. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 51.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporespon-siveness in humans. Nat Genet. 2000;25:187–91. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 52.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–92. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 53.Ameziane N, Beillat T, Verpillat P, Chollet-Martin S, Aumont MC, Seknadji P, Lamotte M, Lebret D, Ollivier V, De Prost D. Association of the Toll-like receptor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler Thromb Vasc Biol. 2003;23:e61–4. doi: 10.1161/01.ATV.0000101191.92392.1D. [DOI] [PubMed] [Google Scholar]
- 54.Yang IA, Holloway JW, Ye S. TLR4 Asp299Gly polymorphism is not associated with coronary artery stenosis. Atherosclerosis. 2003;170:187–90. doi: 10.1016/s0021-9150(03)00286-7. [DOI] [PubMed] [Google Scholar]
- 55.Netea MG, Hijmans A, Van Wissen S, Smilde TJ, Trip MD, Kullberg BJ, De Boo T, Van der Meer JW, Kastelein JJ, Stalenhorf AF. Toll-like receptor-4 Asp299Gly polymorphism does not influence progression of atherosclerosis in patients with familial hyperc-holesterolaemia. Eur J Clin Invest. 2004;34:94–9. doi: 10.1111/j.1365-2362.2004.01303.x. [DOI] [PubMed] [Google Scholar]
- 56.Edfeldt K, Bennet AM, Eriksson P, Frostegard J, Wiman B, Hamsten A, Hansson GK, Faire Ud U, Yan ZQ. Association of hypo-responsive toll-like receptor 4 variants with risk of myocardial infarction. Eur Heart J. 2004;25:1447–53. doi: 10.1016/j.ehj.2004.05.004. [DOI] [PubMed] [Google Scholar]


