Abstract
Angiogenesis, resulting from an imbalance between angiogenic activator factors and inhibitors, is required for tumour growth and metastasis. The determination of the circulating concentration of all angiogenic factors (activators and inhibitors) is not feasible at present. We have evaluated diagnostic and prognostic values of the measurement of serum angiogenic activity in colorectal carcinoma (CRC) patients. Serum proliferative activity (PA) on human umbilical vein endothelial cells (HUVEC) in vitro, and serum vascular endothelial growth factor (VEGF) levels were determined by ELISA in 53 patients with primary CRC, 16 subjects with non-neoplastic gastrointestinal disease (SC) and 34 healthy individuals. Data were compared with clinical outcome of the patients. Although serum from CRC patients significantly increased the PA of HUVEC, compared to culture control (HUVEC in medium + 10% foetal bovine serum (FBS); P < 0.001); our results indicate that serum PA in CRC patients was similar to that of SC or healthy individuals. There was no correlation between serum PA and circulating VEGF concentrations. Surgery produced a decrease of PA at 8 hrs after tumour resection in CRC patients compared to pre-surgery values (186 ± 47 versus 213 ± 41, P < 0.001). However, an increase in serum VEGF values was observed after surgery (280 [176–450] versus 251 [160–357] pg/ml, P = 0.004). Patients with lower PA values after surgery showed a worse outcome that those with higher PA values. Therefore, this study does not support a diagnostic value for serum angiogenic activity measured by proliferative activity on HUVEC but suggests it could have a prognostic value in CRC patients.
Keywords: angiogenesis, colorectal cancer, VEGF, angiogenic activity, prognostic, HUVEC
Introduction
Angiogenesis is the generation of new capillaries by a process of sprouting of pre-existing microvessels. In health, vessel proliferation is under stringent control and occurs only during embryonic development, endometrial regulation, reproductive cycle and wound repair. However, in many pathological conditions such as solid tumour, disease progression appears to be driven by persistent up-regulated angiogenesis [1].
Though the mechanisms leading to pathological angiogenesis are still not obvious, recent evidence indicates that this phenomenon is the result of an imbalance between angiogenic activator factors and inhibitors [2].
Traditionally, the clinical outcome of colorectal cancer (CRC) patients has been predicted by pathological staging, by either Dukes' staging or the UICC-TNM system. However, in some cases, this staging does not reflect the recurrence or the development of metastasis, making the search for new prognostic indicators in CRC a subject of interest [3]. Angiogenesis markers have been considered valuable prognostic factors for cancer patients [4]. In addition, each individual tumour patient's angiogenesis status should be assessed in order to improve the efficacy of a putative anti-angiogenic therapy, therefore identifying the types of tumours most suitable for anti-angiogenic therapy, and the most promising therapies for every group of patients.
The most frequently used methods of assessing tumour angiogenesis are microvessel density counts, immunostaining and reverse transcriptase-polymerase chain reaction (RT-PCR) for angiogenic cytokines [4]. All of these techniques require tumour tissue and are therefore generally performed on post-operative specimens. More recently, measurements of blood levels of angiogenic factors or inhibitors have been used, with the advantage that they are non-invasive, can be performed in situ, and may be used to monitor response to treatment. A number of studies on the role of the measurement of the soluble mediators of angiogenesis in predicting the prognosis of the patients with CRC have been published, but their results are contradictory [5–9]. A possible explanation for these contradictory results could be that both host and tumour cells can generate various positive and negative regulators of angiogenesis, and the final angiogenesis status will be the result of the imbalance between positive and negative regulators of neovascularization. Since the quantification of the concentration of all of these mediators is not feasible at present, an easier experimental approach has been proposed, consisting in the measurement of the angiogenic activity of patients sera by means of their capability to induce proliferation of human endothelial cells in vitro[10]. Serum angiogenic activity has been suggested to correlate to circulating VEGF concentration in patients with breast carcinoma [11], but not in those with renal cell carcinoma [12]. To our knowledge, no obvious indications about the clinical relevance of this new angiogenic parameter in CRC have been reported.
With the purpose of evaluating the diagnostic and prognostic values of the measurement of serum PA in CRC patients, we investigated if serum angiogenesis measured by proliferative activity in CRC patients correlates with their clinicopathological characteristics, their overall survival or serum VEGF levels.
Methods
Patients' samples
Fifty-three patients (25 female and 28 male) with primary CRC, all candidates for surgery resection of primary tumour were included in this study. None of them received radiotherapy or chemotherapy before surgery. Mean age was 67 years (range 30–86 years). Sixteen (8 female and 8 male) subjects with non-neoplastic disease (polyps, adenoma, non-fistulating diverticular disease, cholecystitis and others) that underwent conventional open bowel surgery were included as sex- and age-matched surgery control (SC) group. Mean age was 64 (range 32–80 years). Thirty-four healthy individuals (17 female and 17 male) were recruited as healthy control group. Mean age was 45 years (range 22–70 years). All samples were obtained with patient and control subject consent and local ethical committee approval.
Peripheral blood was drawn pre-operatively and 8 hrs after surgery in both CRC patients and in SC subjects. From healthy individuals a unique sample was obtained. After 20–30 min. of coagulation at room temperature, serum was separated and stored at −80C until in vitro studies.
HUVEC proliferation assay
Human umbilical vein endothelial (HUVE) cells were isolated from fresh human umbilical cords (Hospital Costa del Sol, Marbella, Spain) by collagenase digestion [13] and were grown in Medium 199 containing HEPES (10 mM), L-glutamine (2 mM), heparine (10 mg/ml), penicillin (50 IU/ml), streptomycin (50 μg/ml), and amphoterycin (1.25 μg/ml) (M199), supplemented with 3 mg/l endothelial cell growth supplement (ECGS, Sigma) and 20% foetal bovine serum (M199-ECGS-FBS) in 5% CO2 and 37°C on gelatin-coated plastic plates.
Proliferative activity of HUVEC were estimated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma Chemical Co., St. Louis, MO) dye reduction assay in 96-well microplates was used, essentially as described [14]. Four thousand HUVE cells in a total volume of 100 μl of M199-ECGS-FBS were added to each well of a 96-well plate and they were allowed to attach and grow for 24 hrs in a humidified atmosphere in 5% CO2 at 37°C. Hereafter, the wells were washed with PBS and the test samples were added to the wells at a final concentration of 10% (v/v) in M199. Throughout the whole series of experiments, HUVE cells incubated in M199 + 10% FBS were used as a standard control. After 3 days of incubation (37°C, 5% CO2 in a humid atmosphere) media were aspirated, 100 μl of M199 and 10 μl of MTT (5 mg/ml in PBS) were added to each well, and the plate was incubated for a further 4 hrs (37°C). The resulting formazan was dissolved in 150 μl of 0.04 N HCl-2 propanol and read at 550 nm. The results are expressed as percentage of proliferation versus control grown in M199 + 10% FBS, and are means of eight experimental values.
VEGF immunoassay
Serum VEGF concentrations were measured in duplicate with a commercially available quantitative sandwich enzyme immunoassay kit (R&D Systems, Minneapolis, MN) according to manufacturer's guidelines.
Statistical analyses
Statistical analyses were performed using SPSS version 11.0. Statistical significance of proliferative activity was determined using an analysis of variance (one-way ANOVA) or Student's t-test, as appropriate. Pearson's coefficient of correlation was used to find whether VEGF and proliferative activity correlated. To compare the differences in VEGF values, Kruskal-Wallis test. Mann-Whitney U test and Wilcoxon's test were used. Overall survival of patients was calculated as the number of months from surgery to death or last follow-up. Only deaths from carcinomas were recorded as events. Survival curves were calculated by the Kaplan-Meier method, and the significance of the differences by the log-rank test. Results were considered to be significant when P < 0.05.
Results
Diagnostic value of serum proliferative activity
Although the addition of CRC, SC or healthy individuals serum to HUVEC cultures showed an increase of PA compared to the standard control which was set to 100% (P < 0.001); there was no statistically significant differences in the PA of sera obtained pre-surgery from CRC, SC or from healthys individuals on HUVE cells (Table 1). When CRC and SC patients were matched to groups of high and low PA of sera on HUVE cells, taking the 95th percentile value of healthy individuals as a cut-off, nine out of 53 (16.98%) CRC and 3 out of 16 (18.75%) SC patients showed high proliferative values (Fig. 1). Only sera from one CRC patient (1.81%) and one SC patient (6.25%) showed PA values lower than the lowest value observed in the healthy group.
1.
Pre- and post-surgery serum angiogenic activity measured by proliferative activity on HUVE cells (PA expressed as % of control serum PA) mean ± S.D.) and VEGF levels (pg/ml; median, interquartile range) in patients with colorectal carcinoma (CRC) compared to patients who underwent surgery for non-malignant colorectal diseases (SC) and healthy individuals
| Pre-surgery | 8 hrs post-surgery | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | n | PA | VEGF | n | PA | VEGF | |||||
| CRC | 53 | 213 ± 41† | 251(160–357)†† | 48 | 186 ± 47† | 280(176–450)†† | |||||
| Primary tumour location | |||||||||||
| Colon | 29 | 208 ± 47 | 250(156–301) | 25 | 186 ± 50 | 250(166–449) | |||||
| Rectum | 24 | 220 ± 34 | 260(161–435) | 23 | 186 ± 44 | 297(181–535) | |||||
| Surgery | |||||||||||
| Curative | 42 | 212 ± 42 | 251(158–354) | 39 | 191 ± 41 | 275(174–448) | |||||
| Palliative | 11 | 216 ± 41 | 266(167–493) | 9 | 166 ± 64 | 334(192–850) | |||||
| Lymph node metastases | |||||||||||
| Absent | 36 | 214 ± 42 | 249(113–318) | 34 | 194 ± 40 | 258(141–390) | |||||
| Present | 13 | 214 ± 40 | 266(176–479) | 13 | 159 ± 58§ | 370(204–708) | |||||
| Distant metastases | |||||||||||
| Absent | 45 | 212 ± 41 | 253(160–376) | 42 | 190 ± 41 | 280(179–455) | |||||
| Present | 8 | 218 ± 44 | 215(132–292) | 6 | 155 ± 72 | 281(153–402) | |||||
| Serum proliferative activity | |||||||||||
| High (>250) | 9 | 243(155–372) | 9 | 203(147–362) | |||||||
| Low (<250) | 44 | 252(160–352) | 39 | 310(180–470) | |||||||
| SC | 16 | 197 ± 53‡ | 183(115–467)‡‡ | 15 | 171 ± 51‡ | 190(97–550)‡‡ | |||||
| Healthy individuals | 34 | 201 ± 37 | 162(133–241) | ||||||||
†Pre-surgery versus 8 hrs post-surgery PA values in CRC (P < 0.001) and in ‡SC patients (P= 0.036) (Student's test) §absent versus present lymph node metastases PA values (P= 0.024; Student's test), †† pre-surgery versus 8 hrs post-surgery VEGF levels in CRC (P= 0.004) and in ‡‡SC patients (P= 0.001; Wilcoxon's test).
1.
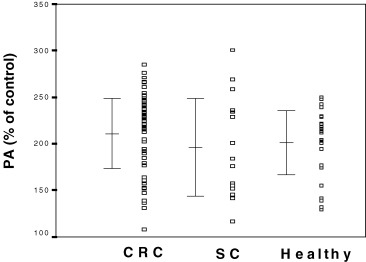
HUVE cell proliferative activity (PA) induced by serum from colorectal cancer patients (CRC, n = 53), gastrointestinal non-malignant diseases patient (SC, n = 16) and healthy individuals (healthy, n = 34). Values represent relative percentages of proliferation compared to control serum (100%). PA in CRC and SC patients and in healthy individuals were increased compared to control values (P < 0.001).
No significant correlation between proliferative activity of pre-surgery sera and clinicopathological variables studied (tumour location, tumour category, histologic grade, TNM stages [UICC, 1987], lymph nodes metastases and distant metastases) was found (Table 2).
2.
Relationship between serum proliferative activity (% of control serum value) (mean±S.D.) and clinicopathologic variables in patients with colorectal cancer
| Patient number (n) | Proliferation (%) | Significance | |
|---|---|---|---|
| Primary tumour location | |||
| Colon | 29 | 208 ± 47 | |
| Rectum | 24 | 220 ± 34 | P= 0.276* |
| TNM stage | |||
| T1/2N0M0 | 9 | 219 ± 46 | |
| T3/4N0M0 | 26 | 209 ± 43 | |
| T1/2N1M0 | 5 | 209 ± 34 | |
| T3/4N1M0 | 3 | 228 ± 56 | |
| T4NxM0 | 2 | 213 ± 20 | |
| T4NxM1 | 8 | 217 ± 44 | P= 0.971** |
| Histologic grade | |||
| 1 Poorly differentiated | 17 | 223 ± 37 | |
| 2 Moderately differentiated | 28 | 206 ± 47 | |
| 3 Well differentiated | 5 | 211 ± 24 | P= 0.404** |
| Unknown | 3 | ||
| Tumour category | |||
| pT1 | 1 | 252 | |
| pT2 | 9 | 207 ± 49 | |
| pT3 | 33 | 214 ± 36 | |
| pT4 | 10 | 212 ± 54 | P= 0.798** |
| Lymph node metastases | |||
| Absent | 36 | 214 ± 42 | |
| Present | 13 | 214 ± 40 | P= 0.962* |
| Unknown | 4 | ||
| Distant metastases | |||
| Absent | 45 | 212 ± 41 | |
| Present | 8 | 218 ± 44 | P= 0.746* |
Student's t-test.
*One-way ANOVA.
Influence of surgery in serum proliferative activity
The possible effect of surgery on the serum proliferative activity was determined by measuring serum PA at 8 post-surgery hours. PA of sera from CRC patients significantly decreased at 8 hrs after tumour resection when compared to pre-surgery values (Table 2; P/0.001). Surgical resection for cure of the primary tumour was performed in 42 out of 53 CRC patients. Eleven out of 53 patients suffering of hepatic metastases, peritoneal dissemination and(or locally advanced unresectable tumours, underwent palliative surgery only. Student's t-test analysis showed no significant differences in sera PA at 8 post-operative hours between curative and palliative surgery groups. Post-surgery sera from patients with lymph node affectation presented lower mean PA than those from patients with unaffected lymph node (Table 2).
In SC patients, a significant decrease was observed in serum PA at 8 hrs after surgery as compared to pre-surgery values (Table 2). No significant differences could be observed in post-operative PA values, irrespective of coming from patients who underwent colorectal resection or not (data not shown). No significant differences in PA pre-surgery and 8-hrs sera from CRC and SC patients were observed.
Association between serum VEGF levels and serum proliferative activity
No significant difference was observed in pre-surgery serum VEGF levels between SC and CRC or healthy groups (Kruskal-Wallis test, Table 1). No significant differences in median serum VEGF values were either observed when patients or healthy individuals were classified according to their age or gender.
As shown in Table 1, serum VEGF levels of CRC patients significantly increased at 8 hrs after tumour resection as compared to pre-surgery values (P = 0.004). When CRC patients were classified according to their serum PA (taking 95th percentile of healthy group, as a cut-off), no significant differences were observed in circulating VEGF levels between patients with high serum PA and those with low PA.
In SC group, at 8 hrs after surgery a significant increase of VEGF levels was observed compared to pre-surgery levels (Table 1, P = 0.001). No significant differences were observed between CRC and SC patients and their pre- or post-surgery VEGF median values.
Prognostic value of serum proliferative activity
To study the association between PA values and clinical outcome, 4 patients who died within 30 days after surgery were excluded. Thirty-four of the 49 patients included in the statistical analysis of prognostic values were alive at the time of the last follow-up evaluation, including 1 patient with local recurrence and 2 with distant metastases. Fifteen patients had died of carcinomas. No significant differences were observed in pre-surgery (PA) values between patients who had survived and those who died (n = 34, 213 ± 42 versus n = 15, 208 ± 45). Serum PA was only determined at 8 hrs after surgery in 45 of the 49 patients. Samples taken 8 hrs after surgery from patients who had survived had PA values significantly higher than those from patients who died (n = 31, 196 ± 37 versus n = 14, 157 ± 57; P = 0.009; Fig. 2).
2.
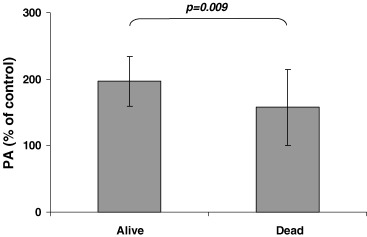
Serum proliferative activity in HUVE cells at 8 hr after surgery tumour removal was lower in CRC patients who died (Dead) during the time of the study compared with patients who were living (Alive) at last follow-up evaluation.
We used a univariate survival analysis considering the patients divided into two groups: PA values less than or equal to median value of PA at 8 hrs after surgery (191, cut-off value), and greater than cut-off value in order to study the differences in overall survival between these two groups. In these groups of patients, considering all patients and only uncensored data, we had a global set of patients and the ranges were (23.14) and (36.77) for uncensored data. After a median global follow-up of 42 months (4–68 months) log-rank test showed a significant difference (P = 0.040) in overall survival rates between both groups (Fig. 3). The percentage of events and values of survival function are shown in Table 3.
3.
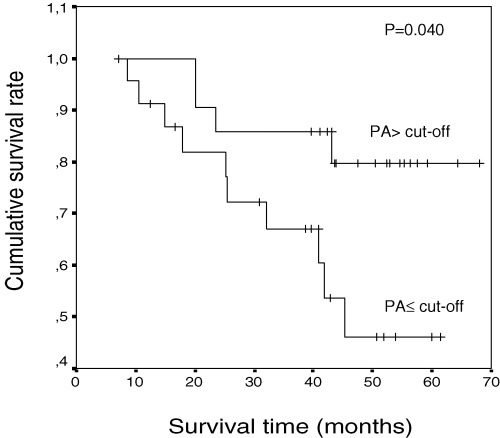
Kaplan-Meier overall survival curves of 45 patients, following PA values at 8 hrs after surgery less than or equal to cut-off level (n = 23) and greater than cut-off level (n = 22). Patients with PA less than or equal to cut-off level had poor prognostic.
3.
Overall survival and PA values at 8 hrs after surgery
| PA values | Number of events (%) | Time of mean survival (months) | S.E. (months) |
|---|---|---|---|
| ≤ Cut-off | 10/23 (43.48) | 44 | 4 |
| Cut-off | 4/22 (18.18) | 60 | 4 |
Discussion
Angiogenesis within colorectal cancer has been proposed to be an important predictor of tumour behaviour that may identify patients at higher risk for recurrence and early death [15]. Increased vessel counts have been associated with a higher risk of metastasis and poor prognosis in colon cancer [16]. Tumour VEGF expression has been shown in some studies to have prognostic significance in CRC, while it did not remain an independent prognostic factor in other studies [3, 5, 16–19]. Determination of circulating levels of angiogenic factors has also yielded conflicting results [5–9]. Although TNM staging system remains the mainstay for the post surgical management of the disease, increasing experimental evidence indicates that the evaluation of the angiogenesis status of CRC patients may be a valuable clinical indicator.
The results of the present study demonstrate that serum proliferative activity in patients suffering from primary CRC is not higher than that of SC patients or healthy individuals. Furthermore, PA values in the pre-surgery sera from 53 CRC patients did not correlate to any of the clinicopathological variables studied. Therefore, according to our results, the measurement of serum ability to stimulate the proliferation of HUVEC in vitro, should not become a diagnostic tool in CRC.
VEGF plays a pivotal role in regulation of normal and pathological angiogenesis. VEGF is secreted by a wide variety of cell types, including neutrophils, platelets and tumor cells in response to hypoxia and inflamation, and by malignant cells after oncogenic activation. The diagnostic value of the measurement of circulating VEGF in CRC has been previously reported by some authors, showing that serum VEGF levels were significantly higher in CRC patients than in SC patients or in normal controls (21–23). However, when circulating VEGF concentrations were evaluated in our group of patients, no significant differences were observed among the three groups of subjects (CRC, SC and healthy groups), probably due to the variability of serum VEGF values in the SC group.
This apparent contradiction between data obtained with the measurements of the angiogenic activity and those of circulating VEGF concentrations is not surprising, since, as previously commented, the global angiogenic activity in serum is the result of a complex mixture of activators and inhibitors of angiogenesis. Recently, Beecken et al.[24] had observed a positive correlation between serum PA on HUVEC and serum VEGF levels in patients with transitional cell carcinoma of the bladder. However, we did not observe a positive association between serum PA and serum VEGF levels in CRC patients. In our hands, HUVE cells are more responsive to the stimulation by bFGF than VEGF (results not shown), what could render that significant changes in VEGF concentrations do not translate into significant differences in the PA in vitro.
We also investigated the effect of surgery on serum PA of CRC and SC patients. In both groups of subjects, a similar time-course was observed, serum PA decreasing at 8 hrs post-surgery. No significant differences were observed in 8-hrs sera from those CRC patients undergoing palliative or curative surgery. It should be noted that, according to our results, serum PA from patients with lymph node affectation was significantly lower than that from patients with unaffected lymph node (Table 1, P = 0.024).
However, the effect of surgery on serum VEGF concentration was very different to that observed on angiogenic activity. An increase in circulating VEGF levels was observed in CRC patients after surgery (Table 1). This increase did not depend on patient clinicopathological status. It has been reported that after colorectal resection for both malign and benign disease there was a transient but significant increase in plasma VEGF at 6–8 hrs after surgery, probably reflecting the release of VEGF in response to surgical trauma [25]. The elevated levels may be associated with the extent of surgery and the angiogenic burst following trauma, but other factors, such as haemolysis related to surgery or concomitant medication, cannot be discarded [26].
In spite of the huge amount of information regarding the diagnostic or prognostic value of serum VEGF levels in cancer patients, very few studies have tried to elucidate the diagnostic or prognostic value of cancer patients' serum mitogenic activity. Morelli et al.[10] evaluated the capacity of sera from patients with breast or gastrointestinal cancer to modulate the proliferation of HUVEC. No correlation of serum PA could be observed with serum VEGF levels, nor with the stage of disease in breast cancer patients. They found high endothelial cell stimulatory activity in 15% of gastrointestinal cancer patients, including in 18% (8 of 43) of intestinal cancer patients. Similar figures arise from our results, since we observed that 17% (9 out of 53) of CRC patients exhibited an increased PA in their sera. On the other hand, we also observed that a similar proportion (3 out of 16, that is 18%) of patients with non-malignant gastrointestinal diseases had increased PA in their sera. The control group in Morelli's study included patients with non-malignant tumour of the breast (fibroadenomas and phyllode tumour), while in our study the control group was formed by patients with gastrointestinal disease (colorectal adenomas, polyps and others) undergoing abdominal laparotomy.
More recently, Beecken et al.[12] demonstrated that serum PA is not elevated in patients suffering from renal cell carcinoma when compared to healthy controls. However, these authors observed a significantly increase of serum PA in patients with transitional cell carcinoma of the bladder compared to healthy individuals and a positive correlation between serum VEGF concentration and serum PA; but they found that patients with low pre-surgery serum angiogenic activity had a higher risk of disease progression [24]. According to their results, the detection of serum PA might become a diagnostic and/or prognostic tool in patients with bladder carcinoma but not in patients with renal cell carcinoma. Our data indicate that CRC patients with lower PA values at 8 hrs after tumour removal can have a worse outcome than those patients with high post-surgery PA values (Fig. 3). To our knowledge, this is the first study devoted exclusively to elucidate the diagnostic and prognostic relevance of the determination of serum mitogenic activity in colorectal cancer. In addition, for the first time results obtained in cancer patients are compared with control patients suffering of benign gastrointestinal disease, and the effect of surgery on their serum angiogenic activity in both populations is determined.
The growing interest in the role of endothelium in physiological and pathological conditions has led to an increased demand for representative in vitro model systems for angiogenesis. To date, HUVE cells have been one of the principal sources of endothelial cells for cell based assays in the field of angiogenesis research. However, the study of genes expressed in human tumour endothelial cells isolated from fresh surgical specimens of human tumours and corresponding normal tissues has pointed out that human tumour endothelium may not be well represented by HUVEC. This can be a limitation of our study, and advises the use of more representative models of tumoural angiogenesis, such as human endothelial precursor cells [27].
In conclusion, the results of our study indicate that there is no correlation between serum angiogenic activity measured by proliferative activity on HUVE cells and serum VEGF concentration in CRC, SC or healthy individuals, and do not support the diagnostic value of serum PA in CRC patients. However, the measurement of post-surgery serum PA may have a prognostic value in CRC patients.
Acknowledgments
This work was supported by grants PETRI 1995/0609 and CTQ2006-15279-C03 (Ministerio de Educacion y Ciemcia, Spain) and from APOMA (Asociación para el Progreso de la Oncología en Malaga). Authors are indebted to Auxiliadora López Jiménez and María José Lozano for their excellent technical assistance.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Liekens S, De Clerk E, Neyst J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253–70. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee JC, Chow NH, Wang ST, Huang SM. Prognostic values of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000;36:748–53. doi: 10.1016/s0959-8049(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 4.Papamichael D. Prognostic role of angiogenesis in colorectal cancer. Anticancer Res. 2001;21:4349–54. [PubMed] [Google Scholar]
- 5.Takahashi Y, Tucker SL, Kitadai Y, Koura AN, Bucana CD, Cleary KR, Ellis LM. Vessel counts and expression of vascular endothelial growth factors as prognostic factors in node-negative colon cancer. Arch Surg. 1997;132:541–6. doi: 10.1001/archsurg.1997.01430290087018. [DOI] [PubMed] [Google Scholar]
- 6.Davies MM, Jonas SK, Kaur S, Allen-Mersh TG. Plasma vascular endothelial but not fibroblast growth factor levels correlate with colorectal liver metastasis vascularity and volume. Br J Cancer. 2000;82:1004–8. doi: 10.1054/bjoc.1999.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werther K, Christensen IJ, Nielsen HJ. Danish RANX05 Colorectal Cancer Study Group. Prognostic impact of matched preoperative plasma and serum VEGF in patients with primary colorectal carcinoma. Br J Cancer. 2002;86:417–23. doi: 10.1038/sj.bjc.6600075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George ML, Tutton MG, Abulafi AM, Eccles SA, Swift RI. Plasma basic fibroblast growth factor levels in colorectal cancer: a clinically useful assay? Clin Exp Metastasis. 2002;19:735–8. doi: 10.1023/a:1021322201816. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama Y, Sako T, Shibao K, Okazaki K, Rempo N, Onitsuka K, Minagawa N, Akahane K, Nagashima N, Nagata N, Itoh H. Prognostic value of plasma vascular endothelial growth factor in patients with colorectal cancer. Anticancer Res. 2002;22:2437–42. [PubMed] [Google Scholar]
- 10.Morelli D, Lazzerini D, Cazzaniga S, Squiccianiri P, Bignami P, Maier AM, Sfondrini L, Menard S, Colnaghi MI, Balsari A. Evaluation of the balance between angiogenic and antiangiogenic circulating factors in patients with breast and gastrointestinal cancers. Clin Cancer Res. 1998;4:1221–4. [PubMed] [Google Scholar]
- 11.Balsari A, Maier JAM, Colnaghi MI, Menard S. Correlation between tumor vascularity, vascular endothelial growth factor production by tumor cells, serum vascular endothelial growth factor levels, and serum angiogenic activity in patients with breast carcinoma. Lab Invest. 1999;79:897–902. [PubMed] [Google Scholar]
- 12.Beecken WD, Bentas W, Glienke W, Linneweber J, Jonas D, Binder J, Kramer W. Serum angiogenic activity: diagnostic relevance in renal cell carcinoma. Eur Urol. 2002;42:364–9. doi: 10.1016/s0302-2838(02)00359-7. [DOI] [PubMed] [Google Scholar]
- 13.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–98. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Frank RE, Saclarides TJ, Leurgans S, Speziale NJ, Drab EA, Rubin DB. Tumor angiogenesis as a predictor of recurrence and survival in patients with node-negative colon cancer. Ann Surg. 1995;222:695–9. doi: 10.1097/00000658-199512000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823–32. doi: 10.1038/sj.bjc.6603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferroni P, Spila A, Martini F, D'Alessandro R, Mariotti S, Del Monte G, Graciano P, Buonomo O, Guadagni F, Roselli M. Prognostic value of vascular endotelial growth factor tumor tissue content of colorectal cancer. Oncology. 2005;69:145–53. doi: 10.1159/000087838. [DOI] [PubMed] [Google Scholar]
- 18.Kang SM, Maeda K, Chung YS. Vascular endothelial growth factor statement correlates with hematogenous metastasis and prognosis in colorectal carcinoma. Oncol Rep. 1997;4:381–4. [PubMed] [Google Scholar]
- 19.Maeda K, Nishiguchi Y, Yashiro M, Yamada S, Onoda N, Sawada T, Kang SM, Hirakawa K. Expression of vascular endothelial growth factor and thrombospondin-1 in colorectal carcinoma. Int J Mol Med. 2000;5:373–8. doi: 10.3892/ijmm.5.4.373. [DOI] [PubMed] [Google Scholar]
- 20.Graziano F, Catalano V, Baldelli AM, Cascinu S. Prognostic biomarkers in resected colorectal cancer: implications for adjuvant chemotherapy. Expert Rev Anticancer Ther. 2001;1:247–57. doi: 10.1586/14737140.1.2.247. [DOI] [PubMed] [Google Scholar]
- 21.Kumar H, Heer K, Lee PW, Duthie GS, MacDonald AW, Greenman J, Kerin MJ, Monson JR. Preoperative serum vascular endothelial growth factor can predict stage in colorectal cancer. Clin Cancer Res. 1998;4:1279–85. [PubMed] [Google Scholar]
- 22.Takeda A, Shimada H, Imaseki H, Okazumi S, Natsume T, Suzuki T, Ochiai T. Clinical significance of serum vascular endothelial growth factor in colorectal cancer patients: correlation with clinicopathological factors and tumor markers. Oncol Rep. 2000;7:333–8. [PubMed] [Google Scholar]
- 23.Karayiannakis AJ, Syrigos KN, Zbar A, Baibas N, Polychronidis A, Simopoulos C, Karatzas G. Clinical significance of preoperative serum vascular endothelial growth factor levels in patients with colorectal cancer and the effect of tumor surgery. Surgery. 2002;131:548–55. doi: 10.1067/msy.2002.123011. [DOI] [PubMed] [Google Scholar]
- 24.Beecken WD, Engl T, Hofmann J, Jonas D, Blatheda R. Clinical relevance of serum angiogenic activity in patients with transitional cell carcinoma of the bladder. J Cell Mol Med. 2005;9:655–61. doi: 10.1111/j.1582-4934.2005.tb00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George ML, Dzik-Jurasz AS, Padhani AR, Brown G, Tait DM, Eccles SA, Swift RI. Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br J Surg. 2001;88:1628–36. doi: 10.1046/j.0007-1323.2001.01947.x. [DOI] [PubMed] [Google Scholar]
- 26.Bondestam J, Salven P, Jaaskela-Saari H, Ikonen T, Lepantalo M, Mattila S, Joensuu H. Major surgery increases serum levels of vascular endothelial growth factor only temporarily. Am J Surg. 2000;179:57–9. doi: 10.1016/s0002-9610(99)00253-6. [DOI] [PubMed] [Google Scholar]
- 27.Bagley RG, Walter-Yohrling J, Cao X, Weber W, Simons B, Cook BP, Chartrand SD, Wang C, Madden SL, Teicher BA. Endothelial precursor cells as a model of tumor endothelium: characterization and comparison with mature endothelial cells. Cancer Res. 2003;63:5866–73. [PubMed] [Google Scholar]


