Abstract
Objective:
Expression of adhesion molecule receptors on venous endothelial cells crucially influences the fate of venous grafts by mediating leukocyte-endothelium interactions. These interactions include adhesion of leuko-cytes to the endothelium, followed by transendothelial migration, leading to neointimal hyperplasia (NIH) and finally graft occlusion. Therefore, inhibition of adhesion molecule expression may be a promising strategy to improve the quality of venous grafts.We tested the efficiency of non-viral transfection of human venous endothe-lial cells (HVEC) with short interfering RNA (siRNA) to specifically down-regulate adhesion molecule expression.
Methods:
Primary cultures of HVEC were examined for expression of the adhesion molecules ICAM1, VCAM1 and E-selectin (SELE) after non viral siRNA transfection. Adhesion molecule expression was measured by flow cytom-etry, real-time polymerase chain reaction and immunoblotting after stimulation with TNF-α, an inflammatory cytokine.
Results:
Non-transfected cells showed a strong increase of adhesion molecule expression following cytokine stimulation (P < 0.01). Upon transfection with specific siRNAs a sixfold decrease in ICAM1 (P < 0.001) and SELE expression and cell positivity (P < 0.05) and a twofold decrease in VCAM1 expression and cell positiv-ity (P < 0.01) Pcould be observed. SiRNA-mediated gene suppression of adhesion molecules was also reflected by corresponding decreases in adhesion protein and transcript levels.
Conclusions:
The expression of adhesion molecules on HVECs can be effectively inhibited by specific siRNAs using a safe, non-viral transfection approach. This is a promising tool to pre-condition venous bypass grafts in order to interfere with endothelium-leukocyte interactions and to prohibit neointima thickening or ath-erosclerosis, which are regarded to be the most important causes of venous graft failure.
Keywords: RNAi, venous graft disease, coronary bypass grafting, adhesion molecules
Introduction
Despite the advances in conservative and catheter intervention treatment surgical bypass grafting remains an outstanding tool for therapy of coronary artery disease. The surgical success is limited by the patency rate of the grafts employed. Although the patency rate of venous conduits is reduced in comparison to arterial grafts, the saphenous vein remains the most commonly used conduit because of its easy disposability. However, the advantage of reduced surgical trauma will disappear, if the patient is at higher risk for redo operation associated with improved perioperative morbidity and mortality [1] caused by a restenosed or occluded graft. Thus, there is a strong interest to develop strategies for improving the patency of venous grafts.
Several main causes are considered to be responsible for the limited patency of venous conduits. Graft thrombosis, which is mainly caused by technical factors such as narrowed anastomosis or improved comparative flow in the bypassed vessel contribute to an early occlusion of the graft. This appears within a few days up to several weeks after surgery. Further changes of venous grafts occur at later time-points, which cannot be influenced by the surgeon. Four to six weeks after implantation, a significant alteration can be observed consisting of an accumulation of smooth muscle cells and deposition of extracellular matrix in the intimal compartment, called NIH. This is commonly accepted as the major pathogenic process in venous grafts within the first year after implantation [2]. NIH leads to a graft alteration in two different ways. Excessive development of the neointima thickening may cause a significant stenosis or an occlusion by itself. Furthermore, it accelerates the development of graft atherosclerosis, superimposed on the previously thickened neointima.
Concerning the described pathomechanisms of NIH and venous graft atherosclerosis, adhesion molecule expression on human venous endothelial cells (HVEC) plays a crucial role [3]. Adhesion molecules represent a group of glycoproteins and carbohydrates expressed on the surface of a wide variety of cell types, including venous endothelial cells. They support an initial adhesive event resulting in the rolling of leukocytes along the endothelium followed by their subsequent firm adhesion [4–6]. Therefore, adhesion molecules enable transendothelial leukocyte migration from the intravascular space into the media of the vasculature, where leukocytes trigger smooth muscle cell proliferation by release of stimulating factors [7]. In conclusion, stress induced expression of adhesion molecules on endothelial cells can be emphasized as a central key factor leading to a limited patency of venous bypass grafts.
Two different groups of adhesion molecules are essential for leukocyte adhesion and migration. Selectins are calcium-dependent transmembrane glycoproteins, which modulate a reversible and weak binding of leukocytes to the endothelium. They are important in the first contact, the so-called tethering. Adhesion molecules belonging to the immunoglobulin superfamily are involved in the following step of firm leukocyte-endothel adhesion [5]. Both adhesion molecule classes are essential for the transendothelial migration of leukocytes, and the incurring NIH.
Several stimuli may lead to an enhanced expression of adhesion molecules in venous endothelial cells. Humoral stimulation by cytokines [8, 9] enforced by the extracorporeal circulation; mechanic manipulation of the venous graft occurring during preparation and harvesting [10] and the modified flow pattern with increased shear stress by arterial pressure in the venous vessel [11].
In summary, various factors contribute to the increased expression of adhesion molecule receptors on HVCS. It is currently impossible to prevent all inductive stimuli leading to adhesion molecule expression on the graft employed. Therefore, at present, surgeons cannot prevent leukocyte-endothelial interactions in the newly implanted venous graft material.
The therapeutic application of small or short interfering ribonucleic acids (siRNAs) may offer a new therapeutic option [12–15]. siRNAs are small double-stranded oligoribonucleotides, which have been originally identified as intermediates of the RNA interference pathway. Upon transfection into cells, they are incorporated into a cytoplasmic ribonucleoprotein complex, the so-called RNA-induced silencing complex (RISC). Upon hybridization to a complementary mRNA sequence, RISC cleaves this transcript leading to its degradation. Thus, despite ongoing tran-scription, no protein can be synthesized because of the RISC-mediated degradation of the mRNA. siRNA may be either transiently delivered by transfection, or can be endogenously expressed as small hairpin RNAs (shRNAs). For both types of delivery, in vivo efficacy has been demonstrated [16].
Concerning an improved protection of venous bypass material, we evaluated the potency of transfected siRNA in reducing adhesion molecule expression on venous endothelial cells in order to protect venous bypass material against NIH.
The aim of the study was to investigate, whether primary HVEC can be transfected with siRNA, and whether specific siRNAs are able to silence the expression of E-selectin (SELE), intercellular adhesion molecule 1 (ICAM1) and vascular adhesion molecule 1 (VCAM1) on HVECs in order to inhibit leukocyte-endothelial interactions.
Material and methods
Patients
Endothelial cells were obtained from vein specimen of the saphenous vein which remained as remnants after elective CABG. All patients gave their written consent and the study was approved by the Ethical Committee of the University of Tuebingen, Faculty of Medicine.
Isolation and cultivation of HVECs
Isolation and cultivation were done as previously described [17]. Briefly, all culture plates and flasks were coated overnight with collagen (0.01%) (Collagen G, Biochrom, IN). After incubation in RPMI 1640 (containing 0.5%/ml gentamycin) the vein was rinsed with buffer solution (137 mM NaCl/5.4 mM KCL/4.2 mM NaHCO3/5 mM D-glucose in 500 ml H2O, pH 7.3). HVECs were harvested by collagenase treatment (0.1% in PBS, PAA Laboratories GmbH, Cölbe, Germany) followed by culture in EGM-2 (+bullet kit, Cambrex Bio Science Verviers, S.p.r.l., Verviers, Belgium). Cells were splitted after reaching confluence. For all experiments, cells from the third or fourth passage were used. HVECs purity was controlled by staining with a FITC-labelled antibody for human CD31 (Immunotools, Friesothye, Germany).
siRNA uptake studies
For siRNA uptake studies, cells were cultured in collagen coated plates without antibiotics. After reaching confluence, they were transfected with 100nM of FITC-labelled nonsense siRNA using a cationic lipid medium (Cellfectin™, Invitrogen GmbH, Karlsruhe, Germany). The amount of positive cells was determined by flow cytometry using a FACScan™ (Becton Dickinson GmbH).
siRNA transfection of human endothelial cells
Before siRNA transfection, cells were cultured in collagen-coated 12-well plates for FACS analysis, in six-well plates for real-time polymerase chain reaction (PCR) or in culture flasks for immunoblotting. After reaching confluence (70–80%), they were transfected with 100 nM of specific siRNA targeting ICAM1, VCAM1 or SELE, using 6.25 μg/ml Cellfectin (Invitrogen GmbH, Karlsruhe) for 2 hrs in serum-free medium, followed by the replacement of the supernatants with EGM-2 (without antibiotics).
Sequences for ICAM1 siRNA:
sense: 5′-GCCUCAGCACGUACCUCUAdTdT-3′ antisense: 5′-UAGAGGUACGUGCUGAGGCdTdT-3′
Sequences for VCAM1 siRNA:
sense: 5′-AAUGCAACUCUCACCUUAAdTdT-3′
antisense: 5′-UUAAGGUGAGAGUUGCAUUdTdT-3′
Sequences for SELE siRNA:
sense: 5′-GGUUGAAUGCACCACUCAAdTdT-3′
antisense: 3′-UUGAGUGGUGCAUUCAACCdTdG-3′
Twenty-four hours later, HVECs were stimulated with 2.5 ng/ml TNF (Immunotools, Friesoythe, Germany) for 14 hrs in case of ICAM1 stimulation, 14 hrs with 10 ng/ml TNF for VCAM1 and 14 hrs with 2.5 ng/ml for SELE detection. The cells were splitted in three groups for FACS analysis, immunoblotting and real-time reverse transcriptase-polymerase chain reaction (RT-PCR).
Flow cytometry
Cells were washed using EGM-2 media followed by incubation in 0.5% FCS. HVECs were stained for specific adhesion molecules with a PE-labelled human ICAM1 antibody (Becton Dickinson GmbH), a FITC-labelled VCAM1 antibody (Becton Dickinson GmbH) and a PE-Cy-5-labelled E-selectin antibody (Becton Dickinson GmbH). After washing and detaching, cells were fixed with 2.5% paraformaldehyde in PBS. FACS analyses (5000 cells/measurement) were performed with the same device described above and evaluated with the CellQuestPro-software (Becton Dickinson GmbH). The results shown represent the averages of three independent experiments. All experiments were performed with primary cells obtained from the same patient.
Immunoblotting
Total cellular protein was extracted from HVECs with the CelLytic MEM protein extraction kit (Sigma-Aldrich, Munich, Germany), containing a protease inhibitor cocktail. Lysates were mixed with two volumes of reducing (ICAM1, VCAM1) or non-reducing (SELE) loading buffer and incubated for 5 min. at 95°C. Protein equivalent to 17,000 cells (ICAM1, SELE) or 60,000 cells (VCAM1) was separated on 8% SDS-PAGE and then transferred by semi-dry blotting (15 V, 30 min.) to nitrocellulose membranes (Bio-Rad Laboratories, Munich). Immunodetection was performed using mouse monoclonal antibodies against human ICAM1 (clone 28, BD Biosciences, Heidelberg, Germany), VCAM1 (clone 1.G11B1, Chemicon, Hampshire, UK) and SELE (clone P2H3, Hölzel Diagnostika, Cologne, Germany). After incubation with the primary antibody, blots were washed three times with TBS and incubated with goat anti-mouse IgG antibody conjugated with alkaline-phosphatase (Coulter-Immunotech, Krefeld, Germany). Bands were visualized using Sigma Fast™ 5-bromo-4-chloro-3-indolyl phosphatase (BCIP)/nitro blue tetrazolium chloride (NBT)
Quantitative real-time RT-PCR
Total RNA from cultured HVEC was purified by Aurum™ total RNA mini kit (Bio-Rad Laboratories, Inc., Hercules, CA). The OD260/OD280 absorption ratio was >1.95. Consecutively, 200 ng of every RNA sample was reverse transcribed by iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer`s instructions. Standard curves were established for the primer pairs as described previously [18]. Primer design was done with the software ‘Primer3’[19] and Primer Premier 5 (PREMIER Biosoft International). All primers were synthesized by Operon Biotechnologies GmbH (Köln, Germany) and MWG-BIOTEC AG (Ebersberg, Germany). PCR was performed as described previously [20]. All PCR reactions contained IQ™SYBR®Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA), 400 nM forward and reverse primer and 2 ng of reverse transcribed RNA in a total volume of 15 μl. All PCR reactions were performed in triplicates. Normalized gene expression was calculated by the ▵C1 method using GAPDH as a reference.
Statistical procedure
The results are expressed as mean ± standard deviation. Statistical significance of differences between the groups was examined by two-sided Student's t-test assuming unequal variances. A P-value of less than 0.05 was considered to be significant.
Results
siRNA uptake tested by flow cytometry
FITC-labelled non-sense siRNAs (so-called scrambled-siRNA) were delivered to HVECs by liposomal transfection using Cellfectin™ (Invitrogen GmbH, Karlsruhe). In comparison to other transfection agents, Cellfectin™ showed a high transfection efficiency combined with a low cell toxicity.Nearly 85% of the cells harboured FITC-labelled siRNA 2 hrs after transfection as judged by FACS-analysis (data not shown).
Screening of different siRNA sequences
First steps within the silencing experiments were to find out from several siRNA sequences those with the highest knockdown potency. For SELE we used four and for VCAM1 five different sequences, respectively (Fig. 1). Sequence 1 (Fig. 1A) showed the highest knockdown for SELE and sequence 5 (Fig. 1B) the best results for VCAM1.Therefore, these siRNA sequences were used for all further experiments. For ICAM1 we used a previously tested sequence [17].
1.
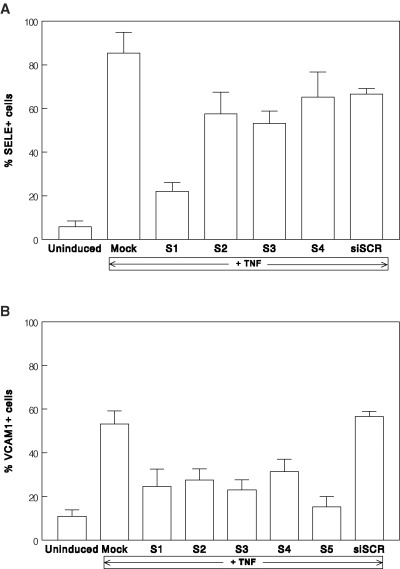
Effects of several siRNA sequences and TNF treatment on the fraction of cells positive for VCAM1 and SELE. Each bar represents the mean of three independent experiments, error bars show standard deviations. (A) Effects of four different sequences (S1–S4) siRNA targeting SELE: S1: sense: (GGU UGA AUG CAC CAC UCA A)dTdT antisense: (UUG AGU GGU GCA UUC AAC C)dTdG S2: sense: (UGG UAG AAU UGG AGA GUA A) dTdT antisense: (UUA CUC UCC AAU UCU ACC A) dTdG S3: sense: (CAG UGU GGU UUG UGU UUG A)dTdT antisense: (UCA AAC ACA AAC CAC ACU G) dGdT S4: sense: (CGG AAG CUA UGA CUU AUG A)dTdT antisense: (UCA UAA GUC AUA GCU UCC G)dTdG (B) Effects of five different sequences (S1–S5) siRNA targeting VCAM1: S1: sense: (GGA GGA UAC GGA UAU GAA A)dTdT antisense: (UUU CAU AUC CGU AUC CUC C)dAdA S2: sense: (GAG CUA AAU UAC ACA UUG A)dTdT antisense: (UCA AUG UGU AAU UUA GCU C)dGdG S3: sense: (CAU CUA CGC UGA CAA UGA A)dTdT antisense: (UUC AUU GCU AGC GUA GAU G)dTdG S4: sense: (CUC UAU AUU UAG AUU GUU A)dTdT antisense: (UAA CAA UCU AAA UAU AGA G)dTdG S5: sense: (AAU GCA ACU CUC ACC UUA A)dTdT antisense: (UUA AGG UGA GAG UUG CAU U)dTdT
Basal receptor expression and increase after TNF stimulation
HVEC showed a low basal expression of each adhesion molecule receptor in culture.
In the uninduced state, 17% of the cells were positive for VCAM1, followed by 15% for ICAM1 and 2.1% for SELE (Fig. 2A and B).
2.
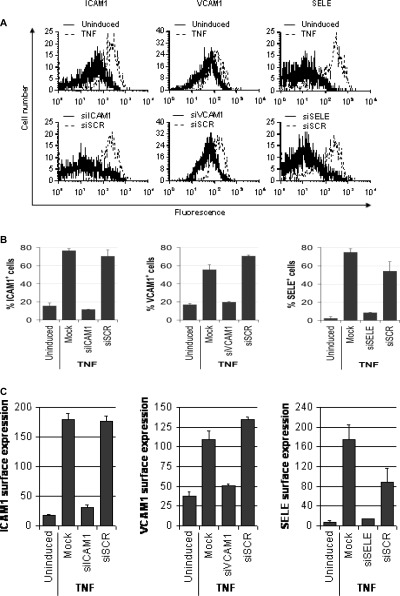
siRNA-mediated interference with adhesion molecule expression. (A) Histograms showing TNF stimulation and siRNA-mediated suppression of adhesion molecule expression. Top row: unstimulated and TNF-stimulated cells; bottom row: TNF-stimulated cells treated beforehand either with the indicated adhesion molecule siRNA or a scrambled control siRNA. Analysed adhesion molecules are indicated on the top. (B) Effects of siRNA and TNF treatment on the fraction of cells positive for ICAM1, VCAM1 and SELE. Each bar represents the mean of three independent experiments, error bars show standard deviations. (C). Effects of siRNA and TNF treatment on the total surface expression of ICAM1, VCAM1 and SELE. The y-axis reflects the geometrical mean of the adhesion molecule expression. Each bar represents the mean of three independent experiments, error bars show standard deviations. Uninduced, untreated cells; TNF, TNF-stimulated cells; Mock, TNF-stimulated untransfected cells; siICAM1, ICAM1 siRNA; siVCAM1, VCAM1 siRNA; siSELE, E-selectin siRNA.
Cytokine treatment and adhesion molecule expression
Incubation with TNF induced a substantial increase of the examined adhesion molecules. Cytokine stimulation of untransfected cells induced a five-fold, threefold and 40-fold increase of ICAM1, VCAM1 and SELE positive cells, and a 10-fold, threefold and 30-fold increase of the surface expression of the corresponding adhesion molecules, respectively (P < 0.01; Fig. 2).
siRNA-transfected cells
Transfection with siRNA strongly reduced the expression of the corresponding adhesion molecules to levels comparable to the uninduced state (Fig. 2A). Transfection with ICAM1 siRNA (siICAM1) decreased the fraction of ICAM1-positive cells sevenfold (P < 0.001; Fig. 2B), and the total ICAM1 surface expression sixfold (P < 0.001; Fig. 2C). PIn the case of VCAM1, the corresponding siRNA (siVCAM1) diminished the fraction of positive cells twofold (P < 0.01; Fig. 2b) and VCAM1 expression threefold (P < 0.01; Fig. 2C). Furthermore, application of the SELE siRNA (siSELE) reduced fraction of SELE-positive cells 10-fold and global SELE expression ninefold. Transfection with scrambled siRNA (siSCR) hardly affected the fractions of cells positive for ICAM1, VCAM1 or SELE. Furthermore, siSCR treatment interfered only marginally with total adhesion molecule expression, when compared to the active siRNAs siICAM1, siVCAM1 and siSELE (P<0.05; Fig. 2B and C).
Western blotting
The Western blot confirmed the FACS results for total adhesion molecule expression (Fig. 3). In all three groups (ICAM1, VCAM1 and SELE) only a weak staining could be observed in the untransfected and non-stimulated cells. After TNF treatment the protein detection was strong. The group of specific siRNA treated cells showed only slight bands after TNF treatment comparable to the group of untransfected and unstimulated cells. In contrast, application of the scramble control siRNA siSCR did not substantially affect adhesion protein levels, when compared to TNF-stimulated untransfected cells.
3.
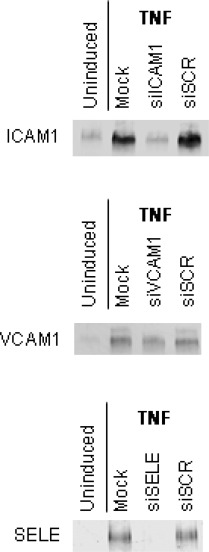
siRNA-dependent effects on adhesion protein levels. Adhesion protein levels were analysed by immunoblotting as described in ‘Material and methods’ section. Cell treatment is shown on top of each blot, the analysed adhesion proteins are indicated on the left. Uninduced, untreated cells; TNF, TNF-stimulated cells; Mock, TNF-stimulated untransfected cells; siICAM1, ICAM1 siRNA; siVCAM1, VCAM1 siRNA; siSELE, E-selectin siRNA.
Real-time PCR
To assess the effects of siRNA transfection on to ICAM1, VCAM1 and SELE mRNAs, transcript levels were analysed by semi-quantitative real-time RT-PCR. In general, the observed effects of siICAM1, siVCAM1 and siSELE on target protein expression were reflected by changes in the corresponding transcript levels (Fig. 4). All three siRNAs siICAM1, siVCAM1 and siSELE reduced the levels of their corresponding target mRNA 10-fold. In contrast, TNF stimulation of the other two adhesion molecule transcripts was affected only twofold to threefold. Furthermore, the control siRNA siSCR caused a twofold drop in SELE transcript levels and did not affect ICAM1 and VCAM1 levels.
4.
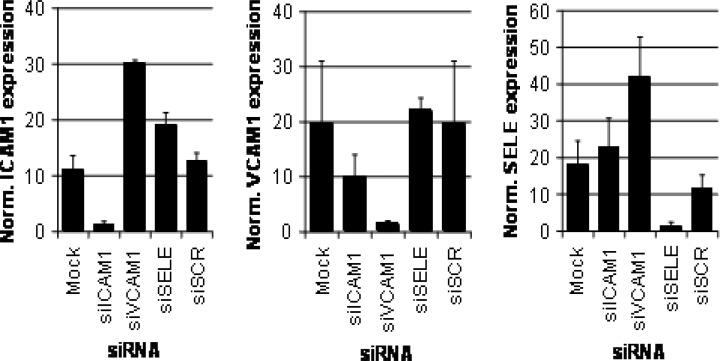
Effects of siRNA and TNF treatment on ICAM1, VCAM1 and SELE mRNA levels.Transcript levels were examined by real-time RT-PCR. Each bar represents the mean of at least two independent experiments, error bars show the range of variation in the case of two experiments and standard deviations in the case of three or more experiments. Mock, TNF-stimulated untransfected cells; siICAM1, ICAM1 siRNA; siVCAM1, VCAM1 siRNA; siSELE, E-selectin siRNA.
Discussion
The 2006 Nobel Prize in Physiology/Medicine was awarded to Andrew Fire and Craig Mello for their discovery of RNA interference, a mechanism for controlling the flow of genetic information. RNAi, which occurs naturally in plants and animals, allows a gene to be specifically ‘silenced’. This process can also be induced experimentally by injecting tailor-made short double-stranded RNA (siRNA) into cells to effect protein knockdown, giving scientists a method for specific silencing a target gene.
The method is now widely used as a basic ge netic tool and is worldwide under most intensive research regarding his potential as a therapeutic entity against numerous diseases itself, including the number one killer in the western countries: cardiovascular disease [21]. Despite medical and interventional procedures like PTCA, coronary artery bypass grafting remains a powerful tool to combat myocardial infarction and disease related symptoms in patients suffering from coronary artery disease. A long-term patency rate of the grafts is one of the pre-conditions for a definite success of surgery and is enormously affected by the material employed. The saphenous vein remains the most commonly used conduit for CABG. However, follow-up investigations demonstrated a reduced patency rate of venous grafts in comparison to arterial conduits. Up to 50% of the venous conduits are occluded within 10 years after implantation [22]. Arterial conduits are accompanied by a prolonged patency rate. However, their availability is reduced and harvesting is commonly associated with an increased operative trauma.
Furthermore vessel length is limited. Concerning these aspects cardiac surgeons are frequently faced with the problem to decide for the best therapy, especially for the best conduit. An excellent graft would combine the advantages of both graft materials: the long-term patency of the arterial conduit and the relatively easy availability of venous material.
Considering the main pathomechanisms leading to graft occlusion in venous bypass material the NIH remains to be the most important alteration after implantation. It alters the newly implanted graft in two ways. The NIH narrows the intraluminal space finally leading to a significant stenosis and occlusion. Furthermore, it facilitates the way for atherosclerotic changes resulting in severe graft alterations within months to years after surgery. One of the initial key factors for NIH is determined by adhesion molecules expression on the endothelial cells of the venous graft. They are modulating leukocyte-endothelium interactions leading to transendothelial migration of leukocytes, thereby stimulating smooth muscle cells for proliferation by a release of paracrine molecules as well as toxic radicals [2]. Regrettably, many different stimuli lead to adhesion molecule expression during CABG: cytokines or the mechanical trauma while harvesting the conduit [10, 23] as well as the hypoxic situation of the graft endothelium while harvesting and storage [24], for example. In summary, there is currently no possibility to reduce gene expression of adhesion molecules in venous bypass material in order to prevent a NIH.
Several efforts were made to inhibit NIH in venous grafts. Gene therapy is considered to be one potential alternative for further therapy strategies. But in most cases it is dependent on a viral carrier, which might be the limitation of the technology. Viral application is limited by a high prevalence of pre-existent immunity to the virus in human beings and a probably deleterious response of the host organism to transduced cells leading to an apoptotic mechanism [25]. Above all, this technology is limited in the practical use by the requirement to security safety level 2 laboratories.
Interfering with the DNA binding activity of the transcription factor E2F by edifoligide [26] transfected in a non-viral manner into the graft has failed to demonstrate any protective effect in preventing neointima thickening and occlusion of venous bypass grafts in recent studies.
Promising results were achieved by the treatment of venous grafts with rapamycin, an immunosuppressive agent, which exhibited a dose dependent reduction of neointima formation in venous bypass grafts [27]. However, further studies demonstrated an increased apoptosis rate in the vascular wall [28]. Therefore, up to now, no widely accepted therapy exists protecting venous bypass grafts against neointima thickening. Prevention continues to present a challenge [29].
This study deals with a novel approach to interfere with gene activation at the posttranscriptional level, namely the impairment of the common end point of the gene activation, the protein-response (e.g. adhesion molecule expression on cell surfaces). A new powerful tool is the application of short interfering RNA (siRNA). This technique promises a high potential for a post-transcriptional knock down of activated genes by degradation of the specific messenger RNA.
We induced adhesion molecule expression with TNF, a cytokine commonly used to induce inflammation-like alterations. TNF is a strong stimulus for adhesion molecule expression on different cell lines. Furthermore, its concentration is markedly elevated by procedures performed under extracorporeal circulatory support [9, 30].
Using fluorescently labelled siRNA in combination with a cationic lipid formulation, we showed that HVEC can be efficiently transfected with siRNA with low con-comitant toxicity. Transfection with siRNAs homologous to ICAM1, VCAM1 and SELE mRNA caused a substantial decrease in both target mRNA and protein levels. The minor effects exerted by the scrambled siRNA emphasizes the sequence specificity of this effect. However, one limitation of siRNA application could be an interference with other, unintended mRNA sequences in addition to the targeted one. In particular, siVCAM1 caused a further twofold to threefold increase in ICAM1 and SELE transcripts levels. The reasons for this off-target effect are currently not obvious, but may be related to the sequence of this siRNA. For a potential clinical application of siRNAs targeting the family of adhesion molecules off-target effects within this family are rather welcome than frightened. Probably, future therapeutics containing siRNA will include application of a cocktail of many different siRNAs to knock down several targets of interest. Nevertheless, we are aware that comprehensive investigations including DNA-chip analyses are obligatory to screen possible off-target effects before clinical application of this new technology.
The transfection of bypass conduits represents an ideal field of therapeutic siRNA application. The conduits can be transfected ex vivo, so that no systemic application of siRNA is required. This is a great advantage because efficient siRNA delivery in vivo in a clinically acceptable fashion is still a major challenge for therapeutic siRNA applications. Furthermore, gene suppression is restricted to the conduit and does not affect other tissues. Finally, with this liposomal technique the graft can be easily transfected inside the operating room without any further biological and genetical security requirements.
In conclusion, our results strongly suggest that the application of siRNA is a promising evolving technique probably resulting in a quality improvement of venous bypass grafts. Applying this novel tool in venous bypass material would result in a conduit, which combines the advantages of venous and arterial graft material, namely the prolonged patency rate comparable to arterial grafts and the unproblematic availability of venous material.
References
- 1.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 2.Shuhaiber JH, Evans AN, Massad MG, Geha AS. Mechanisms and future directions for prevention of vein graft failure in coronary bypass surgery. Eur J Cardiothorac Surg. 2002;22:387–96. doi: 10.1016/s1010-7940(02)00253-1. [DOI] [PubMed] [Google Scholar]
- 3.Asimakopoulos G, Taylor KM. Effects of cardiopulmonary bypass on leukocyte and endothelial adhesion molecules. Ann Thorac Surg. 1998;66:2135–44. doi: 10.1016/s0003-4975(98)00727-9. [DOI] [PubMed] [Google Scholar]
- 4.Chester AH, Morrison KJ, Yacoub MH. Expression of vascular adhesion molecules in saphenous vein coronary bypass grafts. Ann Thorac Surg. 1998;65:1685–9. doi: 10.1016/s0003-4975(98)00274-4. [DOI] [PubMed] [Google Scholar]
- 5.Schurmann G. Cell adhesion. Molecular principles and initial implications for surgery. Chirurg. 1997;68:477–87. doi: 10.1007/s001040050216. [DOI] [PubMed] [Google Scholar]
- 6.Harlan JM, Winn RK. Leukocyte-endothelial interactions: clinical trials of anti-adhesion therapy. Crit Care Med. 2002;30:S214–9. doi: 10.1097/00003246-200205001-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hope SA, Meredith IT. Cellular adhesion molecules and cardiovascular disease. Part I. Their expression and role in atherogenesis. Intern Med J. 2003;33:380–6. doi: 10.1046/j.1444-0903.2003.00378.x. [DOI] [PubMed] [Google Scholar]
- 8.Crook MF, Newby AC, Southgate KM. Expression of intercellular adhesion molecules in human saphenous veins: effects of inflammatory cytokines and neointima formation in culture. Atherosclerosis. 2000;150:33–41. doi: 10.1016/s0021-9150(99)00357-3. [DOI] [PubMed] [Google Scholar]
- 9.Wildhirt SM, Schulze C, Schulz C, Egi K, Brenner P, Mair H, Schutz A, Reichart B. Reduction of systemic and cardiac adhesion molecule expression after off-pump versus conventional coronary artery bypass grafting. Shock. 2001;16(Suppl 1):55–9. doi: 10.1097/00024382-200116001-00011. [DOI] [PubMed] [Google Scholar]
- 10.Chello M, Mastroroberto P, Frati G, Patti G, D'Ambrosio A, Di Sciascio G, Covino E. Pressure distension stimulates the expression of endothelial adhesion molecules in the human saphenous vein graft. Ann Thorac Surg. 2003;76:453–8. doi: 10.1016/s0003-4975(03)00433-8. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson EE, Karlof E, Lundmark K, Rotzius P, Hedin U, Xie X. Powerful inflammatory properties of large vein endothelium in vivo. Arterioscler Thromb Vasc Biol. 2005;25:723–8. doi: 10.1161/01.ATV.0000157578.51417.6f. [DOI] [PubMed] [Google Scholar]
- 12.Tuschl T. RNA interference and small interfering RNAs. Chembiochem. 2001;2:239–45. doi: 10.1002/1439-7633(20010401)2:4<239::AID-CBIC239>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 13.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 14.Ryther RC, Flynt AS, Phillips JA, Patton JG. siRNA therapeutics: big potential from small RNAs. Gene Ther. 2005;12:5–11. doi: 10.1038/sj.gt.3302356. [DOI] [PubMed] [Google Scholar]
- 15.Mahanthappa N. Translating RNA interference into therapies for human disease. Pharmacogenomics. 2005;6:879–83. doi: 10.2217/14622416.6.8.879. [DOI] [PubMed] [Google Scholar]
- 16.Sledz CA, Williams BR. RNA interference in biology and disease. Blood. 2005;106:787–94. doi: 10.1182/blood-2004-12-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker T, Wendel HP, Tetzloff L, Heidenreich O, Ziemer G. Suppression of ICAM-1 in human venous endothelial cells by small interfering RNAs. Eur J Cardiothorac Surg. 2005;28:816–20. doi: 10.1016/j.ejcts.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Simon P. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics. 2003;19:1439–40. doi: 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
- 19.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 20.Simon P, Feldkaemper M, Bitzer M, Ohngemach S, Schaeffel F. Early transcriptional changes of retinal and choroidal TGFbeta-2, RALDH-2, and ZENK following imposed positive and negative defocus in chickens. Mol Vis. 2004;10:588–97. [PubMed] [Google Scholar]
- 21.Agostini F, Dapas B, Farra R. Potential applications of small interfering RNAs in the cardiovascular field. Drugs Future. 2006;31:513–25. [Google Scholar]
- 22.Angelini GD, Jeremy JY. Towards the treatment of saphenous vein bypass graft failure—a perspective of the Bristol Heart Institute. Biorheology. 2002;39:491–9. [PubMed] [Google Scholar]
- 23.Brasil LA, Gomes WJ, Salomao R, Buffolo E. Inflammatory response after myocardial revascularization with or without cardiopulmonary bypass. Ann Thorac Surg. 1998;66:56–9. doi: 10.1016/s0003-4975(98)00181-7. [DOI] [PubMed] [Google Scholar]
- 24.Shreeniwas R, Schulman LL, Narasimhan M, McGregor CC, Marboe CC. Adhesion molecules (E-selectin and ICAM-1) in pulmonary allograft rejection. Chest. 1996;110:1143–9. doi: 10.1378/chest.110.5.1143. [DOI] [PubMed] [Google Scholar]
- 25.Akowuah EF, Sheridan PJ, Cooper GJ, Newman C. Preventing saphenous vein graft failure: does gene therapy have a role? Ann Thorac Surg. 2003;76:959–66. doi: 10.1016/s0003-4975(03)00505-8. [DOI] [PubMed] [Google Scholar]
- 26.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT PREVENTIV Investigators. Efficacy and safety of edi-foligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 27.Schachner T, Zou Y, Oberhuber A, Tzankov A, Mairinger T, Laufer G, Bonatti JO. Local application of rapamycin inhibits neointimal hyperplasia in experimental vein grafts. Ann Thorac Surg. 2004;77:1580–5. doi: 10.1016/j.athoracsur.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Schachner T, Oberhuber A, Zou Y, Tzankov A, Ott H, Laufer G, Bonatti J. Rapamycin treatment is associated with an increased apoptosis rate in experimental vein grafts. Eur J Cardiothorac Surg. 2005;27:302–6. doi: 10.1016/j.ejcts.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Schachner T. Pharmacologic inhibition of vein graft neointimal hyperplasia. J Thorac Cardiovasc Surg. 2006;131:1065–72. doi: 10.1016/j.jtcvs.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 30.Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232–44. doi: 10.1016/s1010-7940(01)01099-5. [DOI] [PubMed] [Google Scholar]


