Abstract
Red blood cell (RBC) membrane proteins undergo progressive pathological alterations during storage. In conditions of increased cellular stress, the cytoskeleton also sustains certain modifications. The hemoglobin (Hb) content and oxidative status of the RBC cytoskeletons as a function of the storage period remain unclear. The possible Hb content and oxidative alterations occurring in the cytoskeletons in the course of storage were monitored in six units, by means of electrophoresis, immunoblotting and protein carbonylation assays. A proportion of the ghost-bound Hb consists of non-reducible crosslinkings of probably oxidized(denatured Hb or hemichromes.The defective Hb-membrane association was strongly affected by the prolonged storage. A progressive accumulation of Hb monomers, multimers and high molecular weight aggregates to corresponding cytoskeletons were also evident. The oxidative index of the cytoskeletal proteins was found increased, signalizing oxidative modifications in spectrin and possibly other cytoskeletal proteins. The reported data corroborate the evidence for oxidative damage in membrane proteins with emphasis to the cytoskeletal components. They partially address the pathophysiological mechanisms underlying the RBC storage lesion, add some new insight in the field of RBC storage as a hemoglobin- and cytoskeleton-associated pathology and suggest the possible use of antioxidants in the units intended for transfusion.
Keywords: red blood cell cytoskeleton, storage in CPDA, membrane-bound hemoglobin, oxidation, carbonylation
Introduction
The membrane skeleton of red blood cells (RBCs) is a structure experimentally defined as the insoluble residue remaining after extraction of the red cell membrane with non-ionic detergents, like the Triton X-100 [1]. The red cell cytoskeleton is organized in a two-dimensional hexagonal network predominantly composed of spectrin, actin and protein 4.1R, along with adaptor proteins like ankyrin, protein 4.2, p55, protein 4.9, adducin, tropomyosin, myosin and tropo-modulin. The cytoskeleton is essential for the maintenance of normal shape, membrane deformability and mechanical stability that are critical for human RBCs to perform their function.This is demonstrated by various congenital abnormalities that result in hemolytic anemia. Furthermore, the cytoskeleton influences several cellular properties like the mobility of integral proteins and the distribution of phospholipids [2].
RBCs undergo major biochemical and mechanical changes during storage in anticoagulant solutions that are collectively referred to as “RBC storage lesion” and that could affect their after-transfusion performance [3]. Reflecting the storage-induced cellular stress, the membrane of stored RBCs is characterized by various modifications in lipid and protein compartments, such as lipid peroxidation and phosphatidylserine externalization [3–5], decline of critical antigenic markers [4, 5], protein aggregation [6], membrane-hemoglobin (Hb) association and oxidation [7]. Furthermore, increase of intracellular calcium and metabolic depletion were also observed [4]. Several of these factors that alter dramatically during storage are potent regulators of membrane skeletal organization. Not surprising, events that are progressively observed in storage include the defective deformability, surface area loss, spheroechinocyte transformation and microvesiculation of red cells that precede the hemolysis of a subpopulation of them [3, 4, 8].
In both in vitro and in vivo conditions of increased metabolic or oxidation stress similar to those found in storage [9], in the aging [10] and in various genetic hemolytic anemias [11], the RBC cytoskeleton sustains Hb-related modifications of pathophysiological significance. Consequently, studies of the Hb-association and oxidative status of the cytoskeletons of stored RBCs intended for transfusion are needed. In this context, our attention was drawn to the possible Hb content and oxidative alterations occurring in RBC cytoskeletal components in the course of storage in citrate-phosphate-dextrose-adenine (CPDA), which have not been described before. The results reported in this work represent the first evidence for a progressive oxidation of cytoskeletal proteins and accretion of denatured Hb proportional to the age of storage and suggest a possible role for these modifications in the phenomenon of RBC storage lesion as an Hb- and cytoskeleton-associated pathology.
Materials and methods
Collection and processing of blood
Whole blood (450 ± 50 ml) from six eligible young blood donors was collected in CPDA double-pack container systems. White blood cells reduction was not performed. After centrifugation most of the plasma was removed and packed RBCs were produced (final Hct 70%). The units were stored at 4°C for 43 days. The units were sampled in sequential time intervals of 2–7 days, beginning from the day of donation (day 0), for the whole storage period (35 days) and 1 week after expiration time. Each bag was fitted with a sterile sampling-site coupler (MacoPharma, Germany) and mixed gently. Aliquots of 6 ml of RBC concentrates were withdrawn through the sampling site at 4°C by use of a 19-gauge needle and attached syringe, in order to avoid any mechanical damage of the cells. As controls (C), ghost membrane samples of days 0–2 of the six packed RBC units, together with ghosts freshly prepared from blood samples of 10 healthy volunteers of matching sex and age, were used.
Preparation of membrane ghosts and cytoskeletons
White ghosts were prepared by hypotonic lysis of RBCs in phosphate buffer as previously described [12], with the addition of 0.3 mmol/L phenyl-methyl-sulfonyl-fluoride to the lysis buffer to inhibit protease activity. Membrane skeletons were prepared from the washed ghosts by Triton X-100 extraction as previously described [10]. Protein concentration was assayed using the Bradford protein assay reagent with bovine serum albumin as a standard (Bio-Rad, Germany).
Gel electrophoresis and immunoblotting analysis
Ghost membranes and membrane skeletons were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions (0.72 M 2-ME) using the discontinuous buffer system of Laemmli and homogenous 11% or 5–15% gradient slab gels. Equal amounts (10 μg) of protein were loaded per track of each gel. The proteins were electrophoretically transferred to nitrocellulose membranes and probed for Hb and cytoskeletal proteins using standard immunoblotting techniques as previously described [13]. The immunoblots were developed using an enhanced chemilumi-nescence (ECL) reagent kit (GE Healthcare, Amersham, UK). Several anti-human erythrocyte proteins-specific antibodies were used as internal loading controls. The anti-human antibodies used were as follows: polyclonal HbA (1/30.000; GR800GAP, Europa Bioproducts, Cambridge, UK), spectrin (1:5.000; S-1515, Sigma), actin (1:1.000; A-2066, Sigma, Germany) and protein 4.1R antiserum developed in rabbit (1:5.000, kindly provided by Prof. J. Delaunay, Service d' Hématologie, Hôpital de Bicetre, Le Kremlin-Bicetre, France). The horseradish peroxidase-conjugated secondary antibodies used were as follows: anti-rabbit (1:8.000; NA 934, Amersham-Pharmacia Biotechnology, Piscataway, NJ, USA), and anti-goat IgG (1:20.000; A-5420, Sigma). Bands were identified and quantified, as required, by scanning densitometry using the Gel Analyzer v.1.0 image-processing program (Biosure, Athens, Greece).
Estimation of cytoskeletal protein oxidative modification
The OxyBlot™ Protein Oxidation Detection Kit from Chemicon (S7150) was employed for the comparative quantitative analysis of the cytoskeleton protein carbonylation during the RBC storage period according to the manufacturer's instructions, with minor modifications as previously described [14]. Briefly, 2,4-dinitrophenylhy-drazine (DNPH) derivatization was carried out for 15 min on 3 μg of Triton-extracted cytoskeletal proteins. Following SDS-PAGE under reducing conditions and immunoblotting, the proteins were incubated with the anti-DNP antibody (specific to the DNP moiety of the proteins), and the blots were developed using a chemilumi-nescence detection system. To quantify the amount of oxidation and allow the comparison between the various samples, the oxidative index was used [15]. The latter is the ratio between densitometric values of the total oxyblot bands and either that stained with Red Ponceau S [15], or those probed with a red cell membrane reference protein-specific antibody, like spectrin or protein 4.1R [14]. The average of the six units examined was used to compare carbonylation levels of each day of storage.
Results
Presence of denatured/oxidized Hb species in the ghost membranes
To determine the nature of the Hb chains interacting with the membranes of stored RBCs and to estimate the proportion of the total membrane-associated Hb that is specifically bound to the cytoskeleton, the non-fractionated ghosts from stored RBCs were probed for Hb by immunoblotting techniques. As expected, the Hb was increasingly associated with the membrane in proportion to the duration of storage (Fig. 1A and C). Our study clearly demonstrated that a proportion of the membrane-bound total Hb consists of non-reducible crosslinkings of probably oxidized/denatured chains or hemichromes. Clearly viewed high molecular weight (MW) Hb-immunopositive bands representing these pathologic multimers were evident after 4 days of storage and were more prominent after a storage period of 22 days (Fig. 1A). Interestingly, the quantity of cross-linked forms of globin were found to increase dramatically as the cells approach the end of the storage period in comparison to the also increased amount of the globin monomers, demonstrating that the defective Hb-membrane association was strongly affected by the prolonged storage. The membrane-bound Hb aggregates are not obviously stabilized solely by disulfide bonds, but rather by non-reducible linkages (such as amides or free radical-generated adducts, e.g. bityro-sine), since they are partly resistant to reduction with beta mercaptoethanol. Despite the fact that the globin multimers were usually present in membranes enriched in globin monomers, in the globin-loaded control ghosts found occasionally, they were always undetectable (see control ghosts in Fig. 1A). On the contrary, stored RBC membrane ghosts with fewer globin monomers in comparison to some controls exhibited aberrant globin-assigned bands (see day 17 preparation in Fig. 1A).
1.
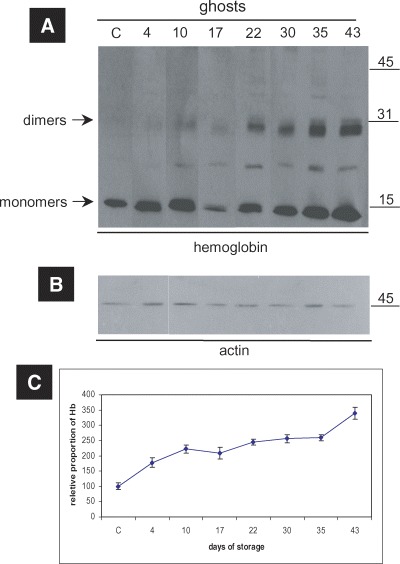
Globin oligomerization/crosslinking events on the membranes of RBCs stored in CPDA. (A) Western blot analysis of the ghosts of a representative donor performed with anti-human Hb polyclonal antibody.The duration of storage is indicated in days starting from blood donation. (B) Western blot analysis of ghosts against human actin (internal control). MW of the proteins is shown in kDa (right-hand side). (C) Densitometry analysis performed on ECL-developed films, regarding the relative proportion of Hb (monomers and oligomers) in the ghosts. The points in the graphs represent the average values among the six tested blood donors, after normalization to control values. Error bars demonstrate the standard deviation between the six donors.
The cytoskeletal component of Hb
To determine if the membrane-bound denatured Hb was associated with the membrane skeletons as well, we achieved a subsequent immunoblotting analysis of the Triton insoluble cytoskeletons after the fractionation of the stored RBC membrane samples with Triton X-100. The present findings showed, for the first time, that the same storage effect of Hb concerns the cytoskeletons of the stored cells as well, since a progressive accumulation of Hb, cross-linked multimers and high MW (< 260 kD) aggregates to them in amounts proportionate to the age of storage was revealed (Fig. 2). Of note, the significant increase in the Hb content of the cytoskeletons extracted from stored RBC ghosts was independent of their protein band 3 content (data not shown). In control membrane preparations (including the RBCs stored for 0 and 2 days in CPDA), the cytoskeletons do not contain detectable amounts of globin. After the extraction with Triton, the globin follows its physical linker, that is, the cytoplasmic site of band 3 [16], in the Triton-soluble fraction of the membrane (data not shown). The cytoskeletons of the six examined RBC units that had been stored even for long periods in CPDA did not exhibit any sign of severe proteolysis or fragmentation, as confirmed by immunoblotting analysis and probing for spectrin, actin and 4.1R proteins (Fig. 2).
2.
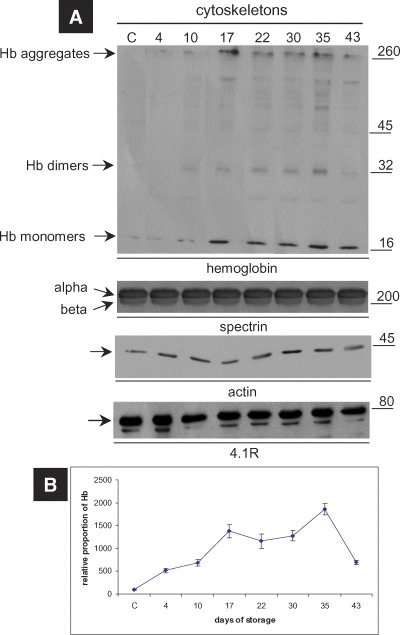
Immunoblotting analysis of the cytoskeletons extracted from the ghost membranes of RBCs stored in CPDA. (A) Western blot analysis of a representative cytoskeleton preparation performed with anti-human Hb and cytoskeletal proteins-specific antibodies. The cytoskeletons under storage accumulate Hb, non-reducible Hb oligomers and high MW aggregates.The duration of storage is indicated in days starting from blood donation. MW of the proteins is shown in kDa (right-hand side). (B) Densitometry analysis of the relative proportion of Hb in the cytoskeletons. The points in the graphs represent the average values and the error bars the standard deviation among the six blood donors tested, after normalization to control values.
Increased RBC cytoskeleton protein carbonylation
The production of carbonyl groups (aldehydes and ketones) on protein side chains (especially of Pro, Arg, Lys and Thr) is a common phenomenon of protein oxidation. The protein carbonyl groups were presently used as biomarkers of protein oxidative stress in order to clarify whether the storage affects the oxidative status of cytoskeletal proteins as well. As shown in Fig. 3, the oxidative index of the cytoskeletal proteins, namely the ratio of the total oxyblot signal (Fig. 3A), either to the immunoblotting signal of a reference protein (like spectrin) (Fig. 3B), or to the sum of the immunoblotted proteins that were revealed after the Red Ponceau staining of each sample (data not shown), was found increased after 10 days of RBC storage, which signalized oxidative modifications of spectrin and possibly other cytoskeletal proteins. The higher levels of cytoskeletal protein carbonyl groups were observed after 35 days of storage in CPDA (Fig. 3C). “Improved” amounts of cytoskeleton-bound Hb (Fig. 2B) and protein carbonylation levels (Fig. 3C) were observed in outdated samples (day 43).
3.
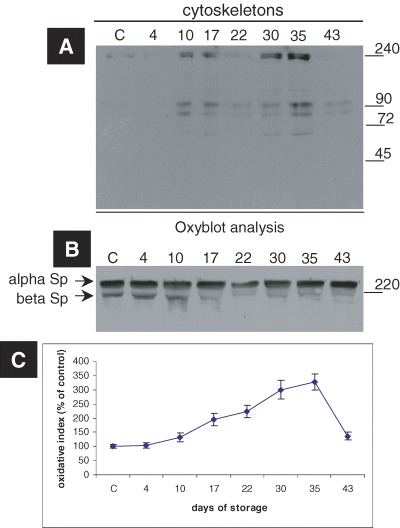
Increased RBC cytoskeletal protein carbonylation induced by storage in CPDA medium. (A) A representative oxyblot analysis, i.e. immunoblot analysis of cytoskeletons from a donor stained with the anti-DNP antibody. The duration of storage is indicated in days starting from blood donation. MW of the proteins is shown in kD (right-hand side). (B) Immunoblot of the cytoskeletons probed with anti-human spectrin antibody (internal loading control). (C) The estimation of the oxidative index of the cytoskeletal samples after normalization to control values. The points in the graphs represent the average values and the error bars the standard deviation among the six tested blood donors.
Discussion
Since the RBC membrane itself represents a primary target of the various pathological influences of storage, it is a major determinant of the clinical out-come of the storing defect. RBC deformability, an energy-dependent process that declines during ex vivo storage, is tightly connected to the structural and functional integrity of the RBC cytoskeleton. Storage-related loss of deformability may account for impaired microvascular oxygenation following transfusion [3]. Recent advances in the study of the RBC storage lesion support the oxidation of the membrane as an important parameter of the pathophysiology of stored cells [14]. The oxidation of the erythrocyte's Hb, a target of the free radicals, may drive some of the steps of this procedure. Previous studies have reported a significant increase in incubated Heinz bodies' formation after prolonged storage in CPDA-1 and an ATP-dependent decline in the antioxidant defense system that is correlated to the defected after-transfusion RBC survival [17]. The documentation of the possible alterations in the Hb content and the oxidation status of the cytoskeletons extracted from CPDA-stored RBCs was the goal of this study.
The finding of increased Hb association with the membrane was not unexpected. In many “stressed” red cells (e.g. ATP depletion, hereditary hemolytic anemias etc.), there can be an accumulation of various cytoplasmic proteins, especially Hb, along the cytoplasmic surface of the membrane [18]. Indeed, previous studies have shown, by means of SDS-PAGE, increased amounts of Hb in ghosts after pro-longed storage [7]. Our study demonstrated for the first time in human, that a part of the membrane-bound Hb in stored erythrocytes consists of non-reducible crosslinkings or hemichromes. Consequently, the amount of membrane-bound Hb in stored red cells is greater than the previously estimated by SDS-PAGE analysis [7], especially regarding the later days of storage and the outdated samples. Similar findings were previously reported in a canine RBC senescence model closely resembling human RBC senescence. The senescent RBCs were found to contain abruptly elevated membrane-bound denatured globin [19]. The oxidative stress induced by various hemolytic agents in RBCs was also associated with extensive binding of oxidized Hb and high MW Hb-immunopositive species to the membrane [20]. The possible oxidation of Hb to hemichromes was expected to exacerbate the influence of the membrane-bound globin to the membrane surface topography and to individual membrane interactions, like the attachment of the band 3 to the membrane skeleton [19]. Normal RBC function has been strongly related to the flexibility of the RBC membrane. The formation of akin Hb crosslinkings in beta thalassemia, sickle cell disease [21] and senescent RBCs [19] is dictated by increased oxidative stress and is positively correlated with increased cell density and membrane rigidity. Both of these distortions are also evident in RBC during storage and are only partially reversible [3].
The finding of cytoskeleton-bound Hb and especially of oxidized Hb species in stored RBCs is of patho-physiological significance. The role of the oxidative protein crosslinking and of cytoskeleton-associated Hb in the pathophysiology of thalassemia and other severe anemias in human [13, 22, 23], as well as during the in vivo red cell aging [10, 24], has been extensively explored. The progressive accumulation of oxidized Hb to the membrane skeleton not only disrupts the membrane organization and promotes localized oxidant damage in cytoskeletal proteins [23], but also threatens phospholipids oxidation and signals macrophage recognition and clearance of affected cells [24].The formation of spectrin-Hb crosslinking represents a kind of widespread oxidant damage in RBC membrane that occurs normally in the more senescent cells in vivo[25]. It can also be reproduced in vitro by treating normal RBCs with hydrogen peroxide [9]. The extent of spectrin-Hb complex formation is associated with progressive echinocyte formation, increased membrane rigidity and increased adherence and phagocytosis [9], as well as with decreased after-transfusion survival of damaged RBCs [26]. Consequently, our results raise the possibility that the RBC storage defect is related to the pathogenic effects of the cytoskeleton-bound Hb and its deleterious effects on membrane structural and functional integrity. In support of this view, it is interesting to notice that the accumulation of Hb to the cytoskeletons that was observed after the second week of storage (Fig. 2B) temporally coincides with the previously reported decrease of CD47 and increase of phosphatidylserine on the RBC membrane during storage [5]. Although our results, according to previous observations, did not imply a functionally intact cytoskeleton, there were no severe quantitative alterations in cytoskeletal proteins nor increased fragmentation of spectrin during storage in our samples, as has been previously described [6]. This is consistent with other studies documenting severe cytoskeletal defects during storage accompanied by normal membrane protein composition [7].
The detection of oxidized/denatured Hb and Hb crosslinkings in the membrane ghosts and cytoskeletons of stored RBCs is in agreement with recent works showing increased carbonylation of red cell membrane proteins with prolonged storage of RBCs in non-leukodepleted CPDA units [14] and further supports the concept of protein oxidation as a part of the storage lesion. The protein carbonylation pattern of the cytoskeletons presented here is similar to one of the ghosts previously described (14), although the increase in the oxidative index is sharper in the case of the cytoskeletal proteins, especially regarding the preparations of a longer storage time. The paradoxical observation of “improved” cytoskeleton-bound Hb and protein carbonylation levels in the outdated sam-ples (day 43) can be explained in part by the microvesiculation and the hemolysis of the most severely damaged cells. As the morphology of stored cells progressively changes from biconcave disks to speculated cells (echinocytes), spicules bud off from the echinocytes in the form of spectrin-free, but 4.1 and 4.2 proteins-containing, microvesicles which are filled by Hb [8, 27].
In conditions of increased RBC susceptibility to oxidative stress, like the ones standing in storage [6, 17], neither the Hb nor the cytoskeletal proteins could be unaffected. The increased carbonylation of cytoskeletal proteins was correlated with the extent of the oxidized Hb accumulation in the membrane and the cytoskeletons after prolonged storage in CPDA. The increased carbonylation has been associated with functional defected proteins and pathological pathways [28]. Consequently, it is not surprising that the oxida-tively injured spectrin detected in stored RBCs [7] seems to have an influential role in the stotage-related vesiculation [27].
In conclusion, the present study corroborates the evidence for the occurrence of oxidative damage in membrane proteins of stored RBCs, with emphasis to the cytoskeletal components. This evidence is substantiated by the observed increase in membrane-and cytoskeleton-bound denatured Hb, the crosslink-ing between spectrin and Hb and the increased oxida-tive index of isolated cytoskeletal components, that were all aggravated with prolonged storage in CPDA. Altogether the data reported here partially address the pathophysiological mechanisms underlying the RBC storage lesion, add some new insight in the field of RBC storage as a Hb- and cytoskeleton-associated pathology and suggest, according to the literature data, the possible use of antioxidants in the RBC stored units intended for transfusion. Under these conditions, our results could be used to screen antioxidant additives for their effectiveness to prevent premature RBC aging.
Acknowledgments
This study was partly supported by the “Empeirikeion Foundation” and the “Special Account for Research Grants of the University of Athens” to I. Papassideri. PhD student A. Kriebardis also thanks the “Hellenic State Scholarship Foundation” for the award of PhD fellowship.
References
- 1.Yu J, Fischman DA, Steck TL. Selective solubilization of proteins and phospholipids from red blood cell membranes by non-ionic detergents. J Supramol Struct. 1973;1:233–48. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]
- 2.Liu S-C, Derick LH. Molecular anatomy of the red blood cell membrane skeleton: structure-function relationships. Semin Hematol. 1992;29:231–43. [PubMed] [Google Scholar]
- 3.Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med. 2003;31:S687–97. doi: 10.1097/01.CCM.0000099349.17094.A3. [DOI] [PubMed] [Google Scholar]
- 4.Bessos H, Seghatchian J. Red cell storage lesion: the potential impact of storage-induced CD47 decline on immunomodulation and the survival of leucofiltered red cells. Trans Aph Sci. 2005;32:227–32. doi: 10.1016/j.transci.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Stewart A, Urbaniak S, Turner M, Bessos H. The application of a new quantitative assay for the monitoring of intefrin-associated protein CD47 on red blood cells during storage and comparison with the expression of CD47 and phosphatidylserine with flow cytometry. Transfusion. 2005;45:1496–503. doi: 10.1111/j.1537-2995.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 6.Messana I, Ferroni L, Misiti G, Girelli G, Pupella S, Castagnola M, Zappacosta B, Giardina B. Blood bank conditions and RBCs: the progressive loss of metabolic modulation. Transfusion. 2000;40:353–60. doi: 10.1046/j.1537-2995.2000.40030353.x. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe LC, Byrne AM, Lux SE. Molecular defect in the membrane skeleton of blood bank-stored red cells. Abnormal spectrin-protein 4.1-actin complex formation. J Clin Invest. 1986;78:1681–6. doi: 10.1172/JCI112762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenwalt TJ. The how and why of exocytic vesicles. Transfusion. 2006;46:143–52. doi: 10.1111/j.1537-2995.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 9.Snyder LM, Fortier NL, Trainor J, Leb L, Lubin B, Chiu D, Shohet S, Mohandas N. Effect of hydrogen peroxide exposure on normal human erythrocyte deformability, morphology, surface characteristics, and spectrin-hemoglobin cross-linking. J Clin Invest. 1985;76:1971–7. doi: 10.1172/JCI112196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller TJ, Jackson CW, Dockter ME, Morrison M. Membrane skeletal alterations during in vivo mouse red cell aging. Increase in the band 4.1a:4.1b ratio. J Clin Invest. 1987;79:492–9. doi: 10.1172/JCI112839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrier SL, Bunyaratvej A, Khuhapinant A, Fucharoen S, Aljurf M, Snyder LM, Keifer CR, Ma L, Mohandas N. The unusual pathobiology of hemoglobin Constant Spring red blood cells. Blood. 1997;89:1762–9. [PubMed] [Google Scholar]
- 12.Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963;100:119–30. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- 13.Antonelou MH, Papassideri IS, Karababa FJ, Stravopodis DJ, Loutradi A, Margaritis LH. Defective organization of the erythroid cell membrane in a novel case of congenital anemia. Blood Cells Mol Dis. 2003;30:43–54. doi: 10.1016/s1079-9796(03)00007-x. [DOI] [PubMed] [Google Scholar]
- 14.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. Membrane protein carbonylation in non-leukode-pleted CPDA-preserved red blood cells. Blood Cells Mol Dis. 2006;36:279–82. doi: 10.1016/j.bcmd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Dalla Libera L, Ravara B, Gobbo V, Betto DD, Germinario E, Angelini A, Vescovo G. Skeletal muscle myofibrillar protein oxidation in heart failure and the protective effect of Carvedilol. J Mol Cell Cardiol. 2005;38:803–7. doi: 10.1016/j.yjmcc.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Walder JA, Chatterje R, Steck TL, Low PS, Musso GF, Kaiser ET, Rogers PH, Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984;259:10238–46. [PubMed] [Google Scholar]
- 17.Lechant NA, Noble NA, Myrhe BA, Tanaka KR. Antioxidant metabolism during blood storage and its relationship to posttransfusion red cell survival. Am J Hematol. 1984;17:237–49. doi: 10.1002/ajh.2830170304. [DOI] [PubMed] [Google Scholar]
- 18.Allen DW, Cadman S, McCann SR, Finkel B. Increased membrane binding of erythrocyte catalase in hereditary spherocytosis and in metabolically stressed normal cells. Blood. 1977;49:113–23. [PubMed] [Google Scholar]
- 19.Rettig MP, Low PS, Gimm JA, Mohandas N, Wang J, Christian JA. Evaluation of biochemical changes during in vivo erythrocyte senescence in the dog. Blood. 1999;93:376–84. [PubMed] [Google Scholar]
- 20.McMillan DC, Powell CL, Bowman ZS, Morrow JD, Jollow DJ. Lipids versus proteins as major targets of pro-oxidant, direct-acting hemolytic agents. Toxicol Sci. 2005;88:274–83. doi: 10.1093/toxsci/kfi290. [DOI] [PubMed] [Google Scholar]
- 21.Fortier N, Snyder LM, Garver F, Kiefer C, McKenney J, Mohandas N. The relationship between in vivo generated hemoglobin skeletal protein complex and increased red cell membrane rigidity. Blood. 1988;71:1427–31. [PubMed] [Google Scholar]
- 22.Shinar E, Shalev O, Rachmilewitz EA, Schrier SL. Erythrocyte membrane skeleton abnormalities in severe β-thalassemia. Blood. 1987;70:158–64. [PubMed] [Google Scholar]
- 23.Advani R, Sorenson S, Shinar E, Lande W, Rachmilewitz E, Schrier SL. Characterization and comparison of the red blood cell membrane damage in severe human α- and β-thalassemia. Blood. 1992;79:1058–63. [PubMed] [Google Scholar]
- 24.Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol. 2000;7:113–6. doi: 10.1097/00062752-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Snyder LM, Leb L, Piotrowski J, Sauberman N, Liu SC, Fortier NL. Irreversible spectrin-haemoglobin crosslinking in vivo: a marker for red cell senescence. Br J Haematol. 1983;53:379–84. doi: 10.1111/j.1365-2141.1983.tb02038.x. [DOI] [PubMed] [Google Scholar]
- 26.McKenney J, Valeri CR, Mohandas N, Fortier N, Giorgio A, Snyder LM. Decreased in vivo survival of hydrogen peroxide-damaged baboon red blood cells. Blood. 1990;76:206–11. [PubMed] [Google Scholar]
- 27.Wagner GM, Chiu DT, Qju JH, Heath RH, Lubin BH. Spectrin oxidation correlates with membrane vasiculation in stored RBCs. Blood. 1987;69:1777–81. [PubMed] [Google Scholar]
- 28.Dalle-Donne I, Rossi R, Giustrarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chem Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]


