Abstract
Cardiovascular disease caused by atherosclerosis is the leading cause of death in the developed world. Narrowing of the vessel lumen, due to atherosclerotic plaque development or the rupturing of established plaques, interrupts normal blood flow leading to various morbidities such as myocardial infarction and stroke. In the clinic endovascular procedures such as angioplasty are commonly performed to reopen the lumen. However, these treatments inevitably damage the vessel wall as well as the vascular endothelium, triggering an excessive healing response and the development of a neointimal plaque that extends into the lumen causing vessel restenosis (re-narrowing). Restenosis remains a major cause of failure of endovascular treatments for atherosclerosis. Thus, preclinical animal models of restenosis are vitally important for investigating the pathophysiological mechanisms as well as translational approaches to vascular interventions. Among several murine experimental models, femoral artery wire injury is widely accepted as the most suitable for studies of post-angioplasty restenosis because it closely resembles the angioplasty procedure that injures both endothelium and vessel wall. However, many researchers have difficulty utilizing this model due to its high degree of technical difficulty. This is primarily because a metal wire needs to be inserted into the femoral artery, which is approximately three times thinner than the wire, to generate sufficient injury to induce prominent neointima. Here, we describe the essential surgical details to effectively overcome the major technical difficulties of this model. By following the presented procedures, performing the mouse femoral artery wire injury becomes easier. Once familiarized, the whole procedure can be completed within 20 min.
Keywords: Medicine, Issue 97, Restenosis, Neointimal Hyperplasia, Mouse Femoral Artery, Wire Injury Model
Introduction
In the era of expanded application of endovascular treatments for various cardiovascular diseases, restenosis after angioplasty is one of the major problems for patients undergoing such treatments. Damage to the vascular endothelium at the time of angioplasty, in concert with the atherosclerotic background, induces excessive smooth muscle cell proliferation in the medial layer, resulting in neointimal hyperplasia.1,2 A viable animal model that recapitulates post-angioplasty neointimal hyperplasia is, thus, important not only for the investigation of disease mechanisms but also for the development of effective therapeutics to treat this pathology.
Mice represent an excellent model animal to recapitulate neointimal hyperplasia for the following reasons: the genetic backgrounds of experimental mice are well established; a wide variety of genetically-modified strains are available;2 obtaining littermates of the same background is easy; and the cost of the animals is relatively low. Arterial ligation model and wire injury model are the two most common mouse models of mechanically-induced neointimal hyperplasia. The arterial ligation model is easy to create, but physiologically dissimilar to the actual angioplasty procedure. The wire injury model closely mimics actual angioplasty procedures but is technically difficult due to the small size of mouse arteries.2,3 Sata et al. first described a wire injury method for mouse femoral arteries based on the anatomical structure of the vasculature and the use of proper-sized flexible wire. Utilizing this technique, they succeeded in reproducibly inducing neointimal hyperplasia in various strains of mice.4
Although femoral wire injury is a well-established model, some of the technical aspects of the technique are highly challenging compared to other models such as ligation.5 The purpose of this paper is to describe our mouse wire injury model procedures in detail, which is a modified version of Sata’s original method. We have made two main modifications: 1) Looping only the arteries, and 2) No lidocaine use.
Protocol
NOTE: Ethics Statement: All procedures conform to the Guide for the Care and Use of Laboratory Animals (National Academies Press, 8th edition, 2011), and protocols approved by the Institutional Animal Care and Use Committee at the University of Wisconsin. All surgeries were performed under isoflurane anesthesia (through inhaling, flow rate 2 ml/min), and all efforts were made to minimize suffering. Animals were euthanized in a chamber gradually filled with CO2.
1. Induction of General Anesthesia and Skin Incision
Use male C57BL/6 mice with an age of 12–16 weeks and weight of 23–28 g. Anesthetize the animal by isoflurane through a closed-circuit anesthetic apparatus. Confirm proper anesthetization by pinching its toe. Apply some artificial tears ointment on eyes to prevent dryness.
Place the mouse in a supine position with the left hind limb slightly abducted and the knee joint slightly flexed. Shave the hair around the left upper thigh region and disinfect the skin with Chlorhexidine, applied three times using sterile cotton tipped applicators. Use a hot bead sterilizer to keep the instruments sterilized during the surgery.
Place a sterilized gauze drape over the left leg. Make a 1.5 cm straight, longitudinal skin incision on the left medial thigh from the knee joint to the groin (Figure 1A).
2. Exposure of Femoral Artery and Branches
NOTE: Figure 1B shows the gross vasculature anatomy in the upper thigh. The superficial femoral artery (SFA) can be observed through a dissecting microscope when the skin incision is properly made along the midline of the medial thigh.
Open up the thin fascia overlying the SFA with fine tweezers and then loop the SFA with 9-0 nylon suture. NOTE: In this process, the femoral nerve is easily put aside, but the adjacent femoral vein requires close attention because its wall is so thin that it easily perforates and bleeds. The key technique is to focus on the artery itself; as long as the dissection is carried out close to the artery, any accidental vein damage is avoidable.
Once the SFA is looped, hold the suture with mosquito forceps and pull it slightly downward. This maneuver makes further dissection easier, but to avoid damaging the artery do not pull too forcefully.
Continue dissecting the artery proximally to expose and loop the common femoral artery (CFA) with 9-0 nylon suture. NOTE: Usually, looping the CFA at a point distal to the epigastric artery (Figure 1B) is enough for wire insertion. Hold the CFA suture loop with mosquito forceps in the same way.
Pull the CFA suture loop upward and the SFA suture loop downward to uncover the bifurcation of SFA and deep femoral artery (DFA). The DFA, the major branch of the CFA, goes toward the thigh muscle (Figure 1B). NOTE: The wire will be inserted from the DFA later.
Loop the DFA with extra caution because the adjacent vein is tangled beneath the artery. In case of bleeding from the vein, a 30- to 60 sec simple compression with gauze is usually sufficient to produce hemostasis.
Once the DFA is looped with 9-0 nylon suture, make sure all three suture loops are at their correct positions (Figure 2A).
Proceed with the dissection of the DFA distally to ligate the artery at a point as far as possible from the bifurcation. Cut extra suture but leave a certain length so that the two ends can be held with mosquito forceps, which stabilize the DFA making the wire insertion easier.
3. Creating Wire Injury
- Insert Wire via DFA
- Strain the CFA, SFA, and DFA sutures to establish a temporary hemostat.
- Make a small hole in the DFA with microscissors. Insert the tips of the curved microtweezers to dilate the hole large enough for a wire with a 0.015 inch diameter (Figure 2B demonstrates the size difference between the artery and the wire).
- Before making a hole in the DFA, loosen the CFA suture loop and gently clamp/squeeze the DFA several times with dull-tipped microtweezers. This maneuver expands the arterial diameter remarkably to ease the wire insertion. Moistening the wire thoroughly with PBS is also important for a smooth insertion.
- When making a hole in the DFA, do not crop the arterial wall, but make a small v-shaped nip and pinch the flap with sharp-tipped microtweezers for manipulation.
- Insert the wire through the adequately-dilated hole on the DFA. Gently advance the wire toward the iliac artery while loosening the suture loop around the CFA. 5–8 mm is enough to cover all the CFA length in most cases (Figure 2C). Do not insert the wire forcefully as an arterial perforation usually results in loss of the mouse. Shortening the wire to about 15-mm in length helps with handling.
- Leave Wire inside for 1 Min
- After inserting the wire to the optimal length, leave it for 1 min to overstretch the arterial wall. Moisten the wire and arteries with PBS to prevent drying. Another 9-0 nylon suture can be placed around the DFA proximal to the wire-insertion point during this waiting time. This suture will be used later to close the hole on the DFA following removal of the wire.
- Retrieve Wire and Resume the Blood Flow
- Hold the wire with vessel cannulation forceps, and pull it back gently; the wire should be retrieved smoothly. To overcome any resistance, usually caused by adherence of the wire to the arterial wall, pulling back the wire with gentle rotation is helpful. Again, a forceful maneuver could result in animal loss.
- After retrieving the wire, ligate the DFA at the point of bifurcation with 9-0 nylon suture. Do not ligate too close to the bifurcation, which would jeopardize the blood outflow to the SFA.
- Loosen the SFA and CFA suture loops to resume the normal blood flow. Note that the CFA is well-expanded by wire insertion (Figure 2D).
4. Skin Closure and Postoperative Care
Control bleeds. Minor oozing can be stopped by simple compression; some major arterial bleeds will require stitches with a 11-0 nylon suture. These sutured arteries will eventually result in arterial occlusion.
Close the skin wound with skin staplers (usually 3–4 staples). Keep the wound area sterilized by applying Chlorhexidine surgical solution.
Apply post-surgery treatments. Control the pain of the mouse with subcutaneous injections of buprenorphine. Keep the mouse on the warming pad until it has regained sufficient consciousness to maintain sternal recumbency. House it in a clean cage without company of other animals until fully recovered.
Representative Results
Four weeks following surgery, harvest the wire-injured femoral artery and make a paraffin block specimen to analyze the neointima formation. Induce general anesthesia as described above. Open the chest immediately and insert a 20-gauge butterfly needle to the left ventricle. Connect the line to an infusion bottle. Perform perfusion fixation via drip infusion of PBS followed by 4 % paraformaldehyde (usually 10–20 ml, each). The infusion bottle is placed approximately 140 cm higher than the heart level so that the animal is perfused at physiological pressure.Wait for 20 min then take out the wire-injured CFA and fix it in 4 % paraformaldehyde overnight at 4 °C. Make a paraffin block of the specimen using standard histological techniques and prepare cross sections of 5-μm thickness for histological staining.
Hematoxylin-eosin staining will allow clear visualization of neointima formation inside the wire-injured femoral artery (Figure 3A, wire-injured femoral artery; 3B, uninjured femoral artery).Use a proper software to measure the lumen area (LA), the internal elastic lamina (IEL) area, and the external elastic lamina (EEL) area from cross section (Figure 3C). Calculate the intima area by subtracting lumen area from IEL area; calculate the media area by subtracting IEL area from EEL area. Intima area/media area ratio is used to compare the neointima formations between different experimental groups.
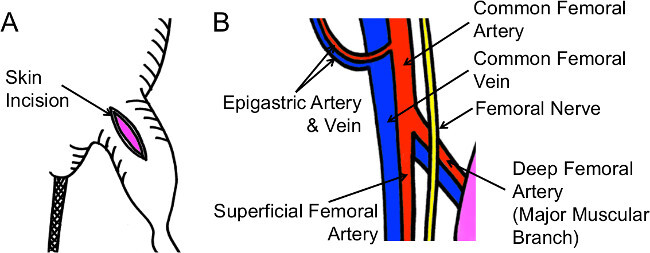 Figure 1: Schematic Illustrations of Skin Incision and Vasculature Anatomy. (A) An appropriate skin incision is about 1.5 cm in length, along the midline of medial upper thigh. (B) Find the superficial femoral artery first, the common femoral artery next, and finally, the deep femoral artery. Dissect each artery carefully without damaging the adjacent vein.
Figure 1: Schematic Illustrations of Skin Incision and Vasculature Anatomy. (A) An appropriate skin incision is about 1.5 cm in length, along the midline of medial upper thigh. (B) Find the superficial femoral artery first, the common femoral artery next, and finally, the deep femoral artery. Dissect each artery carefully without damaging the adjacent vein.
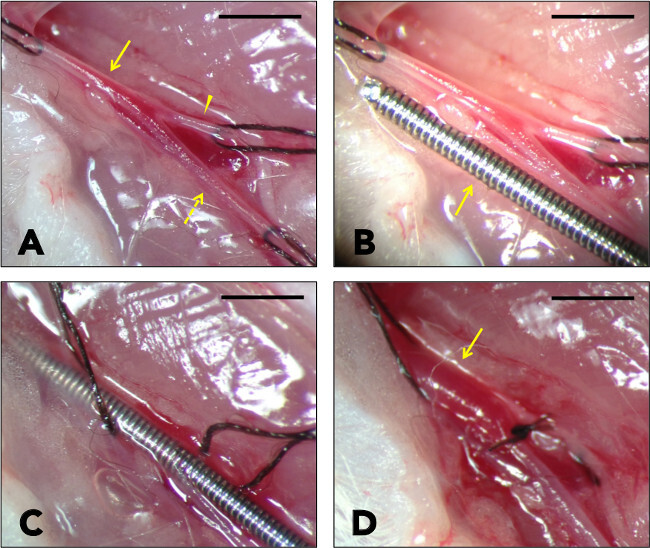 Figure 2: Wire Injury Procedure. (A) The common femoral artery (arrow), the superficial femoral artery (dotted arrow), and the deep femoral artery (arrow head) are exposed and looped with sutures. (B) Note the size difference between the arteries and the 0.015 inch wire. (C) The wire is inserted via the deep femoral artery. Keep the wire inside for 1 min to have the arterial wall overstretched. (D) Blood flow is resumed. The common femoral artery is well-expanded after wire injury (arrow). Bar indicates 1 mm.
Figure 2: Wire Injury Procedure. (A) The common femoral artery (arrow), the superficial femoral artery (dotted arrow), and the deep femoral artery (arrow head) are exposed and looped with sutures. (B) Note the size difference between the arteries and the 0.015 inch wire. (C) The wire is inserted via the deep femoral artery. Keep the wire inside for 1 min to have the arterial wall overstretched. (D) Blood flow is resumed. The common femoral artery is well-expanded after wire injury (arrow). Bar indicates 1 mm.
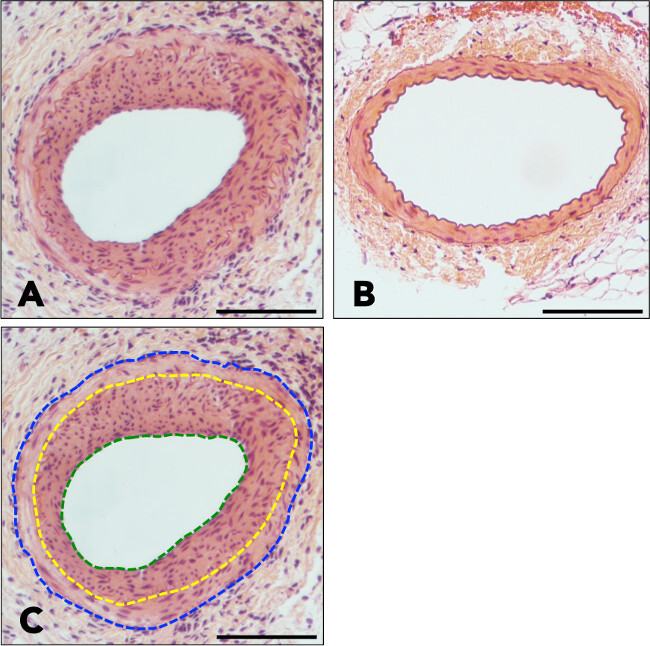
Figure 3: Hematoxylin-eosin staining of wire-injured and uninjured femoral arteries. (A) A cross-section of a wire-injured femoral artery reveals a thick, highly cellular neointima plaque. (B) A cross-section of an uninjured femoral artery demonstrates intact arterial wall structures without any neointima formation. (C) The green line delineates lumen area, the yellow line delineates internal elastic lamina, and the blue line delineates external elastic lamina. Bar indicates 100 μm.
Discussion
The wire injury procedure is applicable to all strains of mice as long as their anatomical structures are similar.4 In this paper, we used male C57BL/6 mice with an age of 12–16 weeks. As we have learned from our preliminary studies, the femoral arteries of mice younger than 10 weeks are often so small that wire insertion is quite challenging. On the other hand, wire insertion in mice older than 16 weeks is technically easier but tends to result in more variable neointimal hyperplasia. Female mice tend to incur less neointimal formation than males. Although the wire injury procedure can be performed on either side of the femoral arteries, we prefer the left side because of the anatomical accessibility for right-handed operators.
Among all the mouse models of post-angioplasty restenosis, arterial wire injury mostly closely mimics angioplasty by endothelial denudation and vessel wall overstreching.2,5,6 There are two major target arteries for wire injury: femoral arteries and carotid arteries. From a technical point of view, wire insertion is easier for carotid arteries because they are wider than femoral arteries. However, neointima formation tends to be less robust in carotid arteries than that in femoral arteries, likely due to less effective denudation. Some authors use a handmade device to create a more aggressive endothelial denudation in carotid arteries.7 On the other hand, the advantage of the femoral artery wire injury model is that it can be performed with homogeneously manufactured, commercially available wires to induce neointimal hyperplasia in a reproducible manner.
To make a successful wire injury in mouse femoral arteries, the key is to operate under a dissecting microscope with sufficient magnification and to use fine instruments – microtweezers and microscissors in particular. Microtweezers with sharp tips are useful to dissect out the tissues surrounding the artery whereas the ones with blunt tips are useful to place a suture loop around the artery and to dilate the wire entrance hole made by a sharp arteriotomy with microscissors.
An anatomical dissection of the artery is another key. Around the arterial adventitia there exists a thin plane where the dissection can be carried out easily and smoothly. Find the plane first, then proceed with the dissection. Also, try to dissect out the adjacent vein only at the site of suture loops to avoid vein damage, which is the major cause of bleeding in this procedure. Although in the original method the artery and vein are looped together,4 we find that looping artery only gives better control for both hemostasis and wire insertion.
Wire insertion is the most difficult part of this procedure. A 0.015-inch wire is roughly three times thicker than the DFA, the access artery. The key techniques here are: dilating the DFA itself before arteriotomy; making a small entrance hole which is gradually dilated with microtweezer tips; and avoiding forceful wire insertion – those techniques are detailed in the protocol section. While in the original method lidocaine is applied to dilate the artery before wire insertion4, we find it unnecessary.
Neointima formation after femoral artery wire injury progresses with time, peaks at 3–4 weeks after injury, and remains stable at 8 weeks.4,8 The authors usually wait for 4 weeks based on our preliminary data that the amount of neointima at 3 weeks is generally less than the amount at 4 weeks after wire injury (data not shown).
Interestingly, the responses to arterial injury differ between various mouse strains. According to several reports investigating differences between mouse strains after femoral or carotid artery injury, FVB/N mice display the highest amount of neointima formation, followed by 129/SvJ, C3H/HeJ, BALB/C, and C57BL/6, respectively.4,9,10 These characteristics should be considered when composing an experimental plan. The femoral injury model can also be utilized for investigation of inflammatory changes after vascular injury. Inflammatory cell infiltration was reported to increase significantly three days after injury.11
In conclusion, the wire injury model using mouse femoral arteries is particularly suitable to investigate post-angioplasty restenosis, and the process can be performed safely and easily using the method presented here. The whole procedure can be finished within 20 min once the techniques are fully familiarized.
Disclosures
The authors declare no competing financial interests.
Acknowledgments
This work was supported by a Wisconsin Partnership Program New Investigator Award (ID 2832), a National Heart, Lung, Blood Institute R01 Grant (HL-068673) and a T32 training Grant (HL-110853). We thank Dr. Melina Kibbe’s group at Northwestern University for providing helpful information.
References
- Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8(11):1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- Hui DY. Intimal hyperplasia in murine models. Curr Drug Targets. 2008;9(3):251–260. doi: 10.2174/138945008783755601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns GA, Avades TY. The mechanisms of coronary restenosis: insights from experimental models. Int J Exp Pathol. 2000;81(2):63–88. doi: 10.1046/j.1365-2613.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sata M, et al. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol Cell Cardiol. 2000;32(11):2097–2104. doi: 10.1006/jmcc.2000.1238. [DOI] [PubMed] [Google Scholar]
- Xu Q. Mouse models of arteriosclerosis: from arterial injuries to vascular grafts. Am J Pathol. 2004;165(1):1–10. doi: 10.1016/S0002-9440(10)63270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L, Meng Q, Ye M, Wang P, Xue G. STAT4 deficiency protects against neointima formation following arterial injury in mice. J Mol Cell Cardiol. 2014;74C:284–294. doi: 10.1016/j.yjmcc.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Zhu B, Kuhel DG, Witte DP, Hui DY. Apolipoprotein E inhibits neointimal hyperplasia after arterial injury in mice. Am J Pathol. 2000;157(6):1839–1848. doi: 10.1016/S0002-9440(10)64823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Duru EA, Davies MG. Effect of metabolic syndrome on the response to arterial injury. J Surg Res. 2014. [DOI] [PMC free article] [PubMed]
- Kuhel DG, Zhu B, Witte DP, Hui DY. Distinction in genetic determinants for injury-induced neointimal hyperplasia and diet-induced atherosclerosis in inbred mice. Arterioscler Thromb Vasc Biol. 2002;22(6):955–960. doi: 10.1161/01.atv.0000017994.77066.75. [DOI] [PubMed] [Google Scholar]
- Cooley BC. Mouse strain differential neointimal response in vein grafts and wire-injured arteries. Circ J. 2007;71(10):1649–1652. doi: 10.1253/circj.71.1649. [DOI] [PubMed] [Google Scholar]
- Ishigami N, et al. Deficiency of CuZn superoxide dismutase promotes inflammation and alters medial structure following vascular injury. J Atheroscler Thromb. 2011;18(11):1009–1017. doi: 10.5551/jat.9324. [DOI] [PubMed] [Google Scholar]


