Abstract
Strategies for attenuating decline in balance function with increasing age are predominantly focused on physical therapies including balance tasks and exercise. However, these approaches do not address the underlying causes of balance decline. Using mice, the impact of near infrared light (NIr) on the metabolism of cells in the vestibular sensory epithelium was assessed. Data collected shows that this simple and safe intervention may protect these vulnerable cells from the deleterious effects of natural aging. mRNA was extracted from the isolated peripheral vestibular sensory epithelium (crista ampullaris and utricular macula) and subsequently transcribed into a cDNA library. This library was then probed for the expression of ubiquitous antioxidant (SOD-1). Antioxidant gene expression was then used to quantify cellular metabolism. Using transcranial delivery of NIr in young (4 weeks) and older (8 - 9 months) mice, and a brief treatment regime (90 sec/day for 5 days), this work suggests NIr alone may be sufficient to improve mitochondrial function in the vestibular sensory epithelium. Since there are currently no available, affordable, non-invasive methods of therapy to improve vestibular hair cell function, the application of external NIr radiation provides a potential strategy to counteract the impact of aging on cellular metabolism inthe vestibular sensory epithelium.
Keywords: Molecular Biology, Issue 97, Vestibular, mitochondria, infrared, neuroprotection, aging, oxidative stress
Introduction
Declining balance performance and subsequent falls are common, and unfortunately often defining features of natural aging1. The impact of this decline can be both physical and social, and significantly reduces quality of life for older people. In response, physical therapies and rehabilitation have been the focus of research into falls but have not been associated with consistent reduction in the prevalence of repeated falls. At the same time, work investigating changes in the peripheral or central vestibular system (the system responsible for maintain balance) is scarce, and potential therapeutic strategies targeting these systems and the underlying causes of imbalance limited.
Recent work on age-associated neurodegenerative disorders including age-related macular degeneration2-4, Alzheimer’s disease models5-8, and Parkinson’s disease9-12 have shown neuroprotective effects of simple non-invasive application of near infrared (NIr) light. Further, in the vestibular system, NIr has been used to increase the activity of vestibular primary afferent neurons in vitro13. While the mechanism of NIr light is not well-understood, most studies using NIr have suggested that NIr stimulates mitochondria complex IV (cytochrome c oxidase)14-17 to facilitate cellular metabolism. In the vestibular sensory epithelium the subcuticular plate of type I hair cells is dense in mitochondria18 and as such may represent a site of action for therapeutic NIr treatment.
Here, a brief, non-invasive treatment regime of transcranially applied NIr that can be used to measure cellular metabolism (and by implication, mitochondrial function) in the mouse vestibular sensory epithelium is described. Also discussed is a preparation of the vestibular sensory epithelium and it is shown that NIr increases the expression of a ubiquitous anti-oxidant (superoxide dismutase 1) in the sensory epithelium – previously shown to be important for cochlea hair cell survival19.
Protocol
Ethics Statement: All procedures outlined below were approved by the University of Sydney Animal Ethics Committee.
1. Animals
NOTE: 1 and 8 - 9 month old mice (C57/BL6) were obtained from the Animal Resources Centre (Perth, Australia). Mice were housed in the Bosch Rodent Facility at the University of Sydney.
House mice in standard mouse cages on a 12/12 hr light/dark cycle with access to food and water ad libitum.
Divide mice in each age group into near infrared (NIr) treated, or sham treated (control) groups for comparison.
2. Near Infrared (NIr) Irradiation and Sham Treatment
Shave the fur on the head and neck region of the mouse with an electric razor as closely as possible to prevent quick regrowth of hair before the completion of the treatment regime. In order to maintain the pathogen-free status of the mice, use 70% ethanol to clean instruments between shaving animals and F10 veterinary disinfectant between animals from separate cages. Do this 2 - 3 days prior to the beginning of treatment so animals are not over-handled.
Restrain mouse by holding the proximal end of the tail and allow it to relax in the palm of one hand or bench top so as to minimize stress which could lead to false results.
Hold the NIr (670 nm) LED (light emitting diode) device 1 - 2 cm away from the exposed (shaved) area and switch on the device for 90 sec (Figure 1A). NOTE: The temperature change as a result of 90 sec exposure was measured as <0.2 °C in 100 ml of water.
Repeat steps 2.2 - 2.3 for the sham treatment group of animals but leave the device switched off (Figure 1B).
Repeat steps 2.2 - 2.3 for the NIr-blocked treatment group of animals but cover the device with aluminium foil.
Repeat steps 2.2 - 2.5 daily at approximate 24 hr intervals for 5 consecutive days.
Extract the vestibular sensory epithelium (3 cristae and 1 utricular macula) from both ears on the fifth day post-treatment (See Section 3 below).
3. Tissue Extraction20
Prepare 300 ml of a glycerol-based artificial cerebrospinal fluid (ACSF) consisting of (in mM): 26 NaHCO3, 11 glucose, 250 glycerol, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, and 2.5 CaCl2. Prior to the addition of CaCl2, gas the solution with carbogen (95% O2 and 5% CO2) to establish a pH of 7.4 and avoid calcium precipitation (cloudiness). Chill the solution in a -80 °C freezer for 45 min so that an ice slurry is formed.
Prepare the RNA isolation lysis buffer in labelled screw top microtubes (stops the popping of lids when tube pressure changes between freeze and thawing in liquid nitrogen) according to the instructions of the manufacturer or usual lab practices. Have liquid nitrogen ready in an aluminium dewar flask. NOTE: Use protective clothing and eyeware when handling liquid nitrogen. Ensure that liquid nitrogen is used in a well-ventilated room as the volume of its gas form is approximately 700 times that of its liquid form and can cause asphyxiation.
Deeply anaesthetize mice with ketamine (400 mg/kg) via an intra-peritoneal injection. Allow the hind-limb reflex to completely subside as indication that mice are fully anaesthetised.
Decapitate mice with sharp stainless steel scissors and make an incision along the sagittal skin of the skull using a razor blade (rounded #22). At this point and throughout steps 3.5 - 3.9, keep the cranial vault, brain, and underlying vestibular apparatus as cool as possible by regular application of ice-cold ACSF over the tissue.
Using the pointed arm of standard pattern scissors make a small incision in the skull at Lambda and cut along the sagittal suture.
Gently slide one arm of a shallow rongeurs beneath the parietal bone keeping the blade as close as possible to the inferior surface of the bone without dragging the brain. Secure and pull away the parietal bone laterally and the occipital bone posteriorly until the brain is exposed.
Use a small stainless steel spatula and lift the brain away from the anterior and middle cranial fossa to expose the vestibulocochlear nerve (CN VIII). Transect the nerve to prevent unnecessary tension on the primary afferent axons that directly innervate the vestibular hair cells.
Remove the brain in toto after transection of CN VIII.
Observe the bony labyrinth containing the cochlea and the peripheral vestibular organs in the middle cranial fossa. Make two small incisions beside each bony labyrinth and excise the entire structure by holding the anterior semicircular canal and pulling laterally.
Immediately immerse excised labyrinths in a dissecting dish containing ice-cold ACSF solution (as described in step 3.1) while continuously perfusing with carbogen.
- Under a stereomicroscope, hold down the labyrinth by the cochlea and secure it to the bottom of the dish with forceps.
- Use straight fine forceps to scratch a small opening in the bone above the anterior semicircular canal (SSC) ampulla.
- Gently enlarge this opening by reaching immediately below the bone and flicking outwards away from the ampulla. Exercise caution here to not push forceps into the opening and damage the fragile membranous labyrinth below. Continue in this fashion until the utricle, anterior and lateral ampullae are all exposed. If possible remove the posterior ampulla also.
Using fine forceps, gently lift the utricle and ampullae away from the bony labyrinth until they are completely detached. Where possible, hold them by their associated semicircular canals to avoid damaging the sensory epithelium. In some cases, the proximal part of the semicircular membranous duct may need to be cut with iris scissors to release the ampullae from the bone.
- Securely grasp the vestibular organs between the tip of forceps and place into the lysis buffer prepared earlier in step 3.2. Gently swirl the forceps around in the buffer to ensure vestibular organs have detached from the forceps. Check this is the case by bringing the forceps under the stereomicroscope.
- Screw on the microtubule lid and freeze the sample in liquid nitrogen immediately.
4. RNA Extraction and RT-PCR
Follow standard methods of messenger RNA (mRNA) extraction according to manufacturer’s instructions or preferred lab protocols. NOTE: A commercial kit that utilized carrier RNAs to help isolate small yields of mRNA was used in this protocol. Lower elution volumes can be used to increase final concentrations of mRNA. NOTE: Negative “no enzyme” controls (NECs) and “no DNA template” controls (NTCs) must also be completed to ensure validity of observed effects.
Apply standard methods of reverse transcription of mRNA to complementary DNA (cDNA) and amplification of target genes21-23.
Representative Results
To compare the impact of NIr treatment in young (4 weeks) and older (8 - 9 months) mice we measured the expression of antioxidant superoxide dismutase 1 (SOD-1) in young (n = 16) and older (n = 20) mice that were NIr-treated, sham-treated, or NIr-blocked. Figure 2 shows a significant increase in β-actin normalized SOD-1 expression of more than 2-fold in young NIr-treated animals compared to young sham-treated animals (p < 0.01) and young NIr-blocked animals (p < 0.01). Older NIr-treated animals also showed more than a 2-fold up-regulation of SOD-1 when compared with older NIr-blocked animals (p < 0.05).
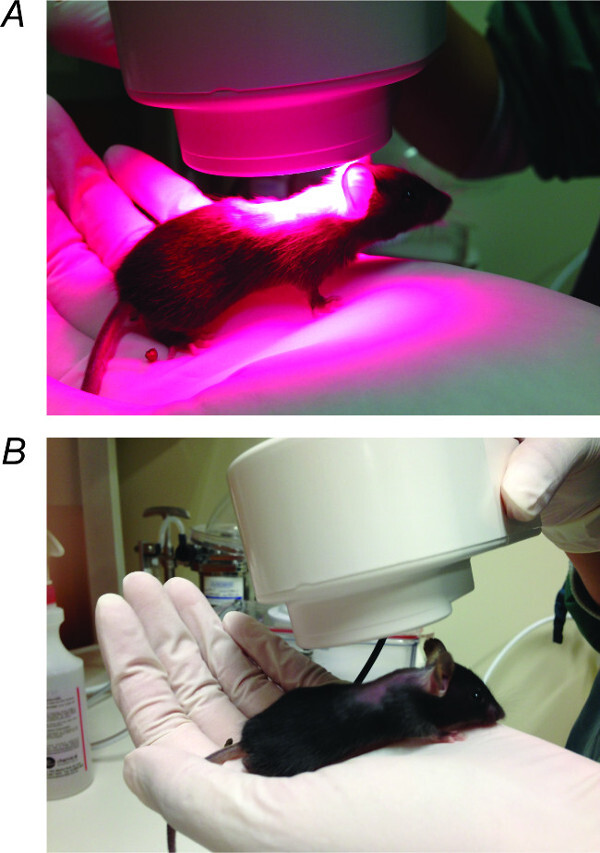 Figure 1. NIr Treatment. (A) NIr LED device held 1 - 2 cm above the shaved region on the head of the mouse for 90 sec per day for 5 consecutive days in irradiation treated mice. (B) NIr LED device held above sham-treated mice in the same way but with the device turned off for the duration of 90 sec. Please click here to view a larger version of this figure.
Figure 1. NIr Treatment. (A) NIr LED device held 1 - 2 cm above the shaved region on the head of the mouse for 90 sec per day for 5 consecutive days in irradiation treated mice. (B) NIr LED device held above sham-treated mice in the same way but with the device turned off for the duration of 90 sec. Please click here to view a larger version of this figure.
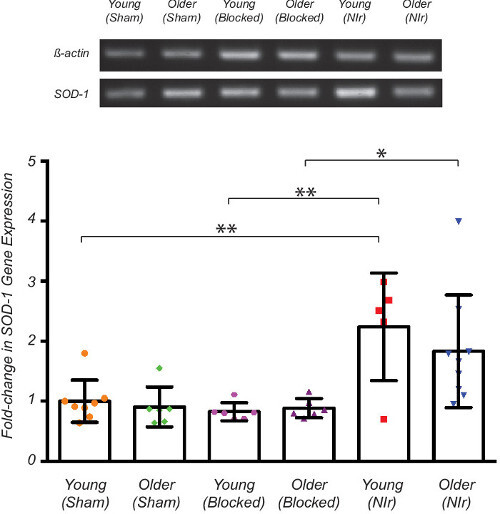 Figure 2. Analysis of SOD-1 gene expression after 5 consecutive days of NIr treatment (90 sec/day). The vestibular sensory epithelium was harvested on the fifth day and specific antioxidant gene probed for using RT-PCR. The SOD-1 gene was normalised to β-actin baseline and densitometry conducted using ImageJ v1.48. Significant up-regulation (p < 0.01) of SOD-1 expression was observed in young NIr-treated animals when compared with both young sham-treated and young NIr-blocked controls (4 weeks). Significant increase (p < 0.05) in SOD-1 expression was also observed in older NIr-treated animals when compared with older NIr-blocked animals (8 - 9 months). All graphs show the fold change of SOD-1 gene expression compared to the young sham-treated control. Data represents mean ± SD. All data were analysed using one-way ANOVA and statistical significance determined using Tukey’s multiple comparison post-hoc test. Please click here to view a larger version of this figure.
Figure 2. Analysis of SOD-1 gene expression after 5 consecutive days of NIr treatment (90 sec/day). The vestibular sensory epithelium was harvested on the fifth day and specific antioxidant gene probed for using RT-PCR. The SOD-1 gene was normalised to β-actin baseline and densitometry conducted using ImageJ v1.48. Significant up-regulation (p < 0.01) of SOD-1 expression was observed in young NIr-treated animals when compared with both young sham-treated and young NIr-blocked controls (4 weeks). Significant increase (p < 0.05) in SOD-1 expression was also observed in older NIr-treated animals when compared with older NIr-blocked animals (8 - 9 months). All graphs show the fold change of SOD-1 gene expression compared to the young sham-treated control. Data represents mean ± SD. All data were analysed using one-way ANOVA and statistical significance determined using Tukey’s multiple comparison post-hoc test. Please click here to view a larger version of this figure.
Discussion
The representative results described here show that brief transcranial delivery of NIr light (90 sec/day for 5 days) is sufficient to raise the levels of antioxidant expression in older mice when compared with sham-treated mice. While emitted heat could represent a source of mitochondrial and/or neuronal activation, as reported for rat vestibular afferents24 – our measurement of heat emitted by the NIr LED device was <0.2°C over 90 sec, and as such is unlikely to cause the changes described here. Further, unlike the report highlighted above, the NIr treatment used here was not applied directly to the sensory epithelium or vestibular afferent neurons, and as such is also unlikely to impact on thermo sensitive channels (e.g., TRPV4). Finally, previous work using the same NIr device in other brain regions have suggested that heat was not the source of observed differences7,9.
While these results show an up-regulation of cellular responses to age-associated oxidative stress on the genetic level, it is not clear whether these changes are further expressed at the protein level, or importantly, whether they are reflected in the overall balance performance of mice. In addition, the up-regulation is shown for a single ubiquitous anti-oxidant. It is likely that multiple markers of cellular metabolism are affected by this treatment regime. Further, since mRNA was extracted from the vestibular apparatus in toto (i.e., hair cells, supporting cells, vestibular afferents and epithelium all present) it is not possible to relate observed changes in cellular metabolism in response to NIr light treatment to a specific vestibular hair cell or primary afferent type. Importantly though, using the described preparation sufficient mRNA can be extracted from a single mouse to allow future correlative studies between cellular metabolism and behavioural analysis of balance performance.
The high intensity LED device used here emits approximately 5 J/cm2 per 90 sec treatment- totalling 25 J of energy over 5 days. While the advantage of the use of long wavelength light (including NIr) is penetrance, it cannot be assumed that the full 25 J of NIr light is delivered to the vestibular sensory epithelium. In the mouse, light must penetrate at least 1 mm through layers of soft tissue and bone that protect the peripheral vestibular apparatus and the brain. In humans this extends to centimetres. Thus the penetrance of light represents a limitation of the described technique in regards to translation of NIr light treatment strategies into the clinical setting. Recent work however has employed optical fibres to deliver NIr light to deep brain structures including the basal ganglia25, and sensory organs including the cochlea26. Based on this and the location of the peripheral vestibular apparatus adjacent to the cochlea (and clinical access to it via the mastoid process), it is feasible to suggest that in the human, direct stimulation of the vestibular sensory epithelium could be achieved through a small optical fibre triggered by an external device – akin to a cochlea implant27.
Several conditions can be modified in the protocol described above to adapt to the interest of the investigator. First, the wavelength used here (670 nm) can be extended to include longer wavelengths in the infrared range as previously reported in other sensory systems and animal models. Second, treatment regimes can also be varied depending on whether short-term or long-term response is in question. Here a very brief treatment regime was utilized, but this could be extended to longer durations, or even shorter durations to measure the dynamics of NIr induced changes.
The main challenge of this protocol is to maintain the viability of the tissue during tissue extraction. Given the time required to remove the bony labyrinth and excise the membranous labyrinth from it, it is critical to reduce metabolic breakdown. This is achieved by bathing the tissue in ice-cold ACSF throughout the entire procedure and perfusing this solution continuously with carbogen. In addition, at the end of the dissection procedure, further tissue degradation can be halted with the use of liquid nitrogen to flash freeze the extracted tissue. When appropriate, the frozen tissue can be thawed and mRNA extracted for further gene analysis.
Although the methods described here do not describe the complete impact of NIr light treatment on cellular metabolism in the vestibular sensory epithelium, further application of this strategy can be modified to include other age-associated markers of mitochondrial function28,29 and/ or cellular metabolic insults such as hypoxia30. Ultimately, the ability to describe the vestibular cellular metabolic profile of an individual mouse will allow the investigation of the correlations between balance performance and subcellular processes during ageing, and the impact of therapeutics including NIr treatment.
Disclosures
The authors declare they have no competing financial interests.
Acknowledgments
The authors wish to acknowledge Dr. Paul Witting and Ms. Genevieve Fong for their assistance with mRNA extraction and PCR, and the Garnett Passe and Rodney Williams Memorial Foundation for support.
References
- Agrawal Y, Carey JP, Della Santina, C C, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Archives of internal medicine. 2009;169:938–944. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- Bessho K, et al. Effect of subthreshold infrared laser treatment for drusen regression on macular autofluorescence in patients with age-related macular degeneration. Retina. 2005;25:981–988. doi: 10.1097/00006982-200512000-00005. [DOI] [PubMed] [Google Scholar]
- Olk RJ, et al. Therapeutic benefits of infrared (810-nm) diode laser macular grid photocoagulation in prophylactic treatment of nonexudative age-related macular degeneration: two-year results of a randomized pilot study. Ophthalmology. 1999;106:2082–2090. doi: 10.1016/S0161-6420(99)90487-6. [DOI] [PubMed] [Google Scholar]
- Rodanant N, et al. Predictors of drusen reduction after subthreshold infrared (810 nm) diode laser macular grid photocoagulation for nonexudative age-related macular degeneration. American journal of ophthalmology. 2002;134:577–585. doi: 10.1016/s0002-9394(02)01691-4. [DOI] [PubMed] [Google Scholar]
- De Taboada L, et al. Transcranial laser therapy attenuates amyloid-beta peptide neuropathology in amyloid-beta protein precursor transgenic mice. Journal of Alzheimer's disease : JAD. 2011;23:521–535. doi: 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- Grillo SL, Duggett NA, Ennaceur A, Chazot PL. Non-invasive infra-red therapy (1072 nm) reduces beta-amyloid protein levels in the brain of an Alzheimer's disease mouse model. TASTPM. Journal of photochemistry and photobiology. B, Biology. 2013;123:13–22. doi: 10.1016/j.jphotobiol.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Purushothuman S, Johnstone DM, Nandasena C, Mitrofanis J, Stone J. Photobiomodulation with near infrared light mitigates Alzheimer's disease-related pathology in cerebral cortex - evidence from two transgenic mouse models. Alzheimer's researc., & therapy. 2014;6:2. doi: 10.1186/alzrt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer AP, et al. 670 nm laser light and EGCG complementarily reduce amyloid-beta aggregates in human neuroblastoma cells: basis for treatment of Alzheimer's disease. Photomedicine and laser surgery. 2012;30:54–60. doi: 10.1089/pho.2011.3073. [DOI] [PubMed] [Google Scholar]
- Moro C, et al. Photobiomodulation preserves behaviour and midbrain dopaminergic cells from MPTP toxicity: evidence from two mouse strains. BMC neuroscience. 2013;14:40. doi: 10.1186/1471-2202-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples C, et al. Photobiomodulation enhances nigral dopaminergic cell survival in a chronic MPTP mouse model of Parkinson's disease. Parkinsonis., & related. 2012;18:469–476. doi: 10.1016/j.parkreldis.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Shaw VE, et al. Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. The Journal of comparative neurology. 2010;518:25–40. doi: 10.1002/cne.22207. [DOI] [PubMed] [Google Scholar]
- Ying R, Liang HL, Whelan HT, Eells JT, Wong-Riley MT. Pretreatment with near-infrared light via light-emitting diode provides added benefit against rotenone- and MPP+-induced neurotoxicity. Brain research. 2008;1243:167–173. doi: 10.1016/j.brainres.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajguru SM, et al. Infrared photostimulation of the crista ampullaris. The Journal of physiology. 2011;589:1283–1294. doi: 10.1113/jphysiol.2010.198333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, et al. The nuts and bolts of low-level laser (light) therapy. Annals of biomedical engineering. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet KD, et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomedicine and laser surgery. 2006;24:121–128. doi: 10.1089/pho.2006.24.121. [DOI] [PubMed] [Google Scholar]
- Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose-response : a publication of International Hormesis Society. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, Gonzalez-Lima F. Low-level light therapy of the eye and brain. Eye and brain. 2011;3:49–67. doi: 10.2147/EB.S21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranceanu F, et al. Striated organelle, a cytoskeletal structure positioned to modulate hair-cell transduction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4473–4478. doi: 10.1073/pnas.1101003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, et al. Separate and combined effects of Sod1 and Cdh23 mutations on age-related hearing loss and cochlear pathology in C57BL/6J mice. Hearing research. 2010;268:85–92. doi: 10.1016/j.heares.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung VW, Di Marco S, Lim R, Brichta AM, Camp AJ. An isolated semi-intact preparation of the mouse vestibular sensory epithelium for electrophysiology and high-resolution two-photon microscopy. Journal of visualized experiments : JoVE. 2013. p. e50471. [DOI] [PMC free article] [PubMed]
- Kirby J, Menzies FM, Cookson MR, Bushby K, Shaw PJ. Differential gene expression in a cell culture model of SOD1-related familial motor neurone disease. Human molecular genetics. 2002;11:2061–2075. doi: 10.1093/hmg/11.17.2061. [DOI] [PubMed] [Google Scholar]
- Parry SN, Ellis N, Li Z, Maitz P, Witting PK. Myoglobin induces oxidative stress and decreases endocytosis and monolayer permissiveness in cultured kidney epithelial cells without affecting viability. Kidney and Blood Pressure Research. 2008;31:16–28. doi: 10.1159/000112921. [DOI] [PubMed] [Google Scholar]
- Saee-Rad S, et al. Analysis of superoxide dismutase 1, dual-specificity phosphatase 1, and transforming growth factor, beta 1 genes expression in keratoconic and non-keratoconic corneas. Molecular vision. 2013;19:2501–2507. [PMC free article] [PubMed] [Google Scholar]
- Albert ES, et al. TRPV4 channels mediate the infrared laser-evoked response in sensory neurons. Journal of neurophysiology. 2012;107:3227–3234. doi: 10.1152/jn.00424.2011. [DOI] [PubMed] [Google Scholar]
- Moro C, et al. Photobiomodulation inside the brain: a novel method of applying near-infrared light intracranially and its impact on dopaminergic cell survival in MPTP-treated mice. Journal of neuroscience. 2014;120:670–683. doi: 10.3171/2013.9.JNS13423. [DOI] [PubMed] [Google Scholar]
- Moreno LE, et al. Infrared neural stimulation: beam path in the guinea pig cochlea. Hearing research. 2011;282:289–302. doi: 10.1016/j.heares.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys IS. A red thread as a guide in the vestibular labyrinth. The Journal of physiology. 2011;589:1241–1241. doi: 10.1113/jphysiol.2011.206763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, et al. Mitochondrial Dysfunction during Brain Aging: Role of Oxidative Stress and Modulation by Antioxidant Supplementation. Aging and disease. 2011;2:242–256. [PMC free article] [PubMed] [Google Scholar]
- Petrosillo G, De Benedictis V, Ruggiero FM, Paradies G. Decline in cytochrome c oxidase activity in rat-brain mitochondria with aging. Role of peroxidized cardiolipin and beneficial effect of melatonin. Journal of bioenergetics and biomembranes. 2013;45:431–440. doi: 10.1007/s10863-013-9505-0. [DOI] [PubMed] [Google Scholar]
- Zhu H, Sun A, Zou Y, Ge J. Inducible metabolic adaptation promotes mesenchymal stem cell therapy for ischemia: a hypoxia-induced and glycogen-based energy prestorage strategy. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:870–876. doi: 10.1161/ATVBAHA.114.303194. [DOI] [PubMed] [Google Scholar]


