Abstract
Seven membrane-spanning, or G protein-coupled receptors were originally thought to act through het-erotrimeric G proteins that in turn activate intracellular enzymes or ion channels, creating relatively simple, linear signalling pathways. Although this basic model remains true in that this family does act via a relatively small number of G proteins, these signalling systems are considerably more complex because the receptors interact with or are located near additional proteins that are often unique to a receptor or subset of receptors. These additional proteins give receptors their unique signalling ‘personalities’. The extracellular Ca-sensing receptor (CaR) signals via Gαi, Gαq and Gα12/13, but its effects in vivo demonstrate that the signalling pathways controlled by these subunits are not sufficient to explain all its biologic effects. Additional structural or signalling proteins that interact with the CaR may explain its behaviour more fully. Although the CaR is less well studied in this respect than other receptors, several CaR-interacting proteins such as filamin, a potential scaffolding protein, receptor activity modifying proteins (RAMPs) and potassium channels may contribute to the unique characteristics of the CaR. The CaR also appears to interact with additional proteins common to other G protein-coupled receptors such as arrestins, G protein receptor kinases, protein kinase C, caveolin and proteins in the ubiquitination pathway. These proteins probably represent a few initial members of CaR-based signalling complex. These and other proteins may not all be associated with the CaR in all tissues, but they form the basis for understanding the complete nature of CaR signalling.
Keywords: calcium-sensing receptor, G protein-coupled receptor, interaction, scaffold, filamin channel, RAMP, cell signalling
Introduction
The calcium-sensing receptor (CaR), a G protein-coupled receptor that signals through Gαi, Gαq and sometimes Gα12/13 pathways, is best known and understood for its role in regulating the secretion and synthesis of parathyroid hormone in response to extracellular Ca in the parathyroid glands [1]. Like many other G protein-coupled receptors, the CaR signals through a defined set G proteins, but also interacts with other proteins that probably give it its unique ‘signalling personality’. This receptor was first cloned as an extracellular Ca sensor using a xeno-pus oocyte expression system. Since that time, it has been detected in most epithelial and mesenchymal cell types including renal and gastrointestinal epithe-lium, endothelial cells, keratinocytes, breast tissue, osteoblasts, cardiac myocytes and cells of the central and peripheral nervous systems where it can be expressed in non-polarized cells and polarized cells on either the apical or basolateral membrane [2]. The CaR appears to have distinct functions in these different cell types, although the functions are not precisely defined. For example, the CaR can either stimulate cell division (rat-1 cells) or inhibit division and promote differentiation (colonocytes or parathyroid cells) [3–6]. Additionally, its biologic effects in tissues such as the kidney or parathyroid glands cannot be explained completely on the basis of known second messenger signalling effects [1, 7].
The original model of signalling by G protein-dependent receptors was relatively simple. The receptors and G proteins through which the intracellular second messenger systems are activated are attached to the plasma membrane of the cell. These proteins could be relatively free in the membrane or loosely associated with each other. Upon activation of a receptor, the protein interactions change, G proteins are activated, which in turn activate effector molecules such as enzymes or ion channels, to generate intracellular signals (Fig. 1). As techniques to identify protein–protein interactions have been developed and as more precise analysis of cell signalling has become possible, the inadequacy of the original model has become clear through demonstration that G protein-coupled receptors interact with many intracellular proteins in addition to G proteins. These interacting proteins include RGS proteins (Regulator of G protein Signalling), scaffolding and structural proteins, ion channels, additional signalling proteins, chaperone and trafficking proteins and others that may not have defined functions yet [8–11]. Receptors that act through similar sets of G proteins may have different signalling or biologic effects in different regions of a cell and in different cells. This finding suggests that they may have common activities based on the G proteins with which they interact, but that they may also have distinct functions based on the unique sets of other proteins with which they interact as well as their unique locations within a cell. Although no one receptor in a native tissue has been characterized completely, enough work has been done with different receptors including the 2-, -adrenergic and metabotropic glutamate receptors in various experimental systems to indicate that such a scenario is not only plausible, but likely (Fig. 1) [9–11].
1.
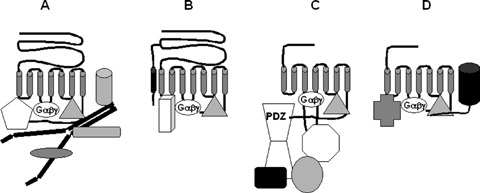
Four scenarios in which all G protein-coupled receptors interact with Gαβγ subunits that regulate standard second messenger generation and other common proteins (grey triangle, e.g. arrestin). They also interact with additional different proteins that give them unique signalling personalities. In A, the receptor interacts with filamin that itself binds additional proteins. The receptor shown in B interacts with an accessory protein (e.g. a RAMP) as well as another unique protein. The receptor shown in C has a long third intracellular loop that interacts with a unique protein (octagon), and a long C-terminus with a PDZ domain that binds a PDZ protein that itself brings additional proteins into the complex. In the scenario shown in D, the C-terminus of another membrane protein (e.g. a channel) interacts with the C-terminus of the receptor, and the receptor binds an additional protein.
Signalling by the CaR
Most work on the signalling pathways controlled by the CaR has focused on traditional G protein-coupled pathways, Gαi, Gαq, and in some cases Gα12 or Gα13[1, 12–16] (Fig. 2). Through Gαi, the CaR inhibits adenylyl cyclase and activates extracellular signal-regulated kinase (ERK), through Gαq it activates phos-pholipase C, increases Cai and DAG (diacyl glycerol) levels, and activates phospholipase A2, and through Gα12/13, it activates Rho and phospholipase D [17]. Although the full physiologic significance is not understood, activation of the CaR initiates Ca oscillations via a Gαq-dependent mechanism that when prolonged or forming a plateau, inhibit adenylyl cyclase activity co-ordinately with Gαi activation [14]. This system serves as an active turn-off system for cAMP signals and depends on the forms of adenylyl cyclase expressed in different tissues. In the intestine, the CaR inhibits cholera toxin and E. coli heat stable enterotox-in-stimulated fluid secretion via activation of cyclic nucleotide phosphodiesterases and inhibition of NKCC1 (sodium, potassium, 2Cl transporter-1) activity [18]. In keeping with studies of other G protein-coupled receptors, the CaR transactivates, the epidermal growth factor receptor presumably via a matrix metal-loproteinase [19, 20]. In different regions of the nephron and gastro-intestinal tract, the CaR is expressed on apical or basolateral membranes of epithelial cells where it is likely to come into contact with distinct sets of proteins that should give the CaR different signalling characteristics and biologic effects. Similarly, the CaR is expressed in many different cell types with different functions, so its signalling and biologic functions could vary from cell type to cell type.
2.
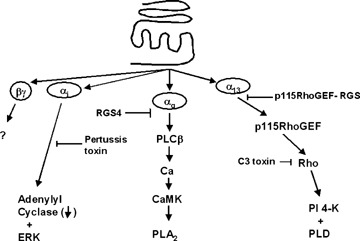
A schematic diagram of the principal second messenger signalling pathways that have been described for the CaR. Most of these studies pathways were identified in heterologous expression systems, and may not all exist in all cells where the CaR is expressed at all times.
Distinct effects of angiotensin II and Ca receptors
A good example of the unique signalling and physiologic activities of the CaR is found in the distal nephron of the kidney where activation of the CaR results in significant Na, K, Ca, Mg, Cl and H2O loss. Although the CaR acts via Gαi, Gαq and Gα12/13, its biologic effects in the distal nephron cannot be explained solely on this basis. A number of G protein-coupled receptors that also act via Gαi, Gαq and Gα12/13 are also expressed in the distal nephron, including those for angiotensin II (AT1), bradykinin (B2) and endothelin (ETB). All of these receptors inhibit cAMP production, stimulate phospholipase C (with increases in Cai and protein kinase C activity, and decreases in PIP2 levels), and stimulate phop-spholipase A2 (with increases in 20-HETE and other arachidonic acid metabolites). In all studies and experimental systems, activation of the CaR results in physiologically significant inhibition of Na transport in the distal nephron, while activation of the AT1 receptor (presumably AT1) can stimulate or inhibit transport depending on the concentration, experimental system and study and the effects are minor [21–31, 31]. These differences between the responses of the distal nephron to Ca and AII indicate that the two receptors have distinct effects on cells despite the fact that they stimulate production of the same second messengers.
The unique signalling characteristics and biologic effects of the CaR may be explained by its interactions with proteins in addition to G protein subunits (Table 1). G protein-coupled receptors have four domains that are exposed to the intracellular space and that are available to interact with other proteins, three intracellular loops that connect transmembrane (TM) domains 1 and 2 (intracellular loop 1 or IC1), TM domains 3 and 4 (IC2), TM domains 5 and 6 (IC3) and the C-terminus. The intracellular loops, particularly IC2 and 3 interact with G proteins as well as other proteins including proteins involved in signalling, such as the arrestins or spinophilin [10]. In G protein-coupled receptors, the size of the loops and C-termini are highly variable. In the CaR, IC1 and 3 are 14 AA, IC2 is 24 AA, all relatively short and the C-terminus is 220 AA, relatively long. Because of its size, the C-terminus is easier to use for techniques to identify interacting protein such as yeast two hybrid cloning. For the CaR, as well as the better-characterized metabotropic glutamate receptors (both have a similar structures), interacting proteins have generally been identified using yeast two hybrid assays with tissue-specific cDNA libraries and the C-terminus as bait. The interactions are verified with co-immunopre-cipitation and the functional interactions are characterized in relatively generic expression systems, such as HEK-293 cells. Although these CaR-interacting proteins have not been characterized completely in native tissue, enough data exist to believe that the interactions are real, and that the biologic functions they contribute are also likely to be real. Interactions with other proteins have been identified presumptively based on the fact that they are receptor interacting proteins and affect the behaviour of the CaR. A number of these interacting proteins such as -arrestin or receptor kinases are common to many other G protein-coupled receptors, or are involved in trafficking and degradation, and so are unlikely to be responsible for distinct signalling features of the CaR. Consequently, we will focus on three sets of proteins, receptor activity modifying proteins (RAMPs), filamin, a potential scaffolding protein, and two inwardly rectifying potassium channels, Kir4.1 and Kir4.2 that through their interactions with the CaR, could begin to explain some of its unique signalling characteristics.
1.
Proteins with which the CaR interacts. Table 1 lists the CaR interacting proteins that have been described so far alphabetically in the first column. The second column lists the function of the interacting protein. The third column lists the experimental basis for the interaction of a protein with the CaR, Y2H for yeast two hybrid, Co-IP for co-immunoprecipitation, GST for GST pull-down assays and functional if expression or mutagenesis studies indicate that the interaction is functionally significant. The fourth column (CaR domain) identifies the region of the CaR that interacts with the protein shown at left. The fifth column states whether co-localization studies of the CaR were performed with the interacting protein. Native means that studies were performed in native tissue, and Het means that studies were performed in heterologous expression systems. The sixth column provides essential references for the observations
| Interacting Protein | Function | Interaction | CaR Domain | Co-localization | Reference |
|---|---|---|---|---|---|
| AMSH | Trafficking/de Ub enzyme | Y2H, GST | C-term | (80) | |
| β-Arrestin | Trafficking/signalling | Functional | ? | (77) | |
| Caveolin-1 | Structural/scaffolding | Co-IP | ? | Native, Het | (81; 84) |
| E3 Ub ligase | Trafficking | Y2H, IP, Functional | C-term | Het | (79) |
| Filamin | Scaffolding/structural/-trafficking | Y2H, Co-IP, GST, functional | C-term | Native, Het | (15; 40; 41) |
| GRK-2 | Signalling | Functional | ? | (77) | |
| GRK-4 | Signalling | Functional | ? | (77) | |
| Kir4.1 | K channel | Y2H, Co-IP, functional | C-term | Native, Het | (54) |
| Kir4.2 | K channel | Y2H, Co-IP, functional | C-term | Het | (54) |
| PI-4-kinase | Signalling | Co-IP | ? | (71) | |
| PKC | Signalling | Functional | C-term | (77) | |
| RAMP1 | Structural/trafficking | Co-IP, Functional | ?ECD and TM | Het | (37) |
| RAMP3 | Structural/trafficking | Co-IP, Functional | ?ECD and TM | Het | (37) |
| RGS proteins | Signalling | Functional | ? | (16; 71) | |
| Rho | Signalling | Co-IP | ? | (71) |
RAMPS (Receptor Activity Modifying Proteins)
The affinity of the CaR for its agonists or calcimimet-ics (agents that sensitize the CaR to activation by agonists), appears to depend on the cell type where it is expressed. In the parathyroid gland and parathy-roid cells, the EC50 for Ca is approximately 1.0 mM, while for the CaR expressed in cultured cells, the EC50 for Ca is on the order of 3.5 mM [32, 33]. In ker-atinocytes, the EC50 for induction of a differentiation marker, involucrin (presumably a CaR-mediated event), by extracellular Ca is approximately 0.1 mM [3]. Parathyroid cells are forty times more sensitive to cinacalcet for inhibition of parathyroid hormone secretion than are thyroid C cells to stimulation of calcitonin release [34]. This variability in CaR responsiveness could be explained by its interaction with different sets of intracellular proteins or accessory proteins such as RAMPs, a family of proteins that affect trafficking, glycosylation, ligand specificity and second messenger production by the receptors with which they interact [35].
The RAMP family currently has three members, RAMPs1–3. RAMP1 was discovered using xenopus oocyte expression cloning to identify an accessory protein for the calcitonin-like receptor (CLR) that could explain differences in ligand binding and sig-nalling observed in vivo and in different expression systems [35]. RAMP1 is a 148 AA protein with a long extracellular domain, a single TM domain and a short cytoplasmic tail that interacts with both the calcitonin receptor (CTR) and CLR. In these settings, RAMP1 acts as a chaperone permitting cell surface expression and alters the affinity as well as the selectivity of these receptors for agonists. RAMP2 and RAMP3 were subsequently identified using database searches and have similar structures and effects on the CTR and CLR. RAMP3 has a C-terminal PDZ domain, so it may have different functions from RAMP2 and 3. The RAMPs interact with their target receptors via their extracellular N-termini and TM domains. The reasons why RAMPs interact with some GPCRs and not others are not known.
This family of proteins is relatively new and has not been studied extensively, so their tissue distributions, subcellular localizations and catalogue of receptors with which they interact are not fully defined, although RAMPs are expressed in all tissues studied to date [35, 36]. The level of RAMP expression changes in physiologic states and disease models, so receptor activity could be modulated by this mechanism [36, 37]. Most work has focused on the CTR, the CLR and their ligands calci-tonin, adrenomedullin, calcitonin gene-related pep-tides (CGRP) 1 and 2 and amylin. Adrenomedullin and the CGRP affect vascular tone and blood pressure [38, 39]. RAMPs interact with other class 2 GPCRs, the vasopressin/pituitary adenylate cyclase-activating peptide (VPAC)-1, parathyroid hormone1, parathyroid hormone 2 and glucagons receptors, but the functional and physiologic consequences of these interactions are not known [35].
The CaR, a class 3 G protein-coupled receptor, interacts with RAMP1 and RAMP3 [37]. The initial observation was that the CaR could not reach the cell surface in COS7 cells. Subsequently, the investigators found that COS7 cells do not express any of the known RAMPs. Interaction of the CaR with one of these RAMPS is required for cell surface expression. In the absence of RAMP1 or 2, the CaR is trapped in the endoplasmic reticulum in an immature core gly-coslyated form. The RAMPs facilitate its exit from the endoplasmic reticulum, and its transit to the Golgi where it is glycosylated and then moves to the cell surface. The functional consequences of interaction with RAMP1 or RAMP3 were not tested, so it is possible that interaction with the two different RAMPs leads to different CaR activation kinetics. The fact that RAMP3 has a C-terminal PDZ domain means that it could also contribute to localization of the CaR in the cell and with other signalling proteins. For example, RAMP-3 interacts with N-ethylmaleimide-sensitive factor (NSF) via its PDZ domain. Interaction with NSF is required for recycling of the agonist-occupied CLR and 2-adrenergic receptor [9, 35].
Filamin
At least two groups identified filamin as a CaR interacting protein that could serve as a scaffold for other signalling proteins [40, 41]. Filamin is a homodimer made up of 280 kD proteins that contain N-terminal actin-binding domains, 24 96 AA IgG-like repeats, a C-terminal dimerization domain (repeat 24) and two hinge regions [42, 43]. Filamin was originally described as an actin cross-linking protein that provides mechanical strength to the actin cytoskeleton. Subsequently, it was found to interact with a number of TM proteins (many of them signalling proteins) and anchor them and the plasma membrane to the cytoskeleton. A partial list of these proteins includes integrins, Ca and K channels, a subset of G protein-coupled receptors (the D2 dopamine receptor, the CTR, some metabotropic glutamate receptors, and the CaR), and the insulin receptor [11, 42]. Filamin also interacts with intracellular signalling proteins including MAP kinases, Rho GTPases, Rho guanine nucleotide exchange factors (GEFs), Rho kinase, SMADS and phosphatases [42, 43]. Filamin appears to be involved in organization of these proteins in the cell and in their trafficking in that under the correct experimental conditions, loss of filamin or interference with its interaction with the protein of interest results in altered membrane expression and lost or reduced function [44, 45].
A problem in understanding the function of filamin is that its distribution in various differentiated cells has not been fully determined. Despite the fact that filamin is generally considered a cytoskeletal protein, most of it is found in the soluble fraction of hepatocytes, endothelial cells and presumably other cell types [46–48]. This distribution suggests that filamin may have a role in processes, such as protein trafficking in addition to anchoring proteins to the cytoskeleton and plasma membrane. One recent study indicates that in polarized epithelial cells, fil-amin may be preferentially expressed in the apical membrane, suggesting that in polarized cells, fil-amin's scaffolding function may be more important at the apical surface [49].
The mid-portion of the CaR C-terminus interacts with the region of filamin that contains repeats 15–17 and hinge 1 based in studies utilizing yeast two hybrid, co-immunoprecipitation and GST-fusion protein-binding assays. In the absence of filamin or when the interaction of filamin and the CaR is blocked, the CaR does not activate ERK or Jun N-terminal kinases (JNK) appropriately [40, 41, 50]. This interaction is also important for CaR-mediated Rho activation because expression of peptides that interfere with the CaR-filamin interaction block CaR-mediated Rho activation [15]. The simplest explanation for these observations is that filamin contributes to cell surface expression of the CaR. This is certainly the case, but even in the absence of filamin some CaR reaches the cell surface [51]. Evidence that fil-amin also provides an important scaffolding function comes from studies of Ca or Phe-induced CaR-mediated intracellular Ca oscillations [12]. Ca and Phe stimulate Ca oscillations with different patterns. The oscillations stimulated by Phe require Gα12/13, Rho, TrpC1, an intact cytoskeleton and filamin, while the Ca-stimulated oscillations persist with disruption of the interactions of filamin and the CaR [12, 52]. Although the precise mechanism by which filamin is involved in CaR-mediated inhibition of parathyroid hormone secretion on the apical surface of parathy-roid cells is not defined, an intact cytoskeleton is required [53]. If filamin is primarily found on the apical surface of epithelial cells, its interaction with the CaR may be important for those cell types (e.g. renal proximal tubule, intestinal and parathyroid cells) where the CaR is expressed on the apical surface. Other interacting proteins may contribute to its function in other cell locations.
Potassium channels
The C-terminus of the CaR interacts with and inactivates two inwardly rectifying K channels, Kir4.1 and Kir4.2 in the kidney that are expressed in the distal nephron (thick ascending limb of Henle and distal convoluted tubule) as well as other tissues [54]. Four Kir subfamilies, Kir2.x, Kir4.x, Kir5.x and Kir7.x, are expressed on the basolateral membrane of the distal nephron where the CaR is also expressed [55–58]. A number of channels undoubtedly contribute to the distal nephron basloateral K conductance, but studies indicate that the biophysical properties of a component of it are compatible with homomeric Kir4.1, Kir4.2, or heteromeric Kir4-Kir5.1 channels [55, 59, 60]. These channels are probably involved in recycling K for Na, K-ATPases (and possibly H, K-ATPases) and regulating membrane potential.
G protein-dependent signalling systems regulate Kir channels by a number of mechanisms that involve protein–protein interactions and second messengers including release of Gβγ subunits that interact directly with Kir3 channels (GIRK) to activate them, and inhibition of Kir3 channels by RGS (regulator of G protein signalling) proteins [61–69]. The 2 adren-ergic and dopamine D2 and D4 receptors interact directly with heteromeric Kir3 channels (Kir3.1/3.4 and Kir3.1/3.2), but the physiologic consequences of these interactions are not defined [70]. Control of inositol lipid metabolism by PLC and PI kinases regulate Kir channel activity by determining the level of PIP2, a lipid that is required for channel activity [69].
The CaR interacts directly and selectively with Kir4.1 and Kir4.2 as demonstrated with yeast two hybrid assays and co-immunoprecipitation. The channels can be co-immunoprecipitated from kidney cortex, and Kir4.2 and the CaR can be co-immuno-precipitated from heart and liver [54]. The reason for this association may be for regulation of channel activity by direct interaction with the receptor, to localize PIP2 metabolism near the channel, (the CaR interacts with and regulates PI4-kinase and regulates PLC) or for trafficking to affect the stochiometry of the receptor channel complex [54, 71]. Kir4.1 and Kir4.2 have C-terminal PDZ domains, and may be organized in multi-protein complexes on that basis.
Other CaR-interacting proteins
Although frequently overlooked in the category of interacting proteins, G protein-coupled receptors interact with each other to form homodimers and with other receptors to form heterodimers [10]. These interactions are important for cell surface expression and determining sensitivity to agonists. The CaR forms homodimers in the ER via interactions of cystienes in the extracellular domain and this dimerization is important for cell surface expression [72–74]. Hetrodimerization with other receptors has not been demonstrated.
Interaction of the three intracellular loops of the CaR with other proteins has not been specifically demonstrated, but can be inferred from functional studies and knowledge of the behaviour of the potential interacting proteins. The CaR must make contact with the G protein subunits through which it acts, Gαi, Gαq and Gα12/13[1]. Mutagenesis of the second and third intracellular loops and naturally occurring mutants demonstrate that these loops contribute to activation of, and presumably interact directly with Gαq and Gαi because mutants are defective in PLC activation, a Gαq-dependent event [75, 76]. These loops are usually sites of phosphorylation by G protein receptor kinases (GRKs) in other receptors, and sites for interaction with proteins like arrestin and spinophilin [10]. GRKs recognize, bind to and phos-phorylate the agonist-occupied receptor. Arrestins then bind to the phosphorylated receptor, block interaction with G proteins and initiate termination of the signal as well as internalization of the receptor [9, 10]. Arrestins are also capable of activating the ERKs via Src kinase in a G protein-independent manner. Based on the fact that over-expression of GRK 2 or 3 and β-arrestins 1 or 2 reduces CaR signalling, these proteins probably interact with the CaR, but the sites of phosphorylation and of interaction were not defined, and interaction was not specifically demonstrated directly [77]. Precisely how interactions with GRKs and arrestins affect CaR signalling and trafficking have not been studied.
In addition to filamin and the K channels, Kir4.1 and Kir4.2, a number of other proteins interact with the CaR C-terminus. The C-terminus is phosphory-lated by, and so must interact with protein kinase C. This phosphorylation at Thr 888 results in reduced intracellular Ca store release [78]. PKC activity is required for PLC-mediated Ca oscillations [12]. PKC-mediated CaR phosphorylation may also be important for mediating the effects of β-arrestins because signalling by a mutant CaR that lacks PKC phospho-rylation sites is not affected by expression of β-arrestins [77]. The degradation of the CaR is mediated by the ubiquitin system through interactions with the E3 ubiquitin ligase, dorfin and AMSH (Associated Molecule with the SH3 domain of STAM), a deubiqui-tinating enzyme [79, 80]. Both interactions were initially identified using yeast two hybrid cloning and co-immunoprecipitation or GST pull-down assays. As expected and consistent with effects of ubiquitination of other G protein-coupled receptors, over-expression of dorfin resulted in reduced levels of CaR protein while a dominant negative dorfin construct increased CaR protein. Dorfin can mediate degradation of immature CaR protein from the ER [79]. Although expression of AMSH would be expected to increase CaR protein expression, it had the opposite effect [80].
The CaR interacts with several other proteins, but the region of the CaR responsible for the interaction is not defined. Caveolin, a protein that also interacts with filamin, co-immunoprecipitates with, and co-localizes with the CaR in parathyroid cells [81, 82]. Caveolae are membrane microdomains enriched in cholesterol and glycosphingolipids that in contrast to lipid rafts also contain caveolin. These structures concentrate the components of G protein signalling systems, and caveolin may be involved in stable membrane expression of these proteins as well as endocytosis and sorting [83]. Precisely how its presence in caveolae or its interactions with caveolin affect CaR function are not defined, but the correlation of reduced caveolin and CaR expression in parathyroid adenomas suggests that the interaction may be important functionally [84]. Filamin may also contribute to these interactions [82]. The CaR co-immunoprecipitates with PI-4-kinase and rho and stimulates PI-4-kinase activity [71]. This arrangement of proteins could result in a signalling module that regulates the metabolism of inositol lipids in a coordinated manner and in a limited region of the cell to precisely control processes such as channel activity, or it could contribute to protein trafficking that can also depend on inositol lipid metabolism [9, 54, 69]. Regulators of G protein signalling (RGS) proteins have been implicated in CaR signalling through expression studies, but interactions of the CaR or a CaR-based signalling complex with specific RGS proteins have not been demonstrated [16, 71].
Conclusions
G protein-coupled receptors act not only through het-erotrimeric G proteins, but though additional proteins that may interact directly with the receptor or be brought into proximity by scaffolding or structural proteins. A long-standing question has been why so many receptors exist to signal through the same G proteins. Part of the answer is that signalling by receptors through subsets of G proteins represents a common characteristic of many receptors, but that the unique signalling and biologic characteristics of receptors are determined by the additional restricted, and possibly unique proteins with which an individual receptor interacts.
Studies of proteins that interact with the CaR are less advanced than studies of α2-, β-adrenergic or metabotropic glutamate receptors, but some common themes are becoming clear. G protein-coupled receptors interact with each other in the ER, an interaction that is important for trafficking and cell-surface expression. These receptors also interact with a restricted set of G proteins, although the location for the initial interaction is not established. Signalling by this class of receptors involves RGS proteins that participate in signal termination, can initiate signals (e.g. Rho GEF), and that can contribute to the specificity of G protein-receptor interactions. G protein-coupled receptors interact with GRKs and second messenger-dependent kinases (e.g. protein kinase C and protein kinase A), and arrestins as a part of the signal termination process and for receptor traffick-ing. The placement of these receptors in cells is specific and is determined by interactions with other proteins that may have structural, scaffolding, signalling, or mixed functions, and that may contain motifs such as PDZ domains. Many interacting proteins such as arrestins have multiple functions that include sig-nalling and trafficking, so these aspects of receptor biology may not be separable.
Based on current information, several interactions may distinguish the CaR from other Gαi, Gαq and Gα12/13-coupled receptors. Filamin appears to serve as a scaffolding protein that also affects CaR traffick-ing. The scaffolding function is probably important in all cells, but particularly so on the apical surface of polarized cells were filamin is enriched. Although the significance of the interaction with RAMPs1 and 3 is not fully established, these proteins have the potential to affect localization and signalling by the CaR. The ability of the CaR to interact directly with ion channels (Kir4.1 and Kir4.2) in tissues could permit tight control over channel activity through direct protein–protein contact or with minimal dispersion of second messengers in the cell. Undoubtedly, additional interacting proteins will be discovered that will provide a more complete understanding ofthe function of the CaR and other similar receptors.
Acknowledgments
This work was supported by grants from the American Heart Association and a VA merit Review to C. Huang, grants from the National Institutes of Health (DK-59985) and a VA Merit Review to R. T. Miller, and the Leonard Rosenberg Research Foundation.
References
- 1.Hofer AM, Brown EM. Extracellular calcium sensing and signaling. Nat Rev Mol Cell Biol. 2003;4:530–8. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 2.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extra-cellular Ca2+-sensing receptor from bovine parathy-roid. Nature. 1993;366:575–80. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 3.Tu C-L, Chang W, Bikle DD. The extracellular Ca-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J Biol Chem. 2001;276:41079–85. doi: 10.1074/jbc.M107122200. [DOI] [PubMed] [Google Scholar]
- 4.Wada M, Furuya Y, Sakiyama J, Kobayashi N, Miyata S, Ishii H, Nagano N. The calcimimetic compound NPS R-568 suppresses parathyroid cell proliferation in rats with renal insufficiency. J Clin Invest. 1997;100:2977–83. doi: 10.1172/JCI119851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin and suppression of -catenin/TCF activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- 6.Mcneil SE, Hobson SA, Nipper V, Rodland KD. Functional calcium-sensing receptors in rat fibrob-lasts are required for activation of SRC kinase and mitogen-activated protein kinase in response to extracellular calcium. J Biol Chem. 1998;273:1114–20. doi: 10.1074/jbc.273.2.1114. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Miller RT. Regulation of renal ion transport by the calcium-sensing receptor: an update. Curr Opin Nephrol Hypertens. 2007;16:437–43. doi: 10.1097/MNH.0b013e3282b974a6. [DOI] [PubMed] [Google Scholar]
- 8.Rebois RV, Hebert TE. Protein complexes involved in heptahelical receptor-mediated signal transduction. Receptors Channels. 2003;9:169–94. [PubMed] [Google Scholar]
- 9.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–82. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Limbird LE. Regulation of 2AR trafficking and signaling by interacting proteins. Biochem Pharmacol. 2007;73:1135–45. doi: 10.1016/j.bcp.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enz R. The trick of the tail: protein-protein interactions of metabotropic glutamate receptors. Bioessays. 2007;29:60–73. doi: 10.1002/bies.20518. [DOI] [PubMed] [Google Scholar]
- 12.Rey O, Young SH, Yuan J, Slice L, Rozengurt E. Amino acid-stimulated Ca oscillations produced by the Ca-sensing receptor are mediated by a phop-sholipase C/Ins (1,4,5) P3-independent pathway that requires G12, Rho, filamin -A, and the actin cytoskeleton. J Biol Chem. 2005;280:22875–82. doi: 10.1074/jbc.M503455200. [DOI] [PubMed] [Google Scholar]
- 13.Kifor O, MacLeod RJ, Diaz R, Bai M, Yamaguchi T, Yao T, Kifor I, Brown EM. Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected cells. Am J Physiol Renal Physiol. 2001;280:F291–302. doi: 10.1152/ajprenal.2001.280.2.F291. [DOI] [PubMed] [Google Scholar]
- 14.Gerbino A, Ruder WC, Curci S, Pozzan T, Zaccolo M, Hofer AM. Termination of cAMP signals by Ca and Gαivia extracellular Ca sensors: a link to intracellular Ca oscillations. J Cell Biol. 2005;171:303–12. doi: 10.1083/jcb.200507054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pi M, Spurney RF, Tu Q, Hinson T, Quarles LD. Calcium-sensing receptor activation of rho involves filamin and rho-guanine nucelotide exchange factor. Endocrinology. 2002;143:3830–8. doi: 10.1210/en.2002-220240. [DOI] [PubMed] [Google Scholar]
- 16.Huang C, Hujer KM, Wu Z, Miller RT. The Ca-sensing receptor couples to Ga12/13 to activate phospho-lipase D in Madin-Darby canine kidney cells. Am J Physiol Cell Physiol. 2004;286:C22–30. doi: 10.1152/ajpcell.00229.2003. [DOI] [PubMed] [Google Scholar]
- 17.Hofer AM, Gerbino A, Caroppo R, Curci S. The extracellular calcium-sensing receptor and cell-cell signaling in epithelia. Cell Calcium. 2004;35:297–306. doi: 10.1016/j.ceca.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Geibel J, Sritharan K, Geibel R, Geibel P, Persing JS, Seeger A, Roepke TK, Deichstetter M, Prinz C, Cheng SX, Martin D, Hebert SC. Calcium-sensing receptor abrogates secretogogue-induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc Natl Acad Sci USA. 2006;103:9387–9. doi: 10.1073/pnas.0602996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano S, Macleod RJ, Chattopadhyay N, Tfelt-Hansen J, Kifor O, Butters RR, Brown EM. Calcium-sensing receptor activation stimulates parathyroid hormone-related protein secretion in prostate cancer cells: role of epidermal growth factor transactivation. Bone. 2004;36:664–72. doi: 10.1016/j.bone.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Schafer B, Gschwind A, Ullrich A. Multiple G-protein-coupled receptor signals converge on the epi-dermal growth factor receptor to promote migration and invasion. Oncogene. 2004;23:991–9. doi: 10.1038/sj.onc.1207278. [DOI] [PubMed] [Google Scholar]
- 21.Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaître X, Paillard M, Planelles G, Déchaux M, Miller RT, Antignac C. Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol. 2002;13:2259–66. doi: 10.1097/01.asn.0000025781.16723.68. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T. Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet. 2002;360:692–4. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Li C, Kwon TH, Miller RT, Knepper MA, Frøkiaer J, Nielsen S. Reduced expression of renal Na transporters in rats with PTH-induced hypercal-cemia. Am J Physiol Renal Physiol. 2004;286:F534–45. doi: 10.1152/ajprenal.00044.2003. [DOI] [PubMed] [Google Scholar]
- 24.De Jesus Ferreira MC, Bailly C. Extracellular Ca decreases chloride reabsorption in rat CTAL by inhibiting cAMP. Am J Physiol. 1998;275:F198–203. doi: 10.1152/ajprenal.1998.275.2.F198. [DOI] [PubMed] [Google Scholar]
- 25.Wang W-H, Lu M, Hebert SC. Cytochrome P-450 metabolites mediate extracellular Ca2+-induced inhibition of apical K+ channels in the TAL. Am J Physiol. 1997;273:F421–9. doi: 10.1152/ajpcell.1996.271.1.C103. [DOI] [PubMed] [Google Scholar]
- 26.Wang W-H, Balazy M, Hebert SC. Phospholipase A2 is involved in mediating the effect of extracellular Ca on apical K channels in rat TAL. Am J Physiol. 1997;273:F421–9. doi: 10.1152/ajprenal.1997.273.3.F421. [DOI] [PubMed] [Google Scholar]
- 27.Kwon T-H, Nielsen J, Kim Y-H, Knepper MA, Frokiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. Am J Physiol Renal Physiol. 2003;285:F152–65. doi: 10.1152/ajprenal.00307.2002. [DOI] [PubMed] [Google Scholar]
- 28.Capasso G, Unwin R, Ciani F, De Santo NG, De Tommaso G, Russo F, Giebisch G. Bicarbonate transport along the loop of Henle: II effects of acid-base, dietary, and neurohumoral determinants. J Clin Invest. 1994;94:830–8. doi: 10.1172/JCI117403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerolle N, Bourgeois S, Leviel F, Lebrun G, Paillard M, Houillier P. Angiotensin II inhibits NaCl absorption in the rat medullary thick ascening limb. Am J Physiol Renal Physiol. 2004;287:F404–10. doi: 10.1152/ajprenal.00265.2003. [DOI] [PubMed] [Google Scholar]
- 30.Good DW, George T, Wang DH. Angiotensin II inhibits HCO3 absorption via a cytochrome p-450-dependent pathway in MTAL. Am J Physiol. 1999;276:F726–36. doi: 10.1152/ajprenal.1999.276.5.F726. [DOI] [PubMed] [Google Scholar]
- 31.Amlal H, Legoff C, Vernimmen C, Soleimani M, Paillard M, Bichara M. Ang II controls Na-K(NH4)-2Cl cotransport via 20-HETE and PKC in medullary thick ascending limb. Am J Physiol. 1998;274:C1047–56. doi: 10.1152/ajpcell.1998.274.4.C1047. [DOI] [PubMed] [Google Scholar]
- 32.Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991;71:371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- 33.Handlogten ME, Shiraishi N, Awata H, Huang C, Miller RT. The extracellular Ca-sensing receptor is a promiscuous polycation sensor that responds to lead. Am J Physiol Renal Physiol. 2000;279:F1083–91. doi: 10.1152/ajprenal.2000.279.6.F1083. [DOI] [PubMed] [Google Scholar]
- 34.Fox J, Lowe SH, Conklin RL, Petty BA, Nemeth EF. Calcimemetic compound NPS R-568 stimulates cal-citonin secretion but selectively targets parathyroid gland Ca2+ receptor in rats. J Pharm and Exp Ther. 1999;290:480–6. [PubMed] [Google Scholar]
- 35.Parameswaran N, Spielman WS. RAMPs:the past, present and future. Trends Biochem Sci. 2006;31:631–8. doi: 10.1016/j.tibs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Udawela M, Hay DL, Sexton PM. The receptor modifying protein family of G protein coupled receptor accessory proteins. Semin Cell Dev Biol. 2004;15:299–308. doi: 10.1016/j.semcdb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Bouschet T, Martin S, Henley JM. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J Cell Sci. 2005;118:4709–20. doi: 10.1242/jcs.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tam CW, Husmann K, Clark NC, Clark JE, Lazar Z, Ittner LM, Götz J, Douglas G, Grant AD, Sugden D, Poston L, Poston R, McFadzean I, Marber MS, Fischer JA, Born W, Brain SD. Enhanced vascular responses to adrenomedullin in mice overexpressing receptor-activity-modifying protein 2. Circ Res. 2006;98:262–70. doi: 10.1161/01.RES.0000200737.63865.58. [DOI] [PubMed] [Google Scholar]
- 39.Dackor R, Fritz-Siz K, Smithies O, Caron K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J Biol Chem. 2007;282:18094–9. doi: 10.1074/jbc.M703544200. [DOI] [PubMed] [Google Scholar]
- 40.Awata H, Huang C, Handlogten ME, Miller RT. Interaction of the Ca-sensing receptor and filamin, a potential scaffolding protein. J Biol Chem. 2001;276:34871–9. doi: 10.1074/jbc.M100775200. [DOI] [PubMed] [Google Scholar]
- 41.Hjalm G, MacLeod J, Kifor O, Chattopadhyay N, Brown EM. Filamin-1 binds to the carboxy-terminal tail of the calcium-sensing receptor (CaR), an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase (MAPK) J Biol Chem. 2001;276:34880–7. doi: 10.1074/jbc.M100784200. [DOI] [PubMed] [Google Scholar]
- 42.Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. Filamins as integrators of cell mechanics and signaling. Nat Rev Mol Cell Biol. 2001;2:138–45. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 43.Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signaling. Nat Cell Biol. 2004;6:1034–8. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- 44.Liu G, Thomas L, Warren RA, Enns CA, Cunningham CC, Hartwig JH, Thomas G. Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and mudu-lates proprotein processing in the endocytic pathway. J Cell Biol. 1997;137:1719–33. doi: 10.1083/jcb.139.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seck T, Baron R, Horne WC. Binding of filamin to the C-terminal tail of the calcitonin receptor controls recycling. J Biol Chem. 2003;278:10408–16. doi: 10.1074/jbc.M209655200. [DOI] [PubMed] [Google Scholar]
- 46.Ramsby ML, Makowski GS. Differential detergent fractionation of eukaryotic cells. Analysis by two-dimensional gel electrophoresis. Methods Mol Biol. 1999;112:53–66. doi: 10.1385/1-59259-584-7:53. [DOI] [PubMed] [Google Scholar]
- 47.Ramsby ML, Makowski GS, Khairallah EA. Differential detergent fractionation of isolated hepatocytes: biochemical, immunological, and two-dimensional gel electrophoresis charactreization of cytoskeletal and noncytoskeletal compartments. Electrophoresis. 1994;15:265–77. doi: 10.1002/elps.1150150146. [DOI] [PubMed] [Google Scholar]
- 48.Shojace N, Patton WF, Chung-Welch N, Su Q, Hechtman HB, Shepro D. Expression and subcellu-lar distribution of filamin isotypes in endothelial cells and pericytes. Electrophoresis. 1998;19:323–32. doi: 10.1002/elps.1150190230. [DOI] [PubMed] [Google Scholar]
- 49.Thelin WR, Chen Y, Gentzsch M, Kreda SM, Sallee JL, Scarlett CO, Borchers CH, Jacobson K, Stutts MJ, Milgram SL. Direct interaction with filamins modulates the stability and plasma membrane expression of CFTR. J Clin Invest. 2007;117:364–74. doi: 10.1172/JCI30376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang C, Wu Z, Hujer KM, Miller RT. Silencing of fil-amin A gene expression inhibts Ca-sensing receptor signaling. FEBS Lett. 2006;580:1795–800. doi: 10.1016/j.febslet.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 51.Zhang M, Breitwieser GE. High affinity interaction with filamin A protects against calcium sensing receptor degradation. J Biol Chem. 2005;280:11140–6. doi: 10.1074/jbc.M412242200. [DOI] [PubMed] [Google Scholar]
- 52.Rey O, Young SH, Papazyan R, Shapiro MS, Rozengurt E. Requirement of the TRPC1 cation channle in the gneration of transient Ca oscillations by the calcium-sensing receptor. J Biol Chem. 2006;281:38730–7. doi: 10.1074/jbc.M605956200. [DOI] [PubMed] [Google Scholar]
- 53.Quinn SJ, Kifor O, Kifor I, Butters RR, Brown EM. Role of the cytoskeleton in extracellular calcium-regulated PTH release. Biochem Biophys Res Commun. 2007;354:8–13. doi: 10.1016/j.bbrc.2006.12.160. [DOI] [PubMed] [Google Scholar]
- 54.Huang C, Sindic A, Hill CE, Hujer KM, Chan KW, Sassen M, Wu Z, Kurachi Y, Nielsen S, Romero MF, Miller RT. Interaction of the Ca-sensing receptor with the inwardly-rectifying potassium channels Kir4.1 and Kir4.2 results in inhibition of channel function. Am J Physiol Renal Physiol. 2007;292:F1073–81. doi: 10.1152/ajprenal.00269.2006. [DOI] [PubMed] [Google Scholar]
- 55.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K+ channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4-Kir5.1 heteromeric channels. J Physiol. 2002;538:391–404. doi: 10.1113/jphysiol.2001.012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, Mori K, Tonosaki A, Tomoike H, Kurachi Y. Immunolocalization of an inwardly rectifying K channel, KAB-2 (Kir4.1), in the basolateral memebrane of renal distal tubular epithelia. FEBS Lett. 1996;388:11–5. doi: 10.1016/0014-5793(96)00502-9. [DOI] [PubMed] [Google Scholar]
- 57.Ookata K, Tojo A, Suzuki Y, Nakamura N, Kimura K, Wilcox CS, Hirose S. Localization of inward rectifier potassium channel Kir7.1 in the basolateral membrane of distal nephron and collecting duct. J Am Soc Nephrol. 2000;11:1987–94. doi: 10.1681/ASN.V11111987. [DOI] [PubMed] [Google Scholar]
- 58.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracel-lular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol. 1998;274:F611–22. doi: 10.1152/ajprenal.1998.274.3.F611. [DOI] [PubMed] [Google Scholar]
- 59.Yoshitomi K, Shimizu T, Taniguchi J, Imai M. Electropysiological characterization of rabbit distal convoluted tubule cell. Pflugers Archiv. 1989;414:457–63. doi: 10.1007/BF00585057. [DOI] [PubMed] [Google Scholar]
- 60.Pessia M, Imbrici P, D'Adamo Mc, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerization with Kir5.1. J Physiol. 2001;532:359–67. doi: 10.1111/j.1469-7793.2001.0359f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C-L, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by bg. Nature. 2004;391:803–6. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 62.Reuveny E, Slesinger PA, Inglese J, Morales JM, Iñiguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein βγ subunits. Nature. 1994;370:143–6. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- 63.Sadja R, Alagem N, Reuveny E. Graded contribution of the Gβγ binding domains to GIRK channel activation. Proc Natl Acad Sci USA. 2002;99:10783–8. doi: 10.1073/pnas.162346199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Core S, Clapham DE. The stoichiometry of Gβγ binding to G protein regulated inwardly rectifying K channels (GIRKS) J Biol Chem. 2001;276:11409–13. doi: 10.1074/jbc.M100058200. [DOI] [PubMed] [Google Scholar]
- 65.Albsoul-Younes AM, Sternweis PM, Zhao P, Nakata H, Nakajima S, Nakajima Y, Kozasa T. Interaction sites of the G protein beta subunit with brain G protein-coupled inward rectifier K+ channel. J Biol Chem. 2001;276:12712–7. doi: 10.1074/jbc.M011231200. [DOI] [PubMed] [Google Scholar]
- 66.Peng L, Mirshahi T, Zhang H, Hirsch JP, Logothetis DE. Critical determinants of the G protein γ subunits in the Gβγ stimulation of G protein-activated inwardly rectifying potassium (GIRK) channel activity. J Biol Chem. 2003;278:50203–11. doi: 10.1074/jbc.M308299200. [DOI] [PubMed] [Google Scholar]
- 67.Grüning W, Arnould T, Jochimsen F, Sellin L, Ananth S, Kim E, Walz G. Modulation of renal tubular cell function by RGS3. Am J Physiol. 1999;276:F535–43. doi: 10.1152/ajprenal.1999.276.4.F535. [DOI] [PubMed] [Google Scholar]
- 68.Doupnik CA, Davidson NA, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proc Natl Acad Sci USA. 1997;94:10461–6. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rohacs T, Lopes CMB, Jin T, Ramdya PR, Molnar Z, Logothetis D. Specificity of activation by phos-phoinositides determines lipid regulation of Kir channels. Proc Natl Acad Sci USA. 2003;100:745–50. doi: 10.1073/pnas.0236364100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lavine N, Ethier N, Oak JN, Pei L, Liu F, Trieu P, Rebois RV, Bouvier M, Hebert TE, Van Tol HH. G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J Biol Chem. 2002;277:46010–9. doi: 10.1074/jbc.M205035200. [DOI] [PubMed] [Google Scholar]
- 71.Huang C, Handlogten ME, Miller RT. Parallel activation of phosphoinositol 4-kinase and phospholipase C by the extracellular calcium-sensing receptor. J Biol Chem. 2002;277:20293–300. doi: 10.1074/jbc.M200831200. [DOI] [PubMed] [Google Scholar]
- 72.Bai M, Trivedi S, Brown EM. Dimerization of the extracellular Calcium-sensing receptor (CaR) on the cell surface of Ca-R-transfected HEK293 cells. J Biol Chem. 1998;273:23605–10. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- 73.Pace AJ, Gama L, Breitwieser GE. Dimerization of the calcium-sensing receptor occurs within the extra-cellular domain and is eliminated by Cys to Ser mutations at Cys101 and Ser236. J Biol Chem. 1999;274:11629–34. doi: 10.1074/jbc.274.17.11629. [DOI] [PubMed] [Google Scholar]
- 74.Ray K, Hauschild BC, Steinbach PJ, Goldsmith PK, Hauache O, Spiegel AM. Identification of the cysteine residues in the amino-teminal extracellular domain of the human Ca2+ receptor critical for dimer-ization. J Biol Chem. 1999;274:27642–50. doi: 10.1074/jbc.274.39.27642. [DOI] [PubMed] [Google Scholar]
- 75.Chang W, Chen T-H, Pratt S, Shoback D. Amino acids in the second and third intracellular loops of the parathyroid Ca2+-sensing receptor mediate efficient coupling to phospholipase C. J Biol Chem. 2000;275:19955–63. doi: 10.1074/jbc.M909613199. [DOI] [PubMed] [Google Scholar]
- 76.Bai M, Quinn S, Trivedi S, Kifor O, Pearce SH, Pollak MR, Krapcho K, Hebert SC, Brown EM. Expression and characterization of inactivating and activating mutations in the human Ca2+o-sensing receptor. J Biol Chem. 1996;271:19537–45. doi: 10.1074/jbc.271.32.19537. [DOI] [PubMed] [Google Scholar]
- 77.Lorenz S, Frenzl R, Paschke R, Breitweisser GE, Meidlich SU. Functional desensitization of the extra-cellular Calcium-sensing receptor is regulated via distinct mechanisms: role of G protein-coupled receptor kinases, protein kinase C, and b-arrestins. Endocrinology. 2007;148:2398–404. doi: 10.1210/en.2006-1035. [DOI] [PubMed] [Google Scholar]
- 78.Bai M, Trivedi S, Lane CR, Yang Y, Quinn SJ, Brown EM. Protein kinase C phosphorylation of threonine at position 888 in the Ca2+-sensing receptor (CaR) inhibits coupling to Ca2+ store release. J Biol Chem. 1998;273:21267–75. doi: 10.1074/jbc.273.33.21267. [DOI] [PubMed] [Google Scholar]
- 79.Huang Y, Niwa J-I, Sobue G, Breitwieser GE. Calcium-sensing receptor ubiquitination and degradation mediated by the E3 ubiquitin ligase dorfin. J Biol Chem. 2006;281:11610–7. doi: 10.1074/jbc.M513552200. [DOI] [PubMed] [Google Scholar]
- 80.Herrera-Vigenor F, Hernandez-Garcia R, Valadez-Sanchez M, Vazquez-Prado J, Reyes-Cruz G. AMSH regulates calcium sensing receptor signaling through direct interactions. Biochem Biophys Res Commun. 2006;347:924–30. doi: 10.1016/j.bbrc.2006.06.169. [DOI] [PubMed] [Google Scholar]
- 81.Kifor O, Diaz R, Butters R, Kifor I, Brown EM. The calcium-sensing receptor is localized to caveolin-rich plasma membrane domains of bovine parathyroid cells. J Biol Chem. 1998;273:21708–13. doi: 10.1074/jbc.273.34.21708. [DOI] [PubMed] [Google Scholar]
- 82.Stahlhut M, Van Deurs B. Identification of filamin as a novel ligand for caveolin-1:evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell. 2000;11:325–37. doi: 10.1091/mbc.11.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Insel PA, Head BP, Patel HH, Roth DM, Bundey RA, Swaney JS. Compartmentation of G-protein-coupled receptors ans thier signaling components in lipid rafts and caveolae. Biochem Soc Trans. 2005;33:1131–4. doi: 10.1042/BST20051131. [DOI] [PubMed] [Google Scholar]
- 84.Kifor O, Kifor I, Moore FD, Jr, Butters RR, Jr, Cantor T, Gao P, Brown EM. Decreased expression of cave-olin-1 and altered regulation of mitogen-activated protein kinase in cultured bovine parathyroid cells and human parathyroid adenomas. J Clin Endocrinol Metab. 2003;88:4455–64. doi: 10.1210/jc.2002-021427. [DOI] [PubMed] [Google Scholar]


