Abstract
This invited review discusses the latest advances stem cell biology, tissue engineering and the transition from bench to bedside. An overview is presented as to which the best cell source might be for cell therapy and tissue engineering applications, best biomaterials currently available and the challenges the field faces to translate basic research into therapies for a large number of human diseases.
Keywords: stem cells, tissue engineering, regenerative medicine, biomaterials, bioreactors, cell expansion
Introduction
Regenerative medicine involves a multidisciplinary effort to replace or repair diseased tissue. Cell therapy and tissue engineering are key components of regenerative medicine. However, each and all are linked by one common aim-to deliver safe, effective and consistent therapies to patients.
As earlier defined by Langer and Vacanti, tissue engineering is an interdisciplinary field that applies to principles of engineering and life sciences towards the design and construction in the laboratory of functional tissues intended for the maintenance, regeneration or replacement of malfunctioning organs [1].
Tissue engineering involves the seeding of appropriate cells into a three-dimensional (3D) scaffold and the culturing of the resulting construct in a bioreactor under conditions which promote cell and tissue growth.
The essentials of tissue engineering
Cells and biomaterials are the pillars of tissue engineering.
Biomaterials
Biomaterials are an important component of tissue engineering. Scaffolds can aid in vivo regeneration of the remaining healthy tissue and also guide the formation of tissue from disassociated implanted cells, ex vivo and in vivo.
A scaffold should be:
-
•
Biocompatible and biodegradable with a controllable degradation rate. Degradation products should be non-toxic
-
•
Highly porous with an interconnected architecture, of controlled shape, size and alignment to facilitate oxygen, nutrients and waste transfer as well as rapid vascularization and tissue in-growth
-
•
Resistant to stress and strain and hold good mechanical properties
-
•
Be clinically compliant (GMP).
It would also be desirable if the scaffold is able to release growth factors, gene and other signals in a time-dependent manner [2, 3].
Scaffolds can be meshes, fibres, porous solids or hydrogels and may have a controlled macro and/or microspacial geometry and surface chemistry. These could encapsulate different cell types in distinct adjacent layers as recently proposed [4].
Ideally, the material should contain signals that recapitulate the normal developmental process and morphogenesis [5, 6].
Microscale technologies provide useful materials for tissue engineering purposes and are also useful for controlling the cellular microenvironment in vitro and for performing high-throughput assays [4].
Other workers have used nanofibrillar networks formed by self-assembly of small building blocks [7], artificial extra cellular matrix networks from protein polymers or peptide conjugated synthetic polymers and combined marrow stromal cell-sheet techniques and high strength biodegradable composites [8].
Cell types for tissue engineering
A variety of cell types have been used for tissue engineering.
Stem cells
Stem cells are cells that have the following capabilities: firstly, they are able to continuously produce daughter cells having the same characteristics as themselves; secondly they can generate daughter cells that have different, more restricted properties, and finally they can re-populate a host in vivo[9]. The first ability is called self-renewal and the second differentiation. There are two kinds of stem cells: embryonic stem cells derived from the inner cell mass (ICM) of pre-implantation embryos and adult/foetal stem cells found in tissues or organs, such as mesenchymal stem cells (MSCs) and haematopoietic stem cells from bone marrow.
Due to their limitless therapeutic potential, stem cells continue to be of enormous public, scientific and clinical interest. Researchers are discovering new sources of stem cells daily. However, the initial excitement generated by the identification of novel stem cell sources must give way to focused efforts on methods to manipulate their differentiation and self-renewal capabilities. It is likely that different stem cell types will be useful in a complementary fashion for different and specific therapies.
Embryonic stem cells
Embryonic stem (ES) cells are derived from the ICM of pre-implantation embryos. These cells have two distinct properties: a capacity for unlimited self-renewal and pluripotent developmental potency as their embryonic founder cells (being able to differentiate into cells and tissues of all three germ layers in vitro and in vivo). These properties render ES cells a novel model system to elucidate molecular signalling pathways required for the development of various lineages. More importantly, the establishment of human ES cells has opened up new opportunities for re-generative medicine. As hES cells can produce most, if not all, the differentiated cell types in a human body, they may provide unlimited cell resources for cell therapy. However, these require intensive studies for a better understanding hES cell properties before they could be used in the clinical applications. For example, we need to know how to efficiently expand human ES cells in culture using defined medium, free of animal products and without introducing genetic abnormalities; how robustly to differentiate human ES cells to specific lineages or cell types as required. In the last several years, some progress has been made in this field, as reviewed in the following sections.
The first ES cells were isolated in 1981 [10, 11] from mouse blastocyst by plating either intact embryos or following immunosurgical ablation of the ICM onto a mitotically inactivated mouse embryonic fibroblast (MEF) feeder layer and culturing them in a conventional tissue culture medium supplemented with 2-mercaptoethanol and 10–20% Foetalfoetal calf serum. Further studies found that adding a single cytokine, leukaemia inhibitory factor (LIF) into the culture medium could sustain mouse ES cell self-renewal in the absence of feeders [12, 13] and in this way, mouse ES cells can be expanded in large quantities.
Similar isolation protocol had been successfully applied to derive human ES cell line in Thomson's laboratory at the University of Wisconsin in 1998. This also involved the use of MEFs as feeder cells [14]. Subsequent studies has found that the mouse fibroblast feeder cells can be replaced by Matrigel matrix, a soluble basement membrane extract of the Engelbreth-Holm-Swarm (EHS) tumour when the culture medium is conditioned by mouse embryonic fibroblast (MEF-CM) and supplemented by basic fibroblast growth factor [15]. bFGF has been reported by several groups to inhibit spontaneous differentiation of hES cells [15–17], thus, has been widely used in human ES cell culture with or without feeder cells. In these culture conditions, human ES cells are able to grow in culture for a long time, and proliferate in large numbers. Meanwhile, they maintain their pluripotency and exhibit normal karyotype. However, these culture conditions are not ideal as the cells in these culture conditions are not homogenous populations that undifferentiated hES cells co-exist with small numbers of differentiated cells and cultures use animal products. Recently, a culture system has been developed in which both matrices and medium are defined, and free of animal products. These culture conditions not only support continuous growth of established hES cell lines, but also can be used to derive new hES cell lines [18]. Notwithstanding this, the molecular mechanisms maintaining hES cell self-renewal and differentiation are still largely unknown.
Successful cell therapy requires the generation of pure populations of defined cell types. This currently remains a challenge for stem cell biologists, despite considerable effort and progress to date. The most commonly used method for differentiating ES cells involves initiating differentiation via‘embryoid body’ formation. Basically, ES cells are plated onto low attachment culture plastic in differentiation culture medium and then form aggregates in suspension, which are called embryoid bodies (EBs). Embryoid body formation in the cell culture resembles, to a certain degree, early embryo development in vivo. The differentiated cells in EBs are heterogeneous, possibly containing all three primitive germ layers, ectoderm, mesoderm and endoderm. Because each of the cells in the EBs have different culture / micro-environmental conditions depending on their location within the EB, they will receive variable autocrine and paracrine signals. This subsequently can lead to different cell fates. Although considerable effort has been made to increase the number of lineage specific types by modifying culture conditions [19–21] it is difficult to achieve more homogenous populations within EBs.
In 2003, scientists at the University of Edinburgh developed a new adherent culture system to differentiate mouse ES cells to neural progenitors in a chemically defined medium [22]. However, hES cells are unable to differentiate into neural lineage efficiently under the same conditions and the resulting cells composed high proportion of extra embryonic endoderm-like cells. Further study found that blocking the BMP signalling pathway can dramatically reduce the formation of endoderm-like cells and increase hES cells differentiation down the neural lineage under adherent culture conditions [23]. Since then, it has been shown by several laboratories that ES cells can be converted into several required cell lineages more efficiently in adherent culture systems without EB formation, such as pancreatic endocrine cells [24], car-diomyocytes and hepatocytes. The adherent culture system not only produces specific cell lineages more efficiently but more importantly, it provides a better model to dissect the molecular signalling pathways required for specific cell fate determination. Co-culturing ES cells with the appropriate tissue, or using a cell-extract derived differentiation protocol also results in an efficient differentiation.
However, it remains a challenge to produce homogenous cell types as required. The alternative strategy is cell purification to enrich the population of appropriate cell type. The most common method is by labelling specific cell lineages with florescent antibodies and then purifying them by FACS. This requires the identification of specific cell surface markers for a particular cell type and the generation of florescent antibodies [25]. However, some cell types do not exhibit any known cell surface marker; therefore, it is not possible to purify them with the antibody method. Another approach is to label particular cell types via genetic modification, in which a florescent reporter gene, such as green florescent protein (GFP), is placed downstream of a cell type specific promoter so that its expression is controlled in a cell type specific manner. Oct4-GFP hES cell reporter lines have been generated in which GFP expression is restricted to undifferentiated hES cells and is dramatically down-regulated after cell differentiation [26]. Therefore, undifferentiated hES cells could be separated from differentiated cells. In another report, a ALB-GFP reporter has been applied to purify early hepatocytes differentiated from mouse ES cells, which were subsequently used to generate a liver-assist device, which when transplanted into mice with liver failure improved liver function [27].
‘Adult’ stem cells
An adult (or postnatal somatic) stem cell is an undifferentiated cell found among differentiated cells in a tissue or organ that can renew itself. Stem cells are distributed around the body in various other ‘niches’. For example, neural stem cells can be isolated from brain tissue, grown in vitro and induced to differentiate into the three cell types of the brain-neurons, astrocytes and oligo-dendrocytes. Similar niche specific stem cells are also thought to reside in other tissues as a repair mechanism against injury. Again, however, these stem cells cannot be grown easily in vitro and are thought to have a limited replicative capacity. In theory, these cells could be removed from a patient, incorporated into a tissue construct and put back into the same individual when repair becomes necessary, thereby removing the need for immunosuppression. Clearly, adult-derived progenitor cells need to be investigated and their clinical usefulness established. However, as with primary mature cells, for many adult stem cell types, problems with accessibility, low frequency (e.g., there is roughly one stem cell per 100,000 bone marrow cells), restricted differentiation potential, and poor growth limit their usefulness for tissue engineering.
‘Adult’ stem cells derived from the bone marrow have been used for more than 40 years for the treatment of haematological disorders. Throughout the 1950s and 1960s, it was shown that transplantations of ‘haematopoietic stem cells’ (HSCs), isolated from the bone marrow, could reconstitute the depleted bone marrow following irradiation. This culminated in 1963 when Mathé demonstrated the long-term survival of a leukaemia patient treated with HSCs. Bone marrow transplantation is now a routine medical procedure [28].
Following this success, Friedenstein et al.[29] noticed another cell type in bone marrow explants, initially called the fibroblast colony-forming cell because it stuck down on cell culture plastic, that was later shown to be a stem cell. They are now referred to as marrow stromal cells or MSCs. These cells resemble cells of the connective tissue (fibroblasts) and, in contrast to HSCs, can be grown easily in cell culture dishes. Mesenchymal stem cells (MSC) can differentiate into mesoderm-derived tissues while HSC can re-constitute the haematopoietic system. By changing the composition of the medium in which they are grown, MSCs can be selectively differentiated into bone cells (osteocytes), fat cells (adipocytes) and cartilage cells (chondrocytes). This property has made them an attractive choice for bone and cartilage tissue engineering, especially since they may be used to treat the person from whom they were isolated-as an ‘autologous’ transplant. There is increasing evidence in the literature that these cells may also differentiate into other lineages, although results are very controversial. Adult MSC also have limitations-they can only divide a finite number of times (depending on the age of the donor), which limits their supply, and they may accumulate genetic changes over time.
MSC can be found at various stages of development during pre- and post-natal life.
Foetal MSC can be isolated not only from foetal blood and haematopoietic organs in early pregnancy [30], but from a variety of somatic organs as well as amniotic fluid and placenta throughout gestation, in liver, spleen, bone marrow, femur, pancreas, lung and circulating in early gestation foetal circulation [31]. In adults, MSC [29] and HSC mainly reside in the bone marrow, but are also niche specific and found in adipose, brain and other tissue sources such as synovial membrane. The blood remaining in an umbilical cord immediately after birth also a rich source of stem cells (cord blood stem cells) that can be collected easily and painlessly without risk to the newborn or mother. Cord blood stem cells have been used therapeutically for nearly 20 years and in more than 10,000 transplants worldwide. Today, they are used successfully to treat a wide range of blood diseases, genetic and metabolic disorders, immunodeficiencies and certain forms of cancer. A number of medical research studies have demonstrated that cord blood stem cells are able to differentiate into multiple cell types and may have potential use in re-generative medical therapies, such as treating diabetes, cardiac disease and several neurological disorders.
The precise identity of MSC remains a challenge due to the lack of single definitive marker. Standard assays to identify MSC rely on their spindle-shaped morphology, selective adherence to a solid surface, proliferative potential, capacity to differentiate and ability to repair tissues. MSC are characterized by their negative expression of haematopoietic antigens (CD45−/34−/14−), and positive expression of stroma-associated markers CD29 (β1-integrin), CD73 (SH3 and SH4), CD105 (SH2), CD44 (HCAM1), the early bone marrow progenitor cell markers CD90 (thy-1) and the extracellular matrix proteins vimentin, laminin and fibronectin.
Foetal MSC are less lineage committed than adult MSC in humans [31–33] and primates. [34]. Human first trimester foetal MSC found in blood, liver, bone marrow, amniotic fluid and trophoblast are more primitive that adult bone marrow MSC, expressing markers associated with pluripotency and having high levels of telomerase activity (Fig. 2) [35].
2.
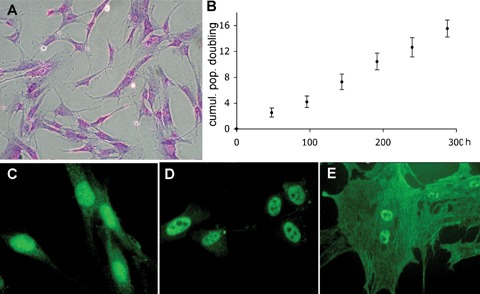
Human first trimester foetal mesenchymal stem cell present a fibroblastic spindle-like morphology visualized by crystal violet staining (A), are readily expandable (B) and express oct-4 (C), nanog (D) and hTERT both in the nucleus (active fraction) and in the cytoplasm (trafficking fraction) (E).
Evidence suggest that both foetal and adult MSC can differentiate in lineages from the three germ layers, and give rise under in vitro permissive conditions to endodermal (i.e. endothelial cells and hepatocytes) and ectodermal (i.e. oligodendrocytes and neurons)-derived lineages. In vivo, MSC can differentiate into chondrocytes [36], adipocytes [36], myocytes [37], cardiomyocytes [36], bone marrow stromal cells [36], thymic stroma [36] and osteoblasts [38].
Transition from bench to bedside
Although ES cells hold great potential for future clinical application, there are still in its infancy. Considerable basic research remains to be done before it can be translated to a clinical setting. In addition to the culture and differentiation challenges mentioned above, other issues also require further investigation. For example, multiple stages are involved for an ES cell to become a terminal functional cell type. It remains to be seen at what stage of differentiation the cells are more suitable for transplantation. If an ES-derived cell is transplanted too early during the differentiation pathway, the cell may not become the required cell type after transplantation as the in vivo environment (niche) in adult tissues may be different from that required for embryonic/-foetal stem cell development and hence may not able to provide the appropriate signals for further differentiation. In addition, it has higher risk of tumour formation. On the other hand, if transplanted too late as a terminally differentiated cell, it may not able to adapt to the in vivo environment and perform the correct function. It will be necessary to have reliable methodology for the accurate and consistent expansion of clinical grade cells.
MSC are able to home in a range of animal models to various tissues after intravenous infusion. In non-injured non-human primates, infused MSC preferentially home to bone marrow [39], and distribute throughout other organs, but the challenge remains their very low engraftment levels [40]. Upon discrete injury, MSC are recruited from remote storage sites to areas of wound healing where they engraft in higher numbers compared to intact tissues [36, 41]. Other examples of MSC preferentially migrating to sites of pathology include swine [42] and rat [43, 44] models of cardiac pathology, where MSC infusion has been also associated with tissue repair and improved organ function. Recently, Boosma et al. showed that MSC engrafted more readily in infarcted mouse hearts than in uninfarcted controls, and improved cardiac function [45].
Therapeutic effects have also been reported after direct injection of MSC at injury sites in animal models (i) of acute and chronic cardiomyopathy, leading to improved cardiac function in rat [46], rabbit [47] and porcine models [48] (ii) of renal failure [49, 50] in mouse and rat models and (iii) of cerebral ischaemia [51] in rat models. In humans, autologous intracoronary injection of MSC into ischaemic hearts improved cardiac mechanical properties [52]. Finally, infusion of allogeneic whole bone marrow [53, 54] or bone marrow MSC [55] in Type III osteogenesis imperfecta children led to a promising phenotypic improvement with reduced fracture frequency and improved growth velocity [38, 53, 55].
The therapeutic potentials of early gestation foetal rather than adult MSC have also been documented in mice in a neonatal muscle injury model [56] and anecdoctically in a human foetus treated pre-nataly for OI, although any clinical benefit for the latter patient was confounded by concomitant biphosphonate treatment [57]. We have recently showed the therapeutic effects of human first trimester foetal blood MSC transplanted in utero in a mouse model of severe osteogenesis imperfecta (oim mice) led to a two-thirds reduction in fracture rate.
Cell expansion/microencapsulation
Traditionally, ES cell culture and differentiation requires the step of embryoid body formation resulting in formation of all the cells in the body, and thus limiting the available cellular material for the desired cell type. Two-dimensionally grown static cultures, the alternative paradigm for nonES cells, differentiate into only a small number of cells, are cumbersome, time consuming and labour intensive. Furthermore, they lack mixing and monitoring.
Ideally 3-D cultures should be carried out to form a cohesive, organized, perfused and functional tissue. Tissue engineering has been greatly aided by the development and use of bioreactors, supplying nutrients, oxygen, removing catabolites, monitoring pH and applying mechanical stresses to stimulate the formation of extracellular matrix [58].
A bioreactor is a device that reproduces the physiological environment (including biochemical and mechanical functions) specific to the tissue that is to be regenerated. Bioreactors can also be used to apply mechanical strength during maturation of the tissue and for studying and understanding the mechanical factors influencing tissue regeneration [59].
In order to obtain a large number of identical cells of a specific phenotype, encapsulated cells should be seeded into a 3D scaffold and the construct cultured in a controlled environment where by nutrients can be provided and waste products removed. 3D dynamic culture conditions are likely to provide an environment more akin to an in vivo situation. Encapsulated undifferentiated cells will grow indefinitely in the bioreactor whereby upon administration of specific growth agents cells can also be maintained differentiated and in large quantity for an unlimited period.
Conclusions
The converging fields of stem cells and tissue engineering for re-generative medicine purposes are reaching maturity. The over excitement and hype of the early years has given way to a more realistic and mature approach to the problems.
It is clear that there are still major challenges that researchers will need to face, as detailed in this review. In brief:
-
a)
It is important that research continues to utilize all possible types of stem cells since there is no clear consensus, and unlikely to be in the near future, as to which is/will be the best cell type for clinical applications.
-
b)
Reliable and robust ways to differentiate stem cells into different phenotypes is still some way away as are methods for obtaining industrial scale of identical cells for clinical applications. It will be important to learn how to encapsulate stem cells, seed them in a 3D biocomposite material, grow them in appropriate bioreac-tors, where non-invasive monitoring systems can help us to determine the nutritional state of the growing tissue.
-
c)
The biomaterials field is also progressing and the need for suitable composite materials with modified nanosurfaces and a 3D configuration is becoming evident.
There is no doubt the field is moving apace. Hardly a day passes where there is not a report on the progress in this field. Examples include the recent report of cell therapy for Type 1 diabetes, the creation of an engineered bladder, implanted in children and then, successfully functioning at least 8 years or the ongoing clinical cardiac trials using either bone marrow stem cells or using cardiac patches.
An exciting recent development that promises significant therapeutic advances is the finding that many, perhaps even most, cancers have an underlying stem cell characteristic. It appears that cells with stem cell features represent a minor component of a tumour, but that they are likely to act as a resistant ‘reserve’ from which the cancer will recur even after the bulk of the tumour has been removed surgically or by chemo- or radio-therapy. The distinct nature of the stem cell-related ‘core’ of a tumour has implications in terms of the design of novel drug therapies that will selectively act on and thereby prevent relapse.
It is possible to predict than in no more than 8–10 years time the field will have reached a degree of maturity which will allow it novel clinical therapies to be offered for a number of unmet clinical problems. But this will not be effortless. Many, many hours of intense research and hopefully better funding will be needed.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Leor J, Amsalem Y, Cohen S. Cells, scaffolds and molecules for myocardial tissue engineering. Pharmacol Ther. 2005;105:151–63. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Levenberg S, Burdick JA, Kraehenbuehl T, Langer R. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tiss Eng. 2005;11:506–12. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- 4.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Nat Acad Sci. USA. 2006;103:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienaime C, Barbotin JN, Nava-Saucedo JE. How to build an adapted and bioactive cell microenvironment? A chemical interaction study of the structure of Ca-alginate matrices and their repercussion on confined cells. J Biomed Mat Res Part A. 2003;67A:376–88. doi: 10.1002/jbm.a.10487. [DOI] [PubMed] [Google Scholar]
- 6.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 7.Harrington DA, Cheng EY, Guler MO, Lee LK, Donovan JL, Claussen RC, Stupp SI. Branched pep-tide-amphiphiles as self-assembling coatings for tissue engineering scaffolds. J Biomed Mat Res Part A. 2006;78A:157–67. doi: 10.1002/jbm.a.30718. [DOI] [PubMed] [Google Scholar]
- 8.Zhou HY, Chen XG, Zhang WF. In vitro and in vivo evaluation of mucoadhensiveness of chitosan/cellulose acetate multimicrospheres. J. Biomed. Mat. Res. Part A. doi: 10.1002/jbm.a.31400. 10.1002/jbm.a.31400. [DOI] [PubMed] [Google Scholar]
- 9.Smith A. A glossary for stem-cell biology. Nature. 2006;441:1060. [Google Scholar]
- 10.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 11.Martin GR. Isolatioin of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Nat Acad Sci USA. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripoten-tial embryonic stem-cell differentiatioin by purified polypeptides. Nature. 1988;336:688–90. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 13.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid-leukemia inhibitory factor maintains the developmental potential of embryonic stem-cells. Nature. 1988;336:684–7. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 14.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blasto-cysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 15.Xu CH, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–4. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 16.Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000;227:271–8. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 17.Xu RH, Peck RM, Li DS, Feng XZ, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Meth. 2005;2:185–90. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, Llanas RA, Thomson JA. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–7. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 19.Buttery LDK, Bourne S, Xynos JD, Wood H, Hughes FJ, Hughes SPF, Episkopou V, Polak JM. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tiss. Eng. 2001;7:89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 20.Bielby RC, Boccaccini AR, Polak JM, Buttery LDK. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tiss Eng. 2004;10:1518–25. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- 21.Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, Bishop AE. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tiss Eng. 2002;8:541–50. doi: 10.1089/107632702760240463. [DOI] [PubMed] [Google Scholar]
- 22.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–86. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 23.Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005a;23:1234–41. doi: 10.1634/stemcells.2005-0110. [DOI] [PubMed] [Google Scholar]
- 24.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 25.Bourne S, Polak JM, Hughes SPF, Buttery LDK. Osteogenic differentiation of mouse embryonic stem cells: differential gene expression analysis by cDNA microarray and purification of osteoblasts by cadherin-11 magnetically activated cell sorting. Tiss Eng. 2004;10:796–806. doi: 10.1089/1076327041348293. [DOI] [PubMed] [Google Scholar]
- 26.Gerrard L, Zhao DB, Clark AJ, Cui W. Stably transfect-ed human embryonic stem cell clones express OCT4-specific green fluorescent protein and maintain self-renewal and pluripotency. Stem Cells. 2005b;23:124–33. doi: 10.1634/stemcells.2004-0102. [DOI] [PubMed] [Google Scholar]
- 27.Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao DB, Okitsu T, Noguchi H, Basma H, Tabata Y, Chen Y, Tanaka K, Narushima M, Miki A, Ueda T, Jun HS, Yoon JW, Lebkowski J, Tanaka N, Fox IJ. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 2006;24:1412–9. doi: 10.1038/nbt1257. [DOI] [PubMed] [Google Scholar]
- 28.Mathe G, Amiel JL, Schwarzenberg L, Cattan A, Schneider M. Haematopoietic chimera in man after allogenic (homologous) bone-marrow transplantation. (Control of the secondary syndrome. Specific tolerance to the chimerism) Br Med J. 1963;28:1633–5. doi: 10.1136/bmj.2.5373.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Rudakow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 30.Campagnoli C, Roberts IAG, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 31.Guillot PV, O'Donoghue K, Kurata H, Fisk NM. Fetal stem cells: betwixt and between. Sem Reprod Med. 2006;24:340–47. doi: 10.1055/s-2006-952149. [DOI] [PubMed] [Google Scholar]
- 32.Gotherstrom C, West A, Liden J, Uzunel M, Lahesmaa R, Le Blanc K. Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Hematol J. 2005;90:1017–26. [PubMed] [Google Scholar]
- 33.Mirmalek-Sani SH, Tare RS, Morgan SM, Roach HI, Wilson DI, Hanley NA, Oreffo ROC. Characterization and multipotentiality of human fetal femur-derived cells: implications for skeletal tissue regeneration. Stem Cells. 2006;24:1042–53. doi: 10.1634/stemcells.2005-0368. [DOI] [PubMed] [Google Scholar]
- 34.Lee CCI, Ye F, Tarantal AF. Comparison of growth and differentiation of fetal and adult rhesus monkey mes-enchymal stem cells. Stem Cells Dev. 2006;15:209–20. doi: 10.1089/scd.2006.15.209. [DOI] [PubMed] [Google Scholar]
- 35.Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripo-tency markers and grow faster and have longer telom-eres than adult MSC. Stem Cells. 2007;25:646–54. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- 36.Liechty KW, Mackenzie TC, Shaaban AF, Radu A, Moseley AB, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–6. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 37.Chan J, Waddington SN, O'Donoghue K, Kurata H, Guillot PV, Gotherstrom C, Themis M, Morgan JE, Fisk NM. Widespread distribution and muscle differentiation of human fetal mesenchymal cells after intrauterine transplantation in dystrophic mdx mouse. Stem Cells. 2007;25:875–84. doi: 10.1634/stemcells.2006-0694. [DOI] [PubMed] [Google Scholar]
- 38.Horwitz EM, Prockop DJ, Gordon PL, Koo WWK, Fitzpatrick LA, Neel MD, Mccarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–31. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 39.Devereux G, Seaton A, Barker RN. In utero priming of allergen-specific helper T cells. Clin Exp Allergy. 2001;31:1686–95. doi: 10.1046/j.1365-2222.2001.01215.x. [DOI] [PubMed] [Google Scholar]
- 40.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 41.Mackenzie TS, et al. Circulating human fetal stromal cells engraft and differentiate in multiple tissues following transplantation into pre-immune fetal lambs. Blood. 2001;98:328a. [Google Scholar]
- 42.Price MJ, Chou CC, Frantzen M, Miyamoto T, Kar S, Lee S, Shah PK, Martin BJ, Lill M, Forrester JS, Chen PS, Makkar RR. Intravenous mesenchymal stem cell therapy early after reperfused acute myocar-dial infarction improves left ventricular function and alters electrophysiologic properties. Int J Cardiol. 2006;111:231–9. doi: 10.1016/j.ijcard.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 43.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchy-mal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol-Heart Circ Physiol. 2004;287:H2670–6. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 44.Chen JL, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–9. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 45.Boomsma RA, Swaminathan PD, Geenen DL. Intraveneously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. Int J Cardiol. 2007;122:17–28. doi: 10.1016/j.ijcard.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 46.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–35. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 47.Wang JA, Fan YQ, Li CL, He H, Sun Y, Lv BJ. Human bone marrow-derived mesenchymal stem cells transplanted into damaged rabbit heart to improve heart function. J Zhejiang Univ Sci. B. 2005;6:242–8. doi: 10.1631/jzus.2005.B0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thor Surg. 2002;73:1919–25. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 49.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 50.Kunter U, Rong S, Djuric Z, Boor P, Muller-Newen G, Yu DH, Floege J. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17:2202–12. doi: 10.1681/ASN.2005080815. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, Huang Z, Xu Y, Zhang S. Differentiation and neurological benefit of the mesenchymal stem cells transplanted into the rat brain following intracerebral hemorrhage. Neurol Res. 2006;28:104–12. doi: 10.1179/016164106X91960. [DOI] [PubMed] [Google Scholar]
- 52.Chen SF, Fang WW, Qian J, Ye F, Liu YH, Shan SJ, Zhang JJ, Lin S, Liao LM, Zhao RCH. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J. 2004;117:1443–8. [PubMed] [Google Scholar]
- 53.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WWK, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchy-mal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 54.Horwitz EH, Gordon PL. Transplanted allogenic gene-marked marrow mesenchymal cells engraft and benefit children with severe osteogenesis imperfecta. Blood. 2000 Abstract no.3456. [Google Scholar]
- 55.Horwitz EM, Gordon PL, Koo WKK, Marx JC, Neel MD, Mcnall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteoge-nesis imperfecta:implications for cell therapy of bone. Proc Nat Acad Sci USA. 2002;99:8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukada S, Miyagoe-Suzuki Y, Tsukihara H, Yuasa K, Higuchi S, Ono S, Tsujikawa K, Takeda S, Yamamoto H. Muscle regeneration by reconstitution with bone marrow or fetal liver cells from green fluorescent protein-gene transgenic mice. J Cell Sci. 2002;115:1285–93. doi: 10.1242/jcs.115.6.1285. [DOI] [PubMed] [Google Scholar]
- 57.Le Blanc K, Gotherstrom C, Ringden O, Hassan M, Mcmahon R, Horwitz E, Anneren G, Axelsson O, Nunn J, Ewald U, Norden-Lindeberg S, Jansson M, Dalton A, Astrom E, Westgren M. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfec-ta. Transplantation. 2005;79:1607–14. doi: 10.1097/01.tp.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- 58.Mantalaris S, Randle W, Polak JM. Stem cell biopro-cessing for clinical applications of regenerative medicine3rd Stem Cell Symposium Proceedings on Repair and Regeneration, Imperial College Press (Publs.) 2007. (submitted)
- 59.Bilodeau K, Mantovani D. Bioreactors for tissue engineering: focus on mechanical constraints. A compari-tive review. Tiss Eng. 2006;12:2367–83. doi: 10.1089/ten.2006.12.2367. [DOI] [PubMed] [Google Scholar]


