Abstract
Vascular grafts are in large demand for coronary and peripheral bypass surgeries. Although synthetic grafts have been developed, replacement of vessels with purely synthetic polymeric conduits often leads to the failure of such graft, especially in the grafts less than 6 mm in diameter or in the areas of low blood flow, mainly due to the early formation of thrombosis. Moreover, the commonly used materials lack growth potential, and long-term results have revealed several material-related failures, such as stenosis, thromboembolization, calcium deposition and infection. Tissue engineering has become a promising approach for generating a bio-compatible vessel graft with growth potential. Since the first success of constructing blood vessels with collagen and cultured vascular cells by Weinberg and Bell, there has been considerable progress in the area of vessel engineering. To date, tissue- engineered blood vessels (TEBVs) could be successfully constructed in vitro, and be used to repair the vascular defects in animal models. This review describes the major progress in the field, including the seeding cell sources, the biodegradable scaffolds, the construction technologies, as well as the encouraging achievements in clinical applications. The remaining challenges are also discussed.
Keywords: vessel substitutes, tissue engineering, smooth muscle cells, endothelial cells, biodegradable scaffold, vessel-reactor
Introduction
Cardiovascular disease remains the leading cause of death in western countries and often requires vascular reconstruction. Autologous arteries or veins are the most commonly used substitutes for coronary and peripheral bypass procedures. However, autologous vessel is not available in over 10% of the patients as a result of trauma, vessel disease or previous surgery [1]. Early attempts to develop blood vessel substitutes have focused on the use of grafts engineered from synthetic material, such as ePTFE (expanded polytetrafluoroethylene) and Dacron (polyethylene terephthalate fibre). However, replacement of vessels with purely synthetic polymeric conduits often leads to the failure of such graft, especially in the small diameter (less than 6 mm) grafts or in the areas of low blood flow, mainly due to the early formation of thrombosis [2–4]. In addition, the materials that are commonly used lack growth potential and long-term results have revealed several material-related failures, such as stenosis, thromboembolization, calcium deposition and infection [5]. To solve these problems, in particular for children who require implantation of dynamic material with growth potential, optimal grafts with biocompatibility and growth potential are desirable.
By seeding functional cells on biodegradable scaffolds, tissue engineering has become a new approach for tissue regeneration [6]. Since Weinberg and Bell reported the construction of blood vessels with collagen and cultured bovine aortic endothelial cells, smooth muscle cells and adventitial fibroblasts in vitro[7], there has been considerable progress in the area of vascular engineering. To date, tissue-engineered blood vessels (TEBVs) could be successfully constructed in vitro, and be used to repair the vascular defects in animal models. However, only a few have achieved clinical success with this approach. This review aims to describe the major progress in the field, including the seeding cell sources, the biodegradable scaffolds, the construction technologies, as well as the encouraging achievements in clinical applications. The remaining problems will also be discussed in an effort to guide future endeavours.
Components of blood vessel
Blood vessels are made of three layers, called from the luminal side outward, the tunica intima, the tunica media and the tunica adventitia. The thickness of these three layers varies greatly depending upon the size and type of vessel (large, medium & small arteries and veins; capillaries, don't have three layers). The vascular wall (except for capillary), with its complicated architecture and unique mechanical properties, is mainly composed by three types of cells: the endothelial cells (ECs) that lined in the tunica intima, the smooth muscle cells (SMCs) that predominantly located in the tunica media and the adventitial fibroblasts in the tunica adventitia. Among them, ECs and SMCs play a pivotal role in keeping the integrity of the vessel and maintaining its mechanical properties. The endothelium layer provides a continuous selective permeable, thrombo-resistant barrier that facilitates laminar blood flow through the blood vessel. It also controls vessel tone, platelet activation, adhesion and aggregation, leukocyte adhesion and SMCs migration and proliferation. Meanwhile, SMCs have secretory capabilities. The collagen fibres, elastic fibres, elastic lamellae and proteoglycans secreted by the SMCs keep the elasticity and radial compliance of the vessel.
Principle of vessel engineering
The general approach of tissue engineering is to seed cells on biodegradable scaffolds first, followed by in vitro culture or in vivo implantation. Ideally, the scaffolds will be gradually resorbed, leaving only the new tissue generated by the cells. Thus, the successful tissue regeneration relies on the seeding cells, the scaffolds and the construction technologies [8, 9]. Functional TEBVs should be non-thrombogenic, non-immunogenic, compatible at high blood flow rates and have similar viscoelasticity to native vessels [10–12]. Moreover, the grafts should be living tissues that could eventually integrate into the body and become indistinguishable from the native vessels. It has been accepted that the functional TEBVs cannot be achieved without ECs, SMCs, biodegradable scaffolds and the unique vessel-engineering techniques (Fig. 1).
1.
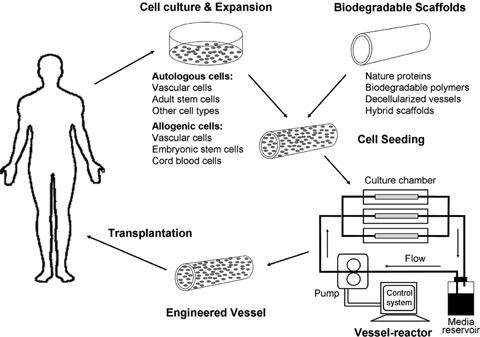
Schematic diagram of engineering blood vessels by tissue-engineering approach for clinical application.
Seeding cell sources
The ideal cell source should be non-immunogenic, functional and easy to achieve and expand in culture. Mature vascular cells, embryonic and adult stem cells, as well as alternative cell types that could possibly replace the ECs and SMCs, have been testified in vessel engineering.
Autologous ECs and SMCs
Non-immunogenic autologous ECs and SMCs isolated from patients themselves are the first choice for vessel engineering. Cells isolated from autologous vessels have been well used for engineering new vessels by many groups [7, 13–15]. In 1986, Weinberg and Bell first constructed TEBVs with cultured bovine aortic ECs, SMCs and adventitial fibroblasts [7]. In our early study, we have performed similar research utilizing ECs and SMCs derived from canine carotid arteries or human umbilical veins (HUV) [16]. Although functional TEBVs could be constructed by seeding those cells on biodegradable scaffolds, the limited proliferation potential of harvested cells makes it impossible to obtain large amount of cells from a small vessel biopsy. It is known that the majority of the cells in adult blood vessel are terminally differentiated. Even the cells isolated from umbilical veins have limited proliferation potential [16]. In addition, cells would lose their function during in vitro expansion. Although Grenier et al. reported that ECs, SMCs and fibroblasts could be isolated simultaneously and expanded in culture from a single and small vein biopsy sample [17], the quality of the cells after expansion were not clear. Many attempts have been tried to improve the proliferation potential of ECs and SMCs. Genetic manipulation is one of the ways that have been tested. Mckee et al. introduced human telomerase reverse transcriptase subunit (hTERT) into human SMCs [18], while Shao et al. utilized the same approach to immortalize the primary human microvascular ECs [19]. Encouraging results approved that the resulting cells could proliferate far beyond their normal lifespan and retained their characteristics of normal control cells. However, the safety of the cells after genetic manipulation is still a great concern. Long-term follow-up of modified cells in vivo is necessary before application of those cells in clinic. Allogeneic ECs and SMCs is another source for vessel engineering. However, immuno-rejection problem could not be avoided in this case, especially for ECs that contact directly with blood cells. To date, there is no promising way to solve the cell proliferation problem. It is of great interest to find alternative cell sources for vessel engineering.
Embryonic stem cells
In the recent few years, stem cell has become a major cell source for tissue engineering [20–22]. Generally there are two types of stem cells based on their origin, the embryonic and adult stem cells. Embryonic stem (ES) cells are able to produce all types of cells, while adult stem cells are normally limited to certain lineages. The merit of utilizing stem cell as a seeding cell source is that those cells are able to self-renew and differentiate into mature cells in the proper conditions, which makes it possible to obtain large amount of functional cells for tissue regeneration.
Differentiation of ES cells into ECs and SMCs has been studied extensively in murine ES cells, including maturation steps, molecular events and growth factor involvement [23–26]. The foetal liver kinase-1 (Flk-1) positive cells from differentiated ES cells, containing EC and SMC progenitors, could participate the neovascular formation when injected into animal bodies [27]. In our early study, we have successfully induced mouse ES cells to differentiate into ECs, and those ECs were further immortalized by transfection with hTERT [28]. The immortalized cells were able to maintain the phenotype of normal ECs, including the expression of Flk-1, von Willebrand factor (vWF) and CD34. Cells could form tubular structures in the presence of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and erythropoietin (EPO). Furthermore, we constructed a blood vessel by using SMCs obtained from rabbit arteries and the ECs derived from ES cells. This is the first work demonstrating that ES cells derived ECs could be a seeding cell source for vessel engineering. Recently, McCloskey et al. demonstrated that high purity of functional ECs could be achieved from differentiated mouse ES cells without genetic manipulation [29]. Moreover, Levenberg et al. showed that human ES cells could be differentiated into ECs that are able to form tube-like structures on matrigel, and form microvessels when they were transplanted into severe combined immune deficiency (SCID) mice [30]. All these achievements support that ES cells could be a good seeding cell source for vessel engineering. However, ES cells are still far away from their clinical application. Besides the ethical issue, immunogenic and tumourgenic problems are still the major obstacles that should be overcome before transplantation of those cells into the body.
Adult stem cells
Comparing with ES cells, adult stem cells can be obtained from patients themselves, which can avoid the immuno-rejection and ethical problems. In addition, adult stem cells are normally limited to certain lineages, which do not have tumorgenic capacity.
Endothelial progenitors cells (EPCs) are one type of the adult stem cells that have the capacity to proliferate, migrate and differentiate into mature ECs [31]. EPCs are mainly located in bone marrow and could be mobilized into peripheral blood by certain growth factors, such as granulocyte macrophage colony stimulating factor (GM-CSF) or VEGF [32–34]. EPCs could be also isolated from umbilical cord blood [35]. Studies have shown that EPCs are able to differentiate into mature ECs which express CD31, vWF and uptake low-density lipoprotein in the presence of VEGF [31]. In addition, EPCs could be expanded for over 20 passages without losing their differentiate potential [31]. No significant differences have been found between EPCs derived from bone marrow, peripheral blood or cord blood in terms of cell proliferation and differentiation [36]. EPCs have been well utilized in the endothelialization of synthetic vessel grafts as well as in vessel engineering [37–39]. Kaushal et al. isolated EPCs from peripheral blood of sheep, expanded and seeded them on decellularized porcine iliac vessels to construct an engineered vascular graft in vitro[38]. EPC-seeded grafts remained patent for 130 days as a carotid interposition graft in sheep. The EPC-explanted grafts exhibited contractile activity and nitric oxide-mediated vascular relaxation that were similar to native carotid arteries. These results indicate that EPCs can function similarly to arterial ECs and they could be a good EC source for vessel engineering.
Regarding SMCs, studies have shown that bone marrow derived mesenchymal stem cells (BMSCs) could be differentiated into SMC phenotypic cells in the presence of certain factors [40–42]. By using bone marrow derived ECs and SMCs from canine, Cho et al. have successfully engineered small-diameter vascular grafts in vitro[43]. The grafts remained patent for up to 8 weeks in the canine carotid artery interposition model. Cells labelled with a fluorescent dye prior to implantation were detected in the retrieved vascular grafts, indicating that the BMSCs participated in the vascular tissue regeneration. This work was confirmed by Koike et al. that networks of long-lasting blood vessels were formed in mice by co-implantation of vascular ECs and mesenchymal precursor cells. The networks were stable and functional for one year in vivo[44]. Moreover, Shin'oka and Mastumura et al. have successfully repaired the vessel defects with bone marrow derived cells in canine model as well as in patients [45–48]. These studies demonstrate that BMSCs could be a good SMC source for vessel engineering.
Adipose tissue is another stem cell source for ECs and SMCs. In 1983, Kern et al. isolated the microvascular ECs from human adipose tissue. These cells could grow readily to confluence and survived serial passages [49]. Arts et al. reported that microvasuclar ECs could be enriched from human adipose tissue by CD34 expression [50]. Martinez-Estrada et al. isolated an endothelial progenitor cell population that expresses Flk-1 from adipose tissue by three-dimensional culture. These cells could differentiate into mature ECs [51]. Meanwhile, Zuk et al. isolated another multi-potent population termed ‘adipose derived stromal cells (ADSCs)’ from adipose tissue, which can differentiate into adipocyte, osteoblasts and muscle cells [52]. Our recent work found that ADSCs isolated from human lipoaspirate could be induced to differentiate into SMCs. In addition, an elastic vessel wall could be successfully constructed in a bioreactor by seeding those cells on polyglycolic acid (PGA) scaffold (unpublished data). However, it should be noted that both BMSCs and ADSCs are multi-potent cells, whether cells would differentiate into other cell types (osteoblasts or adipocytes) and raise pathological problems after transplantation is unclear. Long-term fate of the cells after transplantation needs to be followed up in animal models.
Other cell types
Besides the stem cells, studies have also tried to replace the ECs and SMCs by other type of cells. L'Heureux et al. used adult human fibroblasts extracted from skin biopsies to construct TEBVs, which were further served as arterial bypass grafts in long-term animal models [53]. The TEBVs were antithrombogenic and mechanically stable for 8 months in vivo. Histological analysis showed a smooth muscle-specific α-actin positive cell population developed within the TEBV, indicating a complete re-generation of a vascular media. Campbell et al. took another approach of implanting silastic tubing into the peritoneal cavities of rabbits or rats to generate tissue tubes by an inflammatory response [54]. The tissue tubes that contained layers of myofibroblasts covered by a single layer of mesothelial cells could replace the aorta in rat. A patency rate of 68% was achieved in the absence of any heparin or spasmolytics over a period of up to 4 months. In addition, vascular re-modelling was observed 3 months after transplantation. Similar results have been achieved in other animal model [55]. However, no study has been carried out in human beings.
Biodegradable scaffolds
Scaffold is another key factor for tissue engineering. The 3-dimentional structure of scaffold provide a template for supporting cell growth, migration, differentiation and secretion of extracellular matrix (ECM) proteins, as well as for directing new tissue formation in the tissue regeneration process. Ideally, the scaffolds will be slowly resorbed in culture or after implantation, leaving only the tissue generated by the cells. In order to engineer a biocompatible vessel with growth potential and to avoid material-related side effects, the ideal scaffold for vessel engineering should be biodegradable. Varieties of materials have been utilized for vessel engineering, including the nature proteins, synthetic biodegradable polymers and decellularized vessels. The progresses of each material are discussed bellow.
Nature protein scaffolds
Nature proteins, such as collagen, elastin, fibronectin are the major components of ECM in the body. They are the most ideal substrates for cell attachment and cell signalling. Collagen and elastin are also the major components of blood vessel wall. Collagen gel has been used to create the first tissue-engineered vascular graft by Weinberg and Bell [7]. However, due to the inherent physical weakness of collagen gels and limited extracellular deposition by cultured SMCs, the mechanics of the grafts were not strong enough to support the physical load imposed by the haemodynamic environment. Many strategies have been tried to improve the strength of the collagen gel-based grafts, including the use of glycation to stiffen and strengthen collagen gel construct [56], the use of un-degradable or degradable meshes as ‘sleeves’[57–60], as well as the application of dynamic mechanical stimulation [61, 62]. Wrapping the constructs with Dacron mesh or polyurethane film could improve the strength of the grafts [57, 60]. This was further improved by wrapping with biodegradable materials, such as cross-linked type-I collagen and elastin [58, 59]. Furthermore, Boland et al. have applied the electrospinning technology to develop the biomimetic vascular constructs of micro-and nano-fibrous scaffolds from collagen and elastin, which could withstand the high pressure and pulsatile environment of the bloodstream [63]. As reviewed by Patel et al., elastin is a critical structural and regulatory matrix protein and plays an important and dominant role by conferring elasticity to the vessel wall [64]. Long and Tranquillo found that SMCs secreted more elastin on fibrin gels than on collagen gels [65]. Elastic small-diameter blood vessels were successfully engineered by using fibrin gels as scaffolds [66–69]. The grafts kept patent in jugular veins of lambs up to 15 weeks of observation [69]. Implanted vessels gained significant mechanical strength and reactivity that were comparable to those of native veins, indicating that fibrin-based TEBVs hold significant promise for treatment of vascular disease.
Biodegradable polymer scaffolds
Comparing with nature proteins, sythetic polymers are easily available and cheap. There is little or no batch-to-batch variations. In addition, polymers could be precisely modified to adjust their degradation rate, biocompatibility, elasticity. Several biodegradable synthetic polymer scaffolds have been investigated for their suitability in vascular engineering. Polyglycolic acid (PGA) is one of the most commonly used. By using PGA scaffolds and a biomimetic perfusion system, Niklason et al. produced the first autologous vascular graft and implanted into the arterial system [14]. The grafts were patent in vivo up to 1 month of observation. Although PGA fibre has good biocompatibility, its breakdown products are acidic, which could induce inflammatory response. Higgins et al. found that PGA breakdown products could lead to the dedifferentiation and decreased mitosis in SMC [70]. Moreover, PGA degraded too fast that result in a low mechanical property of engineered graft [14]. Other synthetic polymers with slow degradation rate, such as poly (L-lactic acid) (PLLA) [71], co-polymer of poly(D,L-lactic-co-glycolic acid) (PLGA) [71], poly 4-hydroxybutyrate (P4HB) [72] and co-polymer of PGA and polyhydroxyalkanoate (PHA) [73], have also been testified in vessel engineering. The biocompatibilities of the scaffolds were further improved by physical or chemical surface modifications [74–76]. However, the cellular toxicity of the breakdown products of those materials should be investigated in long-term study.
Decellularized vessels
Decellularized vessels, which are entirely composed of natural ECM, have good biocompatibility and could mostly maintain the mechanical properties of nature vessels [77]. Decellularization is typically accomplished by treating tissues with a combination of detergents, enzyme inhibitors and buffers. Although decellularized porcine carotid arteries followed by heparinization were successfully used to repair the abdominal artery without seeding cells in dog model [78], this approach is more difficult with human beings due to the lack of antithrombogenic EC layer on the lumen of the grafts. Teebken et al. obtained vessel grafts with stable biomechanical properties by seeding ECs and myofibroblasts from human saphenous veins on decellularized porcine aortas [79]. Similar work was performed by seeding human umbilical vein ECs or adult human vascular SMCs onto the decellularized porcine aortas after different decellularization processes [80, 81]. However, studies found that cell migration into these scaffolds was inadequate due to the very tight matrix organization specific to the aortic structure. To address this problem, Simionescu et al. prepared pure elastin scaffolds and pure collagen scaffolds by selectively removing the collagen component or elastin to create more porous scaffolds for cell infiltration [82]. Enhanced potential for repopulation by host cells in vivo was observed after subdermal implantation. In addition, new collagen fibres and bundles were found within the re-modelled elastin scaffolds and new elastin fibres within collagen scaffolds, respectively, indicating that they are able to support de novo ECM synthesis.
Porcine arteries are easy to access. However, the risk of transmission of animal pathogens to human being is still a big concern, even though it has been reported that decellularized porcine vascular scaffolds did not cause cross-species transmission of porcine endogenous retrovirus in a sheep model [83]. To avoid such problem, vessels from human being are the optimal choice [84, 85]. Daniel et al. decellularized HUV using an automated dissection methodology and created a promising scaffold that has excellent potential for cellular integration and maintain the mechanical properties of the native blood vessels [85]. The HUV scaffold could be a good candidate for vascular engineering.
Other materials
Hybrid materials of combining nature proteins and synthetic polymers have been testified for vessel engineering. Li et al. fabricated the vascular graft scaffolds using co-electrospun of PLGA, gelatin and α-elastin [86], while Stitzel et al. modified the approach by co-electrospun of PLGA, type I collagen and elastin [87]. No local or systemic toxic effects were observed when implanted the scaffolds in vivo. The scaffolds possessed tissue composition and mechanical properties similar to native vessels. The electrospun vessel matrix with both nature and synthetic materials could serve as a good scaffold for functional vessel engineering.
Vessel engineering in vitro
Vessel-reactors
Due to the dynamic environment of the cardiovascular system, the engineered vessel should be fully functional at the time of transplantation, which should be non-thrombogenic and have good mechanical strength and vasoreactivity. In addition, the mechanical and haemodynamic properties of vessel grafts are also crucial for their long-term survival [88]. To achieve such a functional graft in culture, a construct technology that could mimic the physiological vessel environment is required. Vessel-reactors have been developed and successfully utilized for vessel engineering by many groups [13, 14, 53, 89–91]. Basically, the vessel-reactor mimics the physiological stimuli that a native vessel received in the body, including the cyclic strain and shear stress [92–97]. Cyclic strain could significantly improve the mechanical property of engineered vessel, while shear stress could change cell alignment and improve endothelial cell adhesion [98–100]. Moore et al. demonstrated that cyclic strain could reduce the cell death [101], while Seliktar et al. found that cyclic strain could increase the matrix re-modelling by overexpression matrix re-modelling enzymes, such as matrix metal-loproteinase 2 (MMP-2) [102, 103]. In addition, Nikolovski et al. reported that cyclic strain could inhibit the switching of SMCs to an osteoblast-like phenotype in culture [104], which may prevent the un-wanted calcification in vascular graft. The important role of mechanics in vascular tissue engineering has been well reviewed by Nerem [100]. Meanwhile, it should be noted that optimal loading of stress to the graft is also critical. Liu reviewed that increased tensile stress and strain may induce vascular hypertrophy, and initiate focal atherosclerosis and intimal hyperplasia [105]. Solan et al. compared different rates of radial distension in vessel engineering, the adult heart rate (90 bpm) and the foetal heart rate (165 bpm) [106]. After 7 weeks of dynamic culture, no significant differences were observed between those two groups in terms of collagen and metallo-proteinase type 1 (MMP-1) expression. The parameters of vessel-reactor, such as pulse rate, deformation rate and pulsatile pressure, need to be optimized in future studies.
Culture additives
Besides the mechanical stimulation, chemical reagents and growth factors in culture media could also regulate the mechanic property of engineered graft. Transforming growth factor β1 (TGF- β1) could dramatically increase ECM production and deposition in plate culture [107]. Combination of TGF- β1, insulin and aprotinin could result in a significant improvement of both mechanical strength and vasoreactivity [68]. Ogle et al. reported that retinoic acid and ascorbic acid treatment could significantly elevate collagen and elastin gene expression, result in more ECM composition and enhanced mechanical properties of engineered graft [108]. Joddar et al. found that fragmented hyaluronan (HA) could stimulate the cell proliferation and synthesis of matrix elastin of SMCs in plate culture [109]. All these studies indicate that proper supplementation in the culture media is also helpful for vessel engineering in vitro.
Clinical applications
To date, TEBVs could be successfully constructed in vitro, and be used to repair the vascular defects in animal models [13, 43, 45, 47, 53, 69, 72, 89, 110]. However, only a few have achieved clinical success with this approach. The first clinical application of using an engineered vessel based on biodegradable scaffold was reported by Shin'oka et al. in Tokyo Women's Medical University [111]. The peripheral pulmonary artery was successfully reconstructed in a 4-year-old girl with the patient's own venous cells seeded onto a polycaprolactone–polylactic acid copolymer tube that was reinforced with woven PGA. After that, three patients were treated with same approach. In their following study, they took another approach of using autologous bone marrow cells as a cell source to avoid the time-consuming cell culturing step [46, 48]. Cells were harvested on the day of surgery, seeded directly on the polymer tube and the grafts were implanted right after 2–4 hrs of in vitro incubation. Twenty-three tissue-engineered conduits and 19 tissue-engineered patches were implanted for the repair of congenital heart defects [48]. They reported over 95% patency at 1 year without evidence of aneurysm formation or calcification. Moreover, there were no complications such as thrombosis, stenosis and obstruction of the tissue-engineered grafts. Long-term follow-up is desired to confirm the durability of this approach.
Different from the general approach, L'Heureux et al. successfully constructed vessel grafts using a novel method termed ‘Sheet-Based Tissue Engineering’[13]. In this approach, SMCs or fibroblasts were cultured in conditions that promote ECM deposition to produce a cohesive sheet that can be detached from the culture flask. The cell sheets were then rolled over a mandrel to form a vascular wall media without synthetic or exogenous scaffolds. After maturation, the inner tube was seeded with ECs. Using adult human fibroblasts extracted from skin biopsies, they successfully constructed TEBVs that could serve as arterial bypass grafts in long-term animal models [13]. This approach has been further testified in patients with haemodialysis [112]. The vessels constructed from autologous dermal fibroblasts and ECs were implanted as arteriovenous fistulas for dialysis access and were allowed to mature in vivo before use. During up to 5 months of implantation, no failures were observed with the first three patients, and the grafts were functioning well for haemodialysis access. These results are extremely encouraging. However, this approach is time consuming that would limit the application of these vessels in urgent cases.
Future perspectives
By seeding vascular cells on biodegradable scaffolds and further maturation of engineered vessel in bioreactor, successful results have been achieved in animal studies (Fig. 1). However, clinic trials demonstrated that the above elements and procedures are not indispensable for vessel regeneration; L'Heureux-engineered vessel grafts without exogenous scaffolds [112], while Shin'oka achieved vessel re-generation without in vitro culture [48]. From a surgeon's point of view, an off-the-shelf graft that available at any time for any patient is preferred. To achieve this goal, alternative ways beyond the traditional tissue engineering approach should be considered. As mentioned early, the basic requirements for a vessel graft should be non-thrombogenic, and have good mechanical strength and vasoreactivity. Those characteristics are accomplished by ECs and SMCs respectively in native vessel. If the scaffold alone could meet both of the characteristics, the cell seeding procedure could be possibly avoided.
Studies have demonstrated that native SMCs could migrate into the scaffold, and participate in the new vessel regeneration when scaffold alone were implanted [78, 110]. Theoretically, if the mechanical property of scaffold alone is close enough to the native vessel at the time of transplantation, the early involvement of SMCs in the graft is not necessary. As far as the vascular media could be completely regenerated by native SMCs before scaffold degradation in the body, seeding of exogenous SMCs could be avoided. Current technologies in material science are feasible to create such a scaffold by delicate design. In addition, certain modifications should also be considered to promote the SMCs migration, such as increasing scaffold porosity or embedding growth factors in the scaffold. Different from SMCs, early involvement of ECs in the graft is extremely important for preventing thrombosis at the time of implantation, which can not be easily achieved by scaffold alone at this moment. Thus, in a short-term plan, engineering vessel graft by seeding of ECs on a proper biodegradable scaffold might be the best way to promote the use of TEBVs in clinical application. In a long-term plan, scaffold that possesses antithrom-bogenic capacity at early stage and could recruit ECs at late time points is expected. Other strategies, such as delivery of therapeutic genes, have become of particular interests in tissue engineering [113, 114].
No matter which approach is taken, from a translational point of view, pre-clinical studies of human TEBVs need to be carried out in immunocompetent large animal models, and long-term outcomes should be followed up. For such experiments, development of an immunologically humanized animal is crucial. Recently, Zeng et al. generated a goat model carrying human cells by transplanting human cord blood cells into foetal goats at 45–55 days of gestation [115]. Long-term engraftment of human cells was detected in haematopoietic and non-haematopoietic organs for up to 2 years. Theoretically, the goat should have been immunologically humanized that would not reject human cells. Detail studies are going on to confirm the speculation. Hopefully, this xeno-transplant goat could provide a unique model for the evaluation of engineered human tissue graft in vivo.
References
- 1.Piccone V. Alternative techniques in coronary artery reconstruction. In: Sawyer PN, editor. Modern Vascular Grafts. New York: McGraw-Hill; 1987. pp. 253–60. [Google Scholar]
- 2.Greisler HP. Interactions at the blood/material interface. Ann Vasc Surg. 1990;4:98–103. doi: 10.1007/BF02042699. [DOI] [PubMed] [Google Scholar]
- 3.Whittemore AD, Kent KC, Donaldson MC, Couch NP, Mannick JA. What is the proper role of polytetra-fluoroethylene grafts in infrainguinal reconstruction? J Vasc Surg. 1989;10:299–305. doi: 10.1067/mva.1989.14116. [DOI] [PubMed] [Google Scholar]
- 4.Faries PL, Logerfo FW, Arora S, Hook S, Pulling MC, Akbari CM, Campbell DR, Pomposelli FB., Jr A comparative study of alternative conduits for lower extremity revascularization:all-autogenous conduit versus prosthetic grafts. J Vasc Surg. 2000;32:1080–90. doi: 10.1067/mva.2000.111279. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JW, Barratt-Boyes BG. Ventricular Septal Defect and Pulmonary Stenosis or Atresia. In: Kirklin JW, Barratt-Boyes BG, editors. Cardiac surgery. 2. New York: Churchill Livingstone; 1993. pp. 861–1612. [Google Scholar]
- 6.Shieh SJ, Vacanti JP. State-of-the-art tissue engineering: from tissue engineering to organ building. Surgery. 2005;137:1–7. doi: 10.1016/j.surg.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 8.Stock UA, Vacanti JP. Tissue engineering: current state and prospects. Annu Rev Med. 2001;52:443–51. doi: 10.1146/annurev.med.52.1.443. [DOI] [PubMed] [Google Scholar]
- 9.Lavik E, Langer R. Tissue engineering:current state and perspectives. Appl Microbiol Biotechnol. 2004;65:1–8. doi: 10.1007/s00253-004-1580-z. [DOI] [PubMed] [Google Scholar]
- 10.Nerem RM, Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3:225–43. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- 11.Vara DS, Salacinski HJ, Kannan RY, Bordenave L, Hamilton G, Seifalian AM. Cardiovascular tissue engineering: state of the art. Pathologie-biologie. 2005;53:599–612. doi: 10.1016/j.patbio.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Heyligers JM, Arts CH, Verhagen HJ, De Groot PG, Moll FL. Improving small-diameter vascular grafts: from the application of an endothelial cell lining to the construction of a tissue-engineered blood vessel. Ann Vasc Surg. 2005;19:448–56. doi: 10.1007/s10016-005-0026-0. [DOI] [PubMed] [Google Scholar]
- 13.L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 14.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 15.Heydarkhan-Hagvall S, Esguerra M, Helenius G, Soderberg R, Johansson BR, Risberg B. Production of extracellular matrix components in tissue-engineered blood vessels. Tissue Eng. 2006;12:831–42. doi: 10.1089/ten.2006.12.831. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Zhang YZ, Chen JJ, Yin DM, Cao Y, Xu ZC, Liu W, Cui L, Cao YL. Experimental study on constructing small-caliber artery by tissue engineering approach. Zhonghua wai ke za zhi. 2003;41:679–83. [PubMed] [Google Scholar]
- 17.Grenier G, Remy-Zolghadri M, Guignard R, Bergeron F, Labbe R, Auger FA, Germain L. Isolation and culture of the three vascular cell types from a small vein biopsy sample. In vitro Cell Dev Biol Anim. 2003;39:131–9. doi: 10.1007/s11626-003-0007-y. [DOI] [PubMed] [Google Scholar]
- 18.McKee JA, Banik SS, Boyer MJ, Hamad NM, Lawson JH, Niklason LE, Counter CM. Human arteries engineered in vitro. EMBO Rep. 2003;4:633–8. doi: 10.1038/sj.embor.embor847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao R, Guo X. Human microvascular endothelial cells immortalized with human telomerase catalytic protein:a model for the study of in vitro angiogenesis. Biochem Biophys Res Commun. 2004;321:788–94. doi: 10.1016/j.bbrc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S, Leshanski L, Itskovitz-Eldor J. Tissue engineering using human embryonic stem cells. Methods Enzymol. 2006;420:303–15. doi: 10.1016/S0076-6879(06)20014-4. [DOI] [PubMed] [Google Scholar]
- 21.Eberli D, Atala A. Tissue engineering using adult stem cells. Methods Enzymol. 2006;420:287–302. doi: 10.1016/S0076-6879(06)20013-2. [DOI] [PubMed] [Google Scholar]
- 22.Levenberg S. Engineering blood vessels from stem cells: recent advances and applications. Curr Opin Biotechnol. 2005;16:516–23. doi: 10.1016/j.copbio.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–32. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 24.Chung YS, Zhang WJ, Arentson E, Kingsley PD, Palis J, Choi K. Lineage analysis of the hemangioblast as defined by FLK1 and SCL expression. Development. 2002;129:5511–20. doi: 10.1242/dev.00149. [DOI] [PubMed] [Google Scholar]
- 25.Hirashima M, Kataoka H, Nishikawa S, Matsuyoshi N, Nishikawa S. Maturation of embryonic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood. 1999;93:1253–63. [PubMed] [Google Scholar]
- 26.Hirashima M, Ogawa M, Nishikawa S, Matsumura K, Kawasaki K, Shibuya M, Nishikawa S. A chemically defined culture of VEGFR2+ cells derived from embryonic stem cells reveals the role of VEGFR1 in tuning the threshold for VEGF in developing endothelial cells. Blood. 2003;101:2261–7. doi: 10.1182/blood-2002-01-0003. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–6. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 28.Shen G, Tsung HC, Wu CF, Liu XY, Wang XY, Liu W, Cui L, Cao YL. Tissue engineering of blood vessels with endothelial cells differentiated from mouse embryonic stem cells. Cell Res. 2003;13:335–41. doi: 10.1038/sj.cr.7290178. [DOI] [PubMed] [Google Scholar]
- 29.McCloskey KE, Gilroy ME, Nerem RM. Use of embryonic stem cell-derived endothelial cells as a cell source to generate vessel structures in vitro. Tissue Eng. 2005;11:497–505. doi: 10.1089/ten.2005.11.497. [DOI] [PubMed] [Google Scholar]
- 30.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:4391–6. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asahara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 32.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powell TM, Paul JD, Hill JM, Thompson M, Benjamin M, Rodrigo M, McCoy JP, Read EJ, Khuu HM, Leitman SF, Finkel T, Cannon RO., 3rd Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- 34.Korbling M, Reuben JM, Gao H, Lee BN, Harris DM, Cogdell D, Giralt SA, Khouri IF, Saliba RM, Champlin RE, Zhang W, Estrov Z. Recombinant human granulocyte-colony-stimulating factor-mobilized and apheresis-collected endothelial progenitor cells: a novel blood cell component for therapeutic vasculogenesis. Transfusion. 2006;46:1795–802. doi: 10.1111/j.1537-2995.2006.00985.x. [DOI] [PubMed] [Google Scholar]
- 35.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 36.Finney MR, Greco NJ, Haynesworth SE, Martin JM, Hedrick DP, Swan JZ, Winter DG, Kadereit S, Joseph ME, Fu P, Pompili VJ, Laughlin MJ. Direct comparison of umbilical cord blood versus bone marrow-derived endothelial precursor cells in mediating neovascularization in response to vascular ischemia. Biol Blood Marrow Transplant. 2006;12:585–93. doi: 10.1016/j.bbmt.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 37.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710–5. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 38.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE., Jr Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–40. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shirota T, He H, Yasui H, Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9:127–36. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- 40.Han YL, Kang J, Li SH. Bone marrow stromal cells of adult mice differentiate into smooth muscle cells in vitro. Zhonghua yi xue za zhi. 2003;83:778–81. [PubMed] [Google Scholar]
- 41.Jerareungrattan A, Sila-asna M, Bunyaratvej A. Increased smooth muscle actin expression from bone marrow stromal cells under retinoic acid treatment:an attempt for autologous blood vessel tissue engineering. Asian Pac J Allergy Immunol. 2005;23:107–13. [PubMed] [Google Scholar]
- 42.Kashiwakura Y, Katoh Y, Tamayose K, Konishi H, Takaya N, Yuhara S, Yamada M, Sugimoto K, Daida H. Isolation of bone marrow stromal cell-derived smooth muscle cells by a human SM22alpha promoter: in vitro differentiation of putative smooth muscle progenitor cells of bone marrow. Circulation. 2003;107:2078–81. doi: 10.1161/01.CIR.0000070082.64414.B5. [DOI] [PubMed] [Google Scholar]
- 43.Cho SW, Lim SH, Kim IK, Hong YS, Kim SS, Yoo KJ, Park HY, Jang Y, Chang BC, Choi CY, Hwang KC, Kim BS. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506–15. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138–9. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 45.Hibino N, Shin'oka T, Matsumura G, Ikada Y, Kurosawa H. The tissue-engineered vascular graft using bone marrow without culture. J Thorac Cardiovasc Surg. 2005;129:1064–70. doi: 10.1016/j.jtcvs.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 46.Matsumura G, Hibino N, Ikada Y, Kurosawa H, Shin'oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24:2303–8. doi: 10.1016/s0142-9612(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 47.Matsumura G, Miyagawa-Tomita S, Shin'oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108:1729–34. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 48.Shin'oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–8. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 49.Kern PA, Knedler A, Eckel RH. Isolation and culture of microvascular endothelium from human adipose tissue. J Clin Invest. 1983;71:1822–9. doi: 10.1172/JCI110937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arts CH, De Groot P, Heijnen-Snyder GJ, Blankensteijn JD, Eikelboom BC, Slaper-Cortenbach IC. Application of a clinical grade CD34-mediated method for the enrichment of microvascular endothelial cells from fat tissue. Cytotherapy. 2004;6:30–42. doi: 10.1080/14653240310004476. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Estrada OM, Munoz-Santos Y, Julve J, Reina M, Vilaro S. Human adipose tissue as a source of Flk-1+ cells: new method of differentiation and expansion. Cardiovasc Res. 2005;65:328–33. doi: 10.1016/j.cardiores.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 52.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue:implica-tions for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 53.L'Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NA, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–5. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell JH, Efendy JL, Campbell GR. Novel vascular graft grown within recipient's own peritoneal cavity. Circ Res. 1999;85:1173–8. doi: 10.1161/01.res.85.12.1173. [DOI] [PubMed] [Google Scholar]
- 55.Chue WL, Campbell GR, Caplice N, Muhammed A, Berry CL, Thomas AC, Bennett MB, Campbell JH. Dog peritoneal and pleural cavities as bioreactors to grow autologous vascular grafts. J Vasc Surg. 2004;39:859–67. doi: 10.1016/j.jvs.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Girton TS, Oegema TR, Grassl ED, Isenberg BC, Tranquillo RT. Mechanisms of stiffening and strengthening in media-equivalents fabricated using glycation. J Biomech Eng. 2000;122:216–23. doi: 10.1115/1.429652. [DOI] [PubMed] [Google Scholar]
- 57.Miwa H, Matsuda T, Iida F. Development of a hierarchically structured hybrid vascular graft biomimicking natural arteries. Asaio J. 1993;39:M273–7. [PubMed] [Google Scholar]
- 58.Berglund JD, Mohseni MM, Nerem RM, Sambanis A. A biological hybrid model for collagen-based tissue engineered vascular constructs. Biomaterials. 2003;24:1241–54. doi: 10.1016/s0142-9612(02)00506-9. [DOI] [PubMed] [Google Scholar]
- 59.Berglund JD, Nerem RM, Sambanis A. Incorporation of intact elastin scaffolds in tissue-engineered collagen-based vascular grafts. Tissue Eng. 2004;10:1526–35. doi: 10.1089/ten.2004.10.1526. [DOI] [PubMed] [Google Scholar]
- 60.He H, Matsuda T. Arterial replacement with compliant hierarchic hybrid vascular graft: biomechanical adaptation and failure. Tissue Eng. 2002;8:213–24. doi: 10.1089/107632702753724987. [DOI] [PubMed] [Google Scholar]
- 61.Isenberg BC, Tranquillo RT. Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng. 2003;31:937–49. doi: 10.1114/1.1590662. [DOI] [PubMed] [Google Scholar]
- 62.Seliktar D, Black RA, Vito RP, Nerem RM. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28:351–62. doi: 10.1114/1.275. [DOI] [PubMed] [Google Scholar]
- 63.Boland ED, Matthews JA, Pawlowski KJ, Simpson DG, Wnek GE, Bowlin GL. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci. 2004;9:1422–32. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 64.Patel A, Fine B, Sandig M, Mequanint K. Elastin biosynthesis: The missing link in tissue-engineered blood vessels. Cardiovasc Res. 2006;71:40–9. doi: 10.1016/j.cardiores.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 65.Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339–50. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 66.Grassl ED, Oegema TR, Tranquillo RT. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J Biomed Mater Res. 2002;60:607–12. doi: 10.1002/jbm.10107. [DOI] [PubMed] [Google Scholar]
- 67.Isenberg BC, Williams C, Tranquillo RT. Endothelialization and flow conditioning of fibrin-based media-equivalents. Ann Biomed Eng. 2006;34:971–85. doi: 10.1007/s10439-006-9101-0. [DOI] [PubMed] [Google Scholar]
- 68.Yao L, Swartz DD, Gugino SF, Russell JA, Andreadis ST. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng. 2005;11:991–1003. doi: 10.1089/ten.2005.11.991. [DOI] [PubMed] [Google Scholar]
- 69.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288:H1451–60. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 70.Higgins SP, Solan AK, Niklason LE. Effects of polyglycolic acid on porcine smooth muscle cell growth and differentiation. J Biomed Mater Res A. 2003;67:295–302. doi: 10.1002/jbm.a.10599. [DOI] [PubMed] [Google Scholar]
- 71.Mooney DJ, Mazzoni CL, Breuer C, McNamara K, Hern D, Vacanti JP, Langer R. Stabilized polyglycol-ic acid fibre-based tubes for tissue engineering. Biomaterials. 1996;17:115–24. doi: 10.1016/0142-9612(96)85756-5. [DOI] [PubMed] [Google Scholar]
- 72.Opitz F, Schenke-Layland K, Cohnert TU, Starcher B, Halbhuber KJ, Martin DP, Stock UA. Tissue engineering of aortic tissue:dire consequence of suboptimal elastic fiber synthesis in vivo. Cardiovasc Res. 2004;63:719–30. doi: 10.1016/j.cardiores.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Shum-Tim D, Stock U, Hrkach J, Shinoka T, Lien J, Moses MA, Stamp A, Taylor G, Moran AM, Landis W, Langer R, Vacanti JP, Mayer JE., Jr Tissue engineering of autologous aorta using a new biodegradable polymer. Ann Thorac Surg. 1999;68:2298–304. doi: 10.1016/s0003-4975(99)01055-3. [DOI] [PubMed] [Google Scholar]
- 74.McGlohorn JB, Holder WD, Jr, Grimes LW, Thomas CB, Burg KJ. Evaluation of smooth muscle cell response using two types of porous polylactide scaffolds with differing pore topography. Tissue Eng. 2004;10:505–14. doi: 10.1089/107632704323061861. [DOI] [PubMed] [Google Scholar]
- 75.Miller DC, Thapa A, Haberstroh KM, Webster TJ. Endothelial and vascular smooth muscle cell function on poly(lactic-co-glycolic acid) with nano-structured surface features. Biomaterials. 2004;25:53–61. doi: 10.1016/s0142-9612(03)00471-x. [DOI] [PubMed] [Google Scholar]
- 76.Ye Q, Zund G, Jockenhoevel S, Schoeberlein A, Hoerstrup SP, Grunenfelder J, Benedikt P, Turina M. Scaffold precoating with human autologous extracellular matrix for improved cell attachment in cardiovascular tissue engineering. ASAIOJ. 2000;46:730–3. doi: 10.1097/00002480-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 77.Dahl SL, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659–66. [PubMed] [Google Scholar]
- 78.Tamura N, Nakamura T, Terai H, Iwakura A, Nomura S, Shimizu Y, Komeda M. A new acellular vascular prosthesis as a scaffold for host tissue regeneration. Int J Artif Organs. 2003;26:783–92. [PubMed] [Google Scholar]
- 79.Teebken OE, Bader A, Steinhoff G, Haverich A. Tissue engineering of vascular grafts: human cell seeding of decellularised porcine matrix. Eur J Vasc Endovasc Surg. 2000;19:381–6. doi: 10.1053/ejvs.1999.1004. [DOI] [PubMed] [Google Scholar]
- 80.Amiel GE, Komura M, Shapira O, Yoo JJ, Yazdani S, Berry J, Kaushal S, Bischoff J, Atala A, Soker S. Engineering of blood vessels from acellular collagen matrices coated with human endothelial cells. Tissue Eng. 2006 doi: 10.1089/ten.2006.12.2355. [DOI] [PubMed] [Google Scholar]
- 81.McFetridge PS, Daniel JW, Bodamyali T, Horrocks M, Chaudhuri JB. Preparation of porcine carotid arteries for vascular tissue engineering applications. J Biomed Mater Res A. 2004;70:224–34. doi: 10.1002/jbm.a.30060. [DOI] [PubMed] [Google Scholar]
- 82.Simionescu DT, Lu Q, Song Y, Lee JS, Rosenbalm TN, Kelley C, Vyavahare NR. Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials. 2006;27:702–13. doi: 10.1016/j.biomaterials.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 83.Kallenbach K, Leyh RG, Lefik E, Walles T, Wilhelmi M, Cebotari S, Schmiedl A, Haverich A, Mertsching H. Guided tissue regeneration: porcine matrix does not transmit PERV. Biomaterials. 2004;25:3613–20. doi: 10.1016/j.biomaterials.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 84.Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, Carabasi RA, Dimuzio PJ. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146–53. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 85.Daniel J, Abe K, McFetridge PS. Development of the human umbilical vein scaffold for cardiovascular tissue engineering applications. ASAIO J. 2005;51:252–61. doi: 10.1097/01.mat.0000160872.41871.7e. [DOI] [PubMed] [Google Scholar]
- 86.Li M, Mondrinos MJ, Chen X, Gandhi MR, Ko FK, Lelkes PI. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering scaffolds. J Biomed Mater Res A. 2006 doi: 10.1002/jbm.a.30833. [DOI] [PubMed] [Google Scholar]
- 87.Stitzel J, Liu J, Lee SJ, Komura M, Berry J, Soker S, Lim G, Van Dyke M, Czerw R, Yoo JJ, Atala A. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27:1088–94. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 88.Greenwald SE, Berry CL. Improving vascular grafts: the importance of mechanical and haemodynamic properties. J Pathol. 2000;190:292–9. doi: 10.1002/(SICI)1096-9896(200002)190:3<292::AID-PATH528>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 89.Hoerstrup SP, Cummings Mrcs I, Lachat M, Schoen FJ, Jenni R, Leschka S, Neuenschwander S, Schmidt D, Mol A, Gunter C, Gossi M, Genoni M, Zund G. Functional growth in tissue-engineered living, vascular grafts:follow-up at 100 weeks in a large animal model. Circulation. 2006;114:I159–66. doi: 10.1161/CIRCULATIONAHA.105.001172. [DOI] [PubMed] [Google Scholar]
- 90.Buttafoco L, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, Feijen J. Physical characterization of vascular grafts cultured in a bioreactor. Biomaterials. 2006;27:2380–9. doi: 10.1016/j.biomaterials.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 91.Engbers-Buijtenhuijs P, Buttafoco L, Poot AA, Dijkstra PJ, De Vos RA, Sterk LM, Geelkerken RH, Vermes I, Feijen J. Biological characterisation of vascular grafts cultured in a bioreactor. Biomaterials. 2006;27:2390–7. doi: 10.1016/j.biomaterials.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 92.Bilodeau K, Couet F, Boccafoschi F, Mantovani D. Design of a perfusion bioreactor specific to the regeneration of vascular tissues under mechanical stresses. Artif Organs. 2005;29:906–12. doi: 10.1111/j.1525-1594.2005.00154.x. [DOI] [PubMed] [Google Scholar]
- 93.McCulloch AD, Harris AB, Sarraf CE, Eastwood M. New multi-cue bioreactor for tissue engineering of tubular cardiovascular samples under physiological conditions. Tissue Eng. 2004;10:565–73. doi: 10.1089/107632704323061924. [DOI] [PubMed] [Google Scholar]
- 94.Narita Y, Hata K, Kagami H, Usui A, Ueda M, Ueda Y. Novel pulse duplicating bioreactor system for tissue-engineered vascular construct. Tissue Eng. 2004;10:1224–33. doi: 10.1089/ten.2004.10.1224. [DOI] [PubMed] [Google Scholar]
- 95.Sodian R, Lemke T, Fritsche C, Hoerstrup SP, Fu P, Potapov EV, Hausmann H, Hetzer R. Tissue-engineering bioreactors: a new combined cell-seeding and perfusion system for vascular tissue engineering. Tissue Eng. 2002;8:863–70. doi: 10.1089/10763270260424222. [DOI] [PubMed] [Google Scholar]
- 96.Thompson CA, Colon-Hernandez P, Pomerantseva I, MacNeil BD, Nasseri B, Vacanti JP, Oesterle SN. A novel pulsatile, laminar flow bioreactor for the development of tissue-engineered vascular structures. Tissue Eng. 2002;8:1083–8. doi: 10.1089/107632702320934173. [DOI] [PubMed] [Google Scholar]
- 97.Williams C, Wick TM. Perfusion bioreactor for small diameter tissue-engineered arteries. Tissue Eng. 2004;10:930–41. doi: 10.1089/1076327041348536. [DOI] [PubMed] [Google Scholar]
- 98.Braddon LG, Karoyli D, Harrison DG, Nerem RM. Maintenance of a functional endothelial cell monolayer on a fibroblast/polymer substrate under physiologically relevant shear stress conditions. Tissue Eng. 2002;8:695–708. doi: 10.1089/107632702760240607. [DOI] [PubMed] [Google Scholar]
- 99.Lee AA, Graham DA, Dela Cruz S, Ratcliffe A, Karlon WJ. Fluid shear stress-induced alignment of cultured vascular smooth muscle cells. J Biomech Eng. 2002;124:37–43. doi: 10.1115/1.1427697. [DOI] [PubMed] [Google Scholar]
- 100.Nerem RM. Role of mechanics in vascular tissue engineering. Biorheology. 2003;40:281–7. [PubMed] [Google Scholar]
- 101.Moore MM, Goldman J, Patel AR, Chien S, Liu SQ. Role of tensile stress and strain in the induction of cell death in experimental vein grafts. J Biomech. 2001;34:289–97. doi: 10.1016/s0021-9290(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 102.Seliktar D, Nerem RM, Galis ZS. The role of matrix metalloproteinase-2 in the remodeling of cell-seeded vascular constructs subjected to cyclic strain. Ann Biomed Eng. 2001;29:923–34. doi: 10.1114/1.1415522. [DOI] [PubMed] [Google Scholar]
- 103.Seliktar D, Nerem RM, Galis ZS. Mechanical strain-stimulated remodeling of tissue-engineered blood vessel constructs. Tissue Eng. 2003;9:657–66. doi: 10.1089/107632703768247359. [DOI] [PubMed] [Google Scholar]
- 104.Nikolovski J, Kim BS, Mooney DJ. Cyclic strain inhibits switching of smooth muscle cells to an osteoblast-like phenotype. FASEB J. 2003;17:455–7. doi: 10.1096/fj.02-0459fje. [DOI] [PubMed] [Google Scholar]
- 105.Liu SQ. Biomechanical basis of vascular tissue engineering. Crit Rev Biomed Eng. 1999;27:75–148. doi: 10.1615/critrevbiomedeng.v27.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 106.Solan A, Mitchell S, Moses M, Niklason L. Effect of pulse rate on collagen deposition in the tissue-engineered blood vessel. Tissue Eng. 2003;9:579–86. doi: 10.1089/107632703768247287. [DOI] [PubMed] [Google Scholar]
- 107.Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–44. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 108.Ogle BM, Mooradian DL. Manipulation of remodeling pathways to enhance the mechanical properties of a tissue engineered blood vessel. J Biomech Eng. 2002;124:724–33. doi: 10.1115/1.1519278. [DOI] [PubMed] [Google Scholar]
- 109.Joddar B, Ramamurthi A. Fragment size- and dose-specific effects of hyaluronan on matrix synthesis by vascular smooth muscle cells. Biomaterials. 2006;27:2994–3004. doi: 10.1016/j.biomaterials.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 110.Borschel GH, Huang YC, Calve S, Arruda EM, Lynch JB, Dow DE, Kuzon WM, Dennis RG, Brown DL. Tissue engineering of recellularized small-diameter vascular grafts. Tissue Eng. 2005;11:778–86. doi: 10.1089/ten.2005.11.778. [DOI] [PubMed] [Google Scholar]
- 111.Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N England J Med. 2001;344:532–3. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 112.L'Heureux N, Dusserre N, Konig G, Fuente L, Marini A, Avila H, Manglano X, Robbins RC, Garrido S. American Heart Association's Scientific Sessions 2005 (Dallas, TX). 2005; abstract 2982.
- 113.Horch RE. Future perspectives in tissue engineering. J Cell Mol Med. 2006;10:4–6. doi: 10.1111/j.1582-4934.2006.tb00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bleiziffer O, Eriksson E, Yao F, Horch RE, Kneser U. Gene transfer strategies in tissue engineering. J Cell Mol Med. 2007;11:206–23. doi: 10.1111/j.1582-4934.2007.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zeng F, Chen MJ, Baldwin DA, Gong ZJ, Yan JB, Qian H, Wang J, Jiang X, Ren ZR, Sun D, Huang SZ. Multiorgan engraftment and differentiation of human cord blood CD34+ Lin- cells in goats assessed by gene expression profiling. Proc Natl Acad Sci USA. 2006;103:7801–6. doi: 10.1073/pnas.0602646103. [DOI] [PMC free article] [PubMed] [Google Scholar]


