Abstract
A considerable body of evidence has revealed that interstitial cells of Cajal (ICC), identified with c-Kit-immunoreactivity, act as gut pacemaker cells, with spontaneous Ca2+ activity in ICC as the probable primary mechanism. Namely, intracellular (cytosolic) Ca2+ oscillations in ICC periodically activate plasmalemmal Ca2+-dependent ion channels and thereby generate pacemaker potentials. This review will, thus, focus on Ca2+-associated mechanisms in ICC in the gastrointestinal (GI) tract, including auxiliary organs.
Keywords: interstitial cells of Cajal, calcium oscillations, smooth muscle, voltage-gated Ca2+channels, non-selective cation channels, transient receptor potential (TRP) homologues, Ca2+-activated Cl−channels, inositol trisphosphate receptors, ryanodine receptors, c-Kit
Introduction
Research has shown that Ca2+-dependent plasmalemmal ion channels are responsible for interstitial cells of Cajal (ICC) pacemaker potentials [1–3], and spontaneous Ca2+ activity in ICC is considered the primary mechanism. Namely, oscillations of the intracellular (cytosolic) Ca2+ concentration ([Ca2+]i) in ICC periodically activate plasmalemmal Ca2+-dependent ion channels, thereby generating pacemaker potentials. This review will, thus, focus on Ca2+-associated mechanisms in ICC in the gastrointestinal (GI) tract including auxiliary organs.
Numerous preparations and methods are used in studies of various types of ICC contained in the GI tract. In the following sections, types of ICC are identified in descriptions of most tissue-level experiments; the term ICC represents interstitial cells expressing c-Kit or other ICC markers in experiments using isolated cells and cultured preparations.
Voltage-gated Ca2+ channels
Voltage-gated Ca2+ channels (VGCC) are thought to play a central role in E-C coupling in smooth muscle cells, including GI smooth muscle. L-type (high voltage-activated [HVA]) Ca2+ channels appear to be predominant in most smooth muscle cells because dihydropyridine (DHP) Ca2+ antagonists, such as nifedipine and nicardipine largely depress contractile activity. It has been shown that the α1-subunit of the smooth muscle L-type Ca2+ channel (Cav1.2b) is a splice variant of the cardiac one (Cav1.2a) and has a higher sensitivity to DHP Ca2+ antagonists [4].
Guinea-pig stomach smooth muscle, frequently used to investigate mechanisms underlying spontaneous electrical activity, referred to as slow waves [5], provides a good example with which we can assess the role of VGCC in smooth muscle tissues showing spontaneous phasic contractions. DHP Ca2+ antagonists completely abolish spontaneous contractile activity, with little effect, however, on pacemaker potentials (and electrical activity recorded from smooth muscle cells, that is, slow waves) [6, 7]. Similar spontaneous electrical activities resistant to the DHP-Ca2+ antagonist have been reported in several other GI smooth muscle tissues [8,9]. It is thus considered that DHP-sensitive L-type Ca2+ channels play an essential role in E–C coupling in GI smooth muscle cells, although these channels are not involved in the generation of pacemaker electrical activity in ICC (Fig. 1). For this reason, DHP-Ca2+ antagonists are frequently used to differentiate pacemaker electrical activity by suppressing smooth muscle activity. However, pacemaker cells in some tissues, for example, sub-mucosal ICC (ICC-SM) in the colon, produce different responses to DHP Ca2+ antagonists: 1 μM nifedipine completely abolishes the spontaneous plateau potentials [10]. Furthermore, in the guinea-pig stomach, a small inhibitory effect was observed when nifedipine was greater than 10 μM [7].
1.
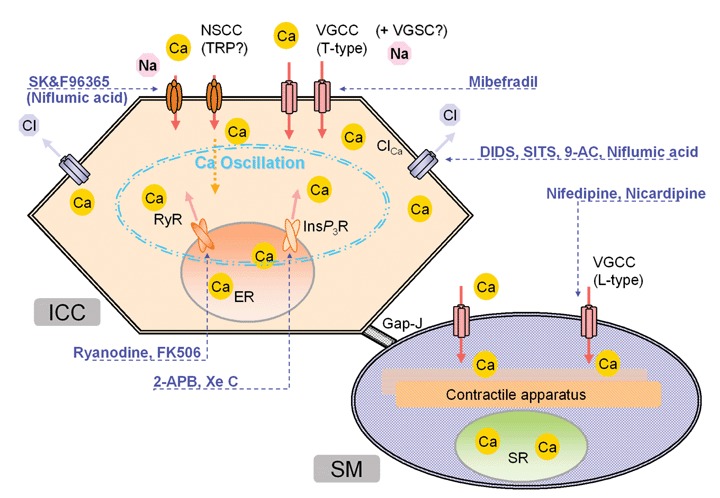
The action sites of drugs on Ca2+-associated mechanisms in ICC pacemaker activity. ICC and SM in this figure represent interstitial cells of Cajal and smooth muscle cells, respectively. For other abbreviations, see text.
In cardiac pacemaker cells, T-type (low voltage-activated [LVA]) Ca2+ channels, known to play an important role in pacemaking, are suppressed with low concentrations (∼40 μM) of Ni2+[11]. Applications of similar concentrations of Ni2+ to guinea-pig stomach smooth muscle tissue (including the smooth muscle layer and myenteric plexus) have little effect on spontaneous electrical activity [7]. On the other hand, in the isolated circular smooth muscle layer, which does not contain myenteric ICC (ICC-MY) but contains only intramuscular ICC (ICC-IM), very low concentrations of Ni2+ (1–10 μM) significantly suppress spontaneous electrical activity. The inhibitory effect is more potent in the plateau phase than in the initial upstroke of pacemaker electrical activity, agreeing well with the notion that ICC-IM produces re-generative potentials forming the plateau phase [12,13]. However, the discrepancy in the Ni2+ concentration range may suggest the existence of Ni2+-sensitive mechanisms other than T-type Ca2+ channels involved in ICC-IM. Recent reverse transcriptase-polymerase chain reaction (RT-PCR) examinations have provided supporting evidence that neither the L- nor the T-type Ca2+ channel gene was detected in ICC-DMP (deep muscular plexus) and ICC-IM of the murine and human small intestine [14].
The existence of VGCC may differ depending on the ICC types, locations of the gut and species. Ward and Sanders [15,16] reported that 40 μM Ni2+ cause sizeable reduction (>50%) in the upstroke velocity of spontaneous electrical activity in canine colon, and a combined application of nifedipine (1 μM) and Ni2+ (40 μM) abolishes it. These results suggest a major contribution of T-type Ca2+ channels. In ICC-SM of the murine colon, Hotta et al.[17] reported that the application of either mibefradil (3 μM) or Ni2+ (100 μM) significantly reduced the rate of rise in the upstroke of pacemaker potentials. Kim et al.[18] recorded VGCC currents either sensitive or resistant to DHP Ca2+ antagonists from ICC in the murine colon and small intestine. The DHP-resistant component of VGCC current is blocked by a higher concentration (100 μM) of Ni2+ or by a T-type Ca2+ channel antagonist, mibefradil. The inhibitory effect of mibefradil may involve the blockade of voltage-gated Na+ channels (VGSC) resistant to tetrodotoxin (TTX) (Nav1.5 encoded by SCN5A), which has been shown to exist in ICC [19,20].
Non-selective cation channels
Non-selective cation channels (NSCC) can carry an electric charge for ICC pacemaking current, and many of these channels can permeate Ca2+. It is well-known that spontaneous electrical activities in GI smooth muscle tissues require extracellular Ca2+[5]. Therefore, NSCC may make a significant contribution to ICC pacemaking.
Under a voltage clamp condition, Thomsen et al.[21] and Koh et al.[22] recorded oscillating inward currents from cultured ICC of the murine small intestine. Removal of extracellular Na+ abolishes the oscillatory inward currents [22], suggesting that oscillating inward currents are produced by the periodic activation of NSCC. Nakayama and Torihashi [23] showed that high concentrations (100–120 μM) of Cd2+ and Ni2+ suppress oscillatory inward currents in cell cluster preparations isolated from the murine small intestine, which contains ICC. Using a special thin muscle layer preparation made by enzymatic treatment under hydrostatic pressure, Goto et al.[24] demonstrated that depolarization steps can evoke large inward currents through NSCC in ICC showing spontaneous electrical activity.
Transient receptor potential (TRP) homologues form NSCC. Epperson et al.[25] and Liu et al.[26] detected mRNA of classical (or canonical) TRP (TRPC), such as TRPC2, TRPC4 and TRPC6 in ICC, using RT-PCR. Torihashi et al.[27] showed immuno-histochemical evidence for the expression of TRPC4 in the caveolae where numerous cellular signals interact. Walker et al.[3] recorded oscillatory inward currents similar to TRPC4: a NSCC inward current inhibited by Ca2+ (see also Note added in proof).
Melastatin-type TRP (TRPM) homologues are channel/enzyme fusion proteins. TRPM6 and TRPM7 (formerly referred to as <TRPC>), which contain a kinase domain in the C-terminus, are well-known to act as Mg2+-permeable channels [28–30]. Kim et al.[31,32] showed mRNA of TRPM2, TRPM4, TRPM7 and TRPM8 in cultured ICC from the murine small intestine, and they reported that TRPM7 channels play an essential role in generating oscillatory currents in ICC;that is, the ionic selectivity and pharmacological properties are essentially the same between TRPM7 and ICC oscillatory currents. The authors also showed that the knockdown of TRPM7 by the use of siRNA suppressed spontaneous electrical activity in ICC. However, the reduction of TRPM7 expression may affect ICC pacemaking through intracellular Mg2+ homeostasis and cell viability [30,33]. The regulation of intracellular Mg2+via TRPM-like Mg2+-permeable channels has been shown in intestinal [34,35] and vascular smooth muscle cells [36–38].
The frequency and duration of GI pacemaker activity are largely modulated by temperature and energy metabolism [23,39,40]. Nakamura et al.[41] suggested that in such modulations of pacemaker activity, several pathways are operating in parallel. Although the mRNA expression of TRPM4 and TRPM8 has been shown in cultured murine ICC [31,32], the existence of vanilloid type (TRPV) and ankyrin-like TRP (TRPA) channels has not yet been assessed. TRPV1, TRPV2, TRPV3, TRPV4, TRPM2, TRPM4 and TRPM5 are heat activated, whereas TRPM8 and TRPA1 are cold activated [42]. Further investigation into TRP homologue channels may, therefore, clarify the mechanisms underlying the characteristic features of GI pacemaker activity. In addition, mitochondria [43] and sulfonylurea receptors (SUR) [44,45] may also contribute to the temperature- and energy-dependence of ICC pacemaking.
Cl− channels
Many reports have suggested that Cl− channels carry pacemaker current in ICC.Spontaneous [Ca2+]i oscillations would periodically activate Ca2+-activated Cl− channels if such channels exist in ICC. In guinea-pig stomach, Dickens et al.[6] demonstrated that ICC-MY can generate giant pacemaker potentials of ∼50 mV in amplitude, reaching −20 mV in the plateau phase. This potential is close to the equilibrium potential of Cl− (ECl:−24 mV) estimated from the intracellular Cl− concentration ([Cl−]i: 42 mM) measured in vas deference smooth muscle cells (i.e., [Cl−]o: 103 mM) [46,47]. Measurements of [Cl−]i in ICC will provide valuable information for the debate over whether Cl− channels or NSCC are responsible for the pacemaker current.
In early ICC research, Tokutomi et al.[1] recorded oscillatory inward currents in ICC ( = c-Kit-positive interstitial cells) isolated from the murine small intes-tine. This current showed Ca2+-dependence and was sensitive to a Cl− channel blocker, 4-acetoamido-4-isothiocyanat-ostilbene-2, 2′-disulfonic acid (SITS). Since then, there has been a growing body of evidence that ICC electrical activity is suppressed by other Cl− channel blockers, such as 4, 4′-diisothio-cyanostilbene-2, 2′-disulfonic acid (DIDS) and anthracene-9-carboxylic acid (9-AC) [9, 20, 48, 49]. Huizinga et al.[2] demonstrated the existence of large-conductance (maxi) chloride channels at the single-channel level of patch-clamp recording. On the other hand, Koh et al.[50] reported that a Cl−channel blocker, niflumic acid, blocks NSCC in ICC.
The existence of small conductance Ca2+-activated K+ channels (SK3 and SK4) has also been shown in ICC of the rat GI tract [51]. In each [Ca2+]i oscillation cycle, these channels may contribute to the repolarization phase of the pacemaker potential.
[Ca2+]i oscillations
[Ca2+]i oscillations in ICC are thought to be a primary mechanism for the generation of pacemaker potentials, which may account for characteristic features of GI pacemaker activity, such as the low voltage sensitivity of the frequency. Publicover et al.[52] reported [Ca2+]i oscillations in freshly dispersed and cultured ICC-like cells from canine colon, although c-Kit-immunoreactivity was unidentified. DHP Ca2+ antagonists suppress [Ca2+]i oscillations in these ICC-like cells. Yamazawa and Iino [53] recorded [Ca2+]i oscillations resistant to DHP Ca2+ antagonists in ICC of the murine small intestine. No such spontaneous [Ca2+]i activity was observed in W/Wv mice lacking ICC.
Using cell cluster preparations from the murine small intestine, Torihashi et al.[27] and Nakayama et al.[23] recorded [Ca2+]i oscillations synchronized with spontaneous electrical and mechanical activities (Fig. 2). These results agree well with the hypothesis that [Ca2+]i oscillations in ICC generate pacemaker electrical activity by periodically activating Ca2+-activated ion channels in the plasma membrane (Scenario 1 in Fig. 3). Moreover, in guinea-pig and mouse stomach ICC, [Ca2+]i oscillations appear to be associated with spontaneous electrical and mechanical activities [26,54–56].
2.
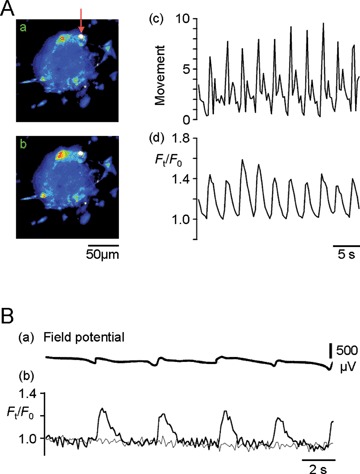
ICC [Ca2+]i oscillations synchronized with mechanical (A) and electrical (B) activities in cell cluster preparations isolated from the muscle layer of the murine small intestine. This figure was made by modifying Figure 2 of [44] and Figure 4 of [27]. (A): Ca2+ images (fluo-3 fluorescence) obtained from a cell cluster preparation with a high intensity area that could be used to monitor mechanical activity. Panels (a) and (b) are pseudo-colour Ca2+ images acquired at basal and peak times of an initial oscillation cycle in normal solution, respectively. The mechanical activity (c: movement) was estimated by tracking the high intensity area indicated by the arrow in (a). The time course of [Ca2+]i oscillations (d) was measured in the square region (red line) of the cell cluster preparation shown in (a). After this recording, using a K+ channel opener to suppress smooth muscle activity, we confirmed that ICC produced the Ca2+ activity in the square region [44]. The fluorescence is expressed relative to that at the initial basal time: Ft/F0.(B) Field potential (a) and fluo-4 fluorescence (b) were measured simultaneously in a cell cluster preparation in the presence of nifedipine which differentiates ICC activity by suppressing smooth muscle activity [27]. Thick and thin lines in (b) represent ICC and non-ICC regions of a cell cluster preparation, respectively.
3.
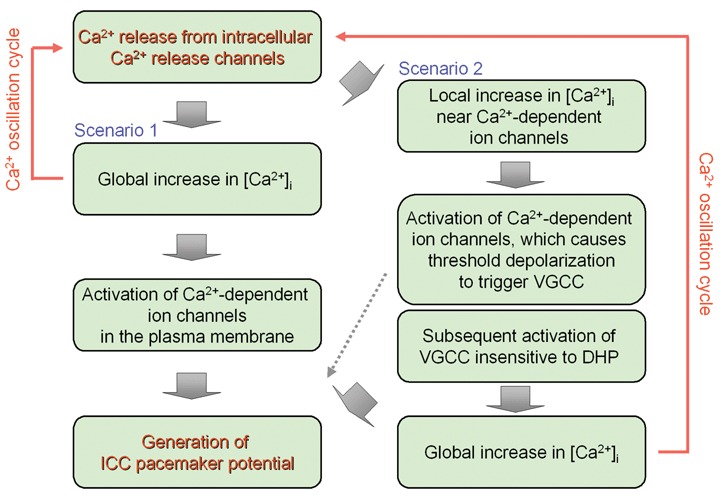
Plausible mechanisms linking spontaneous [Ca2+]i oscillations and pacemaker potentials in ICC.
Using high time-resolution Ca2+ measurements, Park et al.[57] recently showed that the rising phase of [Ca2+]i oscillation in ICC follows the upstroke of the electrical activity recorded from a near-by cell, with a short delay (∼60 ms). VGCC insensitive to DHP or VGSC [18–20] may be responsible for the depolarization preceding the [Ca2+]i rise. Localized elevation of [Ca2+]i in the vicinity of the plasma membrane may first cause the activation of Ca2+-activated ion channels, resulting in depolarization to trigger VGCC and a subsequent global increase in [Ca2+]i (Scenario 2 in Fig. 3). Further investigation is necessary to elucidate the details of mechanisms that link [Ca2+]i oscillationsand pacemaker potentials and to comprehensively address the cell-to-cell coupling among pacemaker cells and smooth muscle cells [58,59].
Ca2+ influx from the extracellular space appears to play an essential role in ICC pacemaker [Ca2+]i activity because the removal of extracellular Ca2+ abolishes it [27]. NSCC may act as this Ca2+ pathway. Applications of Ni2+ and Cd2+ (>100 μM), which competitively block NSCC, suppress [Ca2+]i oscillations in ICC [27,57,60]. Furthermore, SK&F96365 suppresses or terminates [Ca2+]i oscillations [26,27]. SK&F96365 is often used to suppress Ca2+ influx pathways from the extracellular space, including TRP homologue channels, but does not block DHP-sensitive VGCC [61].
ICC can express numerous receptors of neurotransmitters: purinoceptors, neurokinin receptors, muscarinic receptors and prostaglandin receptors [14,63–66]. Furuzono et al.[67] showed dual effects of ATP and analogues on [Ca2+]i oscillations in ICC of the murine small intestine: excitation in the presence of TTX and inhibition in the absence of TTX. It is suggested that NO released from nitrergic nerves via activation of purinoceptors suppresses [Ca2+]i oscillations in ICC, presumably through a cGMP sig-nalling pathway, while in the presence of TTX, a [Ca2+]i transient is evoked through the activation of purinoceptors on the surface of ICC.
Intracellular Ca2+ release channels
Although Ca2+ influx from the extracellular space is likely essential to maintain [Ca2+]i oscillations in ICC, Ca2+ release from intracellular Ca2+ stores, that is, endoplasmic retuculum (ER), appears to make a major contribution to the [Ca2+]i increase. Suzuki et al.[68] and Takano et al.[69] showed that spontaneous electrical and mechanical activities are greatly impaired in the stomach smooth muscle of mice lacking the type-1 inositol trisphosphate receptor (InsP3R1). Liu et al.[26] showed that among three InsP3R isoforms InsP3R1 and InsP3R2 are predominant in ICC in the murine stomach. Aoyama et al.[62] reported that InsP3R2 and InsP3R3 are predominant in the small intestine. Taken together, these findings suggest that InsP3R1 expressed in stomach ICC plays an important role in generating pacemaker activity on its own without using intercellular mechanisms, while the role of InsP3R1 may be substituted by InsP3R2 and/or InsP3R3 in small intestine ICC. Therefore, it is of interest to check whether spontaneous activity is preserved in the small intestine of mutant mice lacking InsP3R1.
There is an increasing body of pharmacological evidence for the involvement of InsP3R in ICC pacemaker activity. The applications of 2-aminoethoxy-diphenyl borate (2-APB) and xestospongin C (Xe C), membrane-permeable blockers for InsP3R, suppress or terminate ICC electrical and [Ca2+]i activities in numerous GI preparations (Table 1) [26, 55, 57, 58, 60, 62, 70–73]. It has also been shown that the application of heparin with a reversible permeabilization loading procedure suppressed depolarization-induced electrical activities reflecting ICC-IM activity [74]. On the other hand, 2-APB affects TRP homologue channels, including TRPM7 [75]. The inhibitory effect of 2-APB on [Ca2+]i oscillations might involve the blockage of TRPM7 because this channel reportedly plays an essential role in generating ICC pacemaker activity [31, 32].
1.
Inhibitory effects of numerous Ca2+-related drugs on pacemaking [Ca2+]i activity in ICC and ICC-like interstitial cells (+) and (−) represent an inhibitory effect and a lack of significant effect, respectively. The numbers in brackets indicate refer-ences. Single crosses (†) indicate examinations of drugs on pacemaker potentials, not on [Ca2+]i oscillations
| DHP Ca2+ blockers | Mibefradil | SK&F96365 | 2-APB | Xe C | Ryanodine | |
|---|---|---|---|---|---|---|
| Murine stomach | (−): [26, 56] | (+):[26] | (+):[26] | (+):[26] | (+):[56] | |
| Murine small intestine | (−):[27, 53, 57,62, 67] | (+):[57] | (+):[27] | (+):[57, 62] | (+):[62, 70] | (+):[62] (−):[57, 70] |
| Murine colon | (+):[10]† | (+):[17]† | ||||
| Canine colon | (+):[52] | (−):[52] | ||||
| Guinea-pig stomach | (−):[55, 80] | (+):[55, 58, 80] | (−):[80] | |||
| Human small intestine | (−):[60] | (+):[60] | (+):[60] | (−):[60] | ||
| Rabbit portal vein | (−):[78] | (+):[78] | (+):[78] | (+):[78] | (+):[78] | |
| Rabbit urethra | (−):[79] | (+):[79] | (+):[79] | (+):[79] |
Another important Ca2+ release channel is the ryanodine receptor (RyR). Using cell cluster preparations from the murine small intestine, Aoyama et al.[62] showed that in addition to blockers for InsP3R and Ca2+ influx, RyR blockers and FK506, which modulates RyR activity through FK506-binding pro-teins (FKBP), all suppress ICC pacemaker [Ca2+]i oscillations. RT-PCR examinations of ICC revealed a predominant expression of RyR3 and a corresponding expression pattern of FKBP isoforms (expression of both FKBP12 and FKBP12.6). Liu et al.[26,56] showed essentially the same results in murine stomach ICC. Also, the application of ryanodine significantly suppresses spontaneous [Ca2+]i oscillations in ICC-like cells of gut-like organ formed from mouse embryonic stem (ES) cells [76]. These results suggest that the coordination of the two families of Ca2+ release channels, that is, RyR and InsP3R, produces ICC pacemaker [Ca2+]i activity under the support of Ca2+ influx from the extracellular space. Furthermore, based on this hypothesis, pacemaker-like cells have been produced by genetic manipulations [62]. Namely, HEK293 cells which express little RyR have yielded spontaneous [Ca2+]i oscillations after the transfection of RyR3. This is also true for RyR2 (Aoyama et al. unpublished observation). Mice lacking RyR3 show apparently normal growth and reproduction [77]. In these mice, RyR2 may compensate for the role of RyR3.
In ICC-like interstitial cells of the rabbit portal vein and urethra, Harhun et al.[78] and Johnston et al.[79], respectively showed essentially the same pharmacological profiles of [Ca2+]i oscillations. In generating [Ca2+]i oscillations, ICC-like cells in these tissues appear to employ both InsP3R and RyR in addition to Ca2+ influx pathways. On the other hand, several studies have reported no significant effect of ryanodine on ICC pacemaker [Ca2+]i activity of the GI tract [57, 60, 70, 80].
Gastrointestinal stromal tumours (GIST), the most common mesenchymal tumours of the human GI tract, are thought to derive from ICC by gain-of-function mutations of c-Kit [81]. The application of the selective c-Kit-receptor inhibitor, imatinib mesylate, which is used to treat advanced GIST, suppresses myogenic activity of the human small intestine [82]. Furuzono et al.[83] reported that isolated ICC-like tumour cells from a human duodenal GIST with the most frequent type of gain-of-function mutation only occasionally produced spontaneous [Ca2+]i activity. These ICC-like GIST cells expressed InsP3R1 and InsP3R2, but RyR2/3 were below detectable levels (Furuzono et al., unpublished observation). The low expression level of RyR may account for the poor spontaneous [Ca2+]i activity in these GIST cells. Evidence is, however, still insufficient to address how intracellular Ca2+ release channels contribute to ICC pacemaking. Comprehensive studies, including the link with Ca2+ influx pathways and other intracellular Ca2+ compartments (e.g. close association of caveolae, ER and mitochondria [84,85]) are required.
ICC-like cells in auxiliary digestive organs
ICC-like cells, that is, c-Kit-positive interstitial cells, exist outside the musculature of the GI tract [86], including the auxiliary organs of the GI tract. Popescu et al.[87] reported the existence of ICC-like cells in the human and rat pancreas. Hinescu et al.[88] and Sun et al.[89] reported ICC-like cells in the human and murine gall bladders, respectively. Lavoie et al.[90] showed spontaneous [Ca2+]i activity in ICC-like cells in the guinea-pig gall bladder and suggested a role of generating spontaneous rhythmicity. Furthermore, ICC-like cells exist in the hepatic portal vein [91,92], which transports nutrient molecules to the liver and is known as a spontaneously active vessel. Harhun et al.[78,93] showed spontaneous [Ca2+]i oscillations associated with membrane depolarizations in ICC-like interstitial cells of the rabbit portal vein and also suggested the contribution of both types of intracellular Ca2+ release channels, that is, InsP3R and RyR. It is of great interest to examine the similarity and dissimilarity of mechanisms underlying spontaneous [Ca2+]i activities between ICC and ICC-like cells distributed over the entire body. Such studies will provide valuable information for planning systematic therapies and for developing tissue- and organ-specific drugs.
Note added in proof
Recently, two research groups reported that spontaneous electrical activity can be still recorded in the GI tract of TRPC4−/− mice (Lee et al., Mol. Cells 2005; 20: 425–31; Sanders et al., Annu. Rev. Physiol. 2006; 68: 307–43). Therapic, it is likely that TRPC4 plays a role in generating ICC pacemaker activity in parallel with after Ca2+- perneable channels.
References
- 1.Tokutomi N, Maeda H, Tokutomi Y, Sato D, Sugita M, Nishikawa S, Nishikawa S, Nakao J, Imamura T, Nishi K. Rhythmic Cl current and physiological roles of the intestinal c-kit-positive cells. Pflügers Arch. 1995;431:169–77. doi: 10.1007/BF00410188. [DOI] [PubMed] [Google Scholar]
- 2.Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterol. 2002;123:1627–36. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- 3.Walker RL, Koh SD, Sergeant GP, Sanders KM, Horowitz B. TRPC4 currents have properties similar to the pacemaker current in interstitial cells of Cajal. Am J Physiol. 2002;283:C1637–45. doi: 10.1152/ajpcell.00266.2002. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann F, Biel M, Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Physiol. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- 5.Tomita T. Electrical activity (spikes and slow waves) in gastrointestinal smooth muscle. In: Bülbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle: An Assessment of Current Knowledge. London: Edward Arnold; 1981. pp. 127–56. [Google Scholar]
- 6.Dickens EJ, Hirst GDS, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514:515–31. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang SM, Nakayama S, Iino S, Tomita T. Voltage sensitivity of slow wave frequency in isolated circular muscle strips from guinea pig gastric antrum. Am J Physiol. 1999;276:G518–28. doi: 10.1152/ajpgi.1999.276.2.G518. [DOI] [PubMed] [Google Scholar]
- 8.Golenhofen K, Lammel E. Selective suppression of some components of spontaneous activity in various types of smooth muscle by iproveratril (verapamil) Pflügers Arch. 1972;331:231–43. [PubMed] [Google Scholar]
- 9.Hirst GDS, Beckett EAH, Sanders KM, Ward SM. Regional variation in contribution of myenteric and intramuscular interstitial cells of Cajal to generation of slow waves in mouse gastric antrum. J Physiol. 2002;540:1003–12. doi: 10.1113/jphysiol.2001.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoneda S, Takano H, Takaki M, Suzuki H. Properties of spontaneously active cells distributed in the submu-cosal layer of mouse proximal colon. J Physiol. 2002;542:887–97. doi: 10.1113/jphysiol.2002.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagiwara K, Irisawa H, Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988;395:233–53. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki H, Hirst GDS. Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J Physiol. 1999;517:563–73. doi: 10.1111/j.1469-7793.1999.0563t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards FR, Hirst GDS, Suzuki H. Unitary nature of regenerative potentials recorded from circular muscle of guinea-pig antrum. J Physiol. 1999;519:235–50. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Redelman D, Ro S, Ward SM, Ördög T, Sanders KM. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol. 2007;292:C497–507. doi: 10.1152/ajpcell.00147.2006. [DOI] [PubMed] [Google Scholar]
- 15.Ward SM, Sanders KM. Dependence of electrical slow waves of canine colonic smooth muscle on calcium gradient. J Physiol. 1992;455:307–19. doi: 10.1113/jphysiol.1992.sp019303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward SM, Sanders KM. Upstroke component of electrical slow waves in canine colonic smooth muscle due to nifedipine-resistant calcium current. J Physiol. 1992;455:321–37. doi: 10.1113/jphysiol.1992.sp019304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotta A, Okada N, Suzuki H. Mibefradil-sensitive component involved in the plateau potential in submucosal interstitial cells of the murine proximal colon. Biochem Biophys Res Commun. 2007;353:170–6. doi: 10.1016/j.bbrc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Kim YC, Koh SD, Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol. 2002;541:797–810. doi: 10.1113/jphysiol.2002.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG, Farrugia G. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol. 2003;285:G1111–21. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- 20.Boddy G, Willis A, Galante G, Daniel EE. Sodium-, chloride-, and mibefradil-sensitive calcium channels in intestinal pacing in wild-type and W/WV mice. Can J Physiol Pharmacol. 2006;84:589–99. doi: 10.1139/y06-009. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen L, Robinson TL, Lee JCF, Farraway LA, Hughes MJG, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998;4:848–51. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- 22.Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 1998;513:203–13. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakayama S, Torihashi S. Spontaneous rhythmicity in cultured cell clusters isolated from mouse small intestine. Jpn J Physiol. 2002;52:217–27. doi: 10.2170/jjphysiol.52.217. [DOI] [PubMed] [Google Scholar]
- 24.Goto K, Matsuoka S, Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol. 2004;559:411–22. doi: 10.1113/jphysiol.2004.063875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epperson A, Hatton WJ, Callaghan B, Doherty P, Walker RL, Sanders KM, Ward SM, Horowitz B. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am J Physiol. 2000;279:C529–39. doi: 10.1152/ajpcell.2000.279.2.C529. [DOI] [PubMed] [Google Scholar]
- 26.Liu H-N, Ohya S, Furuzono S, Wang J, Imaizumi Y, Nakayama S. Co-contribution of IP3R and Ca2+ influx pathways to pacemaker Ca2+ activity in stomach ICC. J Biol Rhythm. 2005;20:15–26. doi: 10.1177/0748730404269572. [DOI] [PubMed] [Google Scholar]
- 27.Torihashi S, Fujimoto T, Trost C, Nakayama S. Calcium oscillation linked to pacemaking of interstitial cells of Cajal. J Biol Chem. 2002;277:19191–7. doi: 10.1074/jbc.M201728200. [DOI] [PubMed] [Google Scholar]
- 28.Montell C. Mg2+ homeostasis:the Mg2+nificent TRPM chanzymes. Curr Biol. 2003;13:R799–801. doi: 10.1016/j.cub.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 29.Voets T, Nilius B, Hoefs S, Van Der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 30.Nadler MJS, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Strokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–5. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 31.Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS, So I, Stanfield PR, Kim KW. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterol. 2005;129:1504–17. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Kim BJ, So I, Kim KW. The relationship of TRP channels to the pacemaker activity of interstitial cells of Cajal in the gastrointestinal tract. J Smooth Muscle Res. 2006;42:1–7. doi: 10.1540/jsmr.42.1. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama S, Nomura H, Smith LM, Clark JF, Uetani T, Matsubara T. Mechanisms for monovalent cation-dependent depletion of intracellular Mg2+: Na+-independent Mg2+ pathways in guinea-pig smooth muscle. J Physiol. 2003;551:843–53. doi: 10.1113/jphysiol.2003.047795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama S, Clark JF. Smooth muscle and NMR review: An overview of smooth muscle metabolism. Mol Cell Biochem. 2003;244:17–30. [PubMed] [Google Scholar]
- 36.Hamaguchi Y, Uetani T, Tatematsu Y, Amano T, Matsubara T, Murohara T, Nakayama S. Properties of Na+-independent passive Mg2+ influx in pig caroid artery. Jpn J Physiol. 2004;54:S122. (228) [Google Scholar]
- 37.He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res. 2005;96:207–15. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- 38.Touyz RM, He Y, Montezano AC, Yao G, Chubanov V, Gudermann T, Callera GE. Differential regulation of transient receptor potential melastatin 6 and 7 cation channels by ANG II in vascular smooth muscle cells from spontaneously hypertensive rats. Am J Physiol. 2006;290:R73–8. doi: 10.1152/ajpregu.00515.2005. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama S, Chihara S, Clark JF, Huang SM, Horiuchi T, Tomita T. Consequences of metabolic inhibition in smooth muscle isolated from guinea-pig stomach. J Physiol. 1997;505:229–40. doi: 10.1111/j.1469-7793.1997.229bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kito Y, Suzuki H. Effects of temperature on pacemaker potentials in the mouse small intestine. Pflügers Arch. 2007;454:263–75. doi: 10.1007/s00424-006-0201-3. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura E, Kito Y, Hashitani H, Suzuki H. Metabolic component of the temperature-sensitivity of slow waves recorded from gastric muscle of the guinea-pig. J Smooth Muscle Res. 2006;42:33–48. doi: 10.1540/jsmr.42.33. [DOI] [PubMed] [Google Scholar]
- 42.Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G. ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium. 2007;42:427–38. doi: 10.1016/j.ceca.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki H, Kito Y, Hashitani H, Nakamura E. Factors modifying the frequency of spontaneous activity in gastric muscle. J Physiol. 2006;576:667–74. doi: 10.1113/jphysiol.2006.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakayama S, Ohya S, Liu HN, Watanabe T, Furuzono S, Wang J, Nishizawa Y, Aoyama M, Murase N, Matsubara T, Ito Y, Imaizumi Y, Kajioka S. Sulphonylurea receptors differently modulate ICC pacemaker Ca2+ activity and smooth muscle contractility. J Cell Sci. 2005;118:4163–73. doi: 10.1242/jcs.02540. [DOI] [PubMed] [Google Scholar]
- 45.Choi S, Yeum CH, Chang IY, You HJ, Park JS, Jeong HS, So I, Kim KW, Jun JY. Activating of ATP-dependent K+ channels comprised of K(ir) 6.2 and SUR 2B by PGE2 through EP2 receptor in cultured interstitial cells of Cajal from murine small intestine. Cell Physiol Biochem. 2006;18:187–98. doi: 10.1159/000097516. [DOI] [PubMed] [Google Scholar]
- 46.Aickin CC, Brading AF. Measurement of intracellular chloridein guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. J Physiol. 1982;326:139–54. doi: 10.1113/jphysiol.1982.sp014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aickin CC, Brading AF. Towards an estimate of chloride permeability in the smooth muscle of guinea-pig vas deferens. J Physiol. 1983;336:179–97. doi: 10.1113/jphysiol.1983.sp014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal dis-tributed in the mouse small intestine. J Physiol. 2003;553:803–18. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, Mucci A, Huizinga JD. Inwardly rectifying chloride channel activity in intestinal pacemaker cells. Am J Physiol. 2005;288:G809–21. doi: 10.1152/ajpgi.00301.2004. [DOI] [PubMed] [Google Scholar]
- 50.Koh SD, Jun JY, Kim TW, Sanders KM. A Ca2+-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002;540:803–14. doi: 10.1113/jphysiol.2001.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujita A, Takeguchi T, Saitoh N, Hanai J, Hata F. Expression of Ca2+-activated K+ channels, SK3, in the interstitial cells of Cajal in the gastrointestinal tract. Am J Physiol. 2001;281:C1727–33. doi: 10.1152/ajpcell.2001.281.5.C1727. [DOI] [PubMed] [Google Scholar]
- 52.Publicover NG, Horowitz NN, Sanders KM. Calcium oscillations in freshly dispersed and cultured interstitial cells from canine colon. Am J Physiol. 1992;262:C589–97. doi: 10.1152/ajpcell.1992.262.3.C589. [DOI] [PubMed] [Google Scholar]
- 53.Yamazawa T, Iino M. Simultaneous imaging of Ca2+ signals in interstitial cells of Cajal and longitudinal smooth muscle cells during rhythmic activity in mouse ileum. J Physiol. 2002;538:823–35. doi: 10.1113/jphysiol.2001.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rich A, Hanani M, Ermilov LG, Malysz J, Belzer V, Szurszewski JH, Farrugia G. Physiological study of interstitial cells of Cajal identified by vital staining. Neurogastroenterol Mot. 2002;14:189–96. doi: 10.1046/j.1365-2982.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 55.Hennig GW, Hirst GD, Park KJ, Smith CB, Sanders KM, Ward SM, Smith TK. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol. 2004;556:585–99. doi: 10.1113/jphysiol.2003.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu HN, Ohya S, Wang J, Imaizumi Y, Nakayama S. Involvement of ryanodine receptors in pacemaker Ca2+ oscillation in murine gastric ICC. Biochem Biophys Res Commun. 2005;328:640–6. doi: 10.1016/j.bbrc.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 57.Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol. 2006;290:C1411–27. doi: 10.1152/ajpcell.00447.2005. [DOI] [PubMed] [Google Scholar]
- 58.Van Helden DF, Imtiaz MS. Ca2+ phase waves: a basis for cellular pacemaking and long-range syn-chronicity in the guinea-pig gastric pylorus. J Physiol. 2003;548:271–96. doi: 10.1113/jphysiol.2002.033720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imtiaz MS, Katnik CP, Smith DW, Van Helden DF. Role of voltage-dependent modulation of store Ca2+ release in synchronization of Ca2+ oscillations. Biophys J. 2006;90:1–23. doi: 10.1529/biophysj.104.058743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterol. 2007;132:1852–65. doi: 10.1053/j.gastro.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 61.Kajioka S, Nakayama S, Asano H, Brading AF. Involvement of ryanodine receptors in muscarinic-receptor-mediated membrane current oscillation in urinary bladder smooth muscle. Am J Physiol. 2005;288:C100–8. doi: 10.1152/ajpcell.00161.2004. [DOI] [PubMed] [Google Scholar]
- 62.Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, Hotta S, Ito Y, Matsubara T, Shimokata K, Chen SRW, Imaizumi Y, Nakayama S. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci. 2004;117:2813–25. doi: 10.1242/jcs.01136. [DOI] [PubMed] [Google Scholar]
- 63.Burnstock G, Lavin S. Interstitial cells of Cajal and purinergic signaling. Autonom Neurosci. 2002;97:68–72. doi: 10.1016/s1566-0702(02)00005-x. [DOI] [PubMed] [Google Scholar]
- 64.Iino S, Ward SM, Sanders KM. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J Physiol. 2004;556:521–30. doi: 10.1113/jphysiol.2003.058792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi S, Park do Y, Yeum CH, Chang IY, You HJ, Park CG, Kim MY, Kong ID, So I, Kim KW, Jun JY. Bradykinin modulates pacemaker currents through bradykinin B2 receptors in cultured interstitial cells of Cajal from the murine small intestine. Br J Pharmacol. 2006;148:918–26. doi: 10.1038/sj.bjp.0706806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jun JY, Choi S, Chang IY, Yoon CK, Jeong HG, Kong ID, So I, Kim KW, You HJ. Deoxycholic acid inhibits pacemaker currents by activating ATP-dependent K+ channels through prostaglandin E2 in interstitial cells of Cajal from the murine small intestine. Br J Pharmacol. 2005;144:242–51. doi: 10.1038/sj.bjp.0706074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furuzono S, Nakayama S, Imaizumi Y. Purinergic modulation of pacemaker Ca2+ activity in interstitial cells of Cajal. Neuropharmacol. 2005;48:264–73. doi: 10.1016/j.neuropharm.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscle obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000;525:105–11. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takano H, Imaeda K, Yamamoto Y, Kato K, Mikoshiba K, Suzuki H. Mechanical responses evoked by nerve stimulation in gastric muscles of mouse lacking inositol trisphosphate receptor. Autonom Neurosci. 2001;87:249–57. doi: 10.1016/S1566-0702(00)00286-1. [DOI] [PubMed] [Google Scholar]
- 70.Ward SM, Ördög T, Koh SD, Abu Baker S, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525:355–61. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirst GDS, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach – a stochastic process. J Physiol. 2001;535:165–80. doi: 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malysz J, Donnelly G, Huizinga JD. Regulation of slow wave frequency by IP3-sensitive calcium release in the murine small intestine. Am J Physiol. 2001;280:G439–48. doi: 10.1152/ajpgi.2001.280.3.G439. [DOI] [PubMed] [Google Scholar]
- 73.Ward SM, Baker SA, De Faoite A, Sanders KM. Propagation of slow waves requires IP3 receptors and mitochondrial Ca2+ uptake in canine colonic muscles. J Physiol. 2003;549:207–18. doi: 10.1113/jphysiol.2003.040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Helden DF, Imtiaz MS, Nurgaliyeva K, Vonder Weid PY, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524:245–65. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bootman MD, Collins TJ, Mackenzie H, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–50. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 76.Ishikawa T, Nakayama S, Nakagawa T, Horiguchi K, Misawa H, Kadowaki M, Nakano A, Inoue S, Komuro T, Takaki M. Characterization of in vitro gut-like organ formed from mouse embryonic stem cells. Am J Physiol. 2004;286:C1344–52. doi: 10.1152/ajpcell.00392.2003. [DOI] [PubMed] [Google Scholar]
- 77.Takeshima H, Ikemoto T, Nishi M, Nishiyama N, Shimuta M, Sugitani Y, Kuno J, Saito I, Saito H, Endo M, Iino M, Noda T. Generation and characterization of mutant mice lacking ryanodine receptor type 3. J Biol Chem. 1996;271:19649–52. doi: 10.1074/jbc.271.33.19649. [DOI] [PubMed] [Google Scholar]
- 78.Harhun M, Gordienko D, Kryshtal D, Pucovsky V, Bolton T. Role of intracellular stores in the regulation of rhythmical [Ca2+]i changes in interstitial cells of Cajal from rabbit portal vein. Cell Calcium. 2006;40:287–98. doi: 10.1016/j.ceca.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 79.Johnston L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565:449–61. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fukuta H, Kito Y, Suzuki H. Spontaneous electrical activity and associated changes in calcium concentration in guinea-pig gastric smooth muscle. J Physiol. 2002;540:249–60. doi: 10.1113/jphysiol.2001.013306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kitamura Y, Hirota S, Nishida T. Gastrointestinal stro-mal tumors (GIST):A model for molecule-based diagnosis and treatment of solid tumors. Cancer Sci. 2003;94:315–20. doi: 10.1111/j.1349-7006.2003.tb01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Popescu LM, Vidulescu C, Curici A, Caravia L, Simionescu AA, Ciontea SM, Simion S. Imatinib inhibits spontaneous rhythmic contractions of human uterus and intestine. Eur J Pharmacol. 2006;546:177–81. doi: 10.1016/j.ejphar.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 83.Furuzono S, Ohya S, Inoue S, Nakao A, Imaizumi Y, Nakayama S. Inherent pacemaker function of a duodenal GIST. Eur J Cancer. 2006;42:243–8. doi: 10.1016/j.ejca.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 84.Popescu LM, Ciontea SM, Cretoiu D, Hinescu ME, Radu E, Ionescu N, Ceausu M, Gherghiceanu M, Braga RI, Vasilescu F, Zagrean L, Ardeleanu C. Novel type of interstitial cell (Cajal-like) in human fallopian tube. J Cell Mol Med. 2005;9:479–523. doi: 10.1111/j.1582-4934.2005.tb00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daniel EE, El-Yazbi A, Cho WJ. Caveolae and calcium handling, a review and a hypothesis. J Cell Mol Med. 2006;10:529–44. doi: 10.1111/j.1582-4934.2006.tb00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huizinga JD, Faussome-Pellegrini MS. About the presence of interstitial cells of Cajal outside the musculature of the gastrointestinal tract. J Cell Mol Med. 2005;9:468–73. doi: 10.1111/j.1582-4934.2005.tb00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Popescu LM, Hinescu ME, Ionescu N, Ciontea SM, Cretoiu D, Ardelean C. Interstitial cells of Cajal in pancreas. J Cell Mol Med. 2005;9:169–90. doi: 10.1111/j.1582-4934.2005.tb00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hinescu ME, Ardeleanu C, Gherghiceanu M, Popescu LM. Interstitial Cajal-like cells in human gallbladder. J Mol Histol. 2007;38:275–84. doi: 10.1007/s10735-007-9099-0. [DOI] [PubMed] [Google Scholar]
- 89.Sun X, Yu B, Xu L, Dong W, Luo H. Interstitial cells of Cajal in the murine gallbladder. Scand J Gastroenterol. 2006;41:1218–26. doi: 10.1080/00365520600708800. [DOI] [PubMed] [Google Scholar]
- 90.Lavoie B, Balemba OB, Nelson MT, Ward SM, Mawe GM. Morphological and physiological evidence for interstitial cell of Cajal-like cells in the guinea pig gallbladder. J Physiol. 2007;579:487–501. doi: 10.1113/jphysiol.2006.122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Povstyan OV, Gordienko DV, Harhun MI, Bolton TB. Identification of interstitial cells of Cajal in the rabbit portal vein. Cell Calcium. 2003;33:223–39. doi: 10.1016/s0143-4160(02)00197-5. [DOI] [PubMed] [Google Scholar]
- 92.Harhun MI, Pucovsky V, Povstyan OV, Gordienko DV, Bolton TB. Interstitial cells in the vasculature. J Cell Mol Med. 2005;9:232–43. doi: 10.1111/j.1582-4934.2005.tb00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harhun MI, Gordienko DV, Povstyan OV, Moss RF, Bolton TB. Function of interstitial cells of Cajal in the rabbit portal vein. Circ Res. 2004;95:619–26. doi: 10.1161/01.RES.0000143014.04535.a3. [DOI] [PubMed] [Google Scholar]


