Abstract
Bullous pemphigoid (BP) is a sub-epidermal autoimmune blistering disease associated with autoantibodies to the dermal–epidermal junction (DEJ). Patients’ autoantibodies induce dermal–epidermal separation when co-incubated with cryosections of human skin and leucocytes from healthy volunteers. IgG autoantibodies trigger complement and/or leucocyte activation resulting in specific pathology in several autoimmune conditions. In these diseases, IgG1 and IgG3 isotypes, but not the IgG4 subclass, are thought to trigger inflammatory pathways resulting in tissue damage. The capacity of IgG4 autoantibodies to mediate tissue damage has not yet been demonstrated. In this study, we isolated IgG1 and IgG4 autoantibodies from bullous pemhigoid patients'serum and analysed their blister-inducing potential in our cryosection assay. As expected, complement-fixing IgG1 autoantibodies induced sub-epidermal splits in this experimental model. Purified IgG4 did not fix complement, but, interestingly, like IgG1, activated leucocytes and induced dermal–epidermal separation. The potential of IgG4 autoantibodies to induce Fc-dependent dermal–epidermal separation was significantly lower compared to IgG1. Our results demonstrate that IgG4 autoantibodies are able to activate leucocytes and point to a hitherto less recognized function of IgG4. Moreover, for the first time, we clearly demonstrate that BP IgG4 autoantibodies have the capacity to induce leucocyte-dependent tissue damage.
Keywords: autoimmunity, complement, inflammation, neutrophils
Introduction
Bullous pemphigoid (BP) is an organ-specific autoimmune disease characterized by sub-epidermal blistering and tissue-bound and circulating autoantibodies against the dermal-epidermal junction (DEJ) [1, 2]. The pathogenic potential of autoantibodies targeting hemidesmosomal proteins associated with BP has been demonstrated both ex vivo and in experimental animals [3–5].
Antibodies are effector molecules of the innate and adaptive immune system secreted by plasmablasts and long-lived plasma cells [6]. Polyspecific, low affinity ‘natural’ IgM antibody may be regarded as a component of the innate immune system whilst protective IgG and IgA antibody responses, mounted following an infection or vaccination, are components of the adaptive immune response. ‘Natural’ antibody can be germ-line encoded, reactive with self-structures, and may have a physiological role. In contrast, autoantibodies encountered in autoimmune disease are thought to result from antigen driven immune responses, resulting in IgG and/or IgA autoantibodies that may mediate the observed immunopathology as a result of specific binding through the antibody variable region and/or indirect effector mechanisms, triggered through the constant regions. The latter mechanisms result from interactions of the Fc regions of antibody/antigen complexes with cellular Fc receptors, expressed on a wide range of leucocytes and/or the C1 component of the classical complement pathway [2, 7–9].
Antibodies of the IgG isotype predominate in the systemic immune response, as reflected in serum immunoglobulin concentration, and activate a wide range of effector functions. Four subclasses of IgG are defined, originally from the antigenic uniqueness of their heavy chains, which are products of distinct genes [10–12]. The subclasses are designated as IgG1, IgG2, IgG3 and IgG4 in order of their serum concentration;∼60%, 25%, 10% and 5%, respectively. Although the heavy chains show > 95% sequence homology, each IgG subclass expresses a unique profile of effector activities [13–18]. Protein antigens characteristically provoke IgG1 and IgG3 responses and these isotypes are able to activate all types of Fc receptors and the C1 component of complement. The IgG4 subclass may be characteristic of chronic antigen stimulation, as in autoimmune disease; it has restricted Fc receptor activating abilities and does not activate C1q. The IgG2 subclass often predominates in responses to carbohydrate antigens; it has restricted Fc receptor and C1 activating abilities [15–18].
It might be expected, therefore, that IgG1 and IgG3 autoantibodies would be mainly involved in the immunopathology associated with IgG-mediated autoimmune inflammatory conditions, including systemic lupus erythematosus, myasthenia gravis, vasculitis and diseases induced by autoantibodies against glomerular basement membrane, such as Goodpasture syndrome [19–23]. However, IgG4 autoantibodies are also found and, sometimes predominate, in several autoimmune diseases, including autoimmune blistering diseases, myasthenia gravis, vasculitis and systemic lupus ythematsus [23–29].
It is established that IgG4 can activate FcγRI and FcγRIIIa, depending on receptor allotype and IgG4 glycoform [30]. Thus, IgG4, like IgG1 and IgG3 has been shown to trigger specific granule release from human neutrophils and to mediate antibody-dependent cell-mediated toxicity in vivo[31–33]; IgG4 anti-neutrophil cytoplasm autoantibodies were shown to stimulate superoxide production from neutrophils [34] and IgG4 antibodies from patients with chronic urticaria to trigger histamine release from basophils [35]. Thus, the capacity of IgG4 autoantibodies to trigger leucocyte activation resulting in pathology is under review. The inability of IgG4 to activate the classical pathway of complement is accepted, however, there is potential for activation through the mannan binding lectin (MBL) pathway [36].
In a group of organ-specific autoimmune diseases, blistering of skin and mucous membranes is induced by autoantibodies against structural epithelial proteins [2]. Interestingly, the mechanisms of autoantibodyinduced blister formation differ markedly. While autoantibodies from patients with pemphigus and mucous membrane pemphigoid cause blisters just by binding to their targets, sub-epidermal blister induction by autoantibodies from patients with BP and epidermolysis bullosa acquisita seem to require subsequent activation of inflammatory pathways [2, 37]. Whereas autoantibodies in pemhigus, BP and epidermolysis bullosa acquisita may belong to all IgG subclasses, the autoantibody response is strongly biased toward IgG4. Blister induction in pemphigus seems to be mediated by a direct binding of IgG4 autoantibodies to desmosomes independent of complement- and leucocyte-activation [2, 38, 39]. However, the pathogenic potential of IgG4 autoantibodies in inflammatory subepidermal blistering diseases has not yet been addressed.
In the present study, we aimed at characterizing the pathogenic effects of IgG4 autoantibodies. For this purpose, we used an ex vivo model of antibody-induced leucocyte-dependent dermal–epidermal separation [4, 40, 41]. IgG4 autoantibodies, purified from patients with BP, induced dermal–epidermal separation in cryosections of human skin, when co-incubated with leucocytes from healthy volunteers. This effect was seen when IgG4 autoantibodies were used at concentrations similar to those in patients’ sera. IgG4 autoantibodies showed, however, a significantly weaker potency in inducing dermal–epidermal separation compared with IgG1 autoantibodies.
Materials and methods
Patients’ sera
Serum samples were obtained from patients with BP (n = 6), before initiation of treatment, as well as from healthy donors (n = 6). Criteria for inclusion of BP patients in this study have been previously published [4]. For the experiments conducted, we obtained institutional approval issued by the ethics committee at the Medical Faculty of the University of Lübeck (Institutional Board Projects 04-061 and 04-144). In adherence to the Helsinki Principles, we obtained informed consent from all patients whose material was used in this study.
Immunofluorescence (IF) microscopy and complement fixation test
The IF microscopy analysis followed standard protocols [4]. Briefly, cryosections of normal human skin were incubated with 10-fold diluted BP serum or IgG subclass fractions. The distribution of the IgG subclasses was detected using fluorescein isothiocyanate (FITC)-conjugated monoclonal mouse antibodies specific to human IgG1, IgG2, IgG3 and IgG4 (clones HP6091, HP6014, HP6050 and HP6025, respectively; all Sigma, Munich, Germany) [42]. To be able to directly compare the titres of IgG1 and IgG4 autoantibodies, we adjusted the dilutions of the FITC-conjugated monoclonal antibodies to human IgG1 and IgG4 by fluorometry, using known concentrations of human myeloma IgG1 and IgG4 proteins (Sigma) and a multi-label counter IgG4 (Victor3; PerkinElmer, Boston, MA, USA). The complement-fixation test was performed as described [43]. Briefly, cryosections of normal human skin were incubated with 10-fold diluted serum or IgG subclass preparations at 37°C, for 30 min, followed by washing with phosphate buffered saline (PBS) twice for 10 min. Subsequently, sections were treated with fresh human serum as a source of complement, diluted 1: 5 with veronal buffered saline (pH 7.4; containing 150 mM NaCl, 3 mM 5,5'-diethylbarbituric acid, 1 mM sodium 5,5′-diethylbarbiturate, 0.15 mM CaCl2 and 0.5 mM MgCl2), at 37°C for 30 min. After washing, complement deposition was visualized with a goat anti-human C3 antibody (Kallestad Diagnostics, Austin, TX, USA).
IgG subclass purifications
Purification of IgG1 and IgG4 antibodies from human serum was described previously [34]. Affinity columns were prepared using subclass-specific monoclonal antibodies for IgG1 and IgG4 (HP6007 and HP6011, respectively) [42] and immobilized to CNBr activated Sepharose-4B (Amersham Biosciences, Buckinghamshire, UK). To deplete the IgG3 fraction, up to 1 ml of serum was loaded onto a HiTrap protein A column (Amersham Biosciences) pre-equilibrated with PBS. The column was washed with PBS and the bound IgG1, 2 and 4 was eluted with 0.1 M glycine, (pH 2.7) and neutralized with 1.5 M Tris (pH 9.5). After extensive dialysis at 4 °C against the equilibration buffer (PBS for HP6011 and Tris-saline pH 8.9 for HP6007, respectively), the eluted IgG was loaded onto the pre-equilibrated IgG1-specific immunoaffinity column. The flow-through fraction was further purified against an IgG4-specific immunoaffinity matrix. Antibodies bound to the affinity matrix were, as before, eluted with 0.1 M glycine buffer (pH 2.7) and the low pH was buffered with 1.5 M Tris-HCl. The eluted IgG subclass fractions were concentrated by ultrafiltration using Ultrafree 4 filters (Millipore, Bradford, MA, USA) under extensive washing with PBS (pH 7.2). Reactivity of serum and eluted fractions was analysed by indirect IF microscopy.
Analysis of the purity of IgG subclass fraction
Sheep red blood cells (SRBC) were sensitized with purified monoclonal antibodies specific for each IgG subclass, by the chromium chloride method [44]. The appropriate IgG subclass agglutinates such cells. Each IgG subclass was titrated in a two-fold dilution series in 2% Hepes buffered Roswell Park Memorial Institute (RPMI) 1640 medium (H-RPMI), in the wells of a 96-well microtitre plate; control wells contained 2% H-RPMI only. Positive control plates were prepared as above using dilutions of known concentrations of purified IgG subclass protein. Sensitized SRBC were added and plates incubated at room temperature for 1 hr. Relative concentrations of the IgG subclass proteins were determined by comparison of the haemagglutination titer end-points obtained for the IgG subclass fractions and the corresponding purified monoclonal subclass protein.
A monoclonal antibody against CD16 (3G8, IgG1; Serotec, Oxford, UK) and purified mouse IgG as control antibody (Sigma) were used at a concentration of 40 μg/ml.
Assessment of the blister-inducing potential of autoantibodies
Blister-inducing capacity of patients’ autoantibodies was evaluated using an ex vivo model of autoantibody-induced dermal–epidermal separation. Isolation of peripheral blood leucocytes and treatment of human skin cryosections were performed as described [4, 40, 41]. Briefly, peripheral blood leucocytes from healthy volunteers were isolated by dextran sedimentation followed by hypotonic red blood cell lysis in 0.2% NaCl. Cells were washed twice in RPMI and re-suspended in the same medium at a density of 3×107 cells/ml. Six-micrometer cryosections of human skin were washed in PBS to remove embedding medium and incubated with 100 μl of diluted serum or IgG subclass preparation for 2 hrs at 37°C. Sections were washed twice in PBS and chambers were prepared as described [4]. Leucocytes suspensions were injected into the chambers and incubated for 3 hrs at 37°C. Subsequently, chambers were disassembled and sections were washed in PBS, air dried and stained with haematoxylin and eosin. To determine the activation status of leucocytes, the cells were re-suspended in medium containing 0.05% nitroblue tetrazolium (NBT; Roth, Karlsruhe, Germany), prior to incubation with the cryosections. Subsequently, unfixed sections were examined by light microscopy for the presence of dark blue formazan precipitates.
Statistical analysis
Differences in leucocyte activation and dermal–epidermal separation were calculated using the Fisher's exact test and the OPENSTAT2 free software for LINUX (http://www.agrivisser.com/cgi-bin/English/OpenStat2.htm) [45].
Results
Affinity purification and characterization of IgG subclass fractions from BP positive sera
BP autoantibodies predominantly belong to the IgG1 and IgG4 subclasses, while IgG2 and IgG3 autoantibodies are only occasionally found [27]. Therefore, in the present study, IgG1 and IgG4 antibodies were purified by immunoaffinity chromatography from serum of 3 and 5 BP patients, respectively. IgG subclass preparations were shown to contain IgG autoantibodies of a single subclass, as determined by haemagglutination assay and/or IF microscopy, respectively (Table 1 and 2). A representative example is depicted in Figure 1. Unless otherwise stated, reactivities of IgG1 and IgG4 autoantibodies used for cryosection experiments were similar in antibody preparations and original BP serum (Table 2).
1.
Measurement of IgG1 and IgG4 in purified antibody preparations
| Patients | Fraction | Haemagglutination assay† | |||
|---|---|---|---|---|---|
| IgG1 (ng/ml) | IgG4 (ng/ml) | ||||
| 1 | IgG1 | 781.25 | 0 | ||
| IgG4 | 0 | 24.4 | |||
| 2 | IgG1 | 1200 | <0.1 | ||
| IgG4 | n.d. | n.d. | |||
| 3 | IgG1 | 1200 | 0 | ||
| 4 | IgG4 | 0 | 12.2 | ||
| 5 | IgG4 | 0 | 6.1 | ||
| 6 | IgG4 | n.d. | n.d. | ||
Relative concentrations of the IgG subclass proteins were determined by comparison of the haemagglutination titer end-points obtained for the IgG subclass fractions and the corresponding purified monoclonal subclass protein. Subsequently, the IgG subclass fractions were concentrated 90–100-fold by ultrafiltration and used for the cryosection experiments. n.d., not done.
2.
IgG1 and IgG4 autoantibodies in serum and purified preparations of BP patients
| Patients | Fraction | Immunofluorescence microscopy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG1† | IgG4 | C3‡ | |||||||||||
| 1 | Serum | 1/80 | 1/640 | Pos | |||||||||
| IgG1 fraction | 1/80 | 0 | Pos | ||||||||||
| IgG4 fraction | 0 | 1/640 | Neg | ||||||||||
| 2 | Serum | 1/80 | 1/1280 | Pos | |||||||||
| IgG1 fraction | 1/80 | 0 | Pos | ||||||||||
| IgG4 fraction | 0 | 1/1280 | Neg | ||||||||||
| 3 | Serum | 1/160 | 1/320 | Pos | |||||||||
| IgG1 fraction | 1/80 | 0 | Pos | ||||||||||
| 4 | Serum | 1/10 | 1/320 | Neg | |||||||||
| IgG4 fraction | 0 | 1/320 | Neg | ||||||||||
| 5 | Serum | 1/10 | 1/320 | Neg | |||||||||
| IgG4 fraction | 0 | 1/320 | Neg | ||||||||||
| 6 | Serum | 1/160 | 1/1280 | Pos | |||||||||
| IgG4 fraction | 0 | 1/1280 | Neg | ||||||||||
IgG1 and IgG4 autoantibodies binding to the dermal–epidermal junction were detected using FITC-conjugated monoclonal antibodies specific to IgG1 and IgG4, respectively. The optimal dilution of secondary antibodies was adjusted by fluorometry against known concentrations of myeloma IgG1 and IgG4.
Presence of complement C3 deposits at the dermal–epidermal junction by the complement-fixation test on human skin.
1.
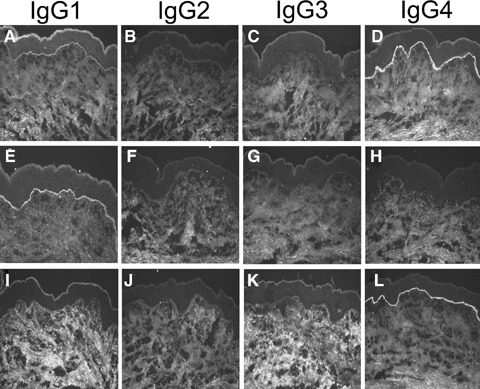
Purification of IgG1 and IgG4 autoantibodies specific to the epidermal basement membrane. IF microscopy study of serum from patient 1 on normal human skin demonstrates deposition of mainly IgG1 (A) and IgG4 (D) and traces of IgG2 (B) and IgG3 (C) at the dermal–epidermal junction. After purification against an affinity matrix containing mon-oclonal antibodies specific for IgG1, IgG1 autoantibodies to the skin basement membrane can be visualized in the purified fraction (E), while IgG2 (F), IgG3 (G) and IgG4 (H) autoantibodies are not detectable. Conversely, the antibody fraction purified against an affinity matrix specific for IgG4 demonstrated binding of autoantibodies to the basement membrane of normal human skin exclusively belonging to the IgG4 subclass (L), whereas no deposits of IgG1 (I), IgG2 (J) or IgG3 (K) were observed (all magnifications, ×200).
IgG1,but not IgG4 autoantibodies, fix complement to the dermal–epidermal junction
Serum samples from BP patients and their purified IgG1 (n = 3) and IgG4 (n = 5) fractions, as well as serum samples from healthy controls, were incubated with cryosections of human skin, in the presence of normal human serum as a source of complement. As expected, like the original sera (Fig. 2A), IgG1 autoantibodies (Fig. 2B) fixed complement to the basement membrane zone. In contrast, in sections treated with IgG4 autoantibodies (Fig. 2C) or serum from a healthy control (Fig. 2D), deposition of complement at the DEJ was not detected.
2.
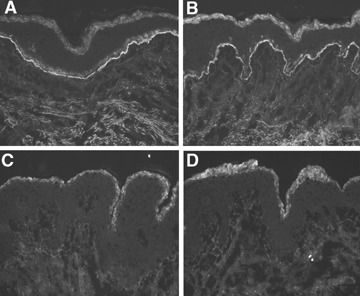
IgG4 autoantibodies, in contrast to IgG1, do not fix complement to the dermal–epidermal junction. In a representative experiment, cryosections of normal human skin were incubated with serum and purified antibody preparations from patient 2 and, subsequently, treated with fresh human serum as a source of complement. Both serum (A) and purified IgG1 autoantibodies (B) fixed complement C3 at the dermal–epidermal junction in a linear fashion. In contrast, incubation of cryosections with IgG4 specific for the dermal–epidermal junction (C) or serum from a healthy control (D) does not result in C3 deposition (all magnifications, ×200).
IgG4 autoantibodies recruit and activate leucocytes to the dermal–epidermal junction
The capacity of IgG1 and IgG4 autoantibodies to activate leucocytes at the basement membrane was examined using frozen sections of human skin incubated with antibody preparations from three and five BP patients, respectively, and, subsequently, with leucocytes from healthy volunteers. Surprisingly, in addition to IgG1 autoantibodies (Fig. 3A), IgG4 (Fig. 3B) also induced an attachment of leucocytes to the DEJ. To determine whether leucocytes that attach to the DEJ become activated, cryosections that had been previously treated with IgG4 and IgG1 autoantibodies were incubated with leucocytes in the presence of nitro blue tetrazolium. After a 90 min incubation, dark-blue precipitates of reduced nitro-blue tetrazolium (formazan) were found at the DEJ of sections treated with IgG1 autoantibodies (Fig. 3D and G). Interestingly, in sections incubated with purified IgG4 autoantibodies, deposition of formazan along the basement membrane was also observed in an antibody dose-dependent manner (Fig. 3E and G). In sections treated with control IgG, no attachment of cells and no precipitates of formazan at the DEJ were found (Fig. 3C and F). Using the Fisher's exact test, IgG4 autoantibodies induced significantly less formazan deposition when compared with IgG1 autoantibodies (P= 0.023).
3.
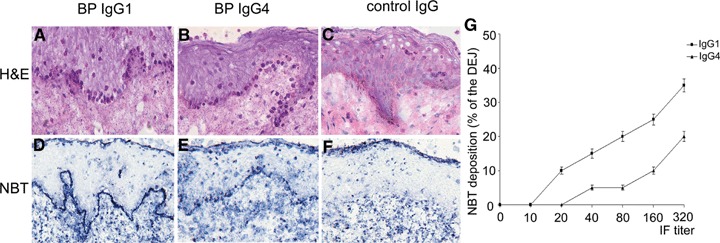
IgG4 autoantibodies recruit and activate leucocytes. Sections of human skin were incubated with IgG1 and IgG4 autoantibodies from a BP patient, as well as with IgG from a healthy control. Subsequent addition of leucocytes from healthy donors leads to leucocyte attachment at the dermal–epidermal junction in sections treated with patient's IgG1 (A) and IgG4 autoantibodies (B), but not in sections incubated with control IgG (C). Activation of leucocytes, as revealed by the reduction of nitro blue tetrazolium (NBT) to formazan (dark precipitates), is induced by purified IgG1 (D) and IgG4 autoantibodies (E), but not by control IgG (F) (all magnifications, ×400). (G) Cryosections of human skin were treated with IgG1 (n = 3) and IgG4 (n = 5) antibodies (four sections/antibody preparation). Subsequently, leucocytes from healthy donors were incubated for 90 min with the cryosections. Deposition of formazan is represented as means ± SEM of the percent of the total length of the dermal–epidermal junction (DEJ) for each section.
IgG4 autoantibodies induce dermal–epidermal separation in cryosections of human skin
When incubated with the cryosections of human skin in the presence of leucocytes purified from healthy donors, both serum (n = 6) (Fig. 4A) and IgG1 autoantibodies (n = 3) (Fig. 4B) from BP patients induced dermal–epidermal separation. Importantly, subsequent addition of leucocytes to cryosections previously treated with non-complement fixing IgG4 autoantibodies (n = 5) also resulted in dermal–epidermal separation (Fig. 4C). Sub-epidermal splits were not observed in frozen skin sections incubated with serum samples from healthy controls (Fig. 4D). Fc RIII (CD16) is constitutively expressed in a high-copy number on neutrophils and was shown to mainly mediate both antibody-induced blistering in a mouse model of BP [46] and autoantibody-induced leucocyte attachment to the basement membrane in the cryosection model [47]. Therefore, to investigate the Fc-dependent leucocyte activation in our model, we blocked the binding of IgG autoantibodies to CD16 on granulocytes using the mAb 3G8. Cryosections of human skin were incubated with IgG, IgG1 and IgG4 purified from two BP patients (n = 4 sections/antibody preparation). Subsequently, granulocytes were treated for 10 min at room temperature with 3G8 or a control antibody prior to incubation with skin sections. The mAb 3G8, but not the control antibody, caused a 86.6, 85 and 85.4% inhibition of dermal–epidermal separation induced by IgG, IgG1 and IgG4, respectively (Fig. 5).
4.
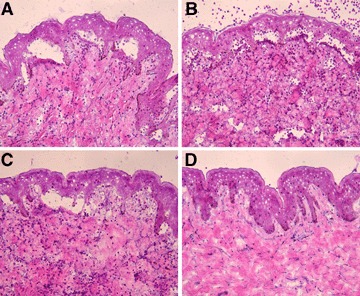
IgG4 autoantibodies induce dermal–epidermal separation in sections of human skin. Results of a representative experiment show that dermal–epidermal separation in sections of normal human skin is induced by serum (A), IgG1 (B) and IgG4 autoantibodies (C) from patient 1. Serum antibodies from a healthy control (D) do not induce sub-epidermal splits (all magnifications, ×200).
5.
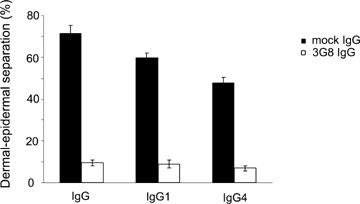
A blocking monoclonal antibody against CD16 significantly inhibits the autoantibody-induced dermal–epidermal separation. Cryo-sections of human skin were incubated with IgG, IgG1 and IgG4 purified from BP patients 1 and 2 (n = 4 sections/antibody preparation). Subsequently, granulocytes were treated for 10 min at room temperature with 3G8 or a control antibody prior to incubation with skin sections. Dermal–epidermal separation is represented as means ± SEM of the percent of the total length of the dermal–epidermal junction (DEJ) for each section.
IgG4 autoantibodies show a significantly lower blister-inducing potential compared to IgG1 autoantibodies
In our patients’ sera, we found lower titres of IgG1 when compared to titres of IgG4 autoantibodies (Table 2). To directly compare the pathogenic potential of autoantibodies of IgG1 and IgG4 subclass, IgG1 and IgG4 antibody preparations from two BP patients were used at similar titres. When incubated with the cryosections, IgG4 autoantibodies induced dermal–epidermal separation to a significantly lower extent when compared with IgG1 autoantibodies as assessed using the Fisher's exact test (P= 0.017) (Fig. 6).
6.
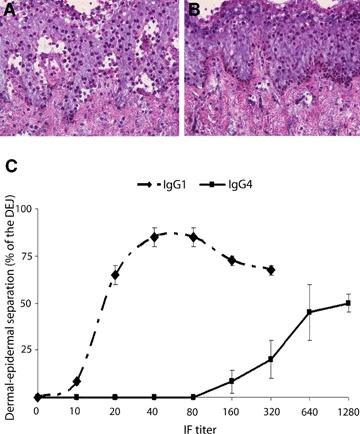
IgG4 autoantibodies show a lower pathogenic capacity compared with IgG1. In the upper panel, cryosections of human skin were incubated with IgG1 or IgG4 autoantibodies purified from patient 2, both adjusted at an end-point titer of 1:80 by IF microscopy. Subsequent addition of leucocytes leads to blister formation in cryosections treated with IgG1 autoantibodies (A). In contrast, IgG4 autoantibodies (B) fail to recruit leucocytes to the dermal–epidermal junction and to induce dermal–epidermal separation (magnification, ×400). The lower panel (C) shows the extent of dermal–epidermal separation after incubating the cryosections with IgG1 and IgG4 preparations from two BP patients (n = 4 sections/antibody preparation). Dermal–epidermal separation is represented as means ± SEM of the percent of the total length of the dermal–epidermal junction (DEJ) for each section. The reactivity of the antibody preparations as determined by their end-point titres by immunofluorescence microscopy is represented on the X-axis.
Discussion
The capacity of IgG4 autoantibodies to induce disease-specific leucocyte-dependent tissue damage has not yet been demonstrated. Here, we show that, after binding to the epidermal basement membrane, IgG4 autoantibodies recruit and activate leucocytes and induce dermal–epidermal separation. Compared to IgG1 against the epidermal basement membrane, IgG4 autoantibodies show a significantly lower pathogenic capacity.
As demonstrated by numerous studies in patients and experimental animals, only certain sub-populations of autoantibodies mediate cellular and tissue injuries in autoimmune diseases. The subclass of IgG autoantibodies is considered as one of the main determinants of their pathogenic potential [17]. Indeed, IgG subclasses show marked differences in their ability to mediate a variety of effector functions, including complement activation and binding to the Fc receptors on leucocytes. Much of our knowledge on the properties of human IgG has been obtained from the study of myeloma proteins [48–50]. However, functional assays using myeloma proteins did not take into account their specificity (i.e.binding to antigens was generally mimicked by using aggregated IgG) and the polyclonal nature of the autoimmune response in human beings. With the advent of recombinant DNA techniques, recombinant human (or usually chimeric mouse/human) antibodies with specificity to known antigens were constructed and their effector properties analysed [51–53]. Generally, these studies found that IgG4 did not bind to and/or activate leucocytes, leading to the proposal that IgG4, in contrast to IgG1 and IgG3, are non-pathogenic.
In a group of autoimmune blistering skin diseases, including BP, IgG autoantibodies against different skin proteins induce blisters and belong predominantly to IgG1 and IgG4 subclasses with a pronounced bias towards IgG4. It has been hypothesized that IgG1 rather than IgG4 autoantibodies are responsible for skin blistering in BP [54]. IgG autoantibodies from patients with BP bind to the cutaneous basement membrane, recruit and activate locally leucocytes and induce dermal–epidermal separation [2, 4]. Interestingly, complement is not required for the induction of dermal–epidermal separation in this model [4]. However, in the cryosection model, in contrast to the in vivo situation, the leucocytes do not have to migrate along a chemotactic gradient from blood vessels to the DEJ. Instead, by incubating the cryosections with leucocytes, the cells are placed in close contact with the DEJ. Therefore, the role of complement for autoantibody-induced blister formation cannot be addressed using this model. On the other hand, leucocytes play a central role for the induction of dermal–epidermal separation in our model: omitting leucocytes results in a lack of split formation, while pepsin-generated F(ab’)2 fragments of the split-inducing antibodies, lacking the effector Fc portion, are not able to induce sub-epidermal cleavage [4]. Cleavage of components of the anchoring complexes by granulocyte-derived proteases results in retraction of the dermis from the epidermis [40]. Bundles of elastic microfibrils of the lamina fibroreticularis anchor the basal lamina to the dermal elastic fibres [55] and a recoil of these elastic fibres could explain the development of sub-epidermal cleavage in cryosections of non-viable skin that strongly adhere to the glass slide.
Therefore, to investigate the pathogenic potential of IgG1 and IgG4 autoantibodies, we used this ex vivo model of dermal–epidermal separation and IgG autoantibodies of different subclasses isolated from BP patients. IgG1 and IgG4 serum fractions were purified by sequential immunoaffinity chromatography and used at concentrations similar to those of IgG1 and IgG4 autoantibodies in serum. Consistent with previous studies [51, 56], in our present study, purified IgG4, in contrast to IgG1 autoantibodies, after binding to the cutaneous basement membrane, did not activate the complement system by the classical pathway as revealed by the complement binding assay.
In a next set of experiments, we evaluated the potential of IgG1 and IgG4 autoantibodies to recruit and activate leucocytes at the DEJ. Interestingly, we found that in addition to IgG1, IgG4 autoantibodies also demonstrate a dose-dependent leucocyte-activating capacity. These findings contradict the current accepted view on the interaction of IgG4 with leucocytes [15, 17], but are in agreement with a few previous reports describing similar functional properties of IgG4 [31, 34, 35]. Importantly, Fc-dependent activation of leucocytes by IgG4 autoantibodies resulted in sub-epidermal cleavage formation in the cryosections. To the best of our knowledge, this is the first report demonstrating disease-specific leucocyte-dependent tissue damage triggered by IgG4 autoantibodies. Our results are in line with recent studies demonstrating that both polyclonal human IgG1 and IgG4 from patients with Wegener's granulomatosis and chronic urticaria can activate leucocytes [34, 35]. To directly compare the pathogenic potential of IgG1 with IgG4 autoantibodies, we used preparations containing similar concentrations of these autoantibodies. Under these conditions, IgG4 autoantibodies induced significantly less separation compared to IgG1 autoantibodies. In addition to the isotype, the molecular specificity of autoantibodies is another major determinant of their pathogenic potential. Using the ex vivo cryosection model, we previously showed that autoantibodies against the immunodominant domain of BP180 induce dermal–epidermal separation [4]. It has been also documented that autoantibodies against this immunodominant region are predominantly of IgG1 subclass, whereas autoantibodies targeting other fragments of B180 mainly belong to IgG4 subclasses [54]. Thus, the lower potential of IgG4 autoantibodies to induce dermal–epidermal separation documented by our present studies may be associated with differential molecular specificity, which, in turn, may be a direct determinant of autoantibody pathogenicity.
Taking into account our present data as well as previous work [31, 34, 35], we speculate that in patients, complement-fixing IgG1 may initiate the inflammatory cascade and recruit leucocytes. Nevertheless, IgG4 autoantibodies, which predominate, may activate the inflammatory cells already infiltrating the upper dermis and thus amplify the recruitment of additional leucocytes and the extent of blister formation. Therefore, when associated with IgG1 and/or IgG3 autoantibodies, IgG4 may significantly contribute to the pathology induced by autoantibodies in antibody-induced granulocyte-mediated autoimmune diseases.
In conclusion, IgG4 autoantibodies induce leucocyte activation and dermal–epidermal separation in cryosections of human skin. IgG4 autoantibodies show a significantly lower pathogenic potential compared to IgG1 autoantibodies.
Acknowledgments
This work was supported by grants SI 1281/1-1 and Zi 439/6-2 (to C.S. and D.Z.) from the Deutsche Forschungsgemeinschaft.
References
- 1.Liu Z. Immunopathology of bullous pemphigoid, an autoimmune and inflammatory skin blistering disease. Keio J Med. 2003;52:128–33. doi: 10.2302/kjm.52.128. [DOI] [PubMed] [Google Scholar]
- 2.Sitaru C, Zillikens D. Mechanisms of blister induction by autoantibodies. Exp Dermatol. 2005;14:861–75. doi: 10.1111/j.1600-0625.2005.00367.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, Giudice GJ. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–8. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sitaru C, Schmidt E, Petermann S, Munteanu SL, Bröcker EB, Zillikens D. Autoantibodies to bullous pemphigoid antigen 180 induce dermal-epidermal separation in cryosections of human skin. J Invest Dermatol. 2002;118:664–71. doi: 10.1046/j.1523-1747.2002.01720.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto K, Inoue N, Masuda R, Fujimori A, Saito T, Imajoh-Ohmi S, Shinkai H, Sakiyama H. Cloning of hamster type XVII collagen cDNA, and pathogenesis of anti-type XVII collagen antibody and complement in hamster bullous pemphigoid. J Invest Dermatol. 2002;118:485–92. doi: 10.1046/j.0022-202x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- 6.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–86. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 7.Nezlin R, Ghetie V. Interactions of immunoglobulins outside the antigen-combining site. Adv Immunol. 2004;82:155–215. doi: 10.1016/S0065-2776(04)82004-2. [DOI] [PubMed] [Google Scholar]
- 8.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–10. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 10.Dray S. Three gamma-globulins in normal human serum revealed by monkey precipitins. Science. 1960;132:1313–4. doi: 10.1126/science.132.3436.1313. [DOI] [PubMed] [Google Scholar]
- 11.Terry WD, Fahey JL. Subclasses of Human Gamma-2-Globulin Based on Differences in the Heavy Polypeptide Chains. Science. 1964;146:400–1. doi: 10.1126/science.146.3642.400. [DOI] [PubMed] [Google Scholar]
- 12.Grey HM, Kunkel HG. H-chain subgroups of myeloma proteins and normal 7 s-gammaglobulins. J Exp Med. 1964;120:253–66. doi: 10.1084/jem.120.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao MH, Smith RI, Morrison SL. Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. J Exp Med. 1993;178:661–7. doi: 10.1084/jem.178.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schur PH. IgG subclasses–a review. Ann Allergy. 1987;58:89–99. [PubMed] [Google Scholar]
- 15.Van De Winkel JG, Capel PJ. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215–21. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 16.Janeway CA, Travers P, Walport M, Shlomchik M. Immunobiology. 5. Garland, New York and London: 2001. [Google Scholar]
- 17.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 18.Woof JM, Burton DR. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat Rev Immunol. 2004;4:89–99. doi: 10.1038/nri1266. [DOI] [PubMed] [Google Scholar]
- 19.Weber M, Lohse AW, Manns M, Zum Buschenfelde M, Kohler H. IgG subclass distribution of autoantibodies to glomerular basement membrane in Goodpasture's syndrome compared to other autoantibodies. Nephron. 1988;49:54–7. doi: 10.1159/000184986. [DOI] [PubMed] [Google Scholar]
- 20.Amoura Z, Koutouzov S, Chabre H, Cacoub P, Amoura I, Musset L, Bach JF, Piette JC. Presence of antinucleosome autoantibodies in a restricted set of connective tissue diseases: antinucleosome antibodies of the IgG3 subclass are markers of renal pathogenicity in systemic lupus erythematosus. Arthritis Rheum. 2000;43:76–84. doi: 10.1002/1529-0131(200001)43:1<76::AID-ANR10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 21.Lieu TS, Reimer CB, Sontheimer RD. Immunoglobulin class and subclass profile of the Ro/SS-A autoanti-body response. J Invest Dermatol. 1988;90:158–64. doi: 10.1111/1523-1747.ep12462142. [DOI] [PubMed] [Google Scholar]
- 22.Rubin RL, Tang FL, Chan EK, Pollard KM, Tsay G, Tan EM. IgG subclasses of autoantibodies in systemic lupus erythematosus, Sjogren's syndrome, and drug-induced autoimmunity. J Immunol. 1986;137:2528–34. [PubMed] [Google Scholar]
- 23.Rodgaard A, Nielsen FC, Djurup R, Somnier F, Gammeltoft S. Acetylcholine receptor antibody in myasthenia gravis: predominance of IgG subclasses 1 and 3. Clin Exp Immunol. 1987;67:82–8. [PMC free article] [PubMed] [Google Scholar]
- 24.French MA, Bernstein RM. Immunoglobulin G subclass distribution of autoantibodies in systemic sclerosis, primary biliary cirrhosis, and overlap syndromes. Ann Rheum Dis. 1987;46:436–40. doi: 10.1136/ard.46.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonfa E, Llovet R, Elkon K. Immunoblot analysis of IgG subclasses of multiple lupus autoantibodies. J Immunol. 1988;140:2231–6. [PubMed] [Google Scholar]
- 26.Jones CC, Hamilton RG, Jordon RE. Subclass distribution of human IgG autoantibodies in pemphigus. J Clin Immunol. 1988;8:43–9. doi: 10.1007/BF00915155. [DOI] [PubMed] [Google Scholar]
- 27.Bird P, Friedmann PS, Ling N, Bird AG, Thompson RA. Subclass distribution of IgG autoantibodies in bullous pemphigoid. J Invest Dermatol. 1986;86:21–5. doi: 10.1111/1523-1747.ep12283737. [DOI] [PubMed] [Google Scholar]
- 28.Yamada H, Hashimoto T, Nishikawa T. IgG subclasses of intercellular and basement membrane zone antibodies: the relationship to the capability of complement fixation. J Invest Dermatol. 1989;92:585–7. doi: 10.1111/1523-1747.ep12709613. [DOI] [PubMed] [Google Scholar]
- 29.Brouwer E, Tervaert JW, Horst G, Huitema MG, Van Der Giessen M, Limburg PC, Kallenberg CG. Predominance of IgG1 and IgG4 subclasses of anti-neutrophil cytoplasmic autoantibodies (ANCA) in patients with Wegener's granulomatosis and clinically related disorders. Clin Exp Immunol. 1991;83:379–86. doi: 10.1111/j.1365-2249.1991.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niwa R, Natsume A, Uehara A, Wakitani M, Iida S, Uchida K, Satoh M, Shitara K. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods. 2005;306:151–60. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Henson PM, Johnson HB, Spiegelberg HL. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972;109:1182–92. [PubMed] [Google Scholar]
- 32.Greenwood J, Clark M, Waldmann H. Structural motifs involved in human IgG antibody effector functions. Eur J Immunol. 1993;23:1098–104. doi: 10.1002/eji.1830230518. [DOI] [PubMed] [Google Scholar]
- 33.Voice JK, Lachmann PJ. Neutrophil Fc gamma and complement receptors involved in binding soluble IgG immune complexes and in specific granule release induced by soluble IgG immune complexes. Eur J Immunol. 1997;27:2514–23. doi: 10.1002/eji.1830271008. [DOI] [PubMed] [Google Scholar]
- 34.Holland M, Hewins P, Goodall M, Adu D, Jefferis R, Savage CO. Anti-neutrophil cytoplasm antibody IgG subclasses in Wegener's granulomatosis: a possible pathogenic role for the IgG4 subclass. Clin Exp Immunol. 2004;138:183–92. doi: 10.1111/j.1365-2249.2004.02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soundararajan S, Kikuchi Y, Joseph K, Kaplan AP. Functional assessment of pathogenic IgG subclasses in chronic autoimmune urticaria. J Allergy Clin Immunol. 2005;115:815–21. doi: 10.1016/j.jaci.2004.12.1120. [DOI] [PubMed] [Google Scholar]
- 36.Abadeh S, Church S, Dong S, Lund J, Goodall M, Jefferis R. Remodelling the oligosaccharide of human IgG antibodies:effects on biological activities. Biochem Soc Trans. 1997;25:S661. doi: 10.1042/bst025s661. [DOI] [PubMed] [Google Scholar]
- 37.Yancey KB. The pathophysiology of autoimmune blistering diseases. J Clin Invest. 2005;115:825–8. doi: 10.1172/JCI24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rock B, Martins CR, Theofilopoulos AN, Balderas RS, Anhalt GJ, Labib RS, Futamura S, Rivitti EA, Diaz LA. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320:1463–9. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 39.Hacker-Foegen MK, Janson M, Amagai M, Fairley JA, Lin MS. Pathogenicity and epitope characteristics of anti-desmoglein-1 from pemphigus foliaceus patients expressing only IgG1 autoantibodies. J Invest Dermatol. 2003;121:1373–8. doi: 10.1111/j.1523-1747.2003.12608.x. [DOI] [PubMed] [Google Scholar]
- 40.Shimanovich I, Mihai S, Oostingh GJ, Ilenchuk TT, Brocker EB, Opdenakker G, Zillikens D, Sitaru C. Granulocyte-derived elastase and gelatinase B are required for dermal-epidermal separation induced by autoantibodies from patients with epidermolysis bul-losa acquisita and bullous pemphigoid. J Pathol. 2004;204:519–27. doi: 10.1002/path.1674. [DOI] [PubMed] [Google Scholar]
- 41.Herrero-Gonzalez JE, Brauns O, Egner R, Ronspeck W, Mascaro JM, Jr, Jonkman MF, Zillikens D, Sitaru C. Immunoadsorption against two distinct epitopes on human type XVII collagen abolishes dermal-epidermal separation induced in vitro by autoantibodies from pemphigoid gestationis patients. Eur J Immunol. 2006;36:1039–48. doi: 10.1002/eji.200535349. [DOI] [PubMed] [Google Scholar]
- 42.Mestecty J, Hamilton RG, Magnussor CG, Jefferis R, Vaerman JP, Goodall M, De Large GG, Moro I, Aucouturier P, Radl J, Carbiaso C, Silvain C, Preud'homne JL, Kusama K, Carlone GM, Biewerga J, Kobayashi K, Skvaril F, Reiner CB. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol Lett. 1985;10:223–52. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- 43.Sitaru C, Powell J, Messer G, Bröcker E-B, Wojnarowska F, Zillikens D. Immunoblotting and enzyme-linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004;103:757–63. doi: 10.1097/01.AOG.0000115506.76104.ad. [DOI] [PubMed] [Google Scholar]
- 44.Ling NR, Bishop S, Jefferis Use of antibody-coated red cells for the sensitive detection of antigen and in rosette tests for cells bearing surface immunoglobulins. J Immunol Methods. 1977;15:279–89. doi: 10.1016/0022-1759(77)90065-5. [DOI] [PubMed] [Google Scholar]
- 45.Sitaru C, Mihai S, Otto C, Chiriac MT, Haußer I, Dotterweich B, Saito H, Rose C, Ishiko A, Zilikens D. Induction of dermal-epidermal separation in mice by passive transfer of antibodies to type VII collagen. J Clin Invest. 2005;115:870–8. doi: 10.1172/JCI21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao M, Trimbeger ME, Li N, Diaz LA, Shapiro SD, Liu Z. Role of FcRs in animal model of autoimmune bullous pemphigoid. J Immunol. 2006;177:3398–405. doi: 10.4049/jimmunol.177.5.3398. [DOI] [PubMed] [Google Scholar]
- 47.Gammon WR, Hendrix JD, Mangum K, Jeffes EW. Recombinant human cytokines stimulate neutrophil adherence to IgG autoantibody-treated epithelial basement membranes. J Invest Dermatol. 1990;95:164–71. doi: 10.1111/1523-1747.ep12477934. [DOI] [PubMed] [Google Scholar]
- 48.Spiegelberg HL. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19:259–94. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]
- 49.Winkelhake JL. Immunoglobulin structure and effector functions. Immunochemistry. 1978;15:695–714. doi: 10.1016/0161-5890(78)90044-5. [DOI] [PubMed] [Google Scholar]
- 50.Burton DR. Immunoglobulin G: functional sites. Mol Immunol. 1985;22:161–206. doi: 10.1016/0161-5890(85)90151-8. [DOI] [PubMed] [Google Scholar]
- 51.Bruggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, Waldmann H, Neuberger MS. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987;166:1351–61. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo J, Jaume JC, Rapoport B, McLachlan SM. Recombinant thyroid peroxidase-specific Fab converted to immunoglobulin G (IgG) molecules: evidence for thyroid cell damage by IgG1, but not IgG4, autoantibodies. J Clin Endocrinol Metab. 1997;82:925–31. doi: 10.1210/jcem.82.3.3831. [DOI] [PubMed] [Google Scholar]
- 53.Vidarsson G, Van Der Pol WL, Van Den Elsen JM, Vile H, Jansen M, Duijs J, Morton HC, Boel E, Daha MR, Cortlésy B, Van De Winkel JG. Activity of human IgG and IgA subclasses in immune defense against Neisseria meningitidis serogroup B. J Immunol. 2001;166:6250–6. doi: 10.4049/jimmunol.166.10.6250. [DOI] [PubMed] [Google Scholar]
- 54.Hofmann S, Thoma-Uszynski S, Hunziker T, Bernard P, Koebnick C, Stauber A, Schuler G, Borradori L, Hertl M. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. J Invest Dermatol. 2002;119:1065–73. doi: 10.1046/j.1523-1747.2002.19529.x. [DOI] [PubMed] [Google Scholar]
- 55.Gammon WR, Lewis DM, Carlo JR, Sams WM, Wheeler CE. Pemphigoid antibody mediated attachment of peripheral blood leucocytes at the dermal-epidermal junction of human skin. J Invest Dermatol. 1980;75:334–9. doi: 10.1111/1523-1747.ep12531082. [DOI] [PubMed] [Google Scholar]
- 56.Lucisano Valim YM, Lachmann PJ. The effect of antibody isotype and antigenic epitope density on the complement-fixing activity of immune complexes: a systematic study using chimaeric anti-NIP antibodies with human Fc regions. Clin Exp Immunol. 1991;84:1–8. doi: 10.1111/j.1365-2249.1991.tb08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


