Abstract
ADAM8 belongs to a family of transmembrane proteins implicated in cell–cell interactions, proteolysis of membrane proteins, and various aspects of carcinogenesis. In the present study, we aimed to evaluate the expression and function of ADAM8 in pancreatic cancer. ADAM8 mRNA levels were analysed by quantitative RT-PCR and correlated to patient survival. Immunohistochemistry was performed to localize ADAM8 in pancreatic tis-sues. Silencing of ADAM8 expression was carried out by transfection with specific siRNA oligonucleotides. Cell growth and invasion assays were used to assess the functional consequences of ADAM8 silencing. SELDI-TOF-MS was performed to detect the proteolytic activity of ADAM8 in pancreatic cancer cells. ADAM8 mRNA was significantly overexpressed in pancreatic ductal adenocarcinoma (PDAC) compared with normal pancreatic tissues (5.3-fold increase; P= 0.0008), and high ADAM8 mRNA and protein expression levels correlated with reduced survival time of PDAC patients (P= 0.048 and P= 0.065, respectively). Silencing of ADAM8 expression did not significantly influence pancreatic cancer cell growth but suppressed invasiveness. In addition, decreased proteolytic activity was measured in cell culture supernatants following silencing of ADAM8. In conclusion, ADAM8 is overexpressed in PDAC, influences cancer cell invasiveness and correlates with reduced survival, suggesting that ADAM8 might be a potential target in pancreatic cancer therapy.
Keywords: pancreatic cancer, ADAM8, invasion, survival
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth most common cause of cancer-related deaths worldwide, having one of the worst prognosis of all malignant tumours [1]. PDAC is relatively resistant to most forms of treatment, such as chemotherapy, radiotherapy, immunotherapy, and targeted therapy [2]. Thus, understanding the molecular biology of PDAC is necessary to find new markers for early diagnosis and to identify potential targets for efficient therapy [3].
ADAMs (A Disintegrin And Metalloprotease) are type I transmembrane glycoproteins that contain metalloprotease and disintegrin domains. They are implicated in cell–cell fusion, cell–cell interaction, and proteolysis of membrane proteins, a process termed ectodomain shedding[4–6]. ADAM8 (CD156a, MS2) is a member of the ADAM family that plays an important role in bone morphogenesis [7]. It encodes a protein of 824 amino acids with a carboxy-terminal transmembrane domain and extracellular adhesion and protease domains [8]. It was originally cloned from monocytic cells and is expressed in granulocytes and B cells, as well as in neurons, reactive glia cells, and oligodendrocytes, and is involved in allergic inflammatory processes [6, 8–10].
ADAM8 is processed by autocatalysis into two forms: one is derived by removal of a prodomain (processed form) and the other is a remnant protein composed of the extracellular region, with a disintegrin domain at the amino terminus [11] (Fig. 1A). It acts as an active metalloprotease in vitro, hydrolyzing myelin basic protein (MBP), β-amyloid precursor protein [12], CD23 [13], interleukins [14] and tumour necrosis factorα (TNF-α) [9]. ADAM8 activity is not inhibited by tissue inhibitors of matrix metalloproteinases (TIMPs), which is in contrast to other proteolytic proteins [15].
1.
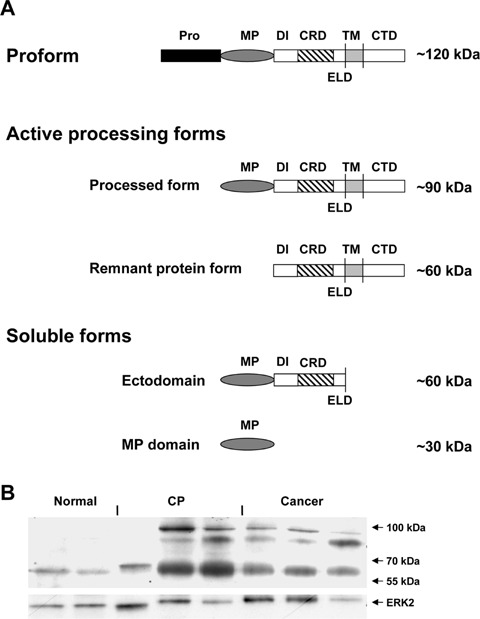
(A) Domain organization and proposed model for ADAM8 processing. The pro-form of ADAM8 is processed by auto-catalytic prodomain removal into two active forms: the processed form and the remnant protein form. Additional proteolytic cleavage results in two soluble forms: the ectodomain and the MP domain (adapted according to ref.[11]). Pro:prodomain;MP: metalloprotease;DI:disintegrin; CRD: cysteine-rich domain; ELD: EGF-like domain; TM: transmembrane; CTD: cyto-plasmic domain. (B) ADAM8 protein expression in pancreatic tissues. Immunoblot analysis of ADAM8 in normal, chronic pancreatitis and pancreatic cancer tissues. Equal loading of the protein samples was confirmed using an ERK-2 anti-body. Size markers are indicated on the right.
Previous studies have shown that ADAM8 is a possible diagnostic serum marker for lung cancer and renal cell carcinoma patients [16, 17], and its overexpression is associated with a higher stage in lung cancer and prostate cancer, with shorter patient survival in renal cell carcinoma, and with increased invasiveness in brain tumours [18, 19]. Recently, two members of the ADAM family; ADAM 9 and ADAM 15 have been shown to be involved in pancreatic car-cinogenesis [20]. In the present study, the expression and release of ADAM8 were analysed in pancreatic tissues. Functional consequences of ADAM8 silencing were assessed in pancreatic cancer cells.
Materials and methods
Cell lines and clinical samples
ASPC-1, Panc-1, BxPC-3, Capan-1 and MiaPaCa-2 cell lines were obtained from American Type Culture Collection (Rockville, MD, USA). Colo-357, Su8686 and T3M4 cell lines were a gift from R.S. Metzgar (Duke University, Durham, NC, USA). Cells were grown in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Karlsruhe, Germany) and were incubated in a 5% CO2 humidified atmosphere. PDAC (n = 99) and CP (n = 36) tissue specimens were obtained from patients in whom pancreatic resections were carried out. Normal human pancreatic tissue samples (n = 28) were obtained through an organ donor program from previously healthy individuals. All samples were confirmed histological. Freshly removed tissues were fixed in 4% paraformaldehyde solution for 12–24 hrs and then paraffin embedded for histological analysis. In addition, portions of the tissue samples were preserved in RNA-later (Ambion Europe Ltd., Huntingdon, UK); other portions were snap-frozen in liquid nitrogen immediately upon surgical removal and maintained at −80°C until use. Serum samples were obtained from 28 PDAC patients (median age: 63 years) and 28 CP patients (median age: 50 years) who were treated at the Department of General Surgery, University of Heidelberg. Twenty-eight serum samples were obtained from healthy volunteers (median age: 35 years). Fresh blood samples were collected and centrifuged. The serum supernatant was collected into polyethylene tubes and kept frozen at −80°C until use. The Human Subjects Committee of the University of Heidelberg, Germany, approved all studies. Written informed consent was obtained from all patients.
Real-time quantitative polymerase chain reaction (qRT-PCR)
All reagents and equipment for mRNA/cDNA preparation were supplied by Roche Applied Science (Mannheim, Germany). mRNA of human pancreatic tissues was prepared by automated isolation performed with the MagNA pure LC instrument and isolation kit I (for cells) and kit II (for tissues). cDNA was prepared using the first-strand cDNA synthesis kit for RT-PCR (AMV) according to the manufacturer's instructions. The primer sequences for ADAM8 were obtained from Search-LC (Heidelberg, Germany). Real-time PCR was performed with the LightCycler FastStart DNA SYBR Green kit. The number of specific transcripts was normalized to the average expression of two housekeeping genes (cyclophilin B and HPRT) and presented as adjusted transcripts/μl cDNA, as described previously [21].
Immunoblot analysis
Cells and tissues samples were lysed in a lysis buffer containing 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 2 mM EDTA pH 8.0, Complete Protease Inhibitor Cocktail Tablet (Roche Diagnostics GmbH, Mannheim, Germany), and 1% SDS. And 17–25 μg proteins were separated on NuPAGE Novex Bis-Tris 4–12% gels (Invitrogen, Karlsruhe, Germany) and electroblotted onto nitrocellulose membranes. Membranes were then incubated in blocking solution (5% milk in 20 mM Tris HCl, 150 mM NaCl, 0.1% Tween-20), followed by overnight incubation with a rabbit polyclonal ADAM8 antibody (dilution 1:250 in blocking solution) (Chemicon International, Temecula, CA, USA). The membranes were then washed in TBS-T and incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibodies (Amersham Bioscience, Buckinghamshire, UK). Antibody detection was performed with an enhanced chemilumines-cence reaction (ECL, Amersham Bioscience). Equal loading and transfer was confirmed using -tubulin and ERK-2 antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). For semiquantitative analysis of the immunoblots, densitometry was carried out and the signal intensity of ADAM8 expression was normalized to its corresponding signal intensity of -tubulin or ERK-2.
Immunohistochemistry
Paraffin-embedded tissue sections (3–5 μm thick) were subjected to immunostaining. Tissue sections were deparaffinized in Roticlear (Carl Roth GmbH, Karlsruhe, Germany) and rehydrated in progressively decreasing concentrations of ethanol. Slides were placed in washing buffer (10 nM Tris-HCl, 0.85% NaCl, 0.1% BSA, pH 7.4) and subjected to immunostaining. After antigen was retrieved by boiling the tissue section in 10 mM citrate buffer for 10 min in the microwave oven, the sections were incubated first with 3% peroxidase (in methanol) for 10 min and then washed and incubated with 3% BSA (in TBS) for 1 hr to block non-specific binding sites. Consecutive normal, CP, and PDAC tissues were incubated with mouse monoclonal anti cytokeratin-19 (CK19) (DAKO Cytomation, Hamburg, Germany), rabbit polyclonal ADAM8 antibody (Chemicon International, Temecula, CA, USA) diluted 1:250 in washing buffer, or with normal rabbit IgG as control at 4°C overnight. The slides were rinsed with washing buffer and incubated with HRP-labeled anti-rabbit antibodies (DAKO Cytomation, Hamburg, Germany) for 45 min at room temperature. The slides were washed in washing buffer and each section was subjected to 100 μl DAB-chromogen substrate mixture (DAKO), and then counterstained with Mayer's haema-toxylin. The sections were washed, dehydrated in progressively increasing concentrations of ethanol, and mounted with xylene-based mounting medium. For semi-quantitative analysis, slides were scored in a blinded manner by two observers (NV, HK). Since the staining pattern within each sample was generally homogenous, staining in the cancer cells of each sample was categorized as absent, weak, moderate or strong. In the cases of divergent scoring, a third observer (JK) decided the final category.
Hypoxia treatment
Cells were grown to ∼70% confluence in 10-cm tissue culture dishes. For hypoxia treatment, cells were incubated in a hypoxic chamber with fresh complete medium (Billups-Rothenberg, Inc., Del Mar, CA, USA) with an 89.25%/10%/0.75% mixture of N2/CO2/O2 for 24 hrs. After the indicated time of hypoxia exposure, RNA and protein were extracted from hypoxic and normoxic pancreatic cancer cells as described above.
ELISA
ADAM8 levels were measured using a commercially available ELISA kit (R&D Systems, Wiesbaden-Nordenstadt, Germany), according to the manufacturer's instructions. Cell culture supernatants were concentrated three-fold using the centrifugal concentrator Vivaspin 6 (Vivascience, Hannover, Germany) at 3000 g for 20 min.
siRNA transfection
Cells were transfected with four different ADAM8 siRNA oligonucleotides (Qiagen, Hilden, Germany) or control siRNA oligonucleotides, using RNAiFect reagent (Life Technologies, Karlsruhe, Germany), according to the manufacturer's instructions. Briefly, 2.5 × 105 pancreatic cancer cells were seeded in six-well plates in complete medium until 70% confluence and subsequently transfected with the indicated siRNA. The ADAM8 target sequences were: #1. AGG CAT CAT CGT CTA CCG CAA; #2. CAA GCT ATA TCA GAA ACT CAA; #3. CCC AGC TTT GTG TGT GTT TAA; #4. CCG GCT ACA CAG AGA CCT ATA. The effects of siRNA silencing were analysed after 24, 48, 72 and 96 hrs. All experiments were repeated four times.
Cell proliferation assay
The 3-(4, 5-methylthiazol-2-yl)-2.5-diphenyltetrazolium-bromide (MTT) (Sigma, Steinheim, Germany) assay was used to assess cell proliferation. The cells were seeded in 96-well plates at a density of 5000 cells/well in 250 μl of complete medium. MTT (5 mg/ml PBS pH 7.4) was added to a final concentration of 0.5 mg/ml after the indicated time. After 4 hrs of incubation, the formazan products were solu-bilized with acidic isopropanol and the optical density was measured at 570 nm. The experiments were carried out in triplicate and repeated three times.
Invasion assay
The invasion activity of pancreatic cancer cells was tested using the BD BioCoat Invasion Assay System (BD Biosciences, Bedford, MA, USA) as previously described [21]. The cells were seeded at a density of 5 × 104 cells/ml in the upper inserts of 24-well invasion chambers and incubated for 24 hrs according to the manufacturer's protocol. Next, the cells were fixed in ice-cold methanol, stained with 0.05% crystal violet in 20% ethanol and the invading cells were quantified under light microscopy. The experiments were repeated three times.
SELDI-TOF-MS analysis
Cell culture supernatants were collected, aliquoted into 1-ml portions, and stored at −80°C until further processing. The supernatants were lyophilized in a speed-vac concentrator and dissolved with 20 μl of aqueous 5% acetonitrile (CAN)/0.1% trifluoroacetic acid (TFA) solution. Aliquots of 10 μl of the eluates were sampled onto NP-20 arrays and processed according to the manufacturer's protocol (Ciphergen Biosystems, Fremont, CA, USA). As an energy absorption matrix, 0.8 μl of saturated sinapinic acid solution (50% v/v CAN, 0.5% v/v TFA) was applied twice per spot. The arrays were then air dried and stored at room temperature in the dark until further processing. SELDI-TOF MS spectra were recorded in the positive ion mode with time lag focusing (focus mass setting 8,000 D with 130 shots of laser intensity, 170 per spot) on a PBS Iic ProteinChip Reader (Ciphergen Biosystems, Fremont, CA, USA) using detector voltage of 2.85 kV and source voltage of 20 kV. Prior to protein analysis, the PBS Iic ProteinChip Reader instrument was externally calibrated using the all-in-one protein molecular mass stan-dard. Qualified mass peaks (signal-to-noise ratio of >5, cluster mass window at 0.3%) within the m/z range of 2,000–75,000 Da were selected automatically. Spectra of all samples were calibrated using external peptide and protein standards, baseline corrected and normalized using the total ion current. With external calibration the observed mass accuracy for the SELDI-MS data was about 0.1–0.15%.
Statistical analysis
For statistical analyses, the non-parametric Mann–Whitney test was used. Survival analysis was performed according to the Kaplan–Meier method. Significance was defined as P < 0.05.
Results
First QRT-PCR was performed to evaluate the expression of ADAM8 mRNA in normal pancreatic tissue samples (n = 20), chronic pancreatitis (CP) samples (n = 28) and PDAC samples (n = 68). This analysis revealed that the median ADAM8 mRNA levels were significantly higher in PDAC than in normal pancreatic tissues (5.3-fold increase; P= 0.0008). In addition, ADAM8 mRNA levels were also higher in CP samples compared to normal pancreatic tissues (2.4-fold increase; P= 0.032) (Fig. 2A). ADAM8 mRNA levels were 2.2-fold higher in PDAC versus CP tissues;how-ever, this difference was not significant.
2.
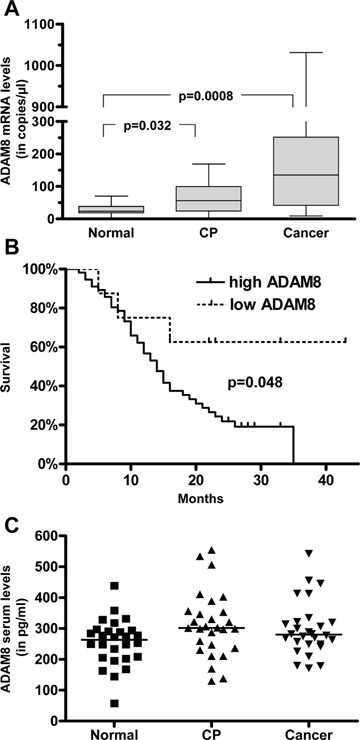
ADAM8 expression in pancreatic tissues and serum release. (A) Box and whisker diagram showing ADAM8 mRNA levels in the normal pancreas (n = 20), chronic pancreatitis (n = 28) and pancreatic cancer tissues (n = 68) were assessed by real-time QRT-PCR as described in the ‘Materials and methods’ section. The values are normalized to housekeeping genes (cyclophilin B and HRPT). (B) Survival curves of PDAC patients (n = 64) with low (<30 copies/μl cDNA) and high (≥30 copies/μl cDNA). (C) Serum levels of ADAM8 in patients and healthy controls. The ELISA analysis was performed for 28 serum samples in each examined group, and the results are presented as single values and median (horizontal line).
To analyse whether increased ADAM8 mRNA levels would also result in increased protein levels, immunoblot analysis was carried out. ADAM8 protein was weakly expressed in the normal pancreas as a 60 kD protein (remnant protein) (Fig. 1B). In line with the QRT-PCR data, there was increased expression in pancreatic cancer tissues and CP tissues compared to the normal pancreas. Interestingly, in contrast to the QRT-PCR data, there was no difference in ADAM8 protein expression between CP and PDAC samples, and some CP tissues exhibited even higher ADAM8 protein levels compared to PDAC samples, suggesting different transcriptional regulation in CP and PDAC tissues (Fig. 1B).
In addition to the 60 kD remnant form of ADAM8, two closely spaced bands with a molecular weight of 85–95 kD were visible in all CP and PDAC cases. One of these bands corresponds to the approximately 90 kDa processed form (Fig. 1A). Whether the other band represents a splice variant or a cross-reactive protein is currently not known. However, the same pattern has already been recently reported by other groups [19].
Interestingly, the 90 kDa processed form contains the metalloprotease (MP) domain responsible for the proteolytic activity of ADAM8, and the 60 kDa remnant form mediates cell adhesion [11] (Fig. 1A).
Since ADAM8 expression has been correlated to stage and survival time in different tumours, we compared the survival data of 64 patients with PDAC in relation to ADAM8 mRNA levels. This analysis revealed that patients with low ADAM8 mRNA expression (below the median ADAM8 expression in normal pancreatic tissues, which was 30 copies/μl cDNA) had significantly longer median survival (>40 months) compared to the patients with elevated ADAM8 mRNA levels (14 months;P = 0.048) (Fig. 2B). However, when the threshold was set to median ADAM8 mRNA levels in PDAC tissues, there was no significant difference in median survival (below median ADAM8 mRNA levels in PDAC tissues:15 months versus 13.5 months for ADAM8 mRNA levels above median; P= 0.87; jdata not shown).
Based on previous studies where it was shown that ADAM8 is a possible prognostic marker for lung ade-nocarcinoma and renal cell carcinoma [16, 17], we decided to measure ADAM8 serum levels in PDAC, CP patients and healthy controls using a commercially available ELISA kit. Interestingly, serum levels of ADAM8 in PDAC patients, CP patients and healthy individuals were not significantly different (Fig. 2C).
In order to determine the exact localization of ADAM8 in CP and PDAC, immunohistochemistry was carried out next. In normal pancreatic tissues (n = 8), ADAM8 exhibited weak cytoplasmic and membranous staining in normal ductal and acinar cells (Fig. 3A, lower inset) and moderate expression in islet cells (Fig. 3A). Ductal cells were confirmed by consecutive sections stained with CK19 as a ductal cell marker (Fig. 3A, upper inset; 3B, inset). ADAM8 expression was also weakly observed in the stroma, nerves and blood vessels of normal pancreatic tis-sues. In CP tissues (n = 8), ADAM8 exhibited moderate to strong staining in ductal cells, tubular complexes and degenerating acinar cells (Fig. 3B) but not in inflammatory cells. In PDAC tissues (n = 99), ADAM8 demonstrated moderate-to-strong staining on the plasma membrane of the cancer cells in 79% of the cases (Fig. 3C, E and F). Pancreatic ductal adeno-carcinoma cells were also positive for CK19 in consecutive sections (Fig. 3D). In addition, ADAM8 expression was detected in tubular complexes of 36% of the cases, and in degenerating acinar cells of 51% of the cases. Weak ADAM8 staining was observed in intra-pancreatic nerves. In lymph node metastases (n = 6) of PDAC, ADAM8 exhibited moderate to strong staining in the metastatic cancer cells of all cases. The specificity of ADAM8 staining was demonstrated by the absent staining in consecutive negative control tissue sections (Fig. 3F, inset). Next, a semi-quantification analysis for the intensity of ADAM8 staining in pancreatic cancer cells in PDAC tissues was performed. Staining was categorized as absent (n = 0), weak (n = 21), moderate (n = 46), and strong (n = 32). There was a tendency towards better survival of PDAC patients exhibiting weak/moderate ADAM8 staining (median survival: 20 months), in comparison to patients exhibiting strong ADAM8 staining in pancreatic cancer cells (median survival: 14 months; P= 0.065) (Fig. 3G).
3.
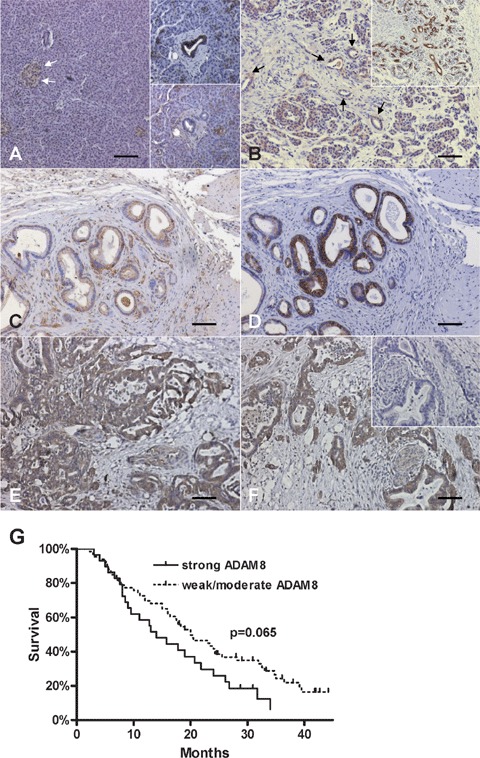
ADAM8 expression and localization in the normal pancreas, CP and PDAC tissues: Immunohistochemistry was performed as described in the ‘Materials and methods’ section. (A) ADAM8 expression in normal pancreatic islets (arrows) and acinar cells and ductal cells (lower inset). Consecutive section stained with CK19 confirming ductal cells (upper inset). (B) ADAM8 expression in CP tissues (arrows indicate tubular complexes). Consecutive section stained with CK19 confirming ductal cells/tubular complexes (inset). (C, D) ADAM8 (C) and CK19 (D) staining in pancreatic cancer cells of PDAC tissues. (E, F) Strong ADAM8 staining in pancreatic cancer cells of PDAC tissues. Note the absent staining in a consecutive negative control tissue section (inset). Horizontal lines represent the scale bar of 50 μm. (G) Survival curves of PDAC patients (n = 99) with weak/moderate ADAM8 staining versus strong ADAM8 staining (P= 0.065).
Next we sought to examine the regulation of ADAM8 mRNA and protein expression in cultured pancreatic cancer cell lines under the effects of hypoxia as has been shown previously for other ADAMs [22, 23]. In addition we sought to test the effects of ADAM8 silencing on pancreatic cancer cell growth, invasion and potential target proteins. Therefore, mRNA levels of ADAM8 were determined in these cells. Different amounts of ADAM8 mRNA with a range of 16–922 copies/μl cDNA were detected in all examined pancreatic cancer cell lines (Fig. 4A). Immunoblot analysis was performed to compare the mRNA data with the corresponding protein expression. As in pancreatic cancer tissues, ADAM8 was present in two forms (60 kD and 85–95 kD) in the investigated cell lines (Fig. 4B). These results were confirmed by ELISA, which demonstrated ADAM8 expression in all pancreatic cancer cell lysates (Fig. 4C). In contrast, the released ADAM8 ectodomain was detectable in only four of eight pancreatic cancer cell supernatants (Fig. 4C).
4.
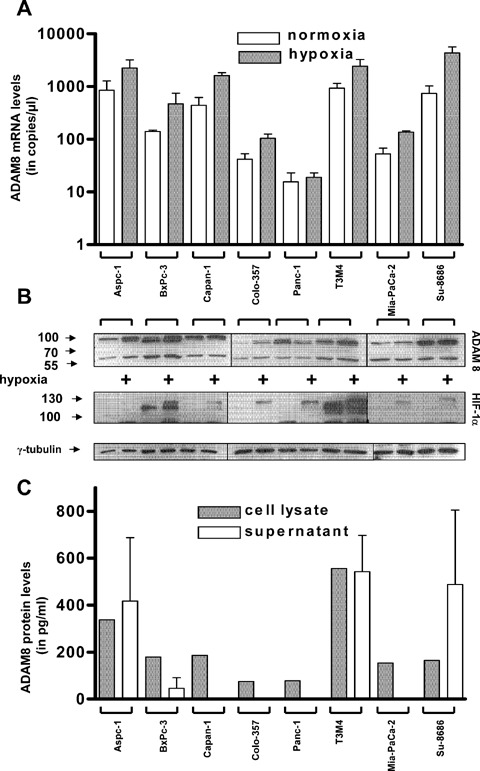
ADAM8 expression under normoxic and hypoxic conditions. ADAM8 mRNA (A) and protein (B) expression in pancreatic cancer cell lines under normal and hypoxic conditions (24 hrs) was determined by qRT-PCR and immunoblotting, respectively. The response towards hypoxic conditions was monitored by HIF-1α expression levels. Equal loading of the protein samples was confirmed using an -tubulin antibody. Size markers are indicated on the left (in kDa). (C) ELISA: ADAM8 expression in the cell lysates (grey bars) and cell culture supernatants (white bars). The values are presented as mean +/− SEM.
Next the effects of hypoxia on ADAM8 levels were examined in pancreatic cancer cells. Hypoxia induced an increase of ADAM8 mRNA levels of 1.5–5.5-fold in the examined pancreatic cancer cell lines except Panc-1 (Fig. 4A). On the protein level, hypoxia induced a 1.2–5.9-fold increase of the ADAM8 prodomain removal form (90 kD) in five of eight pancreatic cancer cells. In Panc-1 cells, there was a 0.4-fold decrease of ADAM8 in response to hypoxia, and in BxPC-3 and Su8686 there were no significant changes observed (Fig. 4B). HIF-1 levels were used as a control for the response towards hypoxic conditions (Fig. 4B). Additionally, hypoxia induced a 1.3–2.0-fold increase of the remnant form ADAM8 in four of eight pancreatic cancer cell lines (Aspc-1, Colo-357, Panc-1, T3M4) (Fig. 4C).
Members of the ADAM family, including ADAM8, are associated with cellular invasion. To investigate the influence of ADAM8 on growth and invasion of pancreatic cancer cells, gene silencing was performed with siRNA oligonucleotides, followed by growth and invasion assays. ASPC-1 pancreatic cancer cells were selected for siRNA transfection experiments since they expressed one of the highest levels of ADAM8 mRNA and protein. The maximum effects of siRNA transfection were observed after 96 hrs, resulting in an 83% reduction of ADAM8 mRNA levels and a 74% reduction in protein levels (Fig. 5A, 5B). Interestingly, silencing of ADAM8 did not significantly influence anchorage-dependent cell growth (Fig. 5C). In contrast, there was significantly reduced invasion (to 36% of control, P < 0.05) (Fig. 5C).
5.
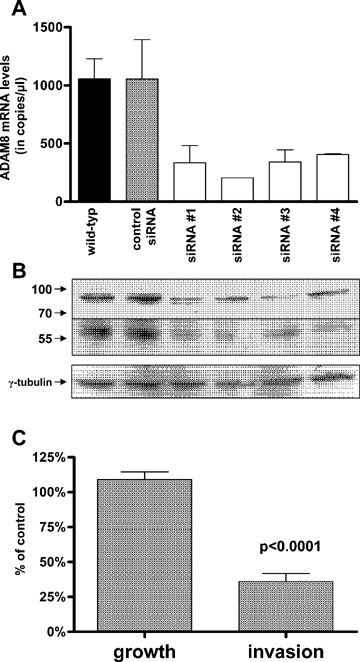
Invasion and growth capacity of ADAM8 siRNA transfected ASPC-1 cells. (A) Real-time quantitative PCR and (B) immunoblot analysis were performed to analyse the effect of four different ADAM8 siRNA oligonucleotides (#1–#4) compared with control trans-fected ASPC-1 cells. Equal loading of the protein samples was confirmed using an -tubulin antibody. Size markers are indicated on the left (in kDa). (C) Cell proliferation and invasion assays were performed as described in the ‘Materials and methods’ section. ADAM siRNA oligonu-cleotides #1 were utilized. The values are expressed as % of control transfected cells and shown as mean ± SEM obtained from four independent experiments.
To analyse whether ADAM8 silencing would result in decreased proteolytic activity, SELDI-TOF-MS analysis was performed with the cell culture super-natants of control siRNA and ADAM8 siRNA trans-fected ASPC-1 cells. Using NP20 protein chip arrays, between 25 and 35 peaks for control siRNA super-natants, and up to 50 protein signals per sample were detected in the ADAM8 siRNA supernatants, covering the molecular weight range from 2.5 to 75 kD.
The data were further analysed by Biomarker Wizard Software, a univariate analysis tool. After analysis of the intensity values for all spectra at a given mass, there were significant differences in distribution of proteins in the control siRNA transfected ASPC-1 cell culture supernatants compared to the ADAM8 siRNA transfected ASPC-1 supernatants. It could be shown (Fig. 6A) that the peak at m/z 39,232 was more than three-fold higher in control super-natants (height from baseline 2.8) compared to siRNA supernatants (height from baseline 0.8). The lower intensity of this protein in ASPC-1 ADAM8 siRNA transfected cell culture supernatants leads to the conclusion that it is probably a shedded part of ADAM8. The differential profiling of ADAM8 and control transfected cell supernatant spectra revealed in the controls not only a decreased intensity of some protein peaks but also a complete disappearance of some proteins, as shown in the boxed area covering the 5–7 kD range (Fig. 6B). The presence of additional peaks in supernatants of the ADAM8 siRNA trans-fected cell cultures indicates the knock-down ADAM8 expression, and consequently a lower proteolytic activity in these cell culture supernatants.
6.
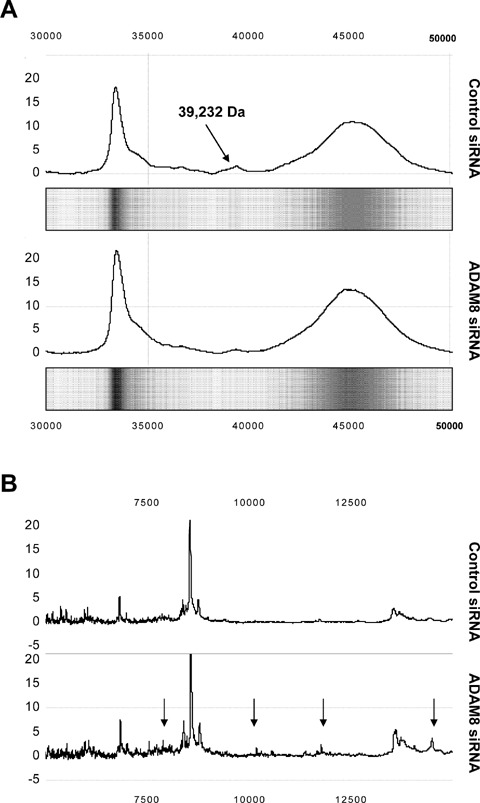
SELDI-TOF-MS analysis. Proteolytic activity of ADAM8 in cell culture super-natants assessed by SELDI-TOF-MS. (A) Trace-view and gel view of the mass spectra between 30 and 50 kD of the control siRNA and ADAM8 siRNA #1 transfected cells. Arrow points to the 39.2 kD protein and its suppression in siRNA transfected cells. (B) Decreased intensities of protein peak (arrows) in control siRNA transfected cells.
Discussion
ADAMs constitute a large family of transmembrane proteins that are involved in proteolysis, making them candidates for mediating the remodelling of the extracellular matrix (ECM). ADAMs influence cell adhesion and cell migration, both of which are important under physiological conditions. During tumour development, these effects might influence the ability of cancer cells to metastasize, making these proteins possible targets for anti-tumour therapy [24, 25]. ADAM8, one of the members of the ADAM family, is overexpressed in various human tumours [16–19]. In pancreatic cancer, aberrant expression of members of the ADAM family–ADAM9, ADAM15 and ADAM17–has been observed, and overexpression of these proteins is related to the invasiveness and aggressiveness of PDAC [20, 26, 27].
In the present study, we observed a significant up-regulation of ADAM8 mRNA and protein levels in PDAC compared to normal pancreatic tissues, suggesting a potential role of ADAM8 in the patho-genesis and evolution of pancreatic cancer. ADAM8 mRNA expression levels in normal pancreatic tissues were within a narrow range, whereas PDAC tissues displayed a wide range of ADAM8 mRNA expression levels. These differences in ADAM8 patterns of normal and cancerous tissues might reflect the rather homogenous composition of normal pancreatic tissues and the heterogeneous composition of PDAC tissues. ADAM8 was localized on the plasma membrane of ductal cells and to a lesser extent of islets and acinar cells in the normal pancreas. In PDAC tissues, ADAM8 was moderately to strongly present in cancer cells and tubular complexes. Thus, the difference in ADAM8 mRNA levels in bulk pancreatic cancer tissues is most likely the sum effect of the percentage of tumour cells within the sample and the amount of ADAM8 expression of the individual tumour cells.
Interestingly, correlation analysis between ADAM8 mRNA and protein levels and survival of PDAC patients demonstrated that patients with low ADAM8 levels had significantly better survival than patients with high levels of ADAM8. This is in agreement with recent studies showing that ADAM8 overexpression is associated with poor prognosis in patients with lung cancers, brain tumours and renal cell carcinomas [16, 17, 19]. Additionally, it has been shown that ADAM8 expression is a good predictor of distant metastases in renal cell carcinoma [17]. The exact mechanism by which ADAM8 influences survival of patients is currently not known, but it has been speculated that enhanced proteolytic activity results in increased cell migration, invasion and metastasis in these tumours [19]. Thus, targeting ADAM8 by specific therapeutic inhibitors might improve the survival of PDAC patients.
Previous studies have shown that ADAM8 might be processed into two soluble forms:the MP-domain form and the ectodomain form. It has also been suggested that soluble ADAM8 forms could be possible serum markers in certain tumours, for example lung adenocarcinoma [11, 16]. In contrast, we did not detect significant differences between ADAM8 levels in the serum of patients with PDAC or CP compared to healthy controls. In addition, in the cell culture supernatants we detected soluble forms of ADAM8 in only four of eight pancreatic cancer cell lines, despite ADAM8 mRNA and protein expression in all examined cell lines. Together, these results suggest that ADAM8 prevails mostly in membrane-associated forms in pancreatic cancer cells. Thus, unlike in other malignancies, ADAM8 cannot be used as a potential marker for pancreatic cancer or CP.
It has been shown that hypoxia influences the expression of some members of the ADAM family, such as ADAM9 and ADAM10 [22, 23]. Likewise, ADAM8 mRNA and protein expression increased in five of eight pancreatic cancer cell lines under hypox-ic conditions. Hypoxia can increase the growth and invasion of pancreatic cancer through different HIF-1α-dependent mechanisms [28, 29]. Indeed, ADAM8 silencing suppressed the invasiveness of pancreatic cancer cells. These data are in agreement with findings in lung and brain cancers [16]. In contrast, ADAM8 silencing did not influence the growth of pancreatic cancer cells, indicating that ADAM8 is an invasion-promoting factor but not a growth-promoting factor.
With the use of SELDI-TOF-MS protein profiling, we were able to detect suppressed extracellular pro-teolytic activity of ADAM8 after siRNA silencing. We have shown that in supernatants of ASPC-1 cells after control transfection some protein peaks decreased below the detection limit and some peaks had significantly lower intensities compared with ADAM8 siRNA transfected cells. We speculate that these peaks correspond to a group of molecules, which might be potential substrates for ADAM8 in pancreatic cancer. Future isolation and purification of such a group of ADAM8 target molecules will shed light on the possible pancreatic cancer cell invasion pathways directed by ADAM8. Nonetheless, our findings imply that ADAM8 has proteolytic activity in pancreatic cancer cells, and that this proteolysis can be effectively blocked by specific ADAM8 siRNA oligonucleotides.
In conclusion, ADAM8 is overexpressed in PDAC and influences pancreatic cancer cell invasion. Increased ADAM8 levels correlate with decreased patient survival, suggesting that ADAM8 might be a potential therapeutic target.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 3.Kleeff J, Michalski C, Friess H, Buchler MW. Pancreatic cancer: from bench to 5-year survival. Pancreas. 2006;33:111–8. doi: 10.1097/01.mpa.0000229010.62538.f2. [DOI] [PubMed] [Google Scholar]
- 4.Naus S, Richter M, Wildeboer D, Moss M, Schachner M, Bartsch JW. Ectodomain shedding of the neural recognition molecule CHL1 by the metal-loprotease-disintegrin ADAM8 promotes neurite outgrowth and suppresses neuronal cell death. J Biol Chem. 2004;279:16083–90. doi: 10.1074/jbc.M400560200. [DOI] [PubMed] [Google Scholar]
- 5.Wolfsberg TG, Primakoff P, Myles DG, White JM. ADAM, a novel family of membrane proteins containing a disintegrin and metalloprotease domain: multi-potential functions in cell-cell and cell-matrix interactions. J Cell Biol. 1995;131:275–8. doi: 10.1083/jcb.131.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto S, Higuchi Y, Yoshiyama K, Shimizu E, Kataoka M, Hijiya N, Matsuura K. ADAM family proteins in the immune system. Immunol Today. 1999;20:278–84. doi: 10.1016/s0167-5699(99)01464-4. [DOI] [PubMed] [Google Scholar]
- 7.Choi SJ, Han JH, Roodman GD. ADAM8: a novel osteoclast stimulating factor. J Bone Miner Res. 2001;16:814–22. doi: 10.1359/jbmr.2001.16.5.814. [DOI] [PubMed] [Google Scholar]
- 8.Yoshiyama K, Higuchi Y, Kataoka M, Matsuura K, Yamamoto S. CD156 (human ADAM8): expression, primary amino acid sequence, and gene location. Genomics. 1997;41:56–62. doi: 10.1006/geno.1997.4607. [DOI] [PubMed] [Google Scholar]
- 9.Schlomann U, Rathke-Hartlieb S, Yamamoto S, Jockusch H, Bartsch JW. Tumor necrosis factor alpha induces a metalloprotease-disintegrin, ADAM8 (CD 156): implications for neuron-glia interactions during neurodegeneration. J Neurosci. 2000;20:7964–71. doi: 10.1523/JNEUROSCI.20-21-07964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida S, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Molecular cloning of cDNA encoding MS2 antigen, a novel cell surface antigen strongly expressed in murine monocytic lineage. Int Immunol. 1990;2:585–91. doi: 10.1093/intimm/2.6.585. [DOI] [PubMed] [Google Scholar]
- 11.Schlomann U, Wildeboer D, Webster A, Antropova O, Zeuschner D, Knight CG, Docherty AJ, Lambert M, Skelton L, Jockusch H, Bartsch JW. The metalloprotease disintegrin ADAM8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. J Biol Chem. 2002;277:48210–9. doi: 10.1074/jbc.M203355200. [DOI] [PubMed] [Google Scholar]
- 12.Naus S, Reipschlager S, Wildeboer D, Lichtenthaler SF, Mitterreiter S, Guan Z, Moss ML, Bartsch JW. Identification of candidate substrates for ectodomain shedding by the metalloprotease-dis-integrin ADAM8. Biol Chem. 2006;387:337–46. doi: 10.1515/BC.2006.045. [DOI] [PubMed] [Google Scholar]
- 13.Fourie AM, Coles F, Moreno V, Karlsson L. Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J Biol Chem. 2003;278:30469–77. doi: 10.1074/jbc.M213157200. [DOI] [PubMed] [Google Scholar]
- 14.King NE, Zimmermann N, Pope SM, Fulkerson PC, Nikolaidis NM, Mishra A, Witte DP, Rothenberg ME. Expression and regulation of a dis-integrin and metalloproteinase (ADAM) 8 in experimental asthma. Am J Respir Cell Mol Biol. 2004;31:257–65. doi: 10.1165/rcmb.2004-0026OC. [DOI] [PubMed] [Google Scholar]
- 15.Amour A, Knight CG, English WR, Webster A, Slocombe PM, Knauper V, Docherty AJ, Becherer JD, Blobel CP, Murphy G. The enzymatic activity of ADAM8 and ADAM9 is not regulated by TIMPs. FEBS Lett. 2002;524:154–8. doi: 10.1016/s0014-5793(02)03047-8. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa N, Daigo Y, Yasui W, Inai K, Nishimura H, Tsuchiya E, Kohno N, Nakamura Y. ADAM8 as a novel serological and histochemical marker for lung cancer. Clin Cancer Res. 2004;10:8363–70. doi: 10.1158/1078-0432.CCR-04-1436. [DOI] [PubMed] [Google Scholar]
- 17.Roemer A, Schwettmann L, Jung M, Stephan C, Roigas J, Kristiansen G, Loening SA, Lichtinghagen R, Jung K. The membrane proteas-es adams and hepsin are differentially expressed in renal cell carcinoma. Are they potential tumor markers? J Urol. 2004;172:2162–6. doi: 10.1097/01.ju.0000144602.01322.49. [DOI] [PubMed] [Google Scholar]
- 18.Fritzsche FR, Jung M, Xu C, Rabien A, Schicktanz H, Stephan C, Dietel M, Jung K, Kristiansen G. ADAM8 expression in prostate cancer is associated with parameters of unfavorable prognosis. Virchows Arch. 2006;449:628–36. doi: 10.1007/s00428-006-0315-1. [DOI] [PubMed] [Google Scholar]
- 19.Wildeboer D, Naus S, Amy Sang QX, Bartsch JW, Pagenstecher A. Metalloproteinase disintegrins ADAM8 and ADAM19 are highly regulated in human primary brain tumors and their expression levels and activities are associated with invasiveness. J Neuropathol Exp Neurol. 2006;65:516–27. doi: 10.1097/01.jnen.0000229240.51490.d3. [DOI] [PubMed] [Google Scholar]
- 20.Yamada D, Ohuchida K, Mizumoto K, Ohhashi S, Yu J, Egami T, Fujita H, Nagai E, Tanaka M. Increased expression of ADAM 9 and ADAM 15 mRNA in pancreatic cancer. Anticancer Res. 2007;27:793–9. [PubMed] [Google Scholar]
- 21.Erkan M, Kleeff J, Esposito I, Giese T, Ketterer K, Buchler MW, Giese NA, Friess H. Loss of BNIP3 expression is a late event in pancreatic cancer contributing to chemoresistance and worsened prognosis. Oncogene. 2005;24:4421–32. doi: 10.1038/sj.onc.1208642. [DOI] [PubMed] [Google Scholar]
- 22.Marshall AJ, Rattray M, Vaughan PF. Chronic hypoxia in the human neuroblastoma SH-SY5Y causes reduced expression of the putative alpha-secretases, ADAM10 and TACE, without altering their mRNA levels. Brain Res. 2006;1099:18–24. doi: 10.1016/j.brainres.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Sung SY, Kubo H, Shigemura K, Arnold RS, Logani S, Wang R, Konaka H, Nakagawa M, Mousses S, Amin M, Anderson C, Johnstone P, Petros JA, Marshall FF, Zhau HE, Chung LW. Oxidative stress induces ADAM9 protein expression in human prostate cancer cells. Cancer Res. 2006;66:9519–26. doi: 10.1158/0008-5472.CAN-05-4375. [DOI] [PubMed] [Google Scholar]
- 24.Arribas J, Bech-Serra JJ, Santiago-Josefat B. ADAMs, cell migration and cancer. Cancer Metastasis Rev. 2006;25:57–68. doi: 10.1007/s10555-006-7889-6. [DOI] [PubMed] [Google Scholar]
- 25.Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer. 2005;115:849–60. doi: 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- 26.Grutzmann R, Luttges J, Sipos B, Ammerpohl O, Dobrowolski F, Alldinger I, Kersting S, Ockert D, Koch R, Kalthoff H, Schackert HK, Saeger HD, Kloppel G, Pilarsky C. ADAM9 expression in pancreatic cancer is associated with tumour type and is a prognostic factor in ductal adenocarcinoma. Br J Cancer. 2004;90:1053–8. doi: 10.1038/sj.bjc.6601645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringel J, Jesnowski R, Moniaux N, Luttges J, Choudhury A, Batra SK, Kloppel G, Lohr M. Aberrant expression of a disintegrin and metalloproteinase 17/tumor necrosis factor-alpha converting enzyme increases the malignant potential in human pancreatic ductal adenocarcinoma. Cancer Res. 2006;66:9045–53. doi: 10.1158/0008-5472.CAN-05-3287. [DOI] [PubMed] [Google Scholar]
- 28.Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, Niizeki H, Kawamura K, Hosokawa M, Asaka M. Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res. 2001;61:6548–54. [PubMed] [Google Scholar]
- 29.Yoon DY, Buchler P, Saarikoski ST, Hines OJ, Reber HA, Hankinson O. Identification of genes differentially induced by hypoxia in pancreatic cancer cells. Biochem Biophys Res Commun. 2001;288:882–6. doi: 10.1006/bbrc.2001.5867. [DOI] [PubMed] [Google Scholar]


