Abstract
Curcumin is a polyphenol derived from the dietary spice turmeric. It possesses diverse anti-inflammatory and anti-cancer properties. Curcumin has been shown to exhibit an inhibitory effect on the production of inflammatory cytokines by human monocytes and has inhibited the animal model of multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE) in association with a decrease in interleukin 12 (IL-12) production and signal transducer and activator of transcription 4 (STAT4) activation. The type I interferon (IFN) IFN-has the ability to suppress IL-12. Both IL-12 and IFN-α/β signal through the activation by phosphorylation of STAT4. Our aim was to investigate the effects of curcumin on the ability of T cells to respond to IL-12 or IFN-α/β. We report that curcumin decreases IL-12-induced STAT4 phosphorylation, IFN-γ production, and IL-12 Rβ1 and β2 expression. IFN-β-induced STAT4 phosphorylation, IL-10 production and IFN receptor (IFNAR) subunits 1 and 2 expression were enhanced by curcumin. Curcumin increased IFN-α-induced IL-10 and IFNAR1 expression. Prior exposure to curcumin decreased IFN-α-induced IFNAR2 expression and did not modify the level of IFN-α-induced pSTAT4 generation. Thus, the effect of curcumin on STAT4 activation in T cells is dependent upon the stimulus to which the T cells have been exposed.
Keywords: human, T cells, curcumin, interleukin-12, interferon, signal transduction
Introduction
Curcumin (diferuloylmethane) is a naturally occurring yellow pigment isolated from the rhizomes of the plant Curcuma Longa Lin, found in south Asia, and is an important ingredient of the dietary spice, turmer-ic. Its medicinal value has been well recognized with its antioxidant, anti-tumour, and anti-inflammatory activities and is under pre-clinical trial for the treatment of cancer and inflammation. Curcumin is a potent inhibitor of tumour initiation in vivo[1] and possesses anti-proliferative and apoptotic activities against tumour cells in vitro[2]. Curcumin has been shown to facilitate apoptosis of cancer cells via inhibition of signal transducer and activator of transcription 3 (STAT3) activation and can also suppress interferon (IFN)-α-induced STAT1 phosphorlaytion, without affecting STAT5 phosphorlyation [3]. Curcumin has been shown to have an inhibitory effect on the production of inflammatory cytokines by human monocytes and alveolar macrophages [4]. Natarajan and Bright (2002) examined the effect and mechanism of action of curcumin on the pathogene-sis of central nervous system (CNS) demyelination in experimental autoimmune encephalomyelitis (EAE), a T cell-mediated demyelinating disease of rodents, widely used as an animal model of multiple sclerosis (MS). They found that curcumin inhibited EAE in association with a decrease in interleukin (IL)-12 production. In vitro treatment of activated T cells with curcumin inhibited IL-12-induced tyrosine phospho-rylation of STAT4 [5].
The type I interferon, IFN-β, is the best characterized and most used disease-modifying treatment for MS. It has been demonstrated that IFN-β significantly increases the expression of the anti-inflammatory cytokine IL-10 [6], a major suppressor of Th1 cytokines [7, 8]. Like IL-12, IFN-β and IFN-α also acts via the STAT4 pathway [9]. In this study, we investigated whether the inhibitory effects of curcum-in were specific to IL-12 or whether similar effects would be observed with the type I interferons IFN-α/β. Here, we report that curcumin has a differential effect on IL-12 and IFN-α/β not only through differential effects on STAT4 phosphorylation, but also upstream at the receptor level. This in turn results in a differential modulation on the level of cytokine induction by IFN-α/β and IL-12.
Materials and methods
Cell preparation
Peripheral blood mononuclear cells (PBMC) from healthy donors were isolated by standard gradient centrifugation with Histopaque 1077 (Sigma-Aldrich, Dorset UK). The mononuclear cells were prepared at 1 × 106 cells/ml in media consisting of Roswell Park Memorial Institute (RPMI) 1640, 2 mM glutamine, 20 mM Hepes, 0.1 mg/ml penicillin and streptomycin and 10% foetal calf serum (Sigma-Aldrich). The cells were co-cultured with 10 μg/ml phytohaemagglutinin (PHA) (Sigma-Aldrich) at 37°C and 5% CO2 for 72 hrs. Following PHA-induced proliferation, the cells were washed with media and stimulated with 100 U/ml IL-2 (R&D systems, Minneapolis, MN, USA) at 37°C and 5%CO2 for a further 24 hrs. The cells were then allowed to rest for 24 hrs in serum-free media under the same conditions. In other experiments, PBMCs were co-cultured with 0.5 μg/ml of anti-CD3 for 72 hrs at 37°C and 5% CO2. Following incubation, the cells were also allowed to rest for 24 hrs in serum-free media under the same conditions. PBMC were also obtained from four patients with relapsing remitting MS who had had no relapses and no steroid treatment for at least 3 months prior to blood donation, and had never had any immunomodulatory or immunosuppressive treatment (including IFN-α/β) at the time of blood collection. The study was approved by the Nottingham Research Ethics Committee.
Cell stimulation
PHA/IL-2-induced T cell blasts (1 × 106 cells/ml) or anti-CD3 stimulated T cells (1 × 106 cells/ml) were either left untreated or pre-treated with 20 μg/ml Curcumin (Sigma-Aldrich) for 30 min at 37°C. Both sets of cells were then either left unstimulated or incubated with 10 ng/ml IFN-β, 10 ng/ml IFN-β or 0.1 μg/ml IL-12 for 30 min at 37°C. The method used was refined so that the optimum concentrations of cytokines and curcumin were used. Varying concentrations of IFN- β1a (a gift from Serono International, London, UK), IFN-α (Sigma-Aldrich) and IL-12 and prior incubation with curcumin were analysed for their effect on STAT4 phospho-rylation; the concentrations used in this experiment were those that produced the peak pSTAT4 value and consistent suppression for curcumin. The effect of the duration of stimulation on STAT4 phosphorylation was also observed. The results generated suggested that the best stimulation was achieved after 30 min. For the ELISA, cells were stimulated for 18 hrs.
Intracellular staining
Following incubation, the cells were fixed in 1 ml of ice cold 70% ethanol and incubated on ice for 20 min. The cells were then washed by centrifugation once in PBA (PBS, 0.5% bovine serum albumin and 1% sodium azide [Sigma-Aldrich]), once in saponin buffer (PBA + 0.1% saponin [Sigma-Aldrich]) and once in 10% foetal calf serum (FCS) in saponin buffer at 300 g for 5 min. The supernatant was poured off and the cells re-suspended in the residue, to which 0.5 μg of the primary antibody, rabbit polyclonal anti-phosphorylated STAT4 or rabbit polyclonal STAT4 (Zymed, San Francisco, CA, USA) (method adapted for intracellular staining specific for STAT4 and phosphorylated STAT4 described by Uzel et al. (2001) [10]) or 10 μl monoclonal anti-human IFN-γ fluorescein isothiocyanate (FITC) antibody or monoclonal anti-human IL-10 phycoerythrin (PE), was added and incubated at room temperature for 30 min. Following incubation the cells were washed by centrifugation with saponin buffer. The cells incubated with non-conjugated primary antibody were incubated with the secondary antibody, 1 μg of PE conjugated goat anti-rabbit IgG for 30 min at room temperature. All cells were washed with 1 ml saponin buffer before resuspension in 500 μl of 0.5% formaldehyde for flow cytometry.
Surface staining
Following incubation, the cells were washed by centrifugation in 2% FCS RPMI, the supernatant was poured off and the cells re-suspended in the residue, to which 10 μl of IFN receptor (IFNAR) 1, or IL-12 Rβ1 PE labelled (R&D systems, Oxford, UK) antibodies were added and incubated on ice for 30 min. The cells were again washed by centrifuga-tion in 2% FCS RPMI. Those cells incubated with the non-conjugated IFNAR antibody were incubated with a secondary antibody, goat antimouse FITC (Caltag, Laboratories, CA, USA) for a further 30 min on ice. All cells were washed twice in 1 ml 2% FCS RPMI before re-suspension in 500 μl of 0.5% formaldehyde for flow cytometry.
Quantitative real-time polymerase chain reaction (PCR)
Quantitative reverse transcriptase real-time PCR was used to assess IL-10, IL-12Rβ1, IL-12Rβ2, IFNAR1 and IFNAR2 mRNA abundance in human PHA/IL-2 T cell blasts that had been stimulated with curcumin and/or IFN-β, IFN-α or IL-12. RNA was extracted using RNeasy miniprep kit (Qiagen, Valencia, CA, USA) following the manufacturers’ instructions. The RNA concentration was determined at 260 nm, and purity was assessed by measuring the 260/280 nm ratio. Only samples within a 1.70–1.95 range were used. First-strand cDNA synthesis was initiated from 0.5 μg total RNA, using random hexamers (Promega, Madison, WI, USA), and avian myeloblastosis virus reverse transcriptase (Promega) using conditions as described by the manufacturer in a final volume of 25 μl. Specific oligonucleotide primers were designed for the published sequence: IL-10, (Genbank accession no. BC104253), IFN-γ (Genbank accession no. NM 000619), IL-12Rβ1, (Genbank accession no. BC029121), IL-12Rβ2 (Genbank accession no. HSU64198), IFNAR1 (Genbank accession no. NM 000629), IFNAR2 (Genbank accession no. NM 207585) and β2 microglobulin (Genbank accession no. AF072097). The primers used were as follows: IL-10 forward 5′ CAACCTGCCTAACATGCTTC 3′; reverse 5′ GGACTCCTTTAACAACAAGTTG 3′; IFN-forward 5′ CGCAAAGCAATACATGAACTC 3′; reverse 5′ GTAATGG TTGTCCTGCCTG 3′; IL-12R β 1 forward 5′ACGAGTGCTCCTGGCAGTAT3′; reverse 5′ TCA-CACTCTG GGTGGAATCCT; IL-12Rβ2 forward 5′ GAGGAT-GCTCATTGATTT 3′; reverse 5′ TACATGCTCTTTGAAGCCCA 3′; IFNAR1 forward 5′ TGCTGCGAAAGTCTTCTTGA 3′; reverse 5′ TGCTTTCAACTTCTGAGGAACA 3′ IFNAR2 forward 5′ CACCAT AGTGACACTG AAATG 3′; reverse 5′ TTG-GAAGCCATGGATATGGT 3′; β2mircoglobulin forward 5′ CTCCGTGGCCTT AGCTGTG 3′ reverse 5′ ATGT-GTCTGGGTTT CATCCATC 3′. All real-time PCR was carried out using the SYBR green fluorescence method with SYBR green qPCR master mix (Stratagene, La Jolla, CA, USA) as specified by the manufacturer. The real-time PCR reactions were carried out in triplicate on a MX4000® Multiplex Quantitative QPCR System (Stratagene) using standard default thermal cycling conditions. Non-template controls were loaded in triplicate and were prepared by replacing the cDNA fraction of the PCR reaction with an equivalent volume of nuclease-free water. Quantification of transcripts was carried out using the relative standard curve method as described by Applied Biosystems (Foster City, CA, USA; 1997) [11]. An equal aliquot of undiluted cDNA from each sample was pooled together. This cDNA pool was serially diluted (neat, 1:2, 1:5, 1:10 1:20) to produce a set of standards, from which the Ct value (cycle number at which the reporter dye emission intensity rises above background noise) of a particular variant could be converted to nanograms of total RNA equivalent used for first-strand synthesis. β2 microglobulin expression was monitored as an internal standard on the cDNA template, therefore mRNA expression for each gene is normalized to the internal standard expression.
Cytokine assay using ELISA
PHA/IL-2-induced T cell blasts (1 × 106 cells/ml) or anti-CD3 stimulated T cells (1 ×1 06 cells/ml) were either left untreated or pre-treated with 20 μg/ml curcumin (Sigma-Aldrich) for 18 hrs at 37°C. Both sets of cells were then either left unstim-ulated or incubated with either 10 ng/ml IFN-β or 0.1 μg/ml IL-12 for a further 18 hrs at 37°C. The cell supernatants were removed for cytokine assay. IFN-γ and IL-10 levels were measured using a solid-phase sandwich ELISA purchased from Pharmingen (BD OptEIA™ BD Biosciences, Franklin Lakes, NJ, USA) and were performed following the manufacturers’ instructions.
Measurements
The PHA/IL-2 blasts were evaluated following antibody staining on an Epics XL flow cytometer (Beckman Coulter, Fullerton, CA, USA), and the results were analysed using the computer software WINMDI 2.8. IFN-γ and IL-10 levels were also measured using a solid-phase sandwich ELISA. Each experiment was repeated, using four independent donors. Statistical analysis was performed using a paired T test.
Results
Curcumin differentially affects the activation of STAT4 by IFN-β, IFN-β and IL-12
We examined whether curcumin affected IFN-β, IFN-α or IL-12-induced STAT4 phosphorylation. As shown in Figure 1, exposure to IFN-β, IFN-α or IL-12 resulted in increased pSTAT4 generation when compared to unstimulated cells. However, pre-treatment of the cells with curcumin produced contrasting results depending on whether the cells were subsequently exposed to IFN-β, IFN-α or IL-12. Pre-incubation with curcumin and then IFN-γ resulted in an increase in pSTAT4 generation (Fig. 1), whilst prior treatment with curcumin followed by IFN-γ resulted with similar pSTAT4 generation compared with IFN-γ alone. However, prior treatment with curcumin resulted in a decrease in pSTAT4 generation (Fig. 1).
1.
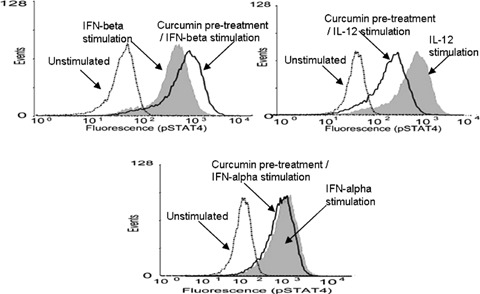
Modulation of pSTAT4 by curcumin. IFN-β (10 ng/ml), IFN-αl (10 ng/ml) and IL-12 (100 ng/ml) induced pSTAT4 expression (shaded curve on each graph). Prior incubation with curcumin (20 μg/ml) and then IFN-β resulted in an increase in pSTAT4 generation, whilst prior stimulation with curcumin (20 μg/ml) followed by incubation with IL-12 resulted in a decrease in pSTAT4 generation. Similar pSTAT4 generation was induced with both curcumin and then IFN-α and IFN-α alone. Dotted line on each graph represents unstimulated cells). Representative results of four independent experiments.
Effects of curcumin on the level of receptor expression
We next investigated whether curcumin also exerted its effects upstream at the receptor level. Quantitative real-time PCR was used to assess IL-12R and IFNAR mRNA expression in PHA/IL-2 T cell blasts. Both IFN-β and IFN-α modestly increased IFNAR1 expression; this was further increased by prior exposure to curcumin (P < 0.05). Prior exposure to cur-cumin also induced a moderate increase of IFNAR2 by IFN-β (Fig. 2). However, although IFN-α alone increased IFNAR2 expression, prior exposure to cur-cumin had no effect on IFN-α-induced IFNAR2 expression. In this study, prior exposure to IL-12 alone up-regulated both IL-12 Rβ1 and β2 expression (Fig. 3). However, pre-exposure to curcumin appeared to reduce this induction (Fig. 3, IL-12Rβ1 P= 0.074, IL-12Rβ2 P < 0.05). Similar results were also obtained when measuring IL-12Rβ1 and IFNAR protein expression using flow cytomtery. The median fluorescence intensity (MFI) for IFNAR was increased from 5.4 (range, 5.4–11.2) to 11.12 (10.35–15.5) when comparing unstimulated cells with cells incubated with IFN-β, respectively. Pretreatment with curcumin followed by IFN-β enhanced the MFI of IFNAR to 18.47 (11.2–28.0). Exposure to IL-12 alone increased β1 receptor whilst pre-exposure to curcumin decreased the IL-12 receptor β1 expression. The MFI was increased from 16.5 (9–28) to 29.0 (25.5–40) when comparing unstimulated cells with cells incubated with IL-12, respectively. Pretreatment with curcumin reduced the MFI of IL-12Rβ 1 to 21.5 (19–31). These medians (range) are from three independent experiments. All these differences above were statistically significant (P < 0.05).
2.
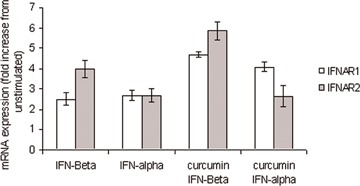
Modulation of IFNAR by curcumin. Quantitative real-time PCR was used to assess IFNAR mRNA expression in PHA/IL-2 T cell blasts. Both IFN-β (10 ng/ml) and IFN-α (10 ng/ml) slightly increased IFNAR1 expression; this induction was increased following prior exposure to curcumin. Prior exposure to curcum-in (20 μg/ml) also increased a moderate induction of IFNAR2 by IFN-β. Although IFN-α alone increased IFNAR2 expression, prior exposure to curcumin (20 μg/ml) decreased IFN-α induced IFNAR2 expression. β2microglobulin expression was monitored as an internal standard on the cDNA template; mRNA expression for each gene is normalized to the internal standard expression. US = unstimulated.
3.
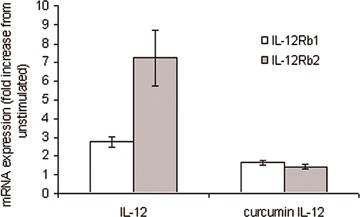
Modulation of IL-12 by curcumin. Quantitative real-time PCR was used to assess IL-12 mRNA expression in PHA/IL-2 T cell blasts. Prior exposure to IL-12 (100 ng/ml) alone up-regulated both IL-12 Rβ1 and β2 expression. This induction, however, was reduced following pre-exposure to curcumin (20 μg/ml). β2microglobulin expression was monitored as an internal standard on the cDNA template; mRNA expression for each gene is normalized to the internal standard expression. US = unstimulated.
Curcumin inhibits IL-12-induced IFN-γ and enhances IFN-β-induced IL-10
In this study, both IFN-β and IFN-α are shown to induce IL-10 production in T cells (Fig. 4), which was enhanced by prior treatment with curcumin (P < 0.05). These results were confirmed by ELISA (Fig. 6A). IL-12 dependent production of IFN-γ is important in the generation of a cell-mediated immune response. Here, we show increased IFN-γ mRNA production by IL-12 in human T cells. However, pre-treatment of these cells with curcumin reduced this induction (Fig. 5A, P < 0.05). We also investigated whether IL-12-induced IFN-γ protein production was also decreased by prior exposure to curcumin; the results were consistent with what we observed at the mRNA level. IL-12 stimulation increased IFN-γ protein expression compared to unstimulated cells, an induction that was reduced by prior treatment of the cells with curcumin (Fig. 5B). These results were confirmed using ELISA (Fig. 6B).
4.
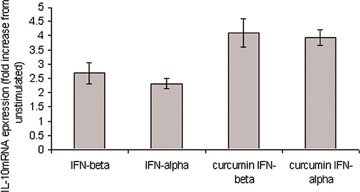
Modulation of type I IFN induced IL-10 by cur-cumin. Quantitative real-time PCR was used to assess IL-10 mRNA expression in PHA/IL-2 T cell blasts. IFN-β (10 ng/ml) and IFN-α (10 ng/ml) induced IL-10 production, which was enhanced by prior treatment with cur-cumin (20 μg/ml). β2microglobulin expression was monitored as an internal standard on the cDNA template, mRNA expression for each gene is normalized to the internal standard expression. US = unstimulated.
6.
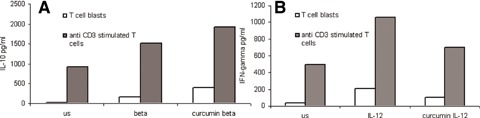
Modulation of IFN-β induced IL-10 and IL-12 induced IFN-γ protein expression by curcumin. IFN-γ and IL-10 levels were measured using a solid-phase sandwich ELISA. IFN-β (10 ng/ml) induces IL-10 production in human T cells (A). IL-10 production is enhanced by prior exposure to curcumin (20 μg/ml). IL-12 (0.1 μg/ml) induces IFN-γ production in human T cells (B). Pre-treatment of these cells with curcumin (20 μg/ml) reduces this induction. The duration of exposure of the cells to IFN-β, IL-12 and curcumin was 18 hrs.
5.
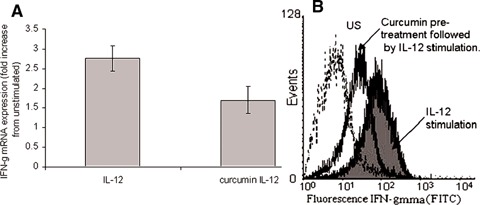
Modulation of IL-12 induced IFN-γ by curcumin. Quantitative real-time PCR and flow cytometry were used to assess IFN-γ mRNA and protein expression, respectively, in PHA/IL-2 T cell blasts. IL-12 (100 ng/ml) increased IFN-g production, however, pre-treatment of these cells with curcumin (20 μg/ml) reduced this induction (A). IL-12 stimulation increased IFN-γprotein expression (shaded curve) compared to unstimulated cells (US), an induction that was reduced by prior treatment of the cells with curcumin (20 μg/ml) (unshaded curve) (B).
To determine whether the immunomodulatory effects of curcumin are similar in T cells from normal donors and patients with MS, we performed the above studies on T cells from PBMC of MS patients. We showed the same effects on IFN-β and IL-12 induction of cytokines, STAT4 phosphorylation (Fig. 7A and B) and IL-10 and IFN-γ modulation (see Fig. 7C and D).
7.
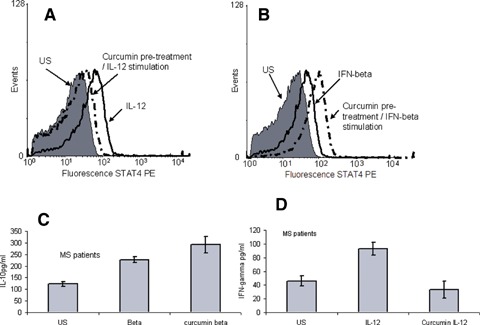
Curcumin modulation in multiple sclerosis (MS) patients. PBMC's were isolated from MS patients (n = 4) and as with the in vitro samples were stimulated with either IFN-β (10 ng/ml) or IL-12 (100 ng/ml). Both induced pSTAT4 expression (unshaded curve on each graph). Prior incubation with curcumin (20 μg/ml) and then IFN-β resulted in an increase in pSTAT4 generation, whilst prior stimulation with curcumin (20 μg/ml) followed by incubation with IL-12 resulted in a decrease in pSTAT4 generation.(Shaded curve on each graph represents unstimulated cells). IFN-β and IL-10 levels were measured using a solid-phase sandwich ELISA. IFN-β (10 ng/ml) induces IL-10 production in human T cells (A). IL-10 production is enhanced by prior exposure to curcumin (20 μg/ml). IL-12 (0.1 μg/ml) induces IFN-γ production in human T cells (B). Pre-treatment of these cells with curcumin (20 μg/ml) reduces this induction. The duration of exposure of the cells to IFN-β, IL-12 and curcumin was 18 hrs.
Discussion
As signal transducers, STAT molecules play an important role in many cellular events, including a role in differentiation, cell growth, apoptosis and inflammation. We have shown previously that the modulation of both STAT1 and STAT4 by glucocorti-coids is dependent on the cytokine by which their activation is induced, and that this modulation is different for IFN-α, IFN-β and IL-12 [12]. This observation is consistent with the results obtained in the current study for modulation by curcumin.
Here, we have shown curcumin to be a potent regulator of IL-12 signalling. The reduction of IL-12-induced STAT4 activation, and IL-12Rβ1 and β2 expression, was also associated with a reduction in IL-12-induced IFN-γ production. However, prior exposure to curcumin appears to increase the ability of T cells to respond to IFN-β by enhancing IFN-β-induced STAT4 activation, IFNAR1 and 2 expression, and IL-10 production. Although IFN-α and IFN-β use the same receptor and sig-nalling pathway, differences in their biological effects have been documented [12, 13] and, therefore it is perhaps not surprising that curcumin modulation of IFN-α signalling, as observed in this study, is slightly different to IFN-β. Although curcumin increases IFN-α-induced IL-10 mRNA and IFNAR1 expression, prior exposure to curcumin decreased IFN-α-induced IFNAR2 expression and resulted in similar pSTAT4 generation comparable to IFN-α alone.
Signal transduction through the IL-12 receptor induces tyrosine phosphorylation of the Janus family kinases JAK2 and TYK2, which in turn phosphorylate STAT4 [14]. Phosphorylated STAT4 (pSTAT4) dimer-izes, translocates to the nucleus and instigates DNA transcription [15]. Curcumin can completely inhibit the expression of JAK2 at the level of transcription [16]. Signal transduction through the IFNAR induces tyrosine phosphorylation of JAK1 and TYK2. The effect of curcumin on JAK1 expression could be an interesting target for future investigation.
Previous studies have looked at the direct effect of curcumin on IL-12 itself [17]. Here we are more interested in the relationship between curcumin and IL-12 with regard to STAT4 activation, how the ability of T cells to respond to IL-12 by producing IFN-γ is affected by prior exposure to curcumin, and the differences between the effects of curcum-in on IL-12, IFN-β and IFN-α signalling, since they all activate STAT4. Interestingly, this modulation was observed to be stimulus dependent as prior exposure to curcumin enhanced the ability of T cells to respond to IFN-β, not only at the level of cytokine induction but also upstream at the sig-nalling pathway and receptor level. The type of modulation by glucocorticoids is dependent upon the T cell phenotype [18, 19], and perhaps this is the same for curcumin. We found that prior treatment with curcumin enhanced IFN-β-induced IL-10 production (an anti-inflammatory cytokine), whilst the induction of IFN-γ (a pro-inflammatory cytokine) by IL-12 in human T cells was reduced by pre-treatment with curcumin.
Deregulated signal transduction pathways, such as activation protein-1 (AP-1) and nuclear factor kappaB (NFkB) contribute to carcinogenesis, [20] and play a critical role in the transcription of several genes involved in immune and inflammatory responses [21]. Curcumin has been shown to inhibit induction of AP-1 and NFkB in bone marrow stromal cells [22]. Curcumin is also thought to down-regulate matrix metalloproteinase-9 (MMP-9) expression, which is implicated in the growth and invasiveness of brain tumours by inhibition of NFkB and AP-1 binding to the DNA promoter region [23].
STAT4 has been demonstrated to recruit AP-1 to the IFN-γ promoter to synergistically enhance IFN-mRNA expression induced by IL-12 and IL-18 [24]. The reduction of IL-12-induced IFN-γ mRNA expression by curcumin as shown in this study may not only be due to a decrease in STAT4 activation but also a reduction of the recruitment of AP-1 to the IFN-γ promoter, perhaps resulting from the decrease in STAT4 phosphorylation.
Although it is clear that the differentiation of Th1 cells is crucial for an effective immunity to a wide variety of intracellular pathogens, Th1 cells and IL-12 may also contribute to the pathogenesis of a variety of immune-mediated inflammatory disorders, including MS and rheumatoid arthritis [25]. It has already been shown that curcumin inhibits IL-12 production and signalling in EAE [5]. This, combined with the inhibition of IL-12 signalling in human T cells and cur-cumin synergistic effect with IFN-β observed in this study on immune cells from both normal volunteers and MS patients, could place curcumin as a potential therapeutic agent in the treatment of MS.
Acknowledgments
Study supported in part by the Multiple Sclerosis Society of the UK and Northern Ireland. We thank Serono International for the gift of IFN-β 1a (Rebif®).
References
- 1.Huang MT, Lou YR, Xie JG, Ma W, Lu YP, Yen P, Zhu BT, Newmark H, Ho CT. Effect of dietary curcumin and dibenzoylmethane on formation of 7,12-dimethylbenz[a]anthracene-induced mammary tumors and lymphomas/leukemias in Sencar mice. Carcinogenesis. 1998;19:1697–700. doi: 10.1093/carcin/19.9.1697. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferu-loylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–62. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 3.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–71. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 4.Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–7. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- 5.Natarajan C, Bright JJ. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus kinase-STAT pathway in T lymphocytes. J Immunol. 2002;168:6506–13. doi: 10.4049/jimmunol.168.12.6506. [DOI] [PubMed] [Google Scholar]
- 6.Rep MH, Schrijver HM, Van Lopik T, Hintzen RQ, Roos MT, Ader HJ, Polman CH, Van Lier RA. Interferon (IFN)-beta treatment enhances CD95 and interleukin 10 expression but reduces interferon-gamma producing T cells in MS patients. J Neuroimmunol. 1999;96:92–100. doi: 10.1016/s0165-5728(98)00271-9. [DOI] [PubMed] [Google Scholar]
- 7.Mosmann TR, Moore KW. The role of IL-10 in cross-regulation of TH1 and TH2 responses. Immunol Today. 1991;12:A49–53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 8.Howard M, O'Garra A. Biological properties of inter-leukin 10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O'Shea JJ, Biron CA. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297:2063–6. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 10.Uzel G, Frucht DM, Fleisher TA, Holland SM. Detection of intracellular phosphorylated STAT-4 by flow cytometry. Clin Immunol. 2001;100:270–6. doi: 10.1006/clim.2001.5078. [DOI] [PubMed] [Google Scholar]
- 11. Applied Biosystems. User Bulletin #2: ABI PRISM 7700 Sequence Detection System, Applied Biosystems 1997.
- 12.Fahey AJ, Robins RA, Kindle KB, Heery DM, Constantinescu CS. Effects of glucocorticoids on STAT4 activation in human T cells are stimulus-dependent. J Leukoc Biol. 2006;80:133–44. doi: 10.1189/jlb.0605296. [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer LM, Constantinescu S. Interferon Therapy in Multiple Sclerosis. ed. New York: Marcel Dekker; 1997. [Google Scholar]
- 14.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–4. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 15.Bacon CM, Petricoin EF, 3rd, Ortaldo JR, Rees RC, Larner AC, Johnston JA, O'Shea JJ. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc Natl Acad Sci USA. 1995;92:7307–11. doi: 10.1073/pnas.92.16.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasius R, Reuter S, Henry E, Dicato M, Diederich M. Curcumin regulates signal transducer and activator of transcription (STAT) expression in K562 cells. Biochem Pharmacol. 2006;72:1547–54. doi: 10.1016/j.bcp.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Kang BY, Chung SW, Chung W, Im S, Hwang SY, Kim TS. Inhibition of interleukin-12 production in lipopolysaccharide-activated macrophages by cur-cumin. Eur J Pharmacol. 1999;384:191–5. doi: 10.1016/s0014-2999(99)00690-1. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a Th2 cytokine response by CD4+ T cells in vitro. Immunol. 1996;156:2406–12. [PubMed] [Google Scholar]
- 19.DeKruyff RH, Fang Y, Umetsu DT. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol. 1998;160:2231–7. [PubMed] [Google Scholar]
- 20.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemopreven-tion by curcumin. AAPS J. 2006;8:E443–9. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Pindolia K, Janakiraman N, Chapman R, Gautam S. Curcumin inhibits IL-1 alpha and TNF-alpha induction of AP-1 and NFkB DNA-binding activity in bone marrow stromal cells. Hematopath Mol Hematol. 1998;11:49–62. [PubMed] [Google Scholar]
- 23.Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH, Kim WK, Kim HS. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signalling pathways in human astroglioma cells. Biochem Biophys Res Commun. 2005;335:1017–25. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- 24.Nakahira M, Ahn HJ, Park WR, Gao P, Tomura M, Park CS, Hamaoka T, Ohta T, Kurimoto M, Fujiwara H. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J Immunol. 2002;168:1146–53. doi: 10.4049/jimmunol.168.3.1146. [DOI] [PubMed] [Google Scholar]
- 25.O'Shea JJ, Visconti R. Type 1 IFNs and regulation of TH1 responses: enigmas both resolved and emerge. Nat Immunol. 2000;1:17–9. doi: 10.1038/76872. [DOI] [PubMed] [Google Scholar]


