Abstract
In vitro expansion of late endothelial progenitor cells (EPCs) might yield a cell therapy product useful for myocardial and leg ischaemia, but the influence of EPC expansion on the angiogenic properties of these cells is unknown. In the present study, we investigated the effect of in vitro EPC expansion on vascular endothelial growth factor (VEGF) receptor expression. EPCs were obtained from CD34+ cord blood cells and expanded for up to 5 weeks. Real-time quantitative reverse-transcription polymerase chain reaction (RT-PCR) showed that VEGFR2 expression, contrary to VEGFR1 and VEGFR3 expression, was significantly higher on expanded EPCs than on freshly isolated CD34+ cells or on human umbilical vein endothelial cells (HUVECs). Quantitative flow cytometry confirmed that VEGFR2 density on EPCs increased during the expansion process and was significantly higher than on HUVECs. The impact of VEGFR2 increase was studied on the three theoretical steps of angiogenesis, i.e., EPC proliferation, migration and differentiation. VEGFR2 up-regulation had no effect on VEGF-induced cell proliferation, but significantly enhanced EPC migration and pseudotubes formation dependent on integrin α6 subunit overexpression. In vitro expansion of late EPCs increases the expression of VEGFR2, the main VEGF receptor, with possible implications for EPC-based angiogenic therapy.
Keywords: endothelial progenitor cells, VEGF receptors, VEGFR2, expansion, integrin α6
Introduction
Neovascularization is a multi-step process requiring timely expression of various growth factors and receptors on endothelial progenitor cells (EPCs) [1–3]. Specific angiogenic factors, such as vascular endothelial growth factor (VEGF), angiopoietins (Ang) 1 and 2 and stromal derived factor 1 (SDF-1) are involved in the regulation of vessel formation. Since Asahara first reported the existence of EPCs in peripheral blood [4], at least two types of cell types have been described, based on the kinetics of their emergence during culture: so-called ‘late EPCs’ that differ from ‘early EPCs’ in that they do not express CD14 or CD45 (leukocyte markers), secrete a smaller array of cytokines but have a high proliferative capacity [5]. Late EPCs can be maintained for up to 12 weeks in culture, and differ from mature endothelial cells in terms of their angiogenic potency in vivo[6], their resistance to oxidative stress [7] and their urokinase expression [8]. Moreover, we have previously reported that specific thrombin receptor.
(PAR)-1 activation on late EPCs induces their proliferation, migration and pseudotube formation in Matrigel far more potently than PAR-1 activation on human umbilical vein endothelial cells (HUVECs) [9, 10]. A major barrier to the development of EPCs as an autologous cell therapy product is their paucity in the peripheral circulation. Pre-clinical studies suggest that it would take more than 10 litres of autologous peripheral blood to produce sufficient EPCs to induce angiogenesis in a patient [11]. In vitro expansion might be able to circumvent this problem, and several methods of cell conditioning are currently being investigated [9, 11]. Late EPCs have a proliferative potential that is theoretically compatible with strong amplification, but the possible impact of such a culture step on the characteristics of these cells, and particularly their VEGF receptor expression, is not known. Activation of VEGFR2 (also called KDR) by VEGF plays a pivotal role in adult angiogenesis by triggering multiple sig-nalling networks leading to endothelial recovery during wound healing [12], cell survival [13], differentiation [14], vascular permeability [15] and EPC mobilization from bone marrow to the peripheral circulation [16]. Ischaemia up-regulates VEGF expression and mobilizes progenitor cells from bone marrow through a matrix metalloproteinase (MMP)-9-dependent mechanism [17–19]. Moreover, VEGF might play a critical role in the development of tumour and progression of diabetic retinopathy [20]. Nowak et al. show that VEGFR2 expression on peripheral blood cells defines functionally competent cell populations that proliferate in vivo and contribute to re-endothelialization [21]. Finally, clinical trials of gene therapy with plasmid DNA encoding VEGF showed an increase in the number of circulating EPCs [22].
The aim of this study was to examine the kinetics of VEGF receptor expression during in vitro expansion of human late EPCs.
Materials and methods
Late-EPC culture
Mononuclear cells were isolated from human cord blood by density gradient centrifugation with Histopaque-1077 (Sigma-Aldrich, Saint-Quentin Fallavier, France). Plastic non-adherent cells were enriched in CD34+ cells by magnetic-activated cell sorting on MiniMacs columns (Miltenyi Biotec, Paris, France) following the manufacturer's instructions. Cells were plated on 0.2% gelatin-coated 24-well plastic culture dishes at a density of 5 × 105/ml and maintained in EGM-2 (BioWhittaker, Cambrex, France) as previously described [9]. Human endothelial cells (HUVECs) were isolated from human umbilical veins as described by Jaffe et al.[23]. HUVECs were maintained in EGM-2 medium and were used at passages 1, 2 and 3, corresponding to a culture of approximately 3 weeks.
Immunofluorescence staining
Cells were seeded on glass coverslips coated with collagen in 12-well plates, then fixed with 4% paraformaldehyde and incubated with 50 mM NH4Cl. Cells were incubated at 37°C for 30 min prior to fixation with 10 μg/ml DiI-Ac-LDL (Molecular Probes). After fixation, cells were permeabilized with 0.1% Triton-X-100 in PBS, and non-specific binding sites were saturated with PBS-10% FBS for 30 min. Cells were then incubated with the VWF antibody in PBS-1% FBS, and then with goat secondary antibodies coupled to either AlexaFluor 488 or AlexaFluor 555 (Molecular Probes). Nuclei were stained with ToPro-3 (Molecular Probes). Coverslips were mounted with Mowiol and observed with a Leica TCS SP2 confocal microscope (Leica Microsystems).
Quantitative flow cytometry
Cultured cells were detached with collagenase (Boehringer Mannheim, Meylan, France), washed in Hank's solution containing 10% FBS, re-suspended in 50 μl of PBS-1% bovine serum albumin (BSA), and incubated for 30 min at 4°C with primary mouse monoclonal antibodies (mAb) against VEGF receptor type 2 (VEGFR2, Sigma-Aldrich) and CD31 (PECAM-1, Immunotech, Marseille, France) at saturating concentrations. Isotype-matched mouse IgG1 was used for negative controls and was purchased from the same manufacturer as the specific antibodies. For quantitative flow cytometry, the staining reagent was a polyclonal FITC-conjugated f(ab’)2 fragment of a goat anti-mouse antibody (Dako, Trappes, France). Ten thousand events were acquired on a FACScan flow cytometer (Becton Dickinson, Le Pont de Claix, France), and data were analysed with CellQuest software (Becton Dickinson). CD31 and VEGFR2 expression on the EPC surface was quantified with a calibrator (Qifikit, Dako, Trappes, France) containing a mixture of five calibration beads coated with increasing densities of mouse IgG (∼3000–600,000 molecules). The numbers of surface molecules were derived from the calibration curve after subtracting the negative isotype control value.
Real-time quantitative reverse-transcription polymerase chain reaction (RT-PCR)
The theoretical and practical aspects of real-time quantitative RT-PCR on the ABI Prism 7700 Sequence Detection System (Perkin-Elmer Applied Biosystems, adresse) are described in detail elsewhere [24]. The parameter Ct (threshold cycle), defined as the fractional cycle number at which the fluorescence generated by SYBR green dye–amplicon complex formation passes a fixed threshold above baseline, is used as a quantitative measurement of the input target.
As an endogenous RNA control, we quantified transcripts of the TBP gene encoding the TATA box-binding protein (a component of the DNA-binding protein complex TFIID). The amount of target transcript (Ntarget) was normalized on the basis of the TBP content of each sample and was subsequently normalized to a basal mRNA level with the equation: Ntarget = 2ΔCtsample where ΔCt is the Ct value of the target gene minus the Ct value of the TBP gene.
The results are presented as ‘normalized mRNA levels’, i.e., the Ntarget value divided by the Ntarget value of the smallest quantifiable amount of target gene mRNA (i.e. target gene Ct value = 35). For each target gene, normalized mRNA levels reflect the variation of the corresponding mRNA content in CD34+ cells, expanded late EPCs at weeks 3 and 5, and HUVECs. As PCR efficiency was optimal and ranged from 90% to 100% in the different target gene RT-PCR assays, the normalized mRNA levels were also used to compare the mRNA content of the four genes of interest (VEGFR1, 2 and 3 and CD31) in each cell type.
Primers for TBP and the target genes were chosen with the assistance of Oligo 5.0 software (National Biosciences, Plymouth, MN, USA). The nucleotide sequences of the primers have been published elsewhere [9, 25].
Cell proliferation assay
To test the effect of VEGF activation on EPCs, all the following experiments were performed after 16 hrs of culture in unsupplemented EBM-2 medium, followed by activation with human recombinant VEGF (isoform 165, R&D systems Europe, Lille, France). The effect of 50 ng/ml VEGF in 2% FBS EBM-2 medium on EPC proliferation was examined by measuring cell phosphatase activity based on the release of paranitrophenol (pNPP, Sigma) recorded at 405 nm (Fluostar optima, BMG labtech, Champigny-sur-Marne, France) after 72 hrs of incubation, by comparison to cells cultured in EGM-2 medium.
Cell migration assay
EPC migration was measured by using 24 well-modified Boyden chambers (Costar, Avon, France) with 8-μm pore-size filters. EPCs were seeded at a density of 5 × 104 per well in 200 μl of migration medium (EBM-2/1% SVF), and were allowed to migrate for 5 hrs at 37°C. Recombinant human VEGF (10 ng/ml, R&D systems) was diluted in EBM-2 medium supplemented with 1% FBS and placed in the lower chamber of the modified Boyden chamber, in a volume of 600 μl.
In vitro angiogenesis assay
Late EPCs (105 cells/well) were seeded on collagen type 1-coated 24-well plates. After 16 hrs of serum and growth factor deprivation, the medium was replaced by EBM-2, 5% FBS containing VEGF (50 ng/ml) or by EGM-2 medium. After 36 hrs, EPCs were detached with non-enzymatic cell dissociation medium (Sigma) to avoid cell membrane antigen proteolysis. After detachment, EPCs were used for the Matrigel angiogenesis assay and for flow cytometric quantification of integrin α6 subunit expression. Cells were seeded on Matrigel (3 × 104 cells/well) and cultured for 18 hrs at 37°C with 5% CO2. Before seeding in Matrigel, EPCs were re-suspended in medium containing 10 μg/ml anti-α6 blocking antibody (clone GoH3, R&D systems) or an irrelevant isotype-matched antibody. Capillary-like structures were examined by phase-contrast microscopy and endothelial cell networks formed by EPCs were quantified by computer-assisted analysis (Videomet 5.4.0).
Statistical analysis
Data are shown as means ± SEM. Significant differences were identified by ANOVA followed by Fisher's protected least-significant-difference test. Intergroup comparisons of CD31 and VEGFR2 density on the EPC surface were based on the Mann and Whitney non-parametric test. All statistical tests were performed using the Stat View software package (SAS, Cary, NC, USA). Differences with P values <0.05 were considered significant.
Result
Characterization of late EPCs
When cultured in the presence of specific endothelial growth factors (EGM-2 medium), human cord blood CD34+ cells yielded small colonies after between 7 and 14 days of culture. At confluence, EPCs exhibited a cobblestone morphology and a monolayer growth pattern typical of the endothelial lineage, expressed VWF and were able to incorporate diL-low-density lipoprotein (LDL) (Fig. 1A) [8, 9, 26]. The endothelial phenotype of expanded EPCs (so-called late EPCs) [6] was further characterized by measuring the expression of endothelial markers, such as CD31 (PECAM), CD146 (S-Endo1) and VEGFR2 (Fig. 1B). Late EPCs expressed CD34 antigen, but not the haematopoietic stem cell antigen CD133 or the leukocyte markers CD45 and CD14.
1.
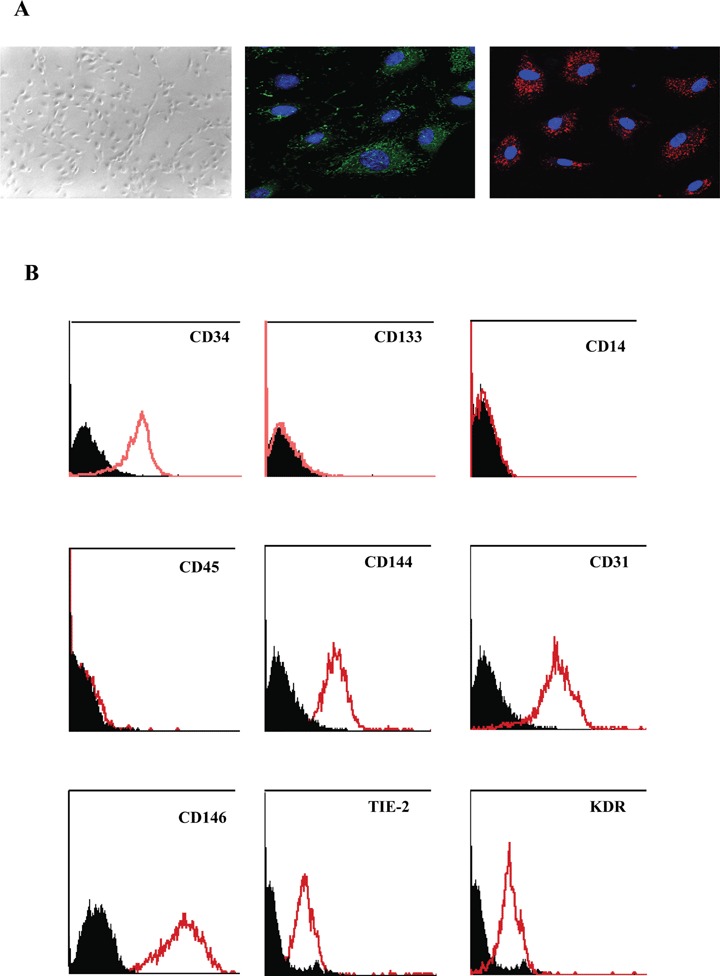
Characterization of late EPCs. A. Morphological aspect and confocal immuno-fluorescence analysis showing VWF expression (green) and Dil-Ac-LDL incorporation (red) of late EPC colonies emerging from CD34+ cord blood cells cultured for 2 weeks in endothelial conditions. Confocal images were acquired with a x63/1.32 PL APO objective. Photomicrographs of EPCs derived from cord blood CD34+ cells are representative of at least three observations. B. Representative histograms based on flow cytometric analysis of detached EPCs after immunolabelling with a control antibody (black line) and specific antibodies (red line) to endothelial markers (CD146, CD31, CD144, KDR and Tie-2), haematopoietic markers (CD34 and CD133) and leukocyte markers (CD45 and CD14). Histograms of EPCs derived from cord blood CD34+ cells are representative of at least three observations.
Expanded EPCs contain abundant VEGFR2 transcripts
Expression of VEGF receptor family genes was measured by real-time quantitative RT-PCR in EPCs expanded for 3 and 5 weeks, and also in freshly isolated CD34+ cells and HUVECs. The results are shown as ‘normalized mRNA levels’, with reference to the smallest quantifiable amount of mRNA defined by a Ct value of 35 (= 1 on the left log ordinate of Fig. 2; see Methods).
2.

VEGFR1, 2 and 3 and CD31 mRNA quantification in CD34+ cells, late EPCs, and HUVECs. mRNA levels were normalized to TBP mRNA levels and to the sample with the lowest quantifiable level (i.e. 1 on the left ordinate, corresponding to a Ct value of 35). Values above 100 represent a strong gene expression. Mean and SEM values of three different colonies are shown at each point.
VEGFR1 and VEGFR3 mRNA levels were moderate in CD34+ cells, and were similarly up-regulated (about 10- and 100-fold, respectively) in expanded EPCs and HUVECs. By contrast, VEGFR2 mRNA levels were low in CD34+ cells but were the most strongly expressed VEGF receptor gene in expanded EPCs, with a 5000-fold increase during expansion. VEGFR2 expression increased slightly (∼3-fold) from week 3 to week 5 of expansion, reaching a value about 5-fold higher than in HUVECs. CD31 transcripts were expressed at a high level in CD34+ cells, expanded EPCs, and HUVECs (Fig. 2).
EPC expansion in vitro increases VEGFR2 expression
Given the strong expression of the VEGFR2 gene during EPC expansion, we quantified the receptor density on the EPC membrane. As a control, we quantified CD31, reported to be the first endothelial antigen to be expressed by progenitor cells committed to the endothelial lineage [27]. EPCs were expanded for 5 weeks after the first passage, corresponding to a total of 45 to 60 days of culture. During the expansion phase, CD31 and VEGFR2 density was measured by means of quantitative flow cytometry, as previously described [9]. As shown in Fig. 3A, CD31 density varied widely among late EPC colonies but was of the same order as that found on HUVECs and remained constant during the 5-week expansion period. In keeping with gene expression analysis, the VEGFR2 protein level increased gradually and significantly throughout the expansion period (trend test = 0.02). VEGFR2 density rose from a mean value of 9200 sites per cell at week 3 to 25,800 sites at week 5 (Fig. 3B). This 3-fold increase from week 3 to week 5 was in line with the concomitant increase in mRNA (Fig. 2). VEGFR2 density was significantly higher on EPCs than on HUVECs at week 4 (P= 0.001) and week 5 (P= 0.03) of expansion, again in keeping with the concomitant 5-fold increase in the mRNA level. This increase was EPC colony-independent, as shown by the kinetics of VEGFR2 expression on cells expanded from single EPC colonies (four typical examples are shown in Fig. 3C). The consequences of this increase in VEGFR2 receptor expression were then evaluated in vitro on the three theoretical steps of angiogenesis, i.e., proliferation, migration and differentiation.
3.

Membrane expression of CD31 and VEGFR2 on late EPCs A and B. CD31 and VEGFR2 surface density on EPCs during a 5-week ex vivo expansion period (corresponding to 45 to 60 days of culture), by comparison to HUVECs. A mean of 15 colonies were tested at each time point. Boxes represent the median values with the 25th and 75th percentiles, and the bar chart shows the 90th and 10th percentiles. EPC surface CD31 and VEGFR2 expression were quantified by flow cytometry using a calibrator (Qifikit, Dako) containing a mixture of five calibration beads coated with increasing densities of mouse IgG (approximately 3000 to 600,000 molecules). The staining reagent was a polyclonal FITC-conjugated f(ab’)2 fragment of a goat antimouse antibody. Surface molecule numbers were derived from the calibration curve, after subtracting the negative isotype control value. C. Time course of VEGFR2 density on EPCs derived from four different colonies of late EPCs. VEGFR2 was quantified by flow cytometry every week during a 5-week expansion period.
In vitro expansion of EPCs does not increase VEGF-induced proliferation
In order to explore the effect of VEGFR2 activation on EPC proliferation, cells were deprived of serum and growth factors (EBM-2 medium) for 16 hrs before adding 50 ng/ml human VEGF. In these conditions, VEGF induced an expected EPC proliferation, as quantified by pNPP release at week 3 of expansion (Fig. 4A). However, the increase in VEGFR2 expression observed at week 5 of expansion did not coincide with an increase in cell proliferation. We then determined at week 3 and 5 of expansion basal expression of mRNA levels of several proliferation or anti-apoptotic factors, including Ki67, Topoisomerase2A (TOP2A), anti-apoptotic members of the bcl-2 protein family bcl-2 and bcl-A1 and an anti-apoptotic protein shown to be a target of p53 GADD45 (Fig. 4B). In vitro expansion of EPCs induced an increase in proliferation markers Ki67 and TOP2A, compared to CD34+ cells. Moreover, we observe a decrease of 50% of these two proliferation markers after 5 weeks of culture. Contrary to proliferation markers, anti-apoptotic markers decrease between CD34+ cells and EPCs after 3 weeks of culture, this phenomenon is also observed between EPCs at week 3 and 5 of culture. Absence of increased proliferation during expansion could also be explained by a decrease of proliferation and anti-apoptotic potential of EPCs during expansion.
4.

Influence of in vitro expansion on EPC proliferation and markers of apoptosis A. Effect of VEGF on EPC proliferation, as evaluated by the release of pNPP (OD at 405 nm) in EBM-2 medium containing 2% FBS (mean ± SEM). VEGF induced late EPC proliferation at week 3 (*P= 0.004) and week 5 (*P= 0.046) compared to control EPCs. No significant difference was observed between week 3 and week 5 of expansion (P= 0.701). The mean and SEM of three experiments are shown. B. Effect of in vitro expansion on mRNA levels of proliferative and anti-apoptotic factors. mRNA levels were normalized to TBP mRNA levels and to the sample with the lowest quantifiable level (i.e. 1 on the left ordinate, corresponding to a Ct value of 35). Values above 100 represent strong gene expression. Mean and SEM of three different colonies are shown at each point.
In vitro expansion of EPCs increases VEGF-induced migration and tube formation in Matrigel via integrin α6
The functional implications of the increase in VEGFR2 density were then investigated in a Boyden chamber migration test with VEGF as chemo-attrac-tant in the lower chamber. We performed previous experiments with increasing VEGF doses (10, 50 and 100 ng/ml). VEGF induced a dose-dependent migration that was statistically significant for all concentrations used (data not shown). However, using 50 and 100 ng/ml of VEGF, the migrating cells were very numerous and the 10 ng/ml VEGF concentration was further chosen to better evaluate any difference in the migration potential. In these conditions, a significant increase in the chemotactic effect of VEGF was observed with EPCs expanded for 5 weeks compared to EPCs expanded for 3 weeks (P= 0.023, Fig. 5).
5.

In vitro expansion modulates VEGF-induced EPC migration VEGF-induced EPC chemotaxis was tested in a Boyden chamber migration assay. Cell expansion increased late EPC migration towards VEGF (10 ng/ml). Data are the numbers of migrating EPCs. The mean and SEM of three experiments are shown (*P= 0.023).
Finally, we used a Matrigel model to examine the capacity of VEGF-activated EPCs to differentiate into capillary-like structures. After 16 hrs of culture in unsupplemented EBM-2 medium, EPCs formed few capillary-like structures (Fig. 6A, top panel). At week 3 of expansion, treatment with VEGF (50 ng/ml) promoted EPC organization into branched structures and pseudotubes with enclosed areas (network length: 1532 ± 41 μm in untreated controls versus 3005 ± 743 μm in VEGF-treated cells, (P= 0.011)) (Fig. 6A and B). At week 5 of expansion, VEGF treatment increased the density of this network 1.5-fold compared to week 3 of expansion (Fig. 6B). To explain this result, we explored the expression of surface proteins potentially involved in tube formation, such as integrin subunits α6 and β1[28], and the adhesion molecule CD31 (PECAM-1) [29]. No significant increase was found in β1 or CD31 expression upon VEGF activation (data not shown). By contrast, integrin α6 subunit expression was up-regulated 1.5-fold upon VEGF treatment after 3 weeks of expansion (P= 0.002, Fig. 6C). After 5 weeks of expansion, VEGF treatment increased integrin α6 subunit expression 3-fold compared to week 3 (P= 0.025), in line with the increase in VEGFR2 expression. An anti-α6 blocking antibody completely inhibited VEGF-induced tube formation (Fig. 6A, bottom), whereas an irrelevant isotype-matched antibody had no effect (data not shown).
6.

In vitro expansion modulates EPC tube formation in Matrigel by up-regulating integrin α6 expression. EPCs were stimulated with VEGF (50 ng/ml) for 36 hrs before being used in the tubule formation assay on Matrigel for 18 hrs. EPCs were plated on Matrigel in the presence or absence of a monoclonal antibody against human α6 (clone GoH3; R&D systems, 10 μg/ml). Results are means ± SEM of three determinations.*: P < 0.05. A. Photographs show pseudotube formation by untreated EPCs and EPCs treated with 50 ng/ml VEGF. Bottom:EPCs treated with VEGF were incubated with 10 μg/ml anti-α6 antibody. Photos (original magnification, x20) are representative of three independent experiments of EPCs after 3 weeks of culture. B. Quantitative analysis of network length of untreated EPCs, EPCs treated with 50 ng/ml VEGF with or without 10 μg/ml anti-α6 antibody at week 3 and week 5 of expansion. Quantitative analysis of network length with Videomet software. (network length of control W3 versus VEGF W3 and VEGF W3 versus VEGF W5, respectively, P= 0.011 and P= 0.009); (network length of VEGF W3 versus VEGF W3 with anti- α6 and VEGF W5 versus VEGF W5 with anti- α6, respectively, P= 0.024 and P= 0.005). C. Effect of VEGF on EPC integrin α6 subunit expression. EPCs were analysed by flow cytometry before and after treatment with VEGF (50 ng/ml). Geometric mean fluorescence intensities are expressed in percentages, 100% corresponding to the control value obtained with VEGF treatment at week 3 of expansion.(Geometric mean fluorescence intensities of control W3 versus VEGF W3 and VEGF W3 versus VEGF W5 respectively P= 0.002 and P= 0.028).
Discussion
Given the importance of VEGF in the angiogenic function of EPCs, we quantified the expression of VEGF receptors 1, 2 and 3 during expansion of late EPCs in vitro, by comparison with freshly isolated CD34+ cells and with HUVECs. We also studied the expression of CD31 (PECAM-1), a 130-kD member of the Ig super-family that is expressed on endothe-lial cells and leukocytes [29] and has an important role in angiogenesis. Time-course analysis of cultured CD133+ cells has shown that CD31 is the earliest marker of endothelial differentiation [27]. In keeping with this observation, we found that CD31 was strongly expressed on expanded EPCs, and that the expression level remained stable during endothe-lial differentiation. VEGFR1, the first VEGF receptor to be identified, was originally found to be expressed only on vascular endothelial cells, but several more recent reports show that it is also expressed on non-endothelial cells, including haematopoietic progenitor cells (for review, [30]). By using quantitative RT-PCR, which is currently the most sensitive and accurate method for quantifying mRNA, we found that VEGFR1 transcript levels were similar in CD34+ cells, EPCs and HUVECs. VEGFR3 signalling in endothelial cells can generate lymphatic endothe-lial-like features. Here, we observed weak VEGFR3 expression on EPCs (at a level similar to that of CD34+ cells and HUVECs), implying that EPCs have poor lymphatic potential when expanded in our experimental conditions.
Interestingly, we found that VEGFR2 was the VEGF receptor family member most strongly expressed by expanded EPCs. VEGFR2 is also a marker of endothelial differentiation, as its transcript levels were much higher in expanded EPCs than in the CD34+ cells from which they derive. We also found that VEGFR2 density was significantly higher on EPCs than on HUVECs. These results agree with those of Yoon et al., who found, by using qualitative flow cytometry (geo mean fluorescence intensity), that adult late EPCs expressed higher VEGFR2 levels than HUVECs [31]. We also observed marked variability among individual EPC colonies, as the number of receptors per cell ranged from 2940 to 28240 sites at week 1 and from 2680 to 30,300 sites at week 5. A VEGFR2 gene polymorphism has been implicated in the onset of coronary artery lesions [32], and the variable cell-surface VEGFR2 density may also be genetically controlled, as we have previously shown for the platelet PAR-1 receptor [33]. One of the explanations of the up-regulation of VEGFR2 during in vitro expansion could be the use of the EGM2 medium for EPC culture. Indeed, this medium contain several growth factors and particularly VEGF, that is known to up-regulate its own receptor VEGFR2 [34, 35].
This study also confirms that VEGF activation increases EPC proliferation, as described by Bompais et al. However, the increase in VEGFR2 expression by EPCs during in vitro expansion did not influence cell proliferation. We have previously shown that PAR-1 activation increases EPC proliferation only during the first 3 weeks of expansion [9]. These results are in keeping with reports that circulating EPCs gradually lose their proliferative potential when expanded in vitro[36], and also that EPCs expanded in culture progressively process an in vitro maturation [38]. Moreover, the decrease in expression of proliferative and anti-apoptotic markers during in vitro expansion might explain the absence of a gain of proliferation observed in EPCs at week 5 after VEGF activation, despite a VEGFR2 increased expression. Recently, Ha et al.[37] explored functional features of EPCs upon aging, and showed that EPCs underwent senescence despite keeping their ability to form capillary tubes.
The loss of CD133 expression could also explain the decrease in proliferative response. When CD34+ cells are committed to the endothelial lineage and acquire a late EPC phenotype, they completely lose CD133 expression on cell surface. CD133 gene expression was only shown at the mRNA level in the first steps of culture. Colonies of EPCs obtained at 5 weeks of culture, neither expressed surface CD133 any longer, nor gene expression. In the present work, the difference in proliferative or apoptotic properties of the different populations cannot be explained by the cell heterogeneity, nor by CD133 expression because cells used have a clonogenic origin. Lack of proliferation should also be explained by a decrease in resistance to apoptosis. Indeed, all our experiments were performed after a culture period of 16 hrs in unsupplemented EBM-2 medium. Thus, the ratio between proliferation at week 3 and week 5 of culture is probably a balance between resistance to apopto-sis and VEGF-induced proliferation.
This study also shows that in vitro expansion of EPCs for at least 5 weeks increases cell migration towards VEGF. The VEGF-165-induced migratory response of bone marrow mononuclear cells (BM-MNC) from patients with chronic cardiac ischaemia is significantly lower than that of BM-MNC from healthy controls [39]. As a different VEGFR2 density is one possible explanation for these findings, an increase in VEGFR2 during in vitro expansion might improve the clinical efficacy of the cell therapy products.
Finally, VEGF promoted in vitro angiogenesis more efficiently after 5 weeks of EPC expansion, as shown by measuring vascular pseudotube formation. We studied cell-surface integrins and adhesion molecules potentially influencing this pseudotube formation, as integrins appear to play a fundamental role in EPC biology [40–42]. Flow cytometry showed integrin α6 overexpression by EPCs after VEGF activation. This α6 overexpression contributed to VEGF-induced pseudotube formation, as the latter was abrogated by an anti- α6 antibody. The integrin α6 subunit combines with the β1 subunit to form the laminin receptor, one of the main constituents of the basal membrane and the most abundant component of Matrigel. The integrin α6 subunit has been previously shown essential for the FGF-2–induced tubular morphogenesis of HUVECs [43].
Vascular tube formation results from a finely tuned balance between proliferation, migration and differentiation. In our study, VEGF treatment of EPCs resulted in overexpression of the integrin α6 subunit and in enhanced tube formation in Matrigel, independently of a gain of proliferation. This effect increased in parallel with the number of VEGFR2 receptors. To our knowledge, this is the first time that a link between VEGF activation on EPCs, integrin α6 subunit overexpression and VEGF-induced pseudo-tube formation has been observed. The key role of integrin α6 in vascular tube formation was underlined by its increased membrane expression between 3 and 5 weeks of expansion.
Recent data showed that expanded EPCs keep in vivo potential despite a maturation of the phenotype [38]. Our findings suggest that EPCs expanded in vitro acquire angiogenic properties resulting from an increase in VEGFR2, making them a promising substrate for autologous cell therapy of ischaemic diseases.
Acknowledgments
This work was supported grants from Inserm (‘Réseau de Recherche sur les Cellules Souches’), Programme Hospitalier de Recherche Clinique OPTIPEC (Ministère Chargé de la Santé, PHRC AOM 03 034, sponsor: AP-HP), and Leducq TransAtlantic Network of Excellence on Atherothrombosis Research (Grant 04CVD01). We thank I. Galy-Fauroux, C. Avignon and A. Lokajczyk for their technical assistance. We are also indebted to the nursing services of Hôpital Jean Rostand (Ivry-sur-Seine) for providing human umbilical cord blood, and to Hôpital Port Royal (Paris) for providing umbilical cords.
References
- 1.Werner N, Nickenig G. Clinical and therapeutical implications of EPC biology in atherosclerosis. J Cell Mol Med. 2006;2:318–32. doi: 10.1111/j.1582-4934.2006.tb00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts N, Jahangiri M, Xu Q. Progenitor cells in vascular disease. J Cell Mol Med. 2005;3:583–91. doi: 10.1111/j.1582-4934.2005.tb00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sata M, Fukuda D, Tanaka K, Kaneda Y, Yashiro H, Shirakawa I. The role of circulating precursors in vascular repair and lesion formation. J Cell Mol Med. 2005;3:557–68. doi: 10.1111/j.1582-4934.2005.tb00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothe-lial cells for angiogenesis. Science. 1997;5302:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Smadja DM, Cornet A, Emmerich J, Aiach M, Gaussem P. Endothelial progenitor cells: Characterization, in vitro expansion, and prospects for autologous cell therapy. Cell Biol Toxicol. 2007;4:223–39. doi: 10.1007/s10565-007-0177-6. [DOI] [PubMed] [Google Scholar]
- 6.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;2:288–93. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 7.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;11:2021–7. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 8.Basire A, Sabatier F, Ravet S, Lamy E, Mialhe A, Zabouo G, Paul P, Gurewich V, Sampol J, Dignat-George F. High urokinase expression contributes to the angiogenic properties of endothelial cells derived from circulating progenitors. Thromb Haemost. 2006;4:678–88. [PubMed] [Google Scholar]
- 9.Smadja DM, Bieche I, Uzan G, Bompais H, Muller L, Boisson-Vidal C, Vidaud M, Aiach M, Gaussem P. PAR-1 activation on human late endothelial progenitor cells enhances angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system. Arterioscler Thromb Vasc Biol. 2005;11:2321–7. doi: 10.1161/01.ATV.0000184762.63888.bd. [DOI] [PubMed] [Google Scholar]
- 10.Smadja DM, Laurendeau I, Avignon C, Vidaud M, Aiach M, Gaussem P. The angiopoietin pathway is modulated by PAR-1 activation on human endothelial progenitor cells. J Thromb Haemost. 2006;4:2051–8. doi: 10.1111/j.1538-7836.2006.02101.x. [DOI] [PubMed] [Google Scholar]
- 11.Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;6:732–8. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 12.Santos SC, Miguel C, Domingues I, Calado A, Zhu Z, Wu Y, Dias S. VEGF and VEGFR-2 (KDR) internalization is required for endothelial recovery during wound healing. Exp Cell Res. 2007;313:1561–74. doi: 10.1016/j.yexcr.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;4:777–94. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellouche S, Mourah S, Bonnefoy A, Schoevaert D, Podgorniak MP, Calvo F, Hoylaerts MF, Legrand C, Dosquet C. Platelets, thrombospondin-1 and human dermal fibroblasts cooperate for stimulation of endothelial cell tubulogenesis through VEGF and PAI-1 regulation. Exp Cell Res. 2007;3:486–99. doi: 10.1016/j.yexcr.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;6:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 16.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;14:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neo-vascularization. Nat Med. 1999;4:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 18.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;23:2776–9. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 19.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;5:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik RA, Li C, Aziz W, Olson JA, Vohra A, McHardy KC, Forrester JV, Boulton AJ, Wilson PB, Liu D, McLeod D, Kumar S. Elevated plasma CD105 and vitreous VEGF levels in diabetic retinopa-thy. J Cell Mol Med. 2005;3:692–7. doi: 10.1111/j.1582-4934.2005.tb00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak G, Karrar A, Holmen C, Nava S, Uzunel M, Hultenby K, Sumitran-Holgersson S. Expression of vascular endothelial growth factor receptor-2 or Tie-2 on peripheral blood cells defines functionally competent cell populations capable of reendothelialization. Circulation. 2004;24:3699–707. doi: 10.1161/01.CIR.0000143626.16576.51. [DOI] [PubMed] [Google Scholar]
- 22.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM, Asahara T. Vascular endothe-lial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;12:1198–202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 23.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;11:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bieche I, Onody P, Laurendeau I, Olivi M, Vidaud D, Lidereau R, Vidaud M. Real-time reverse transcription-PCR assay for future management of ERBB2-based clinical applications. Clin Chem. 1999:1148–56. [PubMed] [Google Scholar]
- 25.Asselah T, Bieche I, Laurendeau I, Paradis V, Vidaud D, Degott C, Martinot M, Bedossa P, Valla D, Vidaud M, Marcellin P. Liver gene expression signature of mild fibrosis in patients with chronic hepatitis C. Gastroenterology. 2005;6:2064–75. doi: 10.1053/j.gastro.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Bompais H, Chagraoui J, Canron X, Crisan M, Liu XH, Anjo A, Tolla-Le Port C, Leboeuf M, Charbord P, Bikfalvi A, Uzan G. Human endothe-lial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood. 2004;7:2577–84. doi: 10.1182/blood-2003-08-2770. [DOI] [PubMed] [Google Scholar]
- 27.Kanayasu-Toyoda T, Yamaguchi T, Oshizawa T, Hayakawa T. CD31 (PECAM-1)-bright cells derived from AC133-positive cells in human peripheral blood as endothelial-precursor cells. J Cell Physiol. 2003;1:119–29. doi: 10.1002/jcp.10229. [DOI] [PubMed] [Google Scholar]
- 28.Davis GE, Camarillo CW. Regulation of endothelial cell morphogenesis by integrins, mechanical forces, and matrix guidance pathways. Exp Cell Res. 1995;1:113–23. doi: 10.1006/excr.1995.1015. [DOI] [PubMed] [Google Scholar]
- 29.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM. Involvement of endothelial PECAM-1/CD31 in angio-genesis. Am J Pathol. 1997;3:671–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost. 2003;7:1356–70. doi: 10.1046/j.1538-7836.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metallopro-teinases. Circulation. 2005;11:1618–27. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 32.Kariyazono H, Ohno T, Khajoee V, Ihara K, Kusuhara K, Kinukawa N, Mizuno Y, Hara T. Association of vascular endothelial growth factor (VEGF) and VEGF receptor gene polymorphisms with coronary artery lesions of Kawasaki disease. Pediatr Res. 2004;6:953–9. doi: 10.1203/01.PDR.0000145280.26284.B9. [DOI] [PubMed] [Google Scholar]
- 33.Dupont A, Fontana P, Bachelot-Loza C, Reny JL, Bieche I, Desvard F, Aiach M, Gaussem P. An intronic polymorphism in the PAR-1 gene is associated with platelet receptor density and the response to SFLLRN. Blood. 2003;5:1833–40. doi: 10.1182/blood-2002-07-2149. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Donner DB, Warren RS. Homeostatic modulation of cell surface KDR and Flt1 expression and expression of the vascular endothelial cell growth factor (VEGF) receptor mRNAs by VEGF. J Biol Chem. 2000;21:15905–11. doi: 10.1074/jbc.M001847200. [DOI] [PubMed] [Google Scholar]
- 35.Herve MA, Buteau-Lozano H, Mourah S, Calvo F, Perrot-Applanat M. VEGF189 stimulates endothelial cells proliferation and migration in vitro and up-regulates the expression of Flk-1/KDR mRNA. Exp Cell Res. 2005;1:24–31. doi: 10.1016/j.yexcr.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Murasawa S, Llevadot J, Silver M, Isner JM, Losordo DW, Asahara T. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation. 2002;9:1133–9. doi: 10.1161/01.cir.0000027584.85865.b4. [DOI] [PubMed] [Google Scholar]
- 37.Ha JM, Kim MR, Oh HK, Lee BH, Ahn HY, Shin JC, Baek SH, Joe YA. Outgrowing endothelial progenitor-derived cells display high sensitivity to angiogenesis modulators and delayed senescence. FEBS Lett. 2007;581:2663–9. doi: 10.1016/j.febslet.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–8. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 39.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;13:1615–22. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 40.Zemani F, Benisvy D, Galy-Fauroux I, Lokajczyk A, Colliec-Jouault S, Uzan G, Fischer AM, Boisson-Vidal C. Low-molecular-weight fucoidan enhances the proangiogenic phenotype of endothelial progenitor cells. Biochem Pharmacol. 2005;8:1167–75. doi: 10.1016/j.bcp.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Chavakis E, Aicher A, Heeschen C, Sasaki K, Kaiser R, El Makhfi N, Urbich C, Peters T, Scharffetter-Kochanek K, Zeiher AM, Chavakis T, Dimmeler S. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;1:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin G, Ii M, Silver M, Wecker A, Bord E, Ma H, Gavin M, Goukassian DA, Yoon YS, Papayannopoulou T, Asahara T, Kearney M, Thorne T, Curry C, Eaton L, Heyd L, Dinesh D, Kishore R, Zhu Y, Losordo DW. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;1:153–63. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chabut D, Fischer AM, Colliec-Jouault S, Laurendeau I, Matou S, Le Bonniec B, Helley D. Low molecular weight fucoidan and heparin enhance the basic fibroblast growth factor-induced tube formation of endothelial cells through heparan sulfate-dependent alpha6 overexpression. Mol Pharmacol. 2003;3:696–702. doi: 10.1124/mol.64.3.696. [DOI] [PubMed] [Google Scholar]


