Abstract
Caveolae are flask-shaped plasma membrane invaginations that mediate endocytosis and transcytosis of plasma macromolecules, such as albumin, insulin and low-density lipoprotein (LDL), as well as certain viruses, bacteria and bacterial toxins. Caveolae-mediated transcytosis of macromolecules is critical for maintaining vascular homeostasis by regulating the oncotic pressure gradient and tissue delivery of drugs, vitamins, lipids and ions. Entrapment of cargo within caveolae induces activation of signalling cascades leading to caveolae fission and internalization. Activation of Src tyrosine kinase is an early and essential step that triggers detachment of loaded caveolae from the plasma membrane. In this review, we examine how Srcmediated phosphorylation regulates caveolae-mediated transport by orchestrating the localization and activity of essential proteins of the endocytic machinery to regulate caveolae formation and fission.
Keywords: caveolae, endocytosis, phosphorylation, Src, caveolin-1, actin cytoskeleton
Introduction
Under normal physiological conditions, endothelial cells form monolayers that line blood vessels and serve as an indispensable barrier that controls the transport of macromolecules from blood to the inter-stitium. Loss of this barrier function results in patho-physiological conditions including oedema [1]. Caveolae-mediated transcytosis is the primary means by which plasma macromolecules cross the endothelium. Caveolae, the omega-shaped plasmalemmal vesicles of 50–80 nm in diameter [2], represent >95% of the endothelial cell vesicles [3]. Transcytosis occurs by the activation of fission and endocytosis of caveolae from the apical membrane [3–5], followed by migration of detached vesicles to the basolateral membrane where they fuse and release their contents into the interstitial space [6–12].
The main structural unit and biological marker of caveolae is the 20–22 kD integral membrane protein, caveolin. To date, several different caveolin iso-forms have been identified: caveolin-1α, caveolin-1β, caveolin-2α, caveolin-2β, caveolin-2γ and caveolin-3 [13] (Fig. 1). Caveolin-1 and -2 are ubiquitously expressed, while caveolin-3 is confined to muscle cells [14–16]. The α and β isoforms of caveolin-1 result from alternative splicing from a single gene; the β form is 32 amino acids shorter than the α form [14]. Caveolin-1 is required for the biogenesis of non-muscle caveolae since the overexpression of caveolin- 1 in cells that lack endogenous caveolin-1 results in the de novo formation of caveolae [17, 18]. In cells that loose caveolin-1 expression during transformation, caveolae are no longer present [19]. Moreover, endothelium and adipose tissue of mice defective in caveolin-1 are devoid of caveolae [20–24]. Suppression of caveolin-1 expression by siRNA leads to a dramatic decrease in the number of caveolae in endothelial cells, which returns to normal following recovery of caveolin-1 expression [25].
1.
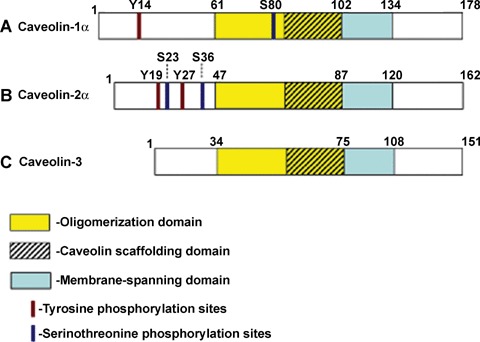
Phosphorylation map of the caveolin family of proteins. Caveolins are highly homologous and conserved proteins. All isoforms contain membrane-spanning, oligomerization and caveolin-scaffolding domains. (A) Caveolin-1α isoform consists of 178 amino acids with phosphorylation sites at tyrosine 14 and serine 80. Caveolin-1β isoform lacks the first 31 amino acids, and thus it does not contain the tyrosine phosphorylation site. Residues 75–158 (part of scaffolding domain, membrane- spanning domain, and most of the C-terminus) are involved in binding Dynamin 2. Scaffolding and membrane-spanning domains of caveolin-1 also participate in homo-oligomerization and formation of heteroligomers with caveolin-2. (B) Caveolin-2 is ∼50% homologues to caveolin-1 and caveolin-2α isoform contains 162 amino acids with phosphorylation sites at tyrosines 19 and 27, and serines 23 and 36. Caveolin-2β isoform, produced by alternative splicing of mRNA, is truncated by 13 N-terminal amino acids, while no information is yet available about the phosphorylation of the caveolin-2α isoform. (C) Caveolin-3 is a muscle-specific isoform, which contains 151 amino acids and is very homologous to caveolin-1. No phosphorylation sites have been demonstrated for caveolin-3 to date.
By tracing the movement of labelled albumin and other molecules from the luminal side of the endothelium by electron microscopy [9, 26–28], it was shown that caveolae are involved in the transcel-lular transport of molecules through endothelial cells. In fibroblasts derived from caveolin-1 knockout mouse embryos, uptake of fluorescent-labelled albu-min was abolished [24]. Both electron microscopy and radio-iodinated albumin uptake studies confirmed the failure of caveolin-1 knockout mouse endothelium to internalize albumin in vivo, further supporting the importance of caveolae in the transport of macromolecules [29]. However, mice lacking caveolae are viable and the albumin concentration in cerebrospinal fluid of caveolin-1 knockout mice is not different from that of wild-type mice [20]. It was subsequently suggested that in the absence of caveolin-1, elevated levels of nitric oxide (NO) leads to destabilization of the normally restrictive paracellular pathway, thus compensating for the loss of caveolae by generating shorter and more permeable transendothelial junctions [25, 29]. Despite our current understanding of the importance of caveolae-mediated transcytosis in maintenance of the structur-al integrity of the endothelial barrier, the signalling mechanisms by which caveolin-1 and transcytosis in general regulate endothelial barrier integrity have not been well established.
Src signalling in caveolae-mediated endocytosis
Selective internalization of certain proteins occurs within caveolae upon ligation of cell surface receptors [10, 30–33]. We and others have shown that endocytosis via caveolae is critically dependent on stimulation of tyrosine kinase signalling [10, 31, 34–41]. Dephosphorylation may also be involved in the control of caveolae-mediated endocytosis [34, 40]. However, the mechanism of caveolae formation and release from the plasma membrane in response to caveolin-1 phosphorylation remains unknown.
Early on, studies showed that the binding of some proteins to their receptors induces receptor clustering in caveolae, which in turn activates phosphorylation cascades [31, 34, 36]. Clustering of albumin receptor gp60 or alkaline phosphatase, induced by cross-linking with antibodies, is sufficient for activation of signalling events associated with caveolae internalization [31, 34, 40]. Virions of SV40 virus, another caveolae marker, initially do not bind to caveolae, but once ligated translocate to caveolae [36, 42, 43]. Major histocompatability complex-I (MHC-I), the surface receptor for SV40, also moves to caveo-lae upon cross-linking with antibodies [44]. Treatment with nystatin, a drug that sequesters cholesterol and flattens caveolae, does not inhibit SV40 binding to the cell surface, but impairs SV40-induced signalling and endocytosis [36], as well as endocytosis of albu-min and glycosphingolipids [39]. These studies suggest that receptor clustering and movement into caveolae is crucial for the induction of endocytosis.
Caveolae are also known to scaffold and concentrate signalling molecules, and entrapment of cargo in caveolae is required for initiation of signalling. As mentioned already, inhibition of phosphatases augments caveolar internalization whereas inhibition of kinases decreases it, implying that phosphorylation events play an essential role in the mechanism of caveolae-mediated endocytosis. While searching for the kinases that could initiate this phosphorylation cascade, it became apparent that Src family non-receptor tyrosine kinases, Src and Fyn, are activated immediately after addition of cargo [31, 40]. Tiruppathi et al., as well as others [39, 40] showed that the tyrosine kinase inhibitors tyrphostin A, genistein and PP2 block albumin uptake. Additionally, expression of dn-Src (dominant-negative Src, Y527F/K295M) inhibited gp60-mediated internalization of caveolae in endothelial cells [10, 41]. Furthermore, a peptide encoding the caveolin-1 scaf-folding domain (CSD) dose-dependently inhibited the auto-activation of purified Src kinases (c-Src and Fyn) [45] and also decreased transcytosis of albumin by 50% when introduced to the cells [46]. Finally, down-regulation of Src with siRNA induced aggregation of caveolae and a decrease in the mobility of caveolae, which resulted in defective endocytosis of SV40 in HeLa cells [47, 48]. A recent screening for kinases important for caveolae-mediated endocytosis revealed a large number of these molecules are involved in co-ordinating caveolae internalization [48]. The present review focuses on Src family kinases as they represent the most well studied signalling mechanism involved in caveolae-mediated endocytosis.
The mechanism by which receptor clustering in caveolae activates Src kinases is not completely understood. Activation of Src kinase and Src-mediated phosphorylation of caveolin-1, caveolin-2 and dynamin-2 represent early and essential steps in this cascade (Fig. 2). The phosphorylation state of these proteins may regulate their three-dimensional structure, activity, localization and set of binding partners. Therefore, the primary function of Src in caveolae-mediated endocytosis has been proposed to be the regulation and assembly of multi-protein complexes responsible for caveolae fission and internalization [10]. Treatment of bovine lung endothelial cells with pertussis toxin or a dominant negative Gαi construct encoding the carboxyl-terminal 11 amino acids of Gαi inhibited endocytosis of iodinated albumin and vesicle formation induced by gp60 cross-linking in endothelial cells, illustrating that caveolar internalization may be regulated by a Gi-linked pathway [10]. Also, both activation and inhibition of Gβγ had a profound effect on Src activation and caveolae-mediated endocytosis of cholera toxin subunit B and albumin [40], suggesting that heterotrimeric G-protein Gi is upstream of Src in the signalling cascade initiated by albumin which is then internalized and transported in caveolae.
2.
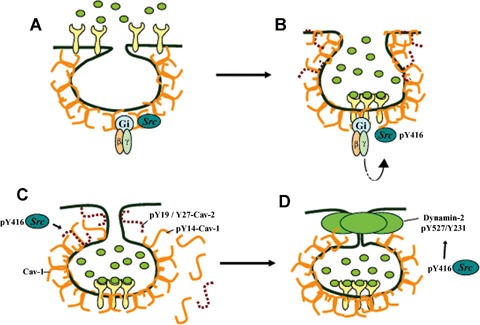
Src-dependent signalling of caveolae-mediated endocytosis. Caveolae are the primary vesicular transporters or ‘carriers’ in endothelial cells. Clustering of various receptors, for example albumin binding protein gp60, initiates endocytosis via caveolae by associating with caveolin-1 and activating Src-family tyrosine kinase signalling (A, B). Caveolin-1 plays a central role as it serves a scaffolding function for components of the signalling machinery responsible for endocytosis and also stabilizes caveolae at the membrane. Gi/βγ-linked Src-family kinase activation (via autophosphorylation of Src Y416) (B) in turn phosphorylates tyrosine residues on caveolin-1 (Y14) and caveolin-2 (Y19, Y27) (C), which may destabilize membrane-associated hetero-oligomers (C), and the GTPase dynamin-2 (Y231, Y597) which is thought to ‘pinch’ caveolae from the plasma membrane (D). Src-dependent phosphorylation is hypothesized to be the trigger that activates caveolar fission by decreasing the rigid structure of the caveolar coat and activating dynamin pinchase function.
When Src is activated, a number of proteins concentrated on the cytoplasmic surface of caveolae are phosphorylated. Caveolin-1, perhaps the primary substrate of Src, is phosphorylated on tyrosine 14 [45] (Fig. 1). It is implied by several studies that phosphorylation of caveolin-1 is essential for caveolae-mediated endocytosis; the possible role phospho-caveolin- 1 plays in this process will be discussed below. Caveolin-2, which forms hetero-oligomers with caveolin-1, represents another Src substrate on caveolae. Caveolin-2 does not appear to be essential for caveolae formation, since caveolin-2 knockout mice were reported to have normal non-muscle caveolae [49]. Additionally, in the absence of caveolin-1, caveolin-2 not only fails to form oligomers, but is also unable to incorporate into lipid rafts, emphasizing the role of caveolin-1 in proper function and localization of caveolin- 2 in vivo[50]. However, caveolin-2 appears to be involved in the regulation of caveolae size since co-expression with caveolin-1 in insect cells lacking endogenous caveolins leads to the formation of smaller and more uniform vesicles than those formed by caveolin-1 alone [51]. Formation of free cytoplasmic caveolae (i.e., vesicles that have detached from the plasma membrane) in HepG2 cells that do not express endogenous caveolins was dependent on co-expression of caveolin-1 and caveolin-2 [52]. Another group showed that caveolae formed by caveolin-1 alone lack the characteristic neck structure and were not connected to the plasma membrane [53], suggesting that expression of both caveolin- 1 and caveolin-2 is necessary for the formation of functional caveolae. In the same study, the authors proposed that caveolin-2 phosphorylation on serines 23 and 36 regulates the attachment of caveolae to the plasma membrane [53]. Caveolin-2 also contains two tyrosines that can be phosphorylated by Src: tyrosine 19 and 27 [54, 55]. In NIH3T3 cells, caveolin- 2 phosphorylated on tyrosine 19 localizes mainly to the cell borders, whereas caveolin-2 phosphorylated on tyrosine 27 was observed in small puncta throughout the membane and cytosol [55]. Despite different cellular localization, both phosphorylated forms of caveolin-2 dissociate from high molecular weight caveolin-1 oligomers but remain in lipid rafts [54, 55]. However, there are no data on the importance of caveolin-2 or its phosphorylated forms in caveolae internalization. It may be possible, by dissociating from caveolin-1 oligomers upon phosphorylation, that caveolin-2 participates in the regulated fission of caveolae. Also, since caveolin-2 is only 36% homologous to caveolin-1 [50], its tyrosine-phosphorylated forms can recruit signalling molecules distinct from those recruited by phosphorylated caveolin-1 [54].
Another target of Src shown to be critical to the regulation of caveolae-mediated endocytosis is the large molecular weight GTPase dynamin-2, which mediates fission of caveolae from the plasma membrane [40, 41, 56–58]. Src phosphorylation of dynamin at Tyr231 and Tyr597 increases its GTPase activity, assembly into oligomers [59, 60], and association with caveolin-1 at the plasma membrane [41, 61]. Dynamin-2 with mutated sites of Src phosphorylation, at tyrosine 231 and 597, fails to migrate to the membrane and also has a reduced ability to bind caveolin-1 [41]. Such binding, which was recently shown to be direct [63], is required for caveolae-mediated endocytosis of albumin and cholera toxin [41]. Interestingly, SV40-induced internalization of caveolae was also shown to be dependent on tyrosine kinase activity [36] and recruitment of dynamin to the membrane [37]. Dynamin-2 localizes to the neck of caveolae and mediates their release from the plasma membrane [56, 62], which is supported by studies that showed expression of the GTPase-defective dynamin mutant (K44A) or microinjection of antibodies against dynamin-2 prevented caveolae-dependent internalization of cholera toxin subunit B, albumin and glycosphingolipid [39, 41, 56].
Potential role of Src-mediated phosphorylation of caveolin-1 in caveolae-mediated endocytosis
Caveolin-1 is a well-known substrate of Src [31, 40, 64–68]. Src phosphorylation of caveolin-1 occurs within the extreme N-terminal region, between residues 6 and 26 which contains three tyrosine residues at positions 6, 14 and 25. Studies involving in vitro phosphorylation of caveolin-1-derived synthetic peptides and site-directed mutagenesis revealed that Tyr14 is the primary residue phosphorylated by Src [45]. Therefore, only caveolin-1α, which contains residues 1-178, can undergo tyrosine phosphorylation since caveolin-1β only contains residues 32–178 (Fig. 1).
The initial approach to understand the role of the caveolin-1 N-terminus included comparisons between α and β caveolin-1 isoforms. Although different by just 32 amino acids, caveolin-1 isoforms play non-redundant roles in zebrafish development [69]. A recent study also detected an alternative promoter in the caveolin-1 gene, suggesting that caveolin- 1 isoforms can be produced by mRNA splicing or perhaps transcribed from two distinct mRNA transcripts [70]. By tracking the level of both mRNA and protein in mouse lungs, it was shown that caveolin-1α is expressed earlier in development and predominates in endothelial cells, while caveolin-1β is expressed primarily in epithelial cells and at a later stage of development [70]. Within each cell, the distribution of caveolin-1α and -1β isoforms only partially overlaps. For example, it was shown that an -isoform specific antibody stains micropatches throughout the cell, whereas a non-selective caveolin-1 antibody that recognizes both α and β caveolin-1 isoforms additionally stains cell borders [14]. Another study suggested that there are different populations of caveolae and that the ratio of caveolin-1α to caveolin-1β was greater in deeply invaginated caveolae [52]. These studies lead to the proposal that the α-isoform possesses a greater internalization potential than the β-isoform.
Different approaches have been used to study the functional role of caveolin-1α phosphorylation in caveolae-mediated endocytosis. Staining of normal rat tissues with an antibody specific to phospho-caveolin-1 (Y14) revealed that in vivo, caveolin-1 is phosphorylated in endothelial cells but not in pericytes, fibroblasts and other cells abundant in caveolin-1 [35]. These data correlate with the constant engagement of endothelial cells in the endocytosis and transcytosis of macromolecules. In cultured cells, vanadate, an inhibitor of protein tyrosine phosphatases, increases the level of caveolin-1 phosphorylation and also stimulates its translocation from peripheral membrane patches to intracellular vesicles [35, 65, 71], enhancing the internalization of albumin [40]. Interestingly, co-transfection of v-Src, which is constitutively active, induces both caveolae internalization and aggregation. Phosphorylation of additional tyrosines in caveolin-1 by v-Src was suggested as an explanation for the differences in response to vanadate versusv -Src [65]. Thus, the N-terminus of caveolin- 1, where tyrosine 14 is located, seems to be critical for caveolae internalization. This point is strengthened by observations that in CV-1 cells expressing caveolin-1 with its N-terminus blocked by a green fluorescent protein (GFP)-tag, SV40 internalization was attenuated, while cells transfected with caveolin-1 tagged with GFP at the C-terminus showed normal SV40 endocytosis [43]. A recent study conducted in epithelial cells illustrated that the Src-dependent increase in the level of caveolin-1 phosphorylation upon epidermal growth factor (EGF) treatment corresponded to caveolae formation and internalization [72]. In the same study, the authors also provided evidence that caveolae formation in response to EGF was dependent on caveolin-1 phosphorylation since a phosphorylation-defective caveolin-1 mutant containing tyrosine 14 substituted with phenylalanine (Y14F) was not able to form visible caveolae. Moreover, we observed that expression of Y14F caveolin-1 mutant in endothelial cells significantly reduced endocytosis and transcytosis of albumin [23, 46] suggesting the caveolin-1 N-terminus plays a regulatory role and its phosphorylation by Src kinase may be crucial for caveolae formation and detachment from the membrane.
Caveolin-1 exists as high-order oligomers consisting of 14–16 monomers. Velocity gradient centrifugation results showed the existence of 350–400 kDa complexes of caveolin-1 in vivo[73, 74]. Residues 60–101 are suggested to be involved in caveolin– caveolin interactions and formation of homo-oligomers, and thus this region has been designated as the caveolin oligomerization domain [74, 75]. Caveolin-1 oligomerization is thought to facilitate the invagination and release of caveolae into the cytoplasm [76]. Therefore, Src-mediated phosphorylation may regulate the state of caveolin-1 oligomerization and subsequently control vesicle formation and fission from the plasma membrane. Another group has shown that caveolin-1 shifts to lighter fractions on a sucrose gradient upon EGF-stimulation in epithelial cells [72]. However, several studies showed that the ability of caveolin-1 to form oligomers incorporate into the lipid rafts, and bind caveolin-2 was not changed by either treatment with vanadate or v-Src co-transfection [54, 65, 77]. Since caveolin-1α and β isoforms co-oligomerize and it is possible that not all caveolin-1α in each oligomeric structure is phosphorylated, it is not yet clear whether caveolin-1 phosphorylation regulates oligomerization state or oligomer stability and thus requires further investigation.
Fernandez et al.[78] assessed the structure of caveolin-1 and its oligomers by studying the biophysical properties of a purified caveolin-1 fragment. This study showed that the short N-terminal sequence adjacent to the caveolin-1 membrane spanning domain forms an α-helix which is required for oligomerization of caveolin [78]. It was also proposed that the rest of the N-terminus wraps around this α-helix, facilitating oligomer formation and stability. In this regard, it is possible that phosphorylation of caveolin-1 changes its tertiary structure and causes destabilization of high molecular weight oligomers [46]. Nevertheless, analysis of caveolin-1-GFP dynamics suggests that the caveolin-1 coating on vesicles do not disassemble and do not exchange with other vesicles during the shuttling of caveolae between the plasma membrane and recycling compartments [79, 80]. Perhaps phosphorylation-mediated destabilization of caveolin-1 oligomers does not result in the complete disassembly of high molecular weight complexes, but rather serves to accommodate an increase in membrane curvature upon formation, fission or closure of caveolae.
Changes in the three-dimensional structure of caveolin oligomers may also affect caveolin-1 binding partners. To date, only two molecules are known to interact with caveolin-1 in a phosphorylation-dependent manner: Csk [81] and adaptor protein Grb7 [77]. Csk is a known regulator of Src family kinases and its recruitment to phosphorylated caveolin-1 provides a negative feedback loop for turning off Src [82]. Grb7 participates in focal adhesion formation and is important for cell migration [77]. Several studies localized caveolin-1 phosphorylated on tyrosine 14 to focal adhesions [77, 83, 84]. del Pozo et al. suggested that phospho-caveolin-1 is sequestered in focal adhesions and it is redistributed to caveolae upon cell detachment, inducing internalization of lipid raft marker GM1 [84]. As discussed above, in adherent cells, caveolae do not exchange caveolin molecules [79] and activation of endocytosis leads to phosphorylation of caveolin-1 in the caveolae coat structure. Thus, phospho-caveolin-1 located in focal adhesions and caveolin-1 phosphorylated in the course of endocytosis are likely to represent two different pools of phospho-caveolin-1 in adherent cells.
Role of actin cytoskeleton in caveolae-mediated endocytosis
The actin cytoskeleton is an essential requirement for endocytosis in several systems [85, 86]. Analysis of endothelial cells by electron microscopy revealed caveolae association with fine actin filaments [87]. This discovery encouraged several research groups to study the role of the actin cytoskeleton in caveolae internalization. Early studies showed that treatment of cells with Cytochalasin D, a drug that disassembles filamentous actin, blocked caveolae-mediated internalization of cholera toxin following treatment of cells with phosphatase inhibitor okadaic acid [34]. Mundy and co-workers illustrated that in Chinese hamster ovary (CHO) cells, cortical actin restricts and organizes caveolae at the plasma membrane since actin depolymerizing drug Latrunculin A induced an increase in lateral mobility of caveolin-1-GFP within the plasma membrane [88]. Other studies showed that the F-actin stabilizing drug Jasplakinolide also blocked caveolae internalization in CV-1 cells [37], implying that dynamic actin cytoskeletal re-modelling is crucial for caveolae-mediated endocytosis. Pelkmans and co-workers proposed a model in which SV40 entrapment in caveolae induces a tyrosine kinase phosphorylation cascade, local disassembly of cortical actin, formation of actin tails on the loaded caveolae and finally vesicle fission and movement into the cytoplasm [37, 89]. SV40 translocation to caveolae was found to be independent of actin or phosphorylation events, whereas virus internalization within caveolae required phosphorylation-mediated actin re-modelling. Furthermore, other kinases that are involved in actin cytoskeletal regulation, such as phosphotidyl inositol (4, 5) kinase, and those that activate the Rho-family small GTPase Cdc42, were shown to inhibit endocytosis by activation of actin polymerization [48]. These and other studies lead to the speculation that caveolae are in fact not involved in constitutive endocytosis, but instead represent a highly stable plasma membrane compartment anchored by the actin cytoskeleton [90] which are mobilized in a phosphorylation dependent manner.
At this point, it is unclear how the actin cytoskeleton connects or communicates with caveolae. Dynamin-2 is known to interact with caveolin-1 [41, 63] as well as actin-binding proteins intersectin and cortactin [91, 92]. Intersectin, a protein with multiple Eps15 homology (EH) and Src homology 3 (SH3) domains, interacts with dynamin at the neck of caveolae in endothelial cells where it is thought to regulate fission and internalization [91]. Another possibility is that actin filaments interact with caveolae through filamin A, an actin cross-linking protein that was shown to directly bind caveolin-1 in vitro[93]. Additionally, Csk that is recruited to phosphorylated caveolin-1 has the ability to regulate actin cytoskeletal dynamics [77, 94, 95]. Src also participates in actin cytoskeletal re-modelling by regulating cortactin [96–98].Therefore, it is possible that Src controls interactions of actin with caveolin-1, thereby regulating caveolar detachment from the membrane and vesicle internalization [88, 98, 99]. Although it is now generally accepted that the actin cytoskeleton plays an important role in caveolae endocytosis, the mechanism of actin re-organization upon activation of caveolae endocytosis and it role in vesicle internalization remains largely unknown.
Conclusion
A large number of kinases are thought to be involved in the regulation of caveolae-mediated endocytosis, including those implicated in integrin and Ca2+ signalling as well as actin cytoskeletal regulation [48]. Among them are Src kinases, which are activated early during initiation of caveolae-mediated endocytosis. Activation of Src represents a general requirement for caveolae internalization as it was shown to regulate caveolae internalization in cells of different origin, such as fibroblasts, endothelial and epithelial cells. Src is known to phosphorylate caveolae-associated proteins caveolin-1, caveolin-2 and dynamin-2, regulating the assembly of multi-protein complexes involved in fission and internalization of caveolae. Src-mediated phoshorylation may also regulate the phosphorylation-dependent changes in the actin cytoskeleton that are necessary for caveolae release and migration through the cortical actin network. Ongoing studies by our group and others will hope-fully provide greater insight into the mechanisms which regulate caveolae-mediated endocytosis, further defining the physiological importance of caveolae trafficking in health and disease.
Acknowledgments
This work was supported by NIH/NHLBI grants HL 71626 and HL 60678.
References
- 1.Minshall RD, Vogel SM. Lung edema and microvascular permeability. In: D S, editor. Microvascular research: biology and pathology. Burlington: Elsevier Science. 2006:471–5. [Google Scholar]
- 2.Palade GE, Bruns RR. Structural modulation of plasmalemmal vesicles. J Cell Biol. 1968;37:633–49. doi: 10.1083/jcb.37.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Predescu D, Palade GE. Plasmalemmal vesiclesrepresent the large pore system of continiousmicrovascular endothelium. Am J Physiol. 1993;265:725–33. doi: 10.1152/ajpheart.1993.265.2.H725. [DOI] [PubMed] [Google Scholar]
- 4.Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu Y-H, Cook RF, Sargiacomo M. Characterization of caveolin-richmembrane domains isolated from an endothelial-rich source: implication for human disease. J Cell Biol. 1994;126:111–26. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RGW. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 6.Ghitescu L, Fixman A, Simionescu M, Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986;102:1304–11. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milici AJ, Watrous NE, Stukenbrok H, Palade GE. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987;105:2603–12. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Predescu D, Horvat R, Predescu S, Palade GE. Transcytosis in the continuous endothelium of the myocardial microvasculature is inhibited by N-ethylmaleimide. Proc Natl Acad Sci USA. 1994;91:3014–8. doi: 10.1073/pnas.91.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Predescu SA, Predescu DN, Palade GE. Plasmalemmal vesicles function as transcytotic carriers for small proteins in the continuous endothelium. Am J Physiol Heart Circ Physiol. 1997;272:H937–49. doi: 10.1152/ajpheart.1997.272.2.H937. [DOI] [PubMed] [Google Scholar]
- 10.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cellsurface gp60 activates vesicle formation and trafficking via Gi-coupled Src kinase signaling pathway. J Cell Biol. 2000;150:1057–69. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel SM, Minshall RD, Pipipovic M, Tiruppathi C, Malik AB. Albumin uptake and transcytosis in endothelial cells in vivo induced by albumin-binding protein. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1512–22. doi: 10.1152/ajplung.2001.281.6.L1512. [DOI] [PubMed] [Google Scholar]
- 12.Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 13.Stan RV. Structure of caveolae. Biochim Biophys Acta. 2005;1746:334–48. doi: 10.1016/j.bbamcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Scherer PE, Tang ZL, Chun M, Sargiacomo M, Lodish HF, Lisanti MP. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. J Biol Chem. 1995;270:16395–401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- 15.Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–61. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- 16.Way M, Parton RG. M-caveolin, a muscle specific caveolin- related protein. FEBS Lett. 1996;378:108–12. doi: 10.1016/0014-5793(96)82884-5. [DOI] [PubMed] [Google Scholar]
- 17.Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes be expression of VIP21-caveolin. Proc Natl Acad Sci USA. 1995;92:8655–9. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelman JA, Wykoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem. 1997;272:16374–81. doi: 10.1074/jbc.272.26.16374. [DOI] [PubMed] [Google Scholar]
- 19.Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA. 1995;92:1381–5. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Birgit Lauterbach, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 21.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, Lisanti MP. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277:8635–47. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 22.You-Yang Z, Liu Y, Stan R-V, Fan L, Gu Y, Dalton N, Chu P-H, Peterson KJR, Jr, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375–80. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minshall RD, Sessa WC, Stan RV, Anderson RGW, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1179–83. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- 24.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Vizio DDHH, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 25.Miyawaki-Shimizu K, Predescu D, Shimizu J, Broman M, Predescu S, Malik AB. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol. 2006;290:L405–13. doi: 10.1152/ajplung.00292.2005. [DOI] [PubMed] [Google Scholar]
- 26.Simionescu N, Simionescu M, Palade GE. Permeability of muscle capillaries to small hemepeptides. J Cell Biol. 1975;64:586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghitescu L, Bendayan M. Transendothelial transport of serum albumin: a quantitative immunocytochemical study. J Cell Biol. 1992;117:745–55. doi: 10.1083/jcb.117.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–32. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schubert W, Frank PG, Razani B, Park DS, Chow C-W, Lisanti MP. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem. 2001;276:48619–22. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- 30.Montesano R, Roth J, Robert A, Orci L. Noncoated membrane invainations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982;296:651–3. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- 31.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinasedependent pathway. J Biol Chem. 1997;272:25968–75. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 32.Schnitzer JE, Carley WW, Palade GE. Albumin interacts specifically with 60-kDa microvascular endothelial glycoprotein. Proc Natl Acad Sci USA. 1988;85:6773–7. doi: 10.1073/pnas.85.18.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.John TA, Vogel SM, Tiruppathi C, Malik AB, Minshall RD. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol. 2003;284:L187–96. doi: 10.1152/ajplung.00152.2002. [DOI] [PubMed] [Google Scholar]
- 34.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoki T, Nomura R, Fujimoto T. Tyrosine phosphorylation of caveolin-1 in the endothelium. Exp Cell Res. 1999;253:629–36. doi: 10.1006/excr.1999.4652. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Norkin LC. Extracellular simian virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp Cell Res. 1999;246:83–90. doi: 10.1006/excr.1998.4301. [DOI] [PubMed] [Google Scholar]
- 37.Pelkmans L, PŸntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40- indueced internalization of caveolae. Science. 2002;296:535–9. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 38.Singh RD, Puri V, Valiyaveettil JT, Marks DL, Bittman R, Pagano RE. Selective caveolin-1- dependent endocytosis of glycosphingolipids. Mol Biol Cell. 2003;14:3254–65. doi: 10.1091/mbc.E02-12-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma DK, Brown JC, Choudhury A, Peterson TE, Holicky E, Marks DL, Simari R, Parton RG, Pagano RE. Selective stimulation of caveloar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell. 2004;15:3114–22. doi: 10.1091/mbc.E04-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shajahan AN, Tiruppathi C, Smrcka AV, Malik AB, Minshall RD. Gβγ activation of Src induces caveolae- mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:48055–62. doi: 10.1074/jbc.M405837200. [DOI] [PubMed] [Google Scholar]
- 41.Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:20392–400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 42.Anderson HA, Chen Y, Norkin LC. Bound Simian Virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–34. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new twostep vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–83. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 44.Stang E, Kartenbeck J, Parton RG. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol Biol Cell. 1997;8:47–57. doi: 10.1091/mbc.8.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S, Couet J, LIsanti M. Src tyrosine kinases, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. J Biol Chem. 1996;271:29182–90. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shajahan AN, Sverdlov M, Hirth AM, Timblin BK, Tiruppathi C, Malik AB, Minshall RD. Src-dependent caveolin-1 phosphoylation destabilizes caveolin-1 oligomers and activates vesicle fission in endothelial cells. The American Society for Cell Biology 44th Annual Meeting. Washington, DC, 2004.
- 47.Pelkmans L, Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 2005;436:128–33. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- 48.Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raftmediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 49.Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Harry Hou J, Christ GJ, Edelmann W, Lisanti M. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329–44. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA. 1996;93:131–5. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S, Galbiati F, Volont_ D, Sargiacomo M, Engelman JA, Das K, Scherer PE, Lisanti MP. Mutational analysis of caveolin-induced vesicle formation. Expression of caveolin-1 recruits caveolin-2 to caveolae membranes. FEBS Lett. 1998;434:127–34. doi: 10.1016/s0014-5793(98)00945-4. [DOI] [PubMed] [Google Scholar]
- 52.Fujimoto T, Kogo H, Nomura R, Une T. Isoforms of caveolin-1 and caveolar structure. J Cell Sci. 2000;113:3509–17. doi: 10.1242/jcs.113.19.3509. [DOI] [PubMed] [Google Scholar]
- 53.Sowa G, Pypaert M, Fulton D, Sessa WC. The phosphorylation of caveolin-2 on serines 23 and 36 modulates caveolin-1-dependent caveolae formation. Proc Natl Acad Sci USA. 2003;100:6511–6. doi: 10.1073/pnas.1031672100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee H, Park DS, Wang XB, Scherer PE, Schwartz PE, Lisanti M. Src-induced phosphorylation of caveolin-2 on tyrosine 19. J Biol Chem. 2002;277:34556–67. doi: 10.1074/jbc.M204367200. [DOI] [PubMed] [Google Scholar]
- 55.Wang XB, Lee H, Capozza F, Marmon S, Sotgia F, Brooks JW, Campos-Gonzales R, Lisanti MP. Tyrosine phosphorylation of caveolin-2 at residue 27: difference in the spatial and temporal behavior of phospho-cav-2 (pY19 and pY27) Biochemistry. 2004;43:13694–706. doi: 10.1021/bi049295+. [DOI] [PubMed] [Google Scholar]
- 56.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–14. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henley JR, Krueger EWA, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–90. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 59.Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for β2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem. 1999;274:1185–8. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- 60.Ahn S, Kim J, Lucaveche CL, Reedy MC, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J Biol Chem. 2002;277:26642–51. doi: 10.1074/jbc.M201499200. [DOI] [PubMed] [Google Scholar]
- 61.Kim Y-N, Bertics PJ. The endocytosis-linked protein dynamin associates with caveolin-1 and is tyrosine phosphorylated in responce to the activation of a noninternalizing epidermal growth factor receptor mutant. Endocrinology. 2002;143:1726–31. doi: 10.1210/endo.143.5.8814. [DOI] [PubMed] [Google Scholar]
- 62.Predescu SA, Predescu DN, Palade GE. Endothelial transcytotic machinery involves supramolecular protein-lipid complexes. Mol Biol Cell. 2001;12:1019–33. doi: 10.1091/mbc.12.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao Q, Chen J, Cao H, Orth JD, McCaffery JM, Stan R-V, McNiven M. Caveolin-1 interacts directly with dynamin-2. J Mol Biol. 2005;348:491–501. doi: 10.1016/j.jmb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Glenney JR. Tyrosine phosphorylation of a 22-kDa protein is correlated wth transformation by Rous Sarcoma virus. J Biol Chem. 1989;264:20163–6. [PubMed] [Google Scholar]
- 65.Nomura R, Fujimoto T. Tyrosine-phosphorylated caveolin-1: immunolocalization and molecular characterization. Mol Biol Cell. 1999;10:975–86. doi: 10.1091/mbc.10.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR, Anderson RGW. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–82. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 67.Ko Y-G, Liu P, Pathak RK, Craig LC, Anderson RGW. Early effects of PP60v-src kinase activation on caveolae. J Cell Biochem. 1998;71:524–35. [PubMed] [Google Scholar]
- 68.Newcomb LF, Mastick CC. Src family kinasedependent phosphrylation of a 29-kDa caveolinassociated protein. Biochem Biophys Res Commun. 2002;290:1447–53. doi: 10.1006/bbrc.2002.6371. [DOI] [PubMed] [Google Scholar]
- 69.Fang P-K, Solomon KR, Zhuang L, Qi M, McKee M, Freeman MR, Yelick PC. Caveolin-1α and -1β perform nonredundant roles in early vertebrate development. Am J Pathol. 2006;169:2209–22. doi: 10.2353/ajpath.2006.060562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kogo H, Aiba T, Fujimoto T. Cell type-specific occurrence of caveolin-1α and -1β in the lung caused by expression of distinct mRNAs. J Biol Chem. 2004;279:25574–81. doi: 10.1074/jbc.M310807200. [DOI] [PubMed] [Google Scholar]
- 71.Botos E, Turi Á, Müllner N, Kovalszky I, Tátrai P, Kiss AL. Regulatory role of kinases and phosphotases on the internalization of caveolae in HepG2 cells. Micron. 2007;38:313–20. doi: 10.1016/j.micron.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 72.Orlichenko L, Huang B, Krueger E, McNiven MA. Epidermal growth factor-induced phosphorylation of caveolin-1 at tyrosine 14 stimulates caveolae formation in epithelial cells. J Biol Chem. 2006;281:4570–9. doi: 10.1074/jbc.M512088200. [DOI] [PubMed] [Google Scholar]
- 73.Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911–27. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sargiacomo M, Scherer PE, Tang Z, Kübler E, Song KS, Sanders MC, Lisanti MP. Oligomeric structure of caveolin: implication for caveolae membrane organization. Proc Natl Acad Sci USA. 1995;92:9407–11. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song KS, Tang Z, Li S, Lisanti MP. Mutational analysis of the properties of caveolin-1. J Biol Chem. 1997;272:4398–403. doi: 10.1074/jbc.272.7.4398. [DOI] [PubMed] [Google Scholar]
- 76.Machleidt T, Li W-P, Liu P, Anderson RGW. Multiple domains in caveolin-1 controls its intracellular traffic. J Cell Biol. 2000;148:12–28. doi: 10.1083/jcb.148.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee H, Volonté D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, Wilson MT, Campos-Gonzales R, Bouzahzah B, Pestell RG, Scherer PE, Lisanti M. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grc7 signaling cassette. Mol Endocrinol. 2000;14:1750–75. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 78.Fernandez I, Ying Y, Albanesi J, Anderson RGW. Mechanism of caveolin filament assembly. Proc Natl Acad Sci USA. 2002;99:11193–8. doi: 10.1073/pnas.172196599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pelkmans L, Bürli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–80. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A. Assembly and trafficking of caveolae domains in the cell: caveolae as stable, cargo-triggered vesicular transporters. J Cell Biol. 2005;170:769–79. doi: 10.1083/jcb.200506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao H, Courchesne WE, Mastick CC. A phosphotyrosine- dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine. J Biol Chem. 2002;277:8771–4. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- 82.Okada M, Nada S, Yamanashi Y, Yamamoto T, Nakagawa H. CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem. 1991;266:24249–52. [PubMed] [Google Scholar]
- 83.Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick J, Giancotti F. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell. 2001;8:115–27. doi: 10.1016/s1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- 84.Del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RGW, Schwartz MA. Phospho-caveolin-1 mediates intergrin-regulated membrane domain internalization. Nature Cell Biology. 2005;7:901–8. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ayscough KR. Endocytosis and the development of cell polarity in yeast require a dynamic F-actin cytoskeleton. Curr Biol. 2000;10:1587–90. doi: 10.1016/s0960-9822(00)00859-9. [DOI] [PubMed] [Google Scholar]
- 86.Apodaca G. Endocytic traffic in polarized epithelial cells. role of actin and microtubule cytoskeleton. Traffic. 2001;2:149–59. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- 87.Izumi T, Shibata Y, Yamamoto T. Quick-freeze, deepetch studies of endothelial components, with special reference to cytoskeletons and vesicle structures. J Electron Microsc Tech. 1991;19:316–26. doi: 10.1002/jemt.1060190307. [DOI] [PubMed] [Google Scholar]
- 88.Dorothy IMundyTM, Yun-shu Ying, Richard GWAnderson, George SBloom. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci. 2002;115:4327–39. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- 89.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–20. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 90.Thomsen P, Roepstorff K, Stahlut M, Van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell. 2002;13:238–50. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Predescu SA, Predescu DN, Timblin BK, Radu VStan, Malik AB. Intersectin regulates fission and internalization of caveolae in endothelial cells. Mol Biol Cell. 2003;14:4997–5010. doi: 10.1091/mbc.E03-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schafer DA, Weed SA, Binns D, Karginov AV, Parsons JT, Cooper JA. Dynamin 2 and cortactin regulate actin assembly and filament organization. Curr Biol. 2002;12:1852–7. doi: 10.1016/s0960-9822(02)01228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stahlut M, Van Deurs B. Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell. 2000;11:325–37. doi: 10.1091/mbc.11.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Radel C, Rizzo V. Intergrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol Heart Circ Physiol. 2005;288:H936–45. doi: 10.1152/ajpheart.00519.2004. [DOI] [PubMed] [Google Scholar]
- 95.Lowry WE, Huang J, Ma Y-C, Ali S, Wang D, Williams DM, Okada M, Cole PA, Huang X-Y. Csk, a critical link of G protein signals to actin cytoskeletal reorganization. Dev Cell. 2002;2:733–44. doi: 10.1016/s1534-5807(02)00175-2. [DOI] [PubMed] [Google Scholar]
- 96.McNiven MA, Kim L, Krueger E, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol. 2000;151:187–98. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptormediated endocytosis. Mol Cell Biol. 2003;23:2162–70. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krueger EW, Orth JD, Cao H, McNiven MA. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol Biol Cell. 2003;14:1085–96. doi: 10.1091/mbc.E02-08-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Deurs B, Roepstorff K, Hommelgaard AM, Sandvig K. Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol. 2003;13:92–8. doi: 10.1016/s0962-8924(02)00039-9. [DOI] [PubMed] [Google Scholar]


