Abstract
Substance P, acting via its neurokinin 1 receptor (NK1 R), plays an important role in mediating a variety of inflammatory processes. Its interaction with chemokines is known to play a crucial role in the pathogenesis of acute pancreatitis. In pancreatic acinar cells, substance P stimulates the release of NFκB-driven chemokines. However, the signal transduction pathways by which substance P-NK1 R interaction induces chemokine production are still unclear. To that end, we went on to examine the participation of mitogen-activated protein kinases (MAPKs) in substance P-induced synthesis of pro-inflammatory chemokines, monocyte chemoanractant protein-1 (MCP-I), macrophage inflammatory protein-lα (MIP-lα) and macrophage inflammatory protein-2 (MIP-2), in pancreatic acini. In this study, we observed a time-dependent activation of ERK1/2, c-Jun N-terminal kinase (JNK), NFκB and activator protein-1 (AP-1) when pancreatic acini were stimulated with substance P. Moreover, substance P-induced ERK 1/2, JNK, NFκB and AP-1 activation as well as chemokine synthesis were blocked by pre-treatment with either extracellular signal-regulated protein kinase kinase 1 (MEK1) inhibitor or JNK inhibitor. In addition, substance P-induced activation of ERK 112, JNK, NFκB and AP-1-driven chemokine production were attenuated by CP96345, a selective NK1 R antagonist, in pancreatic acinar cells. Taken together, these results suggest that substance P-NK1 R induced chemokine production depends on the activation of MAPKs-mediated NFκB and AP-1 signalling pathways in mouse pancreatic acini.
Keywords: MAPK, NFκB, AP-1, substance P
Introduction
The neuropeptide substance P, a member of the tachykinin family, has been shown to play an important role in asthma, inflammatory bowel disease, arthritis and other inflammatory processes [1, 2]. Subsequent to its release from nerve endings, substance P binds to neurokinin 1 receptor (NK1 R) on effector cells, increases microvascular permeability and promotes plasma extravasation from the intravascular to the extravascular space. Pancreatic acinar cells are known to express NKIR, and substance P has been detected within the pancreas [3–5]. It has been suggested that this neuropeptide might play a role in the pathogenesis of a pancreatic inflammatory disease, such as acute pancreatitis [6]. Studies have found that pancreatic levels of substance P and the expression of NK1 R on pancreatic acinar cells are increased during experimental acute pancreatitis [6]. It has also been shown that genetic deletion of NK1R reduces the severity of pancreatitis and pancreatitis-associated lung injury [6]. Furthermore knockout mice deficient in the preprotachykinin-A gene, which encodes for substance P, are protected against acute pancreatitis and associated lung injury [7].
Inflammatory mediators, such as chemokines play a key role in the pathogenesis of acute pancreatitis [6, 8, 9]. The chemokines are afamily of small (8–10 kD) inducible cytokines with activating and chemotactic effects on leucocyte subsets. They can be broadly subdivided on a structural basis into the CC subfamily, in which the first two cysteine residues are adjacent, and the CXC subfamily, in which the first two of the four conserved cysteine residues are separated by another amino acid. CC chemokines, such as monocyte chemoattractant protein-1 (MCP-I), are believed to principally affect monocytes, whereas CXC chemokines that possess the Glutamic acid- Leucine-Arginine (ELR) motif at the amino terminal are believed to act on neutrophils [1]. Recently our group has demonstrated the interaction between CC, CXC chemokines and the inflammatory mediator, substance P in acute pancreatitis and associated lung injury. Blockade of substance P receptor with its potent selective antagonist, CP96345, attenuates the increase in CC chemokines MCP-1, macrophage inflammatory protein (MlP)-1α and CXC chemokine MIP-2 production in both pancreas and lungs in mice induced with acute pancreatitis [10]. Our group also investigated the effect of substance P treatment on chemokine synthesis in mouse pancreatic acinar cells.We demonstrated that substance P stimulates the synthesis of MCP-1, MIP-lα and MIP-2 in pancreatic acinar cells. Furthermore, we found that the increase in the chemokine production is mediated by NFκB activation [11]. Hence substance P plays an important role in the pathogenesis of acute pancreatitis by inducing chemokine production via the NFκB-dependent pathway. However, the signal transduction pathway through which substance P-NKlR interaction induces chemokine production in mouse pancreatic acini has not been elucidated yet. In the present study, we examined the participation of MAPKs in substance P-induced synthesis of chemokines MCP-1, MIP-lα and MIP-2 in pancreatic acini.
Material and methods
Preparation of mouse pancreatic acini
All animal experiments were approved by the Animal Ethics Committee of National University of Singapore and carried out in accordance with established International Guiding Principles for Animal Research.
Pancreatic acini were obtained from mouse pancreas by collagenase treatment as described previously [8]. Briefly, pancreas from three Swiss mice (20–25 g) were removed, infused with buffer A (in mM: 140 NaCl, 4.7 KCl, 1.13 MgCl2, 1 CaCl2, 10 glucose, 10 HEPES, pH 7.2) containing 200 IU/ml collagenase and 0.5 mg/ml soybean trypsin inhibitor and incubated in a shaking water bath for 10 min at 37°C. The digested tissue was passed through 50 mg/ml bovine serum albumin (BSA) and washed twice with buffer A before further experiments.
Viability of mouse pancreatic acinar cells
Viability of the pancreatic acinar cells was determined by trypan blue dye exclusion assay. One drop of 0.4% of trypan blue dye was added to one drop of the isolated acinar cells and the viability was checked under light microscope. In all experiments, cell viability was greater than 95%.
Cell signalling experiments
Pancreatic acini were treated with substance P (Sigma, St Louis, MO, USA) at a dose of 10-6 M (1 μM) for 0, 3, 5, 10, 15, 45, 60 and 120 min at 37°C. After which the cells were subjected to either nuclear extract for NFκB (p65) and AP-1 (c-Jun) detection or cell lysis to detect for MAPKs activation by Western blot analysis. In some experiments, cells were also pre-treated with MAPK kinase (MEK1) inhibitor PD98059 at 10 μM, 30 μM, 50 μM and 100 μM (Calbiochem, Hull, UK) for 1 hr and then stimulated with 1 μM substance P or vehicle (dimethyl sulfoxide [DMSO] for 45 min at 37°C). In other experiments, cells were pre-incubated with JNK inhibitor SP600125 at 10 μM, 25 μM, 50 μM and 100 μM (Calbiochem) for 1 hr followed by treatment with 1 μM substance P or vehicle (DMSO) for 45 min at 37°C). In yet another experiment, cells were pre-incubated with the selective NK1R antagonist, CP96345, at 1 μM (Pfizer Diagnostics, New York, NY, USA) for half an hour followed by treatment with 1 μM substance P or vehicle (saline) for 45 min at 37°C. Subsequently the supernatant was used for chemokine detection and the pellet was used for either nuclear extract, to detect NFκB (p65) and AP-1 (c-Jun) activation, or cell lysis for Western blot analysis. PD98059 or SP600125 stock solutions were prepared by dissolving 5 mg of PD98059 or SP600125 into 100 μl of DMSO. The final concentration of the vehicle was ≤ 0.1% DMSO. CP96345 stock solution was prepared by dissolving 1 mg of CP96345 into 2 ml of saline.
Preparation of cell lysates for Western blot analysis
Pancreatic acini were treated with substance P (Sigma) at a dose of 10-6 M (1 μM) for 0, 3, 5, 10, 15, 45, 60 and 120 min at 37°C. After treatment, pancreatic acinar cells were homogenized on ice in radioimmunoprecipitation (RIPA) buffer supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) and the protease inhibitor cocktail containing pepstatin, leupeptin, chymostatin, antipain and aprotinin (5 μg/ml of each), and centrifuged at 4°C for 15 min at 13,000 rpm. The supernatants were collected and stored at −80°C until use. Protein concentrations were determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA).
Western blot analysis
Cell lysates (50 μg) were separated on 12% SDS-polyacrylamide gel and electrophoretically transferred to nitrocellulose membranes. Non-specific binding was blocked by 1-hour incubation of the membranes in 5% non-fat dry milk in PBST (0.05%Tween 20 in PBS). The blots were then incubated overnight with the primary antibodies phospho-ERK1/2, ERK 1/2, phospho-stress-activated protein kinase (SAPK)/JNK, SAPK/JNK and IκBκ Cell Signalling Technology, Danvers, MA, USA) at 1:1000 dilutions in the buffer containing 2.5% non-fat dry milk in PBST. After which they were washed four times with PBST, and finally incubated for 1 hr with goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:2000 dilutions in the buffer containing 2.5% non-fat dry milk in PBST. The blots were developed for visualization using enhanced chemiluminescence (ECL) detection kit (Pierce, Rockford, IL, USA).
Nuclear cell extract preparation and NFκB DNA-binding activity
Nuclear cell extracts were prepared by employing a kit from Active Motif. In brief, cells were washed, collected in icecold PBS in the presence of phosphatase inhibitors, to limit further protein modifications, and then centrifuged at 24 g for 5 min. The pellets were re-suspended in a hypotonic buffer, treated with detergent and centrifuged at 14,000 g for 30 sec. After collection of the cytoplasmic fraction, the nuclei were lysed and nuclear proteins solubilized in lysis buffer containing proteasome inhibitors. Protein concentrations were determined by the Bio-Rad protein assay (Bio- Rad Laboratories, Hercules, CA, USA).
The binding of NFκB to DNA was measured in nuclear extracts with a fast, user-friendly ELISA-based TransAM NFκB p65 assay kit (Active Motif, Carlsbod, CA, USA). This assay uses multi-well plates coated with an unlabeled oligonucleotide containing the consensus-binding site for NFκB (5′-GGGACTTTCC-3′) [12]. Nuclear proteins (5 μg) were added to each well and incubated for 1 hr to allow NFκB DNA binding. Subsequently, by using an antibody that is directed against NFκB p65 subunit, the NFκB complex bound to the oligonucleotide is detected. Addition of the secondary antibody conjugated to horseradish peroxidase (HRP) provides sensitive colorimetric readout that is easily quantified by spectrophotometry.
AP-1
TransAM AP-1 kits are designed specifically to detect and quantify AP-1 activation. Similar to TransAM NFκB p65, TransAM AP-1 kits contain a 96- well plate on which has been immobilized an oligonucleotide that contains a 12-Otetradecanoylphorbol-13-acetate (TPA)-responsive element TRE (5′-TGAGTCA-3′). AP-1 dimers contained in nuclear extract (5 μg) specifically binds to this oligonucleotide. The primary antibodies used recognize accessible epitopes on c-Jun proteins upon DNA binding. Secondary antibody conjugated to HRP gives the colorimetric reaction. Absorbance was read at 450 nm within 5 min.
Chemokine detection
Pancreatic acinar cell supernatants were assayed for MCP-1, MIP-1α and MIP-2 using a sandwich ELISA, according to the manufacturer's instructions (Duoset kit; R&D Systems, Minneapolis, MN, USA). For example MCP-1, briefly, anti-MCP-1 primary antibody was aliquoted onto ELISA plates and incubated at 4°C overnight. Samples and standards were incubated for 2 hrs, the plates were washed, and a biotinylated anti-MCP-1 antibody was added for 2 hrs. Plates were washed again, and streptavidin bound to HRP was added for 20 min. After a further wash, tetramethylben-zidine was added for colour development, and the reaction was terminated with 2 M H2SO4. Absorbance was meas-ured at 450 nm. The same procedure was followed for the detection of the remaining chemokines MIP-1α and MIP-2.
Statistical analysis
Results are presented as means + SE with six replicates for each condition. Each experiment was repeated at least three times. The significance of changes was evaluated by using ANOVA and Tukey's method was used as a post hoc test for the difference between groups. A P value ≤ 0.05 was taken as the level of significance.
Results
Substance P stimulates ERK1/2 phosphorylation and NFκB activation in a time-dependent manner
To examine whether substance P causes ERK1/2 phosphorylation in pancreatic acini, mouse pancreatic acinar cells were treated with 1 μM substance P for 0, 3, 5, 10, 15, 45, 60, 120 min. Cells were then lysed, and cell proteins were subjected to Western blot analysis using antibodies against both phospho-ERK1/2 and total ERK1/2. As shown in Figure 1A, substance P-induced phosphorylation of ERK1/2 in pancreatic acini that was evident at 3 min and increased in a time-dependent manner up to 120 min. Figure 1B, densitometric analysis of Western blot experiments revealed a significant increase in phosphorylation of ERK2 at all the above mentioned time points when compared to 0 min control. Phosphorylation of ERK1 was significantly higher at 45, 60 and 120 min when compared to 0 min control. The time-dependent increase in phosphorylation of ERK1/2 was in line with the time-dependent degradation of total IκBκ as shown in Figure 1C. In a similar experiment nuclear extract was used, instead of cell lysate, to detect NFκB activation by ELISA. As shown in Figure 1D, treatment with 1 μM substance P caused a time-dependent increase in NFκB activation as early as 3 min. The increase became significant at 10 min and reached maximum at 120 min after substance P treatment.
1.
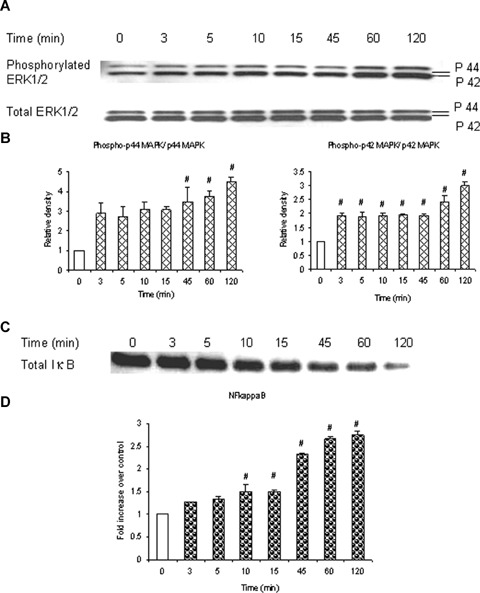
Substance P stimulates ERK1/2 phosphorylation and NFκB activation in a time-dependent manner. Substance P induces a time-dependent phosphorylation of ERK1/2 which coincides with a time-dependent activation of NFκB. Freshly isolated pancreatic acini, obtained from three mice, were incubated with 1 μM substance P for 0, 3, 5, 10, 15, 45, 60, 120 min at 37°C. In some experiments, cells were lysed and cell proteins were subjected to Western blot analysis using antibodies against (A) phospho-ERK1/2, total ERK1/2 (B) Densitometric analysis of Western blot experiments from pancreatic acini. and (C) IκBα. In another experiment, the nuclear extract was used to isolate NFκB and ELISA was carried out to detect activation of (D) NFκB. The results are representative of three independent experiments. Results shown are the means + SE. # P≤0.05 when compared to 0 min control.
ERK1/2-mediated NFκB activation is involved in substance P-induced chemokine synthesis
To verify whether the MEK1 inhibitor PD98059 blocks phosphorylation of ERK1/2 in substance P-treated cells, mouse pancreatic acinar cells were pre-incubated with PD98059 for 1 hr followed by stimulation with 1 μM substance P for 45 min. Cells were then lysed, and cell proteins were subjected to Western blot analysis. As shown in Figure 2A, PD98059 attenuated substance P-induced phosphorylation of ERK1/2 in a dose-dependent manner. In Figure 2B, densitometry showed that the four different doses of PD98059 namely 10 μM, 30 μM, 50 μM and 100 μM significantly blocked phosphorylation of ERK1/2 when compared to substance P-only treated group. To confirm the role of ERK1/2 in substance Pinduced NFκB activation and chemokine production, we pre-treated the pancreatic acini with PD98059 for 1 hr followed by stimulation with 1 μM substance P for 45 min [11]. Our results, in Figure 3A, showed that pre-treatment with PD98059 significantly inhibited substance P-induced NFκB activation in a dosedependent manner which was followed by a dosedependent decrease in CC chemokines (3B) MCP-1, (3C) MIP-1 μ and CXC chemokine (3D) MIP-2. The negative control in which pancreatic acini were pretreated with 10 μM (the dose sufficient to block substance P-mediated activation) of PD98059 for 1 hr followed by stimulation with placebo for 45 min had no significant effect on MAPK, NFκB and chemokine production when compared to unstimulated controls (data not shown). These results indicate that substance P induces the production of chemokines MCP-1, MIP-1α and MIP-2 viathe ERK1/2-mediated NFκB signalling pathway.
2.
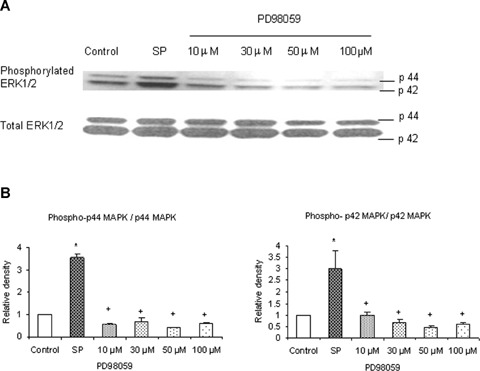
PD98059 dose-dependently decreases phosphorylation of ERK1/2 in pancreatic acini. PD98059 (an inhibitor of MEK1/2) effectively blocked phosphorylation of ERK1/2. Freshly isolated pancreatic acini, obtained from three mice, were pre-incubated with PD98059 at different doses of 10 μM, 30 μM, 50 μM, 100 μM for 1 hr at 37°C followed by stimulation with 1 μM substance P for 45 min at 37°C. Cells were subsequently lysed, and cell proteins were subjected to Western blot analysis using antibodies against (A) phospho-ERK1/2 and total ERK1/2. (B) Densitometric analysis of Western blot experiments from pancreatic acini. The results are representative of three independent experiments. Results shown are the means + SE. * P≤ 0.05 when compared to control, + P≤ 0.05 when compared to substance P (SP).
3.
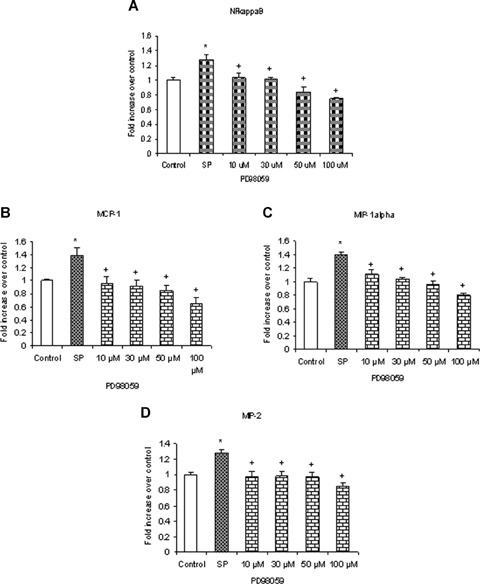
ERK1/2-mediated NFκB activation is involved in substance P-induced chemokine synthesis. Substance Pinduced ERK1/2 phosphorylation and activation mediate NFκB activation and chemokine production. Freshly isolated pancreatic acini, obtained from three mice, were pre-incubated with MEK1 inhibitor PD98059 for 1 hr followed by stimulation with 1 μM substance P for 45 min. Acini were separated from incubation medium by centrifugation. (A) The pellet (acini) was used for NFκB extraction and detection whereas the supernatant was used to measure (B) MCP-1, (C) MIP-1 and (D) MIP-2 levels by ELISA. The results are representative of three independent experiments. Results shown are the means + SE. * P≤0.05 when compared to control, + P≤0.05 when compared to substance P (SP).
Substance P induces phosphorylation of JNK and AP-1 (c-Jun) activation in a time-dependent manner
To examine whether substance P stimulates JNK phosphorylation in pancreatic acini, mouse pancreatic acinar cells were treated with 1 μM substance P for 0, 3, 5, 10, 15, 45, 60, 120 min. Cells were then lysed, and cell proteins were subjected to Western blot analysis using antibodies against both phospho p54 and -p46 JNK and total JNK. As shown in Figure 4A, substance P-induced phosphorylation of phospho-p54 and -p46 JNK in pancreatic acini in a time-dependent manner with the earliest time point being 3 min. Figure 4B, densitometric analysis of Western blot experiments revealed a significant increase in phospho-p54 at 45, 60, 120 min when compared to 0 min control. Densitometric analysis of phospho-p46 could not be carried out as the control for phospho-p46 was undetected.
4.
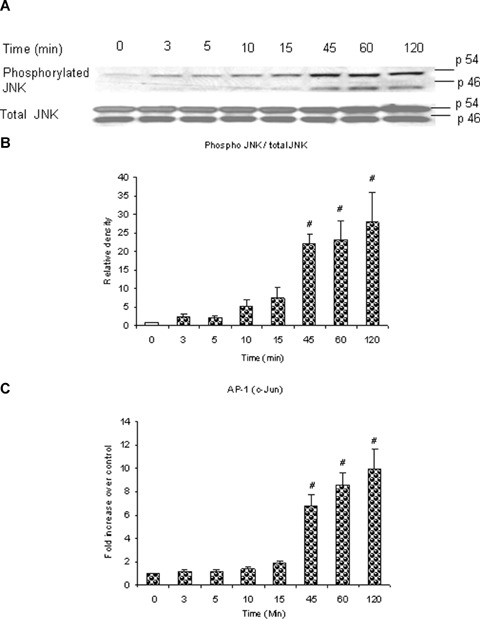
Substance P induces phosphorylation of JNK and AP-1 (c-Jun) activation in a time-dependent manner. Substance P induces a timedependent phosphorylation of JNK which is in line with a timedependent activation of AP-1 (c-Jun). Freshly isolated pancreatic acini, obtained from three mice, were incubated with 1 μM substance P for 0, 3, 5, 10, 15, 45, 60, 120 min at 37°C. In some experiments, cells were lysed, and cell proteins were subjected to Western blot analysis using antibodies against (A) phospho-JNK, total JNK. In another experiment, the nuclear extract was used to isolate AP-1 (c-Jun) and ELISA was carried out to detect activation of (C) AP-1 (c-Jun). (B) Densitometric analysis of Western blot experiments from pancreatic acini. The results are representative of three independent experiments. Results shown are the means + SE. #P ≤0.05 when compared to control (0 min).
In Figure 4C, the time-dependent increase in phosphorylation of phospho-p54 and -p46 JNK was in accord with the time-dependent increase in AP-1 (c-Jun) activation; with a significant maximal intensity ranged from 45 to 120 min when compared to the 0 min control.
JNK is involved in substance P-induced AP-1 (c-Jun) activation and chemokine synthesis
To make sure that SP600125, an inhibitor of JNK which prevents the phosphorylation of JNK substrates by blocking the ATP-binding domain of JNKs, inhibits phosphorylation of JNK in substance P-treated cells, mouse pancreatic acinar cells were pre-incubated with SP600125 for 1 hr followed by stimulation with 1 μM substance P for 45 min. Cells were then lysed, and cell proteins were subjected to Western blot analysis. As shown in Figure 5A, SP600125 attenuated substance P-induced phosphorylation of JNK. In Figure 5B, densitometry showed that 10 μM, 25 μM, 50 μM and 100 μM of SP600125 significantly blocked phosphorylation of JNK when compared to substance P- only treated group. To determine if substance P-induced synthesis of chemokines MCP-1, MIP-1α and MIP-2 and AP-1 (c-Jun) activation are mediated through JNK, pancreatic acini were pretreated with SP600125 for 1 hr followed by stimulation with 1 μM substance P for 45 min. Our data in Figure 6A showed that SP600125 inhibited substance P-induced AP-1 (c-Jun) activation in a dosedependent manner. As shown in Figure 6B, C and D, pre-treatment with SP600125 caused a concentration- dependent attenuation of substance P-induced production of MCP-1, MIP-1α and MIP-2. The negative control in which pancreatic acini were pre-treated with 10 μM (the dose sufficient to block substance P-mediated activation) of SP600125 for 1 hr followed by stimulation with placebo for 45 min had no significant effect on JNK, AP-1 (c-Jun) and chemokine production when compared to unstimulated controls (data not shown). These results demonstrate that substance P-induced synthesis of MCP-1, MIP-1α and MIP-2 is mediated by JNK/AP-1 (c-Jun) signalling pathway.
5.
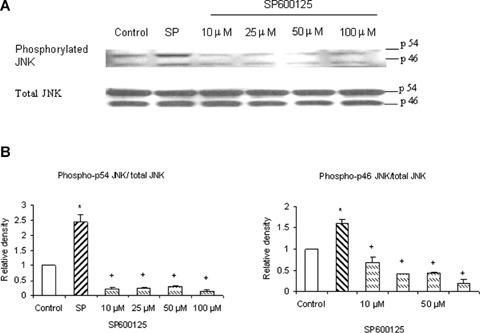
SP600125 decreases phosphorylation of JNK in pancreatic acini. SP600125 (JNK inhibitor) effectively blocked phosphorylation of JNK. Freshly isolated pancreatic acini, obtained from three mice, were pre-incubated with SP600125 at different doses of 10 μM, 25 μM, 50 μM, 100 μM for 1 hr at 37°C followed by stimulation with 1 μM substance P for 45 min at 37°C. Cells were subsequently lysed, and cell proteins were subjected to Western blot analysis using antibodies against (A) phospho-JNK and total JNK. (B) Densitometric analysis of Western blot experiments from pancreatic acini. The results are representative of three independent experiments. Results shown are the means + SE. * P≤0.05 when compared to control, + Pα0.05 when compared to substance P (SP).
6.
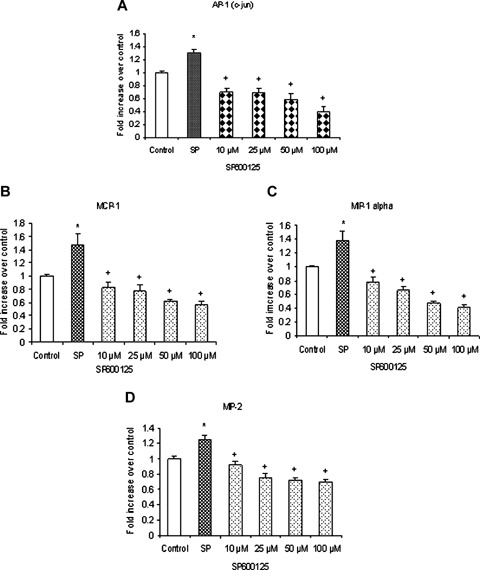
JNK is involved in substance P-induced AP-1 (c-Jun) activation and chemokines synthesis. Substance Pinduced JNK phosphorylation and activation mediate AP-1 (c-Jun) activation and chemokine production. Freshly isolated pancreatic acini, obtained from three mice, were pre-incubated with JNK inhibitor SP600125 for 1 hr followed by stimulation with 1μM substance P for 45 min. Acini were separated from incubation medium by centrifugation. (A) The pellet (acini) was used for AP-1 (c-Jun) extraction and detection whereas the supernatant was used to measure (B) MCP-1, (C) MIP-1κ and (D) MIP-2 levels by ELISA. The results are representative of three independent experiments. Results shown are the means + SE. * P≤0.05 when compared to control, + P≤0.05 when compared to substance P (SP).
Substance P-induced ERK1/2 and JNK cross activate NFκB and AP-1 (c-Jun)
Pancreatic acini were pre-treated with either PD98059 or SP600125 followed by stimulation with 1 μM substance P for 45 min. As shown in Figure 7A, PD98059 given at doses 10 μM, 30 μM, 50 μM and 100 μM significantly blocked AP-1 (c-Jun) activation. SP600125 attenuated NFκB activation at a concentration of 25 μM and above, as shown in Figure 7B. The negative control in which pancreatic acini were pre-treated with either 10 μM of PD98059 or 10 μM or even 25 μM of SP600125 (the doses sufficient to block substance P-mediated activation) for 1 hr followed by stimulation with placebo for 45 min had no significant effect on the activation of AP-1 (c-Jun) and NFκB, respectively, when compared to unstimulated controls (data not shown). Our data suggest that substance P-induced chemokine production also takes place through ERK1/2 mediated AP-1 (c-Jun) activation and JNK- mediated NFκB activation. These results imply that there is a cross-talk between the two classical pathways, ERK1/2-NFκB and JNKAP-1 (c-Jun), to induce synthesis of chemokines MCP-1, MIP-1α and MIP-2 in pancreatic acini.
7.
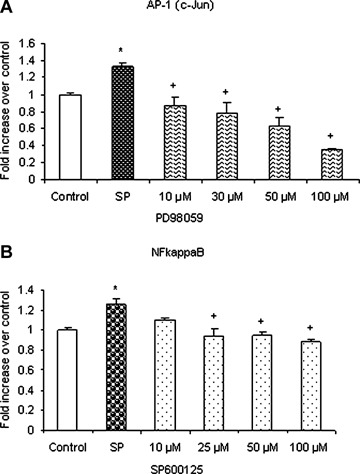
Substance P-induced ERK1/2 and JNK cross activate NFκ B and AP-1 (c-Jun). Freshly isolated pancreatic acini, obtained from three mice, were pre-incubated with either MEK1 inhibitor PD98059 or JNK inhibitor SP600125 for 1 hr followed by stimulation with 1 μM substance P for 45 min. Acini were separated from incubation medium by centrifugation. The pellet (acini) was used for extraction and detection of (A) NFκB activation and (B) AP-1 (c-Jun) activation. The results are representative of three independent experiments. Results shown are the means + SE. *P≤0.05 when compared to control, +P≤0.05 when compared to substance P (SP).
Substance P-NK1R interaction is involved in ERK 1/2 and JNK activation
Our data show that substance P-induced ERK1/2 and JNK activation were mediated through NK1R. We pre-treated the pancreatic acini with 1 μM of CP96345, a selective NK1R antagonist, followed by stimulation with 1 μM of substance P for 45 min. Cells were then lysed, and cell proteins were subjected to Western blot analysis. Our results, in Figure 8, demonstrate that CP96345 significantly reduced substance P-induced ERK1/2 and JNK activation in pancreatic acinar cells when compared to substance P-only treated cells.
8.
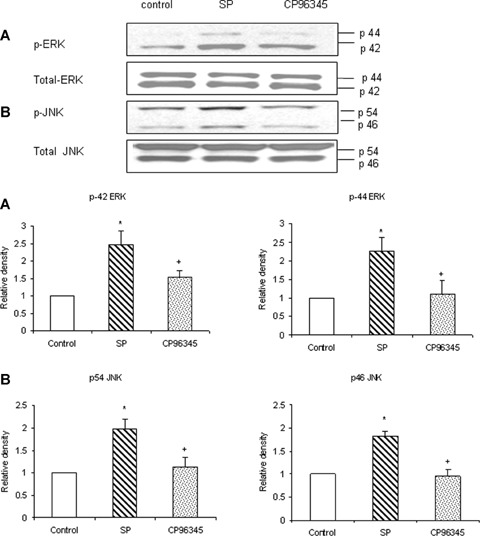
Substance P-NK1R interaction is involved in ERK1/2 and JNK activation. Freshly isolated pancreatic acini, obtained from three mice, were pre-incubated with 1 μM CP96345 for half an hour at 37°C followed by stimulation with 1 μM substance P for 45 min for ERK1/2 and JNK at 37°C. Cells were subsequently lysed, and cell proteins were subjected to Western blot analysis using antibodies against (A) phospho-ERK, total ERK1/2 (B) phospho-JNK, total JNK. Corresponding densitometric analysis of Western blot experiments from pancreatic acini. The results are representative of three independent experiments. Results shown are the means + SE. * P≤ 0.05 when compared to control, + P ≤0.05 when compared to substance P (SP).
Substance P-induced NFκB and AP-1 activation as well as chemokine production are mediated through NK1R
The role of NK1R in substance P-induced NFκB and AP-1 activation and chemokine production was confirmed by pre-treating the cells with 1 μM of CP96345 followed by stimulation with 1 μM of substance P for 45 min. The cells were used for nuclear extraction to determine NFκB and AP-1 activation, whereas the supernatant obtained was used for detection of chemokines MCP-1, MIP-1α and MIP-2 by ELISA Our results, in Figure 9, show that pre-treatment with selective antagonist CP96345 significantly inhibited substance P-induced NFκB and AP-1 activation in pancreatic acinar cells when compared to substance P-only treated cells. As shown in Figure 9, CP96345 significantly attenuated substance P-induced MCP-1, MIP-1α, and MIP-2 synthesis when compared to substance P-only treated cells.
9.
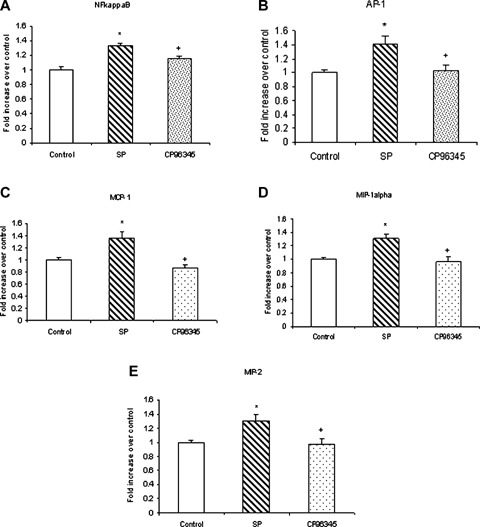
NK1R is involved in substance P-induced NFκ B and AP-1 activation as well as MCP-1, MIP-1α and MIP-2 production. Freshly isolated pancreatic acini, obtained from three mice, were pre-incubated with 1 μgM CP96345 for half an hour followed by stimulation with 1 μM substance P for 45 min. Acini were separated from incubation medium by centrifugation. The pellet was used for (A) NFκB and (B) AP-1 extraction and NFκB (p65) and AP-1 (c-Jun) DN-Abinding assays were carried out. The supernatant was used to measure (C) MCP-1, (D) MIP-1α and (E) MIP-2 levels by ELISA. The results are representative of three independent experiments. Results shown are the means + SE. * P≤0.05 when compared to control, + P≤0.05 when compared to substance P (SP).
Discussion
Previously, we have shown that the substance PNKIR related pathway is involved in mediating the pro-inflammatory effect of H2S in mouse pancreatic acinar cells [13] and that substance P induces chemokine production in pancreatic acinar cells [11]. In the present study, we investigated substance PNKIR induced signalling cascades that lead to increased production of chemokines MCP-1, MIP-1α and MIP-2 in pancreatic acini. Substance P has been shown to stimulate a number of intracellular signalling molecules, such as MAPK members. Ligand binding to NK1R activates MAPKs [14]. There are three well-characterized subfamilies of MAPKs that control an array of physiological processes. It is generally believed that ERKs function in the control of cell division, JNKs are critical regulators of transcription and p38 MAPKs are activated by inflammatory cytokines and environmental stresses. In the present study, we have focussed on the MAPKs ERK1/2 and JNK, as p38 was not activated upon substance P stimulation in our model of isolated acinar cells.
Transcription factor AP-1 is composed of a mixture of heterodimeric complexes of proteins derived from the Fos and Jun families. Only Jun proteins can form transcriptionally active homodimers with AP-1 members or heterodimers with cAMP response elementbinding/activating transcription factor (CREB/ATF) members, to bind the cAMP response element (CRE) (5′-TGACGTCA-3′). Phosphorylation of AP-1 family members by kinases is required for transactivation activity. The transcriptional activity of c-Jun is stimulated by phosphorylation at Ser-63 and -73 within its N-terminal activation domain [15–18]. It was reported that the serine/threonine kinase activity, termed JNK, binds to c-Jun and specifically phosphorylates its Nterminal sites. However, there were also reports that the N-terminal sites of c-Jun are phosphorylated in vitro by ERK1 and ERK2 [16, 19]. Most cells express two isoforms of JNK, 46 and 55 kD in size and termed JNK1 and JNK2, that are highly similar in their modes of regulation [20, 21]. Like activation of ERK1 and ERK2 [22], activation of JNK requires its phosphorylation on adjacent Thr and Tyr residues [23].
In this study, we show that substance P induces JNK phosphorylation which is in accord with AP-1 (c-Jun) activation as well as increased synthesis of MCP-1, MIP-1α and MIP-2. To confirm that production of these chemokines is mediated through the JNK/AP-1(c-Jun) signalling pathway, pancreatic acini were pre-treated with SP600125 followed by stimulation with substance P. SP600125 is a selective inhibitor of JNK. In cells, it dose dependently inhibits the phosphorylation of c-Jun. It competitively and reversibly inhibits JNK1, 2 and 3 and has been shown to have less inhibitory potency on ERK2, p38b and a range of other kinases [24, 25]. Our data shows that SP600125 attenuated the activation of AP-1 (c-Jun) as well as the production of MCP-1, MIP-1α and MIP-2. Moreover, we found that SP600125 attenuated NFκB activation at concentration of 25 μM and above. We also confirmed with the use of Western analysis that SP600125 indeed blocked phosphorylation of JNK in pancreatic acini. Total JNK was used as a control. Our data suggests that substance P-induced chemokine production occurs not only through the classic JNK/AP-1 (c-Jun) pathway, but also through JNK-mediated NFκB activation.
Previously, it has been established that MEKK1 induced activation of both IKK-β and IKK-α leading to NFκB activation [26, 27]. Furthermore ERK1/2 activity and phosphorylation have been associated with degradation of IκB protein leading to NFκB activation. Moreover, it has been shown that MEK-1 and ERK-1 act as intermediates in the cascade of events that regulate AP-1 and NFκB activation. NFκB is located in the cytoplasm in an inducible form, in which the heterodimer is complexed to the inhibitory subunit, IκBα. Upon stimulation of the cell, IκBα is rapidly phosphorylated and degraded; the released nucleophilic heterodimer then moves to the nucleus. Both p50 and p65 contribute to NFκB DNA binding, but only the p65 subunit is responsible for transactivation. Once in the nucleus, NFκB binds to its consensus decameric sequence located in the promoter region of several genes involved in the pro-inflammatory response, encoding various immunoreceptors, cell adhesion molecules, cytokines and chemokines [28–30].
In the present study, we investigated the possible involvement of ERK 1/2 in mediating substance P-induced chemokine synthesis in pancreatic acinar cells, in particular their role in increasing NFκB and AP-1 (c-Jun) activity. We demonstrated that substance P induces ERK 1/2 activation which is in line with NFκB activation as well as with the increased synthesis of chemokines MCP-1, MIP-1α and MIP-2. The ERK pathway-specific inhibitor PD98059 dose dependently inhibited NFκB activation and chemokine production. PD98059 is a potent, selective and cell-permeable inhibitor of MAP kinase kinase. It selectively inhibits the MAPK-activating enzyme, (MEK), without significant inhibitory activity of MAPK itself. Inhibition of MEK by PD98059 prevents activation of MAPK and subsequent phosphorylation of MAPK substrates both in vitroand in intact cells [31, 32]. Here, we found that PD98059 blocked AP-1(c-Jun) activation. Furthermore using Western analysis, we confirmed that PD98059 effectively blocked phosphorylation of ERK1/2 in pancreatic acinar cells.
We have earlier shown that pre-treatment of pancreatic acini with NFκB essential modulator (NEMO)- binding domain peptide (NBD), an NFκB inhibitor, completely attenuated the chemokine synthesis induced by substance P. This shows that the increase in chemokine synthesis induced by substance P was specifically dependent on NFκB activation [11]. Taken together, these results demonstrate that substance P-induced production of MCP-1, MIP-1α and MIP-2 is mediated not only through the classic signalling pathways namely ERK1/2- NFκB and JNK/AP-1 (c-Jun), but also through a cross-talk between the two classic signalling pathways. Similar observations have been made with caerulein/CCK treatment on pancreatic acini. It has been reported in previous studies that caerulein/CCK rapidly activates MAP Kinases, NFκB and the downstream chemokine production in pancreatic acinar cells [11, 33–36].
Pancreatic acini have previously been used as a model cell type to study the mechanisms of protein secretion, hormone action and stimulus-secretion coupling [37]. We have used primary cultures of acinar cells isolated from mouse pancreatic tissue because there are no cell lines that authentically reproduce the function of the acinar cell. It is quite likely that the stress associated with pancreatic acini preparation may affect the signalling pathways in these cells. This explains why very high concentration (100 μM), but not low (10 μM) of PD98059 or SP600125 had lower chemokine production when compared to control. However, 100 μM PD98059 or SP600125 had no effect on pancreatic acinar cell viability. It is probable that at very high concentration (100 μM) these inhibitors are able to inhibit the activation in signalling pathways due to environmental stresses. We therefore suggest that the most appropriate concentrations for the inhibitors are the low doses of 10 μM or 25 μM for PD98059 and 10 μM or 30 μM for SP600125.
To further understand the molecular mechanism and to show that substance P-induced chemokine production was indeed mediated by substance P, and not by some non-specific effects upon acinar cells isolation, we pre-treated the cells with the selective NK1R antagonist, CP96345. In the present study, CP96345 decreased the activation of ERK1/2, JNK, NFκB and AP-1 mediated chemokine production, hence showing that substance P-induced chemokine production is dependent on NK1R in pancreatic aci-nar cells. Based on our results, we proposed the mechanism by which substance P induces chemokine production in mouse pancreatic acinar cells (Fig. 10).
10.
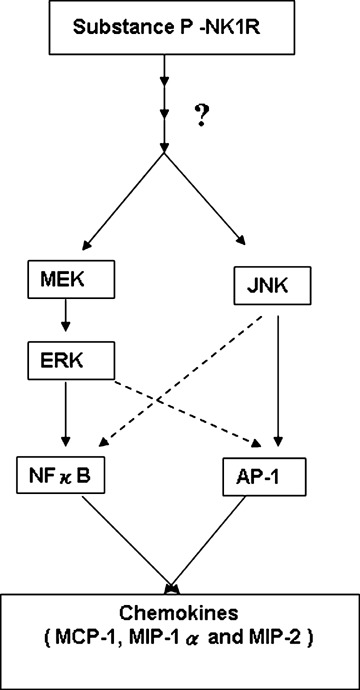
A schematic representation of the signalling cascade that mediates substance P-NK1-R induced chemokine production in pancreatic acinar cells. Substance P (SP) induces activation of MAP kinases ERK and JNK in pancreatic acinar cells. Moreover, substance P-induced phosphorylation of ERK and JNK mediates the activation of transcription factors NFκB and AP-1, thus resulting in increased secretion of pro-inflammatory mediators MCP- 1, MIP-1α and MIP-2 in acinar cells. Substance P-induced ERK1/2, JNK, NFκB, AP-1 activation and chemokine synthesis are depended on NK1R.
To our knowledge, this is the first study that shows the involvement of ERK1/2, JNK, AP-1(c-Jun) and NFκB in substance P-induced chemokine production in pancreatic acini. The present study provides evi-dence that activation of p42/p44 MAPK pathways by substance P is necessary for production of MCP-1, MIP-1α and MIP-2. Furthermore, JNK seems to be the other MAPK required for substance P-induced chemokine production in these cells. These results show that substance P-induced activation of both p42/p44 MAPK and JNK cascades are essential for NFκB and AP-1 activation, resulting in increased production of chemokines MCP-1, MIP-1α and MIP-2 in mouse pancreatic acini. The current study gives us an insight into the mechanism by which substance P contributes to the inflammatory responses in acute pancreatitis.
Acknowledgments
The authors thank Dr Zhi Liang for his comments and Abel Damien Ang and Mei Leng Shoon for assistance with the experiments. This work was supported by Academic Research Fund Grant No. R-184-000-054- 112 and Biomedical Research Council Grant No. R-184-000-069-305.
References
- 1.Bowden JJ, Garland AM, Baluk P, Lefevre P, Grady EF, Vigna SR, Bunnett NW, McDonald DM. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci USA. 1994;91:8964–8. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thurgston G, Baluk P, Hirata A, McDonald DM. Permeability-related changes revealed at endothelial cell borders in inflamed venules by lectin binding. Am J Physiol. 1996;271:H2547–62. doi: 10.1152/ajpheart.1996.271.6.H2547. [DOI] [PubMed] [Google Scholar]
- 3.Sjodin L, Gylfe E. A selective and potent antagonist of substance P receptors on pancreatic acinar cells. Biochem Int. 1992;27:145–53. [PubMed] [Google Scholar]
- 4.Jensen RT, Jones SW, Lu YA, Xu JC, Folkers K, Gardner JD. Interaction of substance P antagonists with substance P receptors on dispersed pancreatic acini. Biochim Biophys Acta. 1984;804:181–91. doi: 10.1016/0167-4889(84)90148-4. [DOI] [PubMed] [Google Scholar]
- 5.Patto RJ, Vinayek R, Jensen RT, Gardner JD. Carbachol does not down-regulate substance P receptors in pancreatic acini. Pancreas. 1992;7:447–52. doi: 10.1097/00006676-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C, Steer ML. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci USA. 1998;95:4760–5. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia M, Slavin J, Cao Y, Basbaum AI, Neoptolemos JP. Preprotachykinin-A gene deletion protects mice against acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2003;284:G830–6. doi: 10.1152/ajpgi.00140.2002. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia M, Brady M, Kang YK, Costello E, Newton DJ, Christmas SE, Neoptolemos JP, Slavin J. MCP-1 but not CINC synthesis is increased in rat pancreatic acini in response to cerulean hyperstimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G77–85. doi: 10.1152/ajpgi.00031x.2002. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia M, Ramnath RD, Chevali L, Guglielmotti A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1259–65. doi: 10.1152/ajpgi.00435.2004. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Bhatia M. Blockade of neurokinin 1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2007;292:G143–53. doi: 10.1152/ajpgi.00271.2006. [DOI] [PubMed] [Google Scholar]
- 11.Ramnath RD, Bhatia M. Substance P treatment stimulates chemokine synthesis in pancreatic acinar cells via the activation of NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1113–9. doi: 10.1152/ajpgi.00177.2006. [DOI] [PubMed] [Google Scholar]
- 12.Parry GC, Mackman N. A set of inducible genes expressed by activated human monocytic and endothelial cells contain kappa B-like sites that specifically bind c-Rel-p65 heterodimers. J Biol Chem. 1994;269:20823–5. [PubMed] [Google Scholar]
- 13.Tamizhselvi R, Moore PK, Bhatia M. Hydrogen sulfide acts as a mediator of inflammation in acute pancreatitis: in vitro studies using isolated mouse pancreatic acinar cells. J Cell Mol Med. 2007;2:315–26. doi: 10.1111/j.1582-4934.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo W, Sharif TR, Sharif M. Substance P-induced mitogenesis in human astrocytoma cells correlates with activation of the mitogen-activated protein kinase signalling pathway. Cancer Res. 1996;56:4983–99. [PubMed] [Google Scholar]
- 15.Binetruy B, Smeal T, Karin M. Ha-Ras augments c- Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991;351:122–7. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- 16.Pulverer BJ, Kyriakis JM, Avruch J, Nikolakaki E, Woodgett Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:670–4. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 17.Smeal T, Binetruy B, Mercola D, Birrer M, Karin M. Oncogenic and transcriptional cooperation with Ha- Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991;354:494–6. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- 18.Smeal T, Binetruy B, Mercola D, Grover-Bardwick A, Heidecker G, Rapp UR, Karin M. Oncoproteinmediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol Cell Biol. 1992;12:3507–13. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulverer BJ, Hughes K, Franklin CC, Kraft AS, Leevers SJ, Woodgett Co-purification of mitogen- activated protein kinases with phorbol esterinduced c-Jun kinase activity in U937 leukaemic cells. Oncogene. 1993;7:407–15. [PubMed] [Google Scholar]
- 20.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein and UV responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–48. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 21.Su B, Jacinto E, Hibi M, Kallunk T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–36. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 22.Ahn NG, Seger R, Krebs EG. The mitogen-activated protein kinase activator. Curr Opin Cell Biol. 1992;4:992–9. doi: 10.1016/0955-0674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- 23.Drijard B, Hibi M, Wu I-H, Barrett T, Su B, Karin M, Davis R. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–37. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 24.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin M, Yan C, Boyd D. An inhibitor of c-jun aminoterminal kinase (SP600125) represses c-Jun activation, DNA-binding and PMA-inducible 92-kDa type IV collagenase expression. Biochim Biophys Acta. 2002;1589:311–6. doi: 10.1016/s0167-4889(02)00195-7. [DOI] [PubMed] [Google Scholar]
- 26.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh S, May MJ, Kopp EB. NFκB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 28.Akira S, Kishimoto T. NF-IL6 and NF-B in cytokine gene regulation. Adv Immunol. 1997;65:1–46. [PubMed] [Google Scholar]
- 29.Grimm S, Baeuerle PA. The inducible transcription factor NF-kB: structure-function relationship of its protein subunits. Biochem J. 1993;290:297–308. doi: 10.1042/bj2900297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baeuerle PA, Baichwal VR. NFκB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111–37. [PubMed] [Google Scholar]
- 31.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–94. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 32.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–9. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabrowski A, Grady T, Logsdon CD, Williams JA. Jun kinases are rapidly activated by cholecystokinin in rat pancreas both in vitroand in vivo. J Biol Chem. 1996;271:5686–90. doi: 10.1074/jbc.271.10.5686. [DOI] [PubMed] [Google Scholar]
- 34.Duan RD, Williams JA. Cholecystokinin rapidly activates mitogen-activated protein kinase in rat pancreatic acini. Am J Physiol. 1994;267:G401–8. doi: 10.1152/ajpgi.1994.267.3.G401. [DOI] [PubMed] [Google Scholar]
- 35.Duan RD, Zheng CF, Guan KL, Williams JA. Activation of MAP kinase kinase (MEK) and Ras by cholecystokinin in rat pancreatic acini. Am J Physiol. 1995;268:G1060–5. doi: 10.1152/ajpgi.1995.268.6.G1060. [DOI] [PubMed] [Google Scholar]
- 36.Perides G, Sharma A, Gopal A, Tao X, Dwyer K, Ligon B, Steer ML. Secretin differentially sensitizes rat pancreatic acini to the effects of supramaximal stimulation with caerulein. Am J Physiol Gastrointest Liver Physiol. 2005;289:G713–21. doi: 10.1152/ajpgi.00519.2004. [DOI] [PubMed] [Google Scholar]
- 37.Williams JA. Regulation of pancreatic acinar cell function. Curr Opin Gastroenterol. 2006;22:498–504. doi: 10.1097/01.mog.0000239863.96833.c0. [DOI] [PubMed] [Google Scholar]


