Abstract
Bone is the most common site of breast cancer metastasis. Although it is widely accepted that the microenvironment influences cancer cell behavior, little is known about breast cancer cell properties and behaviors within the native microenvironment of human bone tissue.We have developed approaches to track, quantify and modulate human breast cancer cells within the microenvironment of cultured human bone tissue fragments isolated from discarded femoral heads following total hip replacement surgeries. Using breast cancer cells engineered for luciferase and enhanced green fluorescent protein (EGFP) expression, we are able to reproducibly quantitate migration and proliferation patterns using bioluminescence imaging (BLI), track cell interactions within the bone fragments using fluorescence microscopy, and evaluate breast cells after colonization with flow cytometry. The key advantages of this model include: 1) a native, architecturally intact tissue microenvironment that includes relevant human cell types, and 2) direct access to the microenvironment, which facilitates rapid quantitative and qualitative monitoring and perturbation of breast and bone cell properties, behaviors and interactions. A primary limitation, at present, is the finite viability of the tissue fragments, which confines the window of study to short-term culture. Applications of the model system include studying the basic biology of breast cancer and other bone-seeking malignancies within the metastatic niche, and developing therapeutic strategies to effectively target breast cancer cells in bone tissues.
Keywords: Medicine, Issue 97, Metastatic niche, bone microenvironment, breast cancer metastasis, human bone, osteotropism, ex vivo model, explant culture system, bioluminescence imaging
Introduction
The tumor microenvironment is widely recognized as a critical determinant of cancer cell behavior during breast cancer progression and metastatic spread1-3. The goal of the method presented here is to facilitate the study of breast cancer cells within the microenvironment of human bone tissue, the most frequent site of breast cancer metastasis4-6. Bone is comprised of mineralized and marrow compartments7-9, both of which harbor cells and secreted factors implicated in metastatic progression10,11. Although widely used in vivo mouse models facilitate systemic studies of the metastatic process12-15, cell interactions within the skeleton are not readily accessible for observation and direct perturbation. Model systems for studying and culturing mouse bone tissues and mouse marrow cells provide better access and have yielded many insights regarding crosstalk and mechanisms underlying breast cancer cell metastasis of the murine skeleton16-22. However, studies have suggested that there may be species-specific patterns of osteotropism for bone tissue23,24. These patterns could reflect inherent species-specific differences in bone tissue properties, differences resulting from altered bone marrow populations in immune-deficient mice11, and/or the relatively young age of mice used in experiments, all of which are likely determinants of the bone microenvironment.
In vitro approaches for studying breast cancer cells in human bone co-cultures have typically focused on specific bone cell types such as marrow-derived osteoblasts or stromal cells cultured as monolayers or within engineered 3-dimensional model systems25-35. Although 2-dimensional cell culture models have formed the mainstay of in vitro approaches for cancer research, it has long been recognized that cell behavior is fundamentally altered in monolayer culture systems18,36,37. This has led to the development of engineered microenvironments that mimic the complexity of 3-dimensional living tissues, including matrix- and scaffold-based models composed of natural materials such as collagen, or synthetic polymers seeded with specific cell types to create tissue-like microenvironments36-41. Engineered approaches have also included the use of bioreactor platforms to control and study the hormonal milieu of the microenvironment18,19,41-43. Although biomimetic models and bioreactors provide many elements of a complex tissue microenvironment in a controlled setting, and have been successfully applied to advance the study of breast cancer metastasis to bone18,19,35,42,43, engineered model systems do not generally incorporate the full spectrum of cell types and extracellular matrix components present within the native bone environment.
Until recently, breast cancer cells have not been studied within the native, intact, 3-dimensional microenvironment of human bone tissues. We recently reported the development of a co-culture model using human bone tissue fragments isolated from total hip replacement (THR) surgery specimens44. These specimens harbor both the mineralized and marrow compartments necessary to study mechanisms underlying micro-metastasis in short term culture. In previous work we established proof-of-principle for using bioluminescence imaging (BLI) to monitor the proliferation of luciferase-expressing breast cancer cells (MDA-MB-231-fLuc) co-cultured with bone fragments44. Here we present detailed experimental protocols for studying breast cell cancer proliferation, colonization, and migration within the context of the native microenvironment using human bone tissue explants.
First we present a protocol for co-culturing breast cancer cells adjacent to bone fragments to measure breast cell proliferation using BLI. In this section, breast cell suspensions are seeded as cell spots in 6-well plates adjacent to bone fragments, which are immobilized by pieces of bone wax. Control wells contain bone wax, but no bone fragment. Once cell spots attach, medium is added and the plate is cultured for 24 hr, after which bioluminescent signal intensity (associated with cell number) is measured with BLI. In the next step we present methods for co-culturing breast cancer cells seeded directly onto bone fragments to study colonization and proliferation. Here the breast cell suspensions are pipetted directly onto the bone tissue fragments, which are monitored in culture by BLI and fluorescence microscopy over time to track colonization and cell number. In this method, the marrow compartment can be flushed from the bone fragments at any time point for analysis of colonized breast cancer cells by flow cytometry, or marrow viability assays.
In addition, we describe two different approaches for measuring breast cancer cell migration. In the first method steps are outlined for measuring migration toward bone tissue culture supernatants. In this protocol the breast cancer cells are seeded onto the inner, upper surfaces of Transwell insert membranes with an 8 µm pore size (as shown in Figure 1A). The inserts are then placed into a receiver plate with wells containing bone tissue supernatant or control medium. Over the course of a 20 hr incubation period, small numbers of breast cancer cells migrate through the insert membranes down into the lower tissue culture wells where they attach for detection by BLI. In the second migration method the breast cancer cells are seeded onto the lower, outer surfaces of Transwell insert membranes. Bone tissue fragments are placed into the insert cups and the breast cancer cells migrate up through the membranes to colonize the bone fragments (as shown in Figure 1B), which are then imaged using BLI.
Protocol
Femoral heads were collected from patients undergoing elective THR surgery in the Department of Orthopaedic Surgery at the Stanford University School of Medicine. All tissues were collected as de-identified specimens in accordance with regulations of the Stanford University Research Compliance Office.
1. Selecting a Breast Cancer Cell Line(s)
Obtain or transfect the desired breast cancer cell line(s) with a luciferase-GFP reporter construct. The following experiments were performed using the MDA-MB-231 and MCF-7 breast cancer cell lines engineered for the stable expression of firefly luciferase (fLuc) and enhanced green fluorescent protein (EGFP) by transfection with the Sleeping Beauty transposon plasmid pKT2/LuBiG and the transposase plasmid pK/hUbiC-SB1145,46. NOTE: The transfected cell lines are referred to as MDA-MB-231-fLuc-EGFP, and MCF-7-fLuc-EGFP.
2. Isolating Tissue Fragments from Femoral Heads
Following THR surgery, collect the discarded femoral head in a sterile container filled with physiological saline. Transfer the specimen to the laboratory and process it within 1-3 hr of surgery. Note: if there is a delay in processing, place the specimen at 4 °C.
Prepare a working area by placing the following items on an absorbent mat in a laminar flow tissue culture hood: sterile gloves, surgical rongeur, forceps, beaker with 70% isopropanol to rinse instruments, and tissue culture vessels as stipulated below for each type of experiment.
Wearing a sterile surgical glove to hold the round femur head in one hand, use the rongeur in the other hand to extract trabecular bone fragments of desired size (~2-5 mm) from the exposed upper shaft of the femur. Transfer bone fragments into the culture vessel (e.g., 6-, 12-, or 24-well tissue culture plate or migration chamber), depending on the type of proliferation, colonization or migration experiment, as described in steps 3, 4 or 5. NOTE: Bone tissue fragments should be harvested as described above after completing the initial steps for each specific proliferation, colonization or migration assay. Fragments will range in size as shown in Figure 2. Fragments can be weighed before or after experiments for the purpose of standardization.
3. Co-culturing Breast Cancer Cells Adjacent to Bone Fragments to Measure Breast Cell Proliferation Using BLI
Prior to the experiment, prepare a suspension containing 2 x 105 breast cancer cells/50 µl, and prepare plugs of bone wax for immobilizing bone fragments in culture. Cut a P200 micropipette tip into 3 pieces and use the narrow end of the largest piece in a “cookie cutter” manner to cut small (~35 mg) pieces of bone wax. Use the wide end of the smallest piece to eject the bone wax plugs into a Petri dish for storage.
Place a piece of bone wax at the 12 o’clock position of each well and gently press down with a sterile-gloved index finger.
Pipette 50 µl of cell suspension containing 1 x 105 breast cancer cells in DMEM-10% FBS + Pen-Strep in the center of each well of a 6-well plate. (Alternatively, seed cells in a dispersed pattern if desired.) Place the plate in a 37 °C tissue culture incubator for 45 min to promote cell attachment.
Place 1 bone fragment onto 1 piece of bone wax for each experimental well and apply gentle pressure with the rongeur to secure it. Perform experiments in triplicate such that 3 bone fragments from a given THR specimen are placed into the top three wells, while the bottom three wells contain bone wax only as controls.
Using a 5 ml pipette, gently and slowly deliver 5 ml of DMEM-10% FBS to the side of each well to avoid dislodging the breast cells or bone fragments. Place the plate in a 37 °C tissue culture incubator for 20-24 hr.
To perform bioluminescence imaging (BLI) remove the plate from the incubator and add luciferin substrate (to achieve a concentration of 300 µg/ml) to each well. Image the plate immediately on an IVIS Imaging Platform using the following parameters: f-stop of 1, small binning, exposure time of 1 sec, level D. Measure the signal intensity of each well as average radiance (photons/second/square centimeter/steradian).
Use imaging software to define Regions of Interest (ROI) to quantitate the average radiance for all experimental and control wells. Export average radiance values to an Excel sheet to perform statistics and generate graphs.
4. Co-culturing Breast Cancer Cells Seeded Directly Onto Bone Fragments to Study Colonization and Cell Number
Use forceps to place bone fragments directly into the empty wells of a 24-well tissue culture plate (1 fragment/well).
Pipette a 50 µl cell suspension droplet of DMEM-10% FBS containing 1 X 103-6 breast cancer cells directly on top of each bone fragment. Place the plate in a 37 °C tissue culture incubator for 45 min to promote cell attachment. Gently add 1-2 ml DMEM-10% FBS into the side of each well such that the fragment is entirely submerged in medium. Incubate the plate in a 37 °C tissue culture incubator for 20-24 hr.
After incubation, use forceps to transfer each bone tissue fragment to a fresh 24-well plate containing 1 ml DMEM-10% FBS in each well. To perform BLI, follow steps 3.6-3.7 above. Additionally, fragments may be viewed using a fluorescence microscope, or flushed to obtain the marrow population for analyses of breast cancer cell numbers and properties as described in step 6.
To prepare the intact fragments for qualitative evaluation by fluorescence microscopy following BLI, pipette 5 µl of fluorescent bisphosphonate labeling solution (prepared according to the manufacturer’s instructions) into each well to label the mineralized spicules of the bone fragments. Incubate the plate in the tissue culture incubator for another 24 hr.
Following 24 hr of culture in fluorescent bisphosphonate labeling solution, use forceps to transfer the fragments to tissue culture plates containing phenol red-free medium and view using a fluorescence microscope configured to image GFP (485 nm) and bisphosphonate label (680 nm).
5. Measuring Migration of Breast Cancer Cells to Bone Tissue Fragments and Cultured Bone Fragment Supernatants
- Migration toward bone tissue culture supernatant. NOTE: Perform experiments in triplicate such that 3 fragments from a given THR specimen are used to generate 3 supernatants. For each experiment, migration is compared across three wells containing bone supernatants vs. three wells containing control medium only.
- Generate bone tissue supernatants by placing bone fragments into the wells of a 12-well plate containing 2.2 ml DMEM-10% FBS per well. Culture in a 37 °C tissue culture incubator for 24 hr. Withdraw the medium at 24 hr and replace with 2.2 ml fresh DMEM-10% FBS. Treat control wells containing DMEM-10% FBS the same way. NOTE: The medium is changed at 24 hr to remove factors secreted in response to tissue trauma resulting from surgery. Because bone tissue supernatants are generated in FBS-containing medium, wells containing FBS-containing medium only are included to control for the contributions of FBS to breast cancer cell migration.
- At 48 hr, collect 0.9 ml of each supernatant/control medium and pipette into the wells of a receiver plate. The inserts will later be placed into these wells. Incubate the receiver plate in the tissue culture incubator while seeding the inserts. Note: The remaining supernatant (~1 ml after evaporation) can be collected for analysis of secretome factors if desired44.
- To seed the Transwell inserts, place them into a standard, empty 24-well tissue culture plate. Pipette 350 µl cell suspension containing 1 x 105 breast cancer cells in DMEM-10% FBS onto the upper, inner membrane surface of each insert as shown in Figure 1A. Place the 24-well plate containing the seeded inserts into the 37 °C tissue culture incubator for 45 min to promote attachment.
- Once the cells have attached to the inserts, use forceps to transfer the inserts to the receiver plate according to the manufacturer’s instructions. Angle each insert when placing it into the receiver well to prevent bubble formation between the insert membrane and the receiver well supernatant. Incubate the receiver plate with inserts for 20 hr in a 37 °C tissue culture incubator. NOTE: Check the bottom side of the receiver plate for bubbles between the insert membrane and the receiver well supernatant. If bubbles are visible, remove the specific inserts and place them again into the receiver well until no bubbles are present.
- After 20 hr use forceps to remove the inserts from the receiver plate. Using the procedures in steps 3.6-3.7 perform BLI to image the cells that have migrated into and attached to the bottom of the receiver plate wells.
- Migration toward bone tissue fragments. NOTE: Perform experiments in triplicate such that 3 fragments from a given THR specimen are placed into 3 separate inserts, and 3 glass beads are placed into 3 separate inserts as controls.
- Prepare the receiver plate by pipetting 0.9 ml DMEM-10% FBS into each well. Place it into the tissue culture incubator while preparing the inserts.
- To seed the inserts, invert them on the surface of a plastic microfuge tube box that has a lid. Pipette a 50 µl cell suspension droplet of DMEM-10% FBS containing 1 x 105 breast cancer cells directly onto the outer, bottom surface of each insert membrane (which is facing up on the inverted inserts). Cover the microfuge tube box with the lid cracked open and transfer it to the 37 °C tissue incubator for 45 min to promote cell attachment.
- Transfer the microfuge tube box to the tissue culture hood, and use forceps to turn the seeded inserts right side up and transfer them into the wells of the receiver plate. Pipette 0.450 ml DMEM-10% FBS into each insert cup. Using forceps, transfer one bone tissue fragment (~3 mm) into each prepared insert cup. Optionally, use glass beads as negative controls in additional wells. Place the plate in a tissue culture incubator for 20 hr.
- To measure migration, prepare a standard 24-well tissue culture plate containing 1 ml DMEM-10% FBS per well. Remove the receiver plate from the incubator and use forceps to transfer the bone fragments and beads from each insert into separate wells of the standard plate. Add luciferin substrate (to achieve a final concentration of 300 µg/ml) to each well and perform BLI using the procedures in steps 3.6-3.7.
6. Additional Pre- and Post-experimental Analyses
- Analysis of flushed marrow.
- Transfer a bone fragment into a 70 µm cell strainer placed at the top of a 50 ml conical tube. Use a 10 ml syringe with a 25 G needle to vigorously flush the fragment with 10 ml of PBS to obtain a suspension of marrow cells in the conical tube. Centrifuge the cell suspension for 3 min at 300 x g and 21 °C, aspirate the supernatant and resuspend the pelleted cells in PBS or other desired solution for flow cytometry. NOTE: Resuspension volumes will vary depending on the size of the cell pellet after centrifugation, and application.
- For viability assays ~75-300 µl volumes of PBS are appropriate, and these suspensions can be analyzed using commercially available fluorescence based viability assays according to the manufacturer’s instructions, pipetted into a conventional hemocytometer, and counted using an inverted fluorescence microscope.
- For flow cytometric analysis or sorting based on detection of GFP-positive breast cancer cells, resuspend the cell pellet in 1 ml PBS containing 2% FBS, 2mM EDTA, and 10 mM HEPES such that the concentration is 1 x 106 cells/ml.
- H & E Staining and Immunohistochemistry.
- To process the fragments for hematoxylin & eosin (H&E) staining and/or immunohistochemistry, place the fragments in plastic cassettes labeled with a number 2 pencil, and submerge in a container filled with 10% buffered formalin for 24 hr.
- Process tissues for paraffin embedding, sectioning and staining using routine protocols that include a decalcification step to account for the mineralized spicules within each fragment.
Representative Results
Isolating Tissue Fragments from Femoral Heads
Femoral heads were collected from equal numbers of male and female patients (44-90 years of age) undergoing elective total hip replacement surgery in the Department of Orthopaedic Surgery at the Stanford University School of Medicine. Each discarded femoral head contains a small portion of the upper femur, which harbors trabecular bone tissue composed of mineralized spicules and marrow. This tissue is accessible through the surgically exposed cross section of the shaft (Figure 2A) using a surgical rongeur to extract small fragments. The histology of the extracted pieces is shown in Figure 2B, revealing the mineralized and marrow components. The appearance of the extracted pieces varies across femoral heads obtained from different patients, ranging from yellow to red, depending on the adipocyte content of the marrow as shown in Figure 2C. We demonstrate the use of these bone tissue fragments in short term assays to measure dynamic interactions with breast cancer cells engineered for luciferase and EGFP expression.
Co-culturing Breast Cancer Cells adjacent to Bone Fragments to Measure Breast Cell Proliferation using Bioluminescence Imaging
The culture plate setup for co-culturing MDA-MB-231-fLuc-EGFP breast cancer cells adjacent to immobilized bone fragments to measure breast cell proliferation using BLI is shown in Figure 3A. BLI of the plate at 24 hr is illustrated in Figure 3B, and BLI signal from 3 experiments, demonstrating enhanced cell proliferation in the presence vs. absence of bone fragments is shown in Figure 3C.
Co-culturing Breast Cancer Cells Seeded directly onto Bone Fragments to Study Colonization and Cell Number
Colonization of MCF-7-fLuc-EGFP cells seeded directly onto bone tissue fragments as measured via BLI at 24 hr is illustrated in Figure 4A, demonstrating decreased signal associated with declining seeding density. A directly-seeded fragment is shown at 48 hr in Figure 4B, after labeling with fluorescent biphosophonate reagent, as imaged by fluorescence microscopy to reveal colonization of the breast cancer cells within the mineralized and marrow compartments. These colonization patterns are also revealed in directly-seeded fragments processed post-experimentally for immunohistochemical staining with cytokeratin antibody (Figures 4C and 4D).
Measuring Migration of Breast Cancer Cells to Bone Tissue Fragments and Cultured Bone Fragment Supernatants
Migration of MDA-231-fLuc-EGFP cells across porous migration membranes toward bone tissue culture supernatants and into bone fragments is detected with BLI as shown in Figure 5. A robust migration pattern toward culture supernatants derived from three fragments of a given THR specimen is shown in Figure 5A, which depicts increased migration into wells containing bone tissue supernatant vs. control medium. Although not all migration patterns are this robust, migration toward bone supernatants is typically greater than toward control medium in most experiments. A typical migration pattern into bone fragments from a given THR specimen is shown in Figure 5B.
Analysis of Flushed Marrows
In addition, we illustrate that marrow cells can be flushed from colonized fragments, and that flushed breast cancer cells can be detected among these cells by flow cytometry (Figure 6B), BLI (Figure 6C), and/or fluorescence microscopy (Figure 6D).
Measuring Viability
Marrow cells can also be flushed from fragments to determine marrow cell viability immediately following collection, and at various time points during experiments. Typical viabilities for marrow cells flushed from bone tissue fragments from 4 THR specimens immediately after collection and after 24, 48, 72 hr, and 6 days of culture in DMEM-10%FBS are shown in Figure 7.
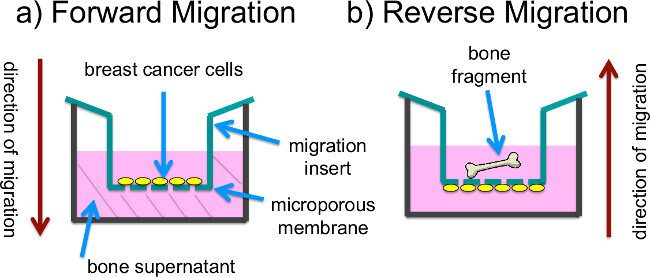
Figure 1: Set up of migration experiments. (A) In “forward migration” experiments, the receiver plate wells are filled with previously generated bone tissue culture supernatants. Breast cancer cells that are seeded onto the inner, upper surfaces of Transwell membranes in insert cups containing DME-10% FBS migrate through the membranes and attach to the bottom of the wells for detection by BLI. (B) In “reverse migration” experiments, the receiver plate wells are filled with DME-10% FBS and bone fragments are placed into the insert cups, which also contain DME-10% FBS. Breast cancer cells are seeded onto the outer, lower surface of the insert membranes, and migrate through the membranes to colonize the fragments, which are transferred to fresh plates for BLI.
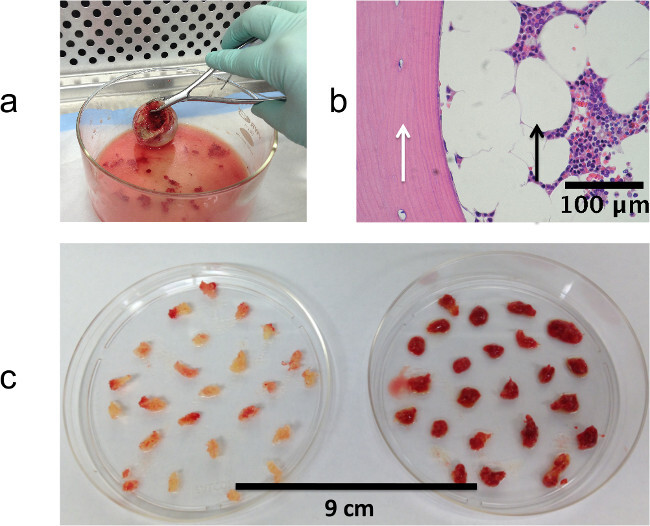 Figure 2: Isolating tissue fragments from femoral heads. (A) Extracting fragments of trabecular bone using a surgical rongeur. (B) Histology of fragment fixed immediately after extraction from femur tissue showing mineralized (white arrow) and marrow (black arrow) compartments. (C) Trabecular bone fragments extracted from two different femoral heads demonstrating variation in red vs. yellow marrow content. (A) and (B) reproduced with permission44.
Figure 2: Isolating tissue fragments from femoral heads. (A) Extracting fragments of trabecular bone using a surgical rongeur. (B) Histology of fragment fixed immediately after extraction from femur tissue showing mineralized (white arrow) and marrow (black arrow) compartments. (C) Trabecular bone fragments extracted from two different femoral heads demonstrating variation in red vs. yellow marrow content. (A) and (B) reproduced with permission44.
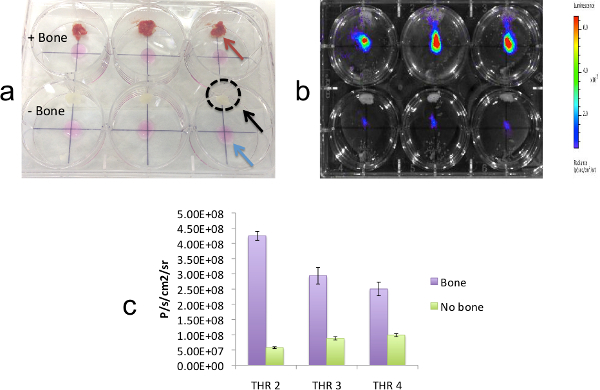 Figure 3: Co-culturing breast cancer cells adjacent to bone fragments to measure breast cell proliferation using bioluminescence imaging. (A) Culture plate setup for co-culturing MDA-MB-231-fLuc-EGFP breast cancer cells adjacent to immobilized bone fragments, as indicated by arrows: cell spots (blue), bone wax (black), and bone fragment (red). The position of the bone wax is also noted by a black dotted circle. The plate is shown before addition of medium. The top 3 wells contain bone fragments tethered to bone wax and the bottom three wells contain bone wax only. (B) BLI of the plate at 24 hr. (C) Quantitation of BLI signals for MDA-MB-231-fLuc-EGFP cells growing in the presence (purple bars) vs. absence (green bars) of fragments from three THR specimens cultured as described. For each THR specimen, bars represent average BLI values measured across 3 fragment-containing - vs. 3 control wells. This result demonstrates higher levels of proliferation in the presence vs. absence of bone fragments. Error bars represent standard error (p <0.01). Reproduced with permission44 .
Figure 3: Co-culturing breast cancer cells adjacent to bone fragments to measure breast cell proliferation using bioluminescence imaging. (A) Culture plate setup for co-culturing MDA-MB-231-fLuc-EGFP breast cancer cells adjacent to immobilized bone fragments, as indicated by arrows: cell spots (blue), bone wax (black), and bone fragment (red). The position of the bone wax is also noted by a black dotted circle. The plate is shown before addition of medium. The top 3 wells contain bone fragments tethered to bone wax and the bottom three wells contain bone wax only. (B) BLI of the plate at 24 hr. (C) Quantitation of BLI signals for MDA-MB-231-fLuc-EGFP cells growing in the presence (purple bars) vs. absence (green bars) of fragments from three THR specimens cultured as described. For each THR specimen, bars represent average BLI values measured across 3 fragment-containing - vs. 3 control wells. This result demonstrates higher levels of proliferation in the presence vs. absence of bone fragments. Error bars represent standard error (p <0.01). Reproduced with permission44 .
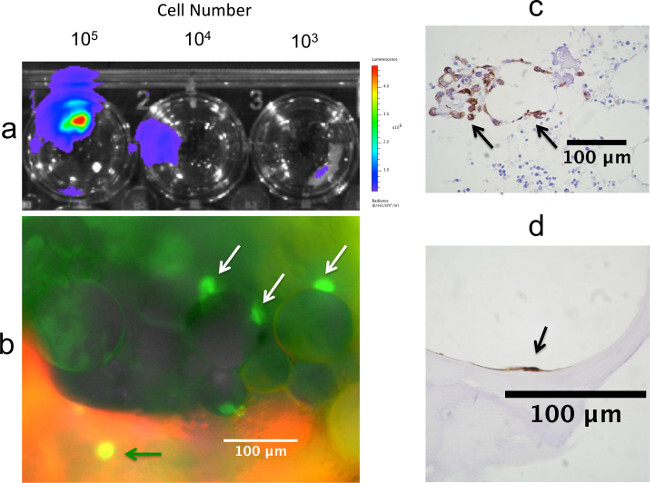 Figure 4: Detection of breast cancer cells seeded directly onto bone fragments. (A) Colonization of MCF-7-fLuc-EGFP cells seeded directly onto bone tissue fragments as detected by BLI at 24 hr, showing decreased signal associated with declining seeding density. (B) Directly-seeded fragment at 48 hr post seeding, and 24 hr post labeling with fluorescent biphosophonate reagent. Fluorescence microscopy reveals EGFP+ breast cancer cells associated with a mineralized spicule (green arrow), and with adipose cells in the marrow compartment (white arrows). These colonization patterns are also revealed in directly-seeded fragments processed for immunohistochemical staining with an AE1/AE3 pan-cytokeratin antibody following 72 hr of co-culture (C) and (D). In (C) cytokeratin-positive breast cancer cells, denoted by arrows, are observed adjacent to adipocytes in the marrow compartment. In (D) a cytokeratin-positive breast cancer cell is observed attached to a mineralized spicule. (C) and (D) reproduced with permission 44 .
Figure 4: Detection of breast cancer cells seeded directly onto bone fragments. (A) Colonization of MCF-7-fLuc-EGFP cells seeded directly onto bone tissue fragments as detected by BLI at 24 hr, showing decreased signal associated with declining seeding density. (B) Directly-seeded fragment at 48 hr post seeding, and 24 hr post labeling with fluorescent biphosophonate reagent. Fluorescence microscopy reveals EGFP+ breast cancer cells associated with a mineralized spicule (green arrow), and with adipose cells in the marrow compartment (white arrows). These colonization patterns are also revealed in directly-seeded fragments processed for immunohistochemical staining with an AE1/AE3 pan-cytokeratin antibody following 72 hr of co-culture (C) and (D). In (C) cytokeratin-positive breast cancer cells, denoted by arrows, are observed adjacent to adipocytes in the marrow compartment. In (D) a cytokeratin-positive breast cancer cell is observed attached to a mineralized spicule. (C) and (D) reproduced with permission 44 .
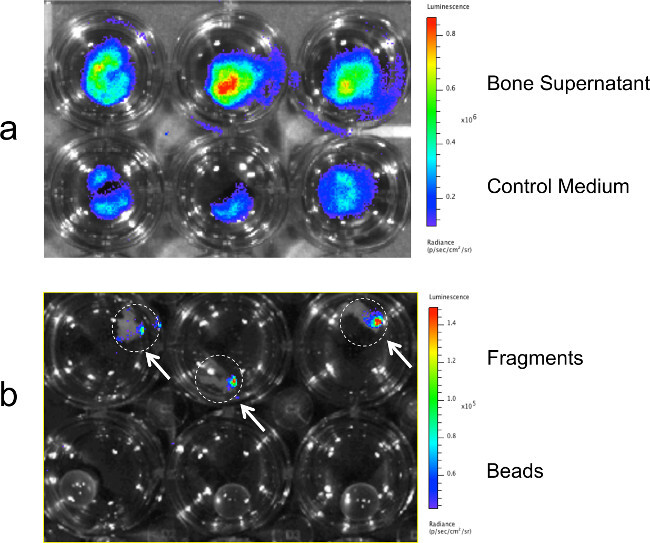 Figure 5: Measuring migration of breast cancer cells to bone tissue fragments and cultured bone fragment supernatants. Migration of MDA-MB-231-fLuc-EGFP cells across porous migration membranes toward bone tissue culture supernatants and into bone fragments as detected at 24 hr with BLI. (A) BLI signal revealing enhanced migration toward culture supernatants derived from a given THR specimen vs. control medium. (B) BLI signal demonstrating migration pattern of breast cancer cells into 3 bone fragments from a single THR specimen. Bone tissue fragments are denoted by white dotted circles. Control wells containing glass beads are shown on the bottom.
Figure 5: Measuring migration of breast cancer cells to bone tissue fragments and cultured bone fragment supernatants. Migration of MDA-MB-231-fLuc-EGFP cells across porous migration membranes toward bone tissue culture supernatants and into bone fragments as detected at 24 hr with BLI. (A) BLI signal revealing enhanced migration toward culture supernatants derived from a given THR specimen vs. control medium. (B) BLI signal demonstrating migration pattern of breast cancer cells into 3 bone fragments from a single THR specimen. Bone tissue fragments are denoted by white dotted circles. Control wells containing glass beads are shown on the bottom.
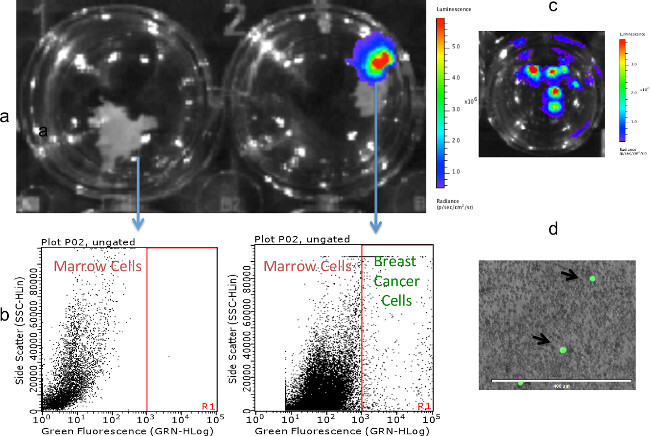 Figure 6: Detection of MDA-MB-231fLUC-EGFP cells in marrows flushed from colonized fragments. (A) Fragments without (left) and with (right) colonized breast cancer cells, as detected by BLI. (B) Analysis of marrows flushed from fragments (A) by flow cytometry. EGFP-positive breast cancer cells were detected in marrow flushed from the BLI-positive fragment, but not the BLI-negative fragment. (C) BLI detection of breast cancer cell colonies resulting from the culture of marrow flushed from a BLI-positive fragment. (D) Fluorescence microscopy of the marrow compartment flushed from a BLI-positive fragment, showing MDA-MB-231fLUC-EFGP cells in a field of marrow cells.
Figure 6: Detection of MDA-MB-231fLUC-EGFP cells in marrows flushed from colonized fragments. (A) Fragments without (left) and with (right) colonized breast cancer cells, as detected by BLI. (B) Analysis of marrows flushed from fragments (A) by flow cytometry. EGFP-positive breast cancer cells were detected in marrow flushed from the BLI-positive fragment, but not the BLI-negative fragment. (C) BLI detection of breast cancer cell colonies resulting from the culture of marrow flushed from a BLI-positive fragment. (D) Fluorescence microscopy of the marrow compartment flushed from a BLI-positive fragment, showing MDA-MB-231fLUC-EFGP cells in a field of marrow cells.
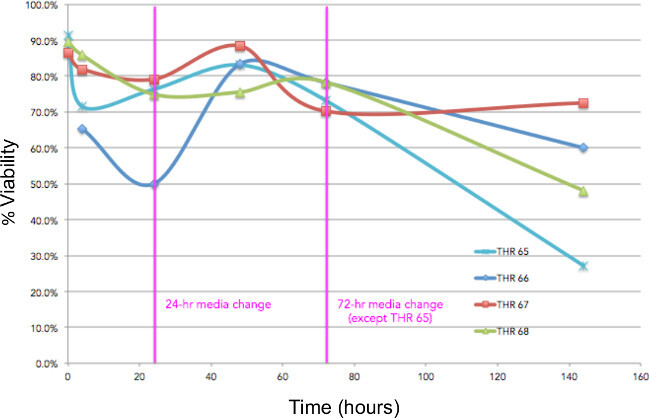 Figure 7: Measuring viability. Viabilities for marrow cells flushed from tissue fragments isolated from 4 THR specimens immediately after collection and at 24, 48, 72 hr, and 6 days of culture in DMEM-10%FBS. Medium was changed at 24 and 72 hr.
Figure 7: Measuring viability. Viabilities for marrow cells flushed from tissue fragments isolated from 4 THR specimens immediately after collection and at 24, 48, 72 hr, and 6 days of culture in DMEM-10%FBS. Medium was changed at 24 and 72 hr.
Discussion
Mehra et al. have previously described the postmortem collection and analysis of bone specimens from metastatic prostate cancer patients harvested at the time of autopsy, revealing high quality tissues and validating the use of human bone samples to study the metastatic process47. Here we have described protocols for collecting, processing and using human bone tissue fragments obtained from discarded femoral heads following THR surgery in a short-term co-culture system to study breast cell migration, colonization and proliferation. Approximately 300,000 hip replacement surgeries are performed annually in the U.S. (American Academy of Orthopaedic Surgeons; Orthoinfo.aaos.org), and these specimens are typically discarded. One of the most frequent indications for this surgery is osteoarthritis of the hip joint, leading to gradual breakdown of the cartilage surfaces covering the femoral head, which results in joint pain. During total hip replacement surgery a small portion of the upper femur is removed along with the femoral head. The femur is a common site of breast cancer metastasis48, and these specimens contain trabecular bone tissue, the site of metastatic breast cell colonization. Most patients who undergo hip replacement are 50-80 years old, an age range that brackets the years of peak breast cancer incidence. Thus, these tissues constitute a valuable resource for studying the breast cancer bone metastatic niche.
There are several critical steps and considerations associated with these techniques. First, a good quality surgical ronguer is essential for extracting the small trabecular bone fragments while holding the femoral head in the opposite, gloved hand. Forceps will not work because they are not sturdy enough to extract trabecular tissue containing mineralized spicules. Another important consideration in these assays pertains to breast cell line handling and passaging, particularly in regard to the migration assays. Long-term passage and/or variability in trypsinization procedures can lead to the sequential selection of more/less tenacious cell populations, potentially altering the migratory responses in experiments. Another issue relates to the standardization of experiments, given the variability of surgical tissues obtained from patients of different ages and disease states. To address this, we employ an intra-subject experimental design in which all variables are tested in triplicate (e.g., across three fragments/experimental wells vs. three fragments/control wells) using fragments from a given patient’s surgical specimen. Variables can be tested using this design on specimens from multiple patients, and analyzed for statistical significance using the paired T test for data averaged over replicates, and/or a within-subjects one-way ANOVA for data over replicates. And finally, a primary limitation of this method is the short window of viability during explant culture.
Our measurements revealed marrow viabilities of ~90% on the day of collection, in keeping with viability measurements obtained from bone marrow aspirates49. Over time, however, we observed a gradual drop over days two and three, with dramatic loss of viability by day 6. Although we have not measured the viability of the mineralized compartment, qualitative observations suggest that there is a similar decline in the viability of bone-lining osteoblasts and osteocytes, as seen in Figure 4D. Based on these experiments, we have limited our culture experiments to short term studies performed over 48 or 72 hr. Preliminary experiments (not included) indicated that viability may be improved using commercially available media formulated to sustain marrow cells. However we have not characterized the marrow cell populations in these experiments, and do not know if the improved viability results from the selected outgrowth of certain marrow cell subpopulations. The development of protocols to sustain and extend the viability of these explants will be important to advance this model system.
Although this model is limited to short time periods, its key advantages in relation to other methods include the following features: 1) intact 3-dimensional tissues harboring relevant cell types that constitute the human bone microenvironment, 2) accessibility to the bone microenvironment for monitoring and perturbing dynamic interactions, 3) high numbers of fragments per specimen for replicate experiments, and 4) low cost compared to animal models.
While we have described assays for studying breast cancer metastasis to bone, these specimens and approaches can readily be extended to the study of other bone seeking malignancies such as prostate cancer, as well as other bone pathologies. Although we have used the discarded femoral heads primarily as a source of tissue fragments, these specimens also constitute an additional source of human bone marrow, which is traditionally collected through bone marrow aspiration from young volunteers. And finally, while we have described approaches using cell lines engineered for bioluminescence and fluorescence, most of the procedures can be modified for use with other cells if these cell lines and/or imaging platforms are not available.
Disclosures
No disclosures to report.
Acknowledgments
These studies were funded, in part, by grants from the Alternative Research and Development Foundation (107588), the National Institute of Health (1U54CA136465-04S1), and the California Breast Cancer Research Program (201B-0141). We thank Drs. Andrew Wilber and R. Scott McIvor for the generous donation of their transponson and pK/hUbiC-SB11 transposase plasmids. We gratefully acknowledge John Tamaresis, Ph.D. for advice on statistical methods, Timothy Brown for performing flow cytometry, Georgette Henrich for advice on migrations assays, and Nancy Bellagamba for facilitating the collection of THR specimens.
References
- Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13:227. doi: 10.1186/bcr2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. British journal of cancer. 1987;55:61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- Hess KR, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherholt AM, Fuchs RK, Warden SJ. Specialized connective tissue: bone, the structural framework of the upper extremity. Journal of hand therapy : official journal of the American Society of Hand Therapists. 2012;25:123–131. doi: 10.1016/j.jht.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travlos GS. Normal structure, function, and histology of the bone marrow. Toxicologic pathology. 2006;34:548–565. doi: 10.1080/01926230600939856. [DOI] [PubMed] [Google Scholar]
- Casimiro S, Guise TA, Chirgwin J. The critical role of the bone microenvironment in cancer metastases. Molecular and cellular endocrinology. 2009;310:71–81. doi: 10.1016/j.mce.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Patel LR, Camacho DF, Shiozawa Y, Pienta KJ, Taichman RS. Mechanisms of cancer cell metastasis to the bone: a multistep process. Future Oncol. 2011;7:1285–1297. doi: 10.2217/fon.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RH, Weinberg RA, Rosenblatt M. Of mice and (wo)men: mouse models of breast cancer metastasis to bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:431–436. doi: 10.1002/jbmr.68. [DOI] [PubMed] [Google Scholar]
- Kim IS, Baek SH. Mouse models for breast cancer metastasis. Biochemical and biophysical research communications. 2010;394:443–447. doi: 10.1016/j.bbrc.2010.03.070. [DOI] [PubMed] [Google Scholar]
- Fantozzi A, Christofori G. Mouse models of breast cancer metastasis. Breast Cancer Res. 2006;8:212. doi: 10.1186/bcr1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaudeau L, et al. Mimicking breast cancer-induced bone metastasis in vivo: current transplantation models and advanced humanized strategies. Cancer metastasis reviews. 2014;33:721–735. doi: 10.1007/s10555-014-9499-z. [DOI] [PubMed] [Google Scholar]
- Schiller KR, et al. Secretion of MCP-1 and other paracrine factors in a novel tumor-bone coculture model. BMC Cancer. 2009;9:45. doi: 10.1186/1471-2407-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin P, Youm H, Salih E. Three-dimensional cancer-bone metastasis model using ex-vivo co-cultures of live calvarial bones and cancer cells. Biomaterials. 2012;33:1065–1078. doi: 10.1016/j.biomaterials.2011.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, et al. Dynamic interaction between breast cancer cells and osteoblastic tissue: comparison of two- and three-dimensional cultures. Journal of cellular physiology. 2011;226:2150–2158. doi: 10.1002/jcp.22550. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Vogler EA, Sosnoski DM, Mastro AM. In vitro mimics of bone remodeling and the vicious cycle of cancer in bone. Journal of cellular physiology. 2014;229:453–462. doi: 10.1002/jcp.24464. [DOI] [PubMed] [Google Scholar]
- Bussard KM, Venzon DJ, Mastro AM. Osteoblasts are a major source of inflammatory cytokines in the tumor microenvironment of bone metastatic breast cancer. Journal of cellular biochemistry. 2010;111:1138–1148. doi: 10.1002/jcb.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnoski DM, Krishnan V, Kraemer WJ, Dunn-Lewis C, Mastro AM. Changes in Cytokines of the Bone Microenvironment during Breast Cancer Metastasis. International journal of breast cancer. 2012;2012:160265. doi: 10.1155/2012/160265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussard KM, et al. Localization of osteoblast inflammatory cytokines MCP-1 and VEGF to the matrix of the trabecula of the femur, a target area for metastatic breast cancer cell colonization. Clinical & experimental metastasis. 2010;27:331–340. doi: 10.1007/s10585-010-9330-3. [DOI] [PubMed] [Google Scholar]
- Kuperwasser C, et al. A mouse model of human breast cancer metastasis to human bone. Cancer research. 2005;65:6130–6138. doi: 10.1158/0008-5472.CAN-04-1408. [DOI] [PubMed] [Google Scholar]
- Rosenblatt M. A tale of mice and (wo)men: development of and insights from an 'all human' animal model of breast cancer metastasis to bone. Transactions of the American Clinical and Climatological Association. 2012;123:135–150. [PMC free article] [PubMed] [Google Scholar]
- Oh HS, et al. Bone marrow stroma influences transforming growth factor-beta production in breast cancer cells to regulate c-myc activation of the preprotachykinin-I gene in breast cancer cells. Cancer research. 2004;64:6327–6336. doi: 10.1158/0008-5472.CAN-03-3122. [DOI] [PubMed] [Google Scholar]
- Moharita AL, et al. SDF-1alpha regulation in breast cancer cells contacting bone marrow stroma is critical for normal hematopoiesis. Blood. 2006;108:3245–3252. doi: 10.1182/blood-2006-01-017459. [DOI] [PubMed] [Google Scholar]
- Koro K, et al. Interactions between breast cancer cells and bone marrow derived cells in vitro define a role for osteopontin in affecting breast cancer cell migration. Breast cancer research and treatment. 2011;126:73–83. doi: 10.1007/s10549-010-0889-9. [DOI] [PubMed] [Google Scholar]
- Pohorelic B, et al. Role of Src in breast cancer cell migration and invasion in a breast cell/bone-derived cell microenvironment. Breast cancer research and treatment. 2012;133:201–214. doi: 10.1007/s10549-011-1753-2. [DOI] [PubMed] [Google Scholar]
- Nicola MH, et al. Breast cancer micrometastases: different interactions of carcinoma cells with normal and cancer patients' bone marrow stromata. Clinical & experimental metastasis. 2003;20:471–479. doi: 10.1023/a:1025462417256. [DOI] [PubMed] [Google Scholar]
- Korah R, Boots M, Wieder R. Integrin alpha5beta1 promotes survival of growth-arrested breast cancer cells: an in vitro paradigm for breast cancer dormancy in bone marrow. Cancer research. 2004;64:4514–4522. doi: 10.1158/0008-5472.CAN-03-3853. [DOI] [PubMed] [Google Scholar]
- Martin FT, et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT) Breast cancer research and treatment. 2010;124:317–326. doi: 10.1007/s10549-010-0734-1. [DOI] [PubMed] [Google Scholar]
- Kapoor P, Suva LJ, Welch DR, Donahue HJ. Osteoprotegrin and the bone homing and colonization potential of breast cancer cells. Journal of cellular biochemistry. 2008;103:30–41. doi: 10.1002/jcb.21382. [DOI] [PubMed] [Google Scholar]
- Chen X, Lu J, Ji Y, Hong A, Xie Q. Cytokines in osteoblast-conditioned medium promote the migration of breast cancer cells. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:791–798. doi: 10.1007/s13277-013-1109-0. [DOI] [PubMed] [Google Scholar]
- Bersini S, et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35:2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow R, et al. A novel model of dormancy for bone metastatic breast cancer cells. Cancer research. 2013;73:6886–6899. doi: 10.1158/0008-5472.CAN-13-0991. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hutmacher DW, et al. Can tissue engineering concepts advance tumor biology research. Trends in biotechnology. 2010;28:125–133. doi: 10.1016/j.tibtech.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nature reviews. Molecular cell biology. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- Hutmacher DW. Biomaterials offer cancer research the third dimension. Nature. 2010;9:90–93. doi: 10.1038/nmat2619. [DOI] [PubMed] [Google Scholar]
- Kim JB. Three-dimensional tissue culture models in cancer biology. Seminars in cancer biology. 2005;15:365–377. doi: 10.1016/j.semcancer.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Xu X, Farach-Carson MC, Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnology advances. 2014;32:1256–1268. doi: 10.1016/j.biotechadv.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhurjati R, Krishnan V, Shuman LA, Mastro AM, Vogler EA. Metastatic breast cancer cells colonize and degrade three-dimensional osteoblastic tissue in vitro. Clinical & experimental metastasis. 2008;25:741–752. doi: 10.1007/s10585-008-9185-z. [DOI] [PubMed] [Google Scholar]
- Mastro AM, Vogler EA. A three-dimensional osteogenic tissue model for the study of metastatic tumor cell interactions with bone. Cancer research. 2009;69:4097–4100. doi: 10.1158/0008-5472.CAN-08-4437. [DOI] [PubMed] [Google Scholar]
- Contag CH, et al. Monitoring dynamic interactions between breast cancer cells and human bone tissue in a co-culture model. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2014;16:158–166. doi: 10.1007/s11307-013-0685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhaup M, et al. Cytotoxicity associated with artemis overexpression after lentiviral vector-mediated gene transfer. Human gene therapy. 2010;21:865–875. doi: 10.1089/hum.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne SH, et al. CNOB/ChrR6, a new prodrug enzyme cancer chemotherapy. Mol Cancer Ther. 2009;8:333–341. doi: 10.1158/1535-7163.MCT-08-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra R, et al. Characterization of bone metastases from rapid autopsies of prostate cancer patients. Clin Cancer Res. 2011;17:3924–3932. doi: 10.1158/1078-0432.CCR-10-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio AI, Wodajo FM, Malawer M. Metastatic carcinoma of the long bones. Am Fam Physician. 2007;76:1489–1494. [PubMed] [Google Scholar]
- Ahmann GJ, et al. Effect of tissue shipping on plasma cell isolation, viability, and RNA integrity in the context of a centralized good laboratory practice-certified tissue banking facility. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:666–673. doi: 10.1158/1055-9965.EPI-07-2649. [DOI] [PubMed] [Google Scholar]


