Abstract
A set of behavioral tasks for assessing perceptual and sensorimotor timing abilities in the general population (i.e., non-musicians) is presented here with the goal of uncovering rhythm disorders, such as beat deafness. Beat deafness is characterized by poor performance in perceiving durations in auditory rhythmic patterns or poor synchronization of movement with auditory rhythms (e.g., with musical beats). These tasks include the synchronization of finger tapping to the beat of simple and complex auditory stimuli and the detection of rhythmic irregularities (anisochrony detection task) embedded in the same stimuli. These tests, which are easy to administer, include an assessment of both perceptual and sensorimotor timing abilities under different conditions (e.g., beat rates and types of auditory material) and are based on the same auditory stimuli, ranging from a simple metronome to a complex musical excerpt. The analysis of synchronized tapping data is performed with circular statistics, which provide reliable measures of synchronization accuracy (e.g., the difference between the timing of the taps and the timing of the pacing stimuli) and consistency. Circular statistics on tapping data are particularly well-suited for detecting individual differences in the general population. Synchronized tapping and anisochrony detection are sensitive measures for identifying profiles of rhythm disorders and have been used with success to uncover cases of poor synchronization with spared perceptual timing. This systematic assessment of perceptual and sensorimotor timing can be extended to populations of patients with brain damage, neurodegenerative diseases (e.g., Parkinson’s disease), and developmental disorders (e.g., Attention Deficit Hyperactivity Disorder).
Keywords: Behavior, Issue 97, rhythm, timing, synchronization, disorders, beat deafness, perception and action
Introduction
Humans are particularly efficient at processing the duration of events occurring in their environment1. In particular, the ability to perceive the beat of music or the regular ticking of a clock and the ability to move along with it (e.g., in dance or synchronized sports) is widespread in the general population (i.e., in individuals who have not received musical training)2,3. These abilities are underpinned by a complex neuronal network involving cortical brain regions (e.g., the premotor cortex and the supplementary motor area) and subcortical structures, such as the basal ganglia and the cerebellum4-7.
Disruption of this network and consequent poor temporal processing can result from brain damage8-10 or neuronal degeneration, as observed in patients with Parkinson’s disease11. However, poor perception of duration and poor synchronization to the beat of music can also manifest in healthy individuals in the absence of brain damage. In spite of the fact that the majority can perceive auditory rhythms and synchronize the movement to the beat (e.g., in music), there are notable exceptions. Some individuals have major difficulties in synchronizing their body movements or finger tapping to the beat of music and can exhibit poor beat perception, showing difficulties in discriminating melodies with notes of different duration. This condition has been referred to as “beat deafness” or “dysrhythmia” 2,12-14. For example, beat deafness was described in a recent study13, in which the case of a patient named Mathieu was reported. Mathieu was particularly inaccurate at bouncing to the beat of rhythmical songs (e.g., a Merengue song). Synchronization was still possible, but only to the sounds of a simple isochronous sequence (e.g., a metronome). Poor synchronization was associated with poor beat perception, as revealed by the Montreal Battery of Evaluation of Amusia (MBEA)15. In an additional task, Mathieu was asked to match the movements of a dancer to the music; interestingly, Mathieu exhibited unimpaired pitch perception.
Poor rhythm perception and poor synchronization, in beat-deaf individuals with spared pitch perception, were observed in further studies2,12,14, thus providing compelling evidence that rhythm disorders can occur in isolation. Beat deafness is therefore distinct from the typical description of congenital amusia (i.e., tone deafness), a neurodevelopmental disorder affecting pitch perception and production16-19. Interestingly, poor rhythm perception and production can co-occur with poor pitch processing in congenital amusia12,16,20. Nevertheless, poor rhythm perception in this case depends on the ability of an individual to perceive pitch variation. When pitch variations in melodies are removed, congenital amusics can successfully discriminate rhythm differences21.
Important individual differences have been observed in beat deafness; this fact deserves particular attention. In most cases, both rhythm perception and synchronization to the beat of music are deficient2,12-14; however, poor synchronization can also occur when rhythm perception is spared2. This dissociation between perception and action in the timing domain has been shown using synchronized tapping tasks with a variety of rhythmic auditory stimuli (e.g., a metronome and music) and using different rhythm perception tasks (e.g., the discrimination of melodies based on different note durations and the detection of deviations from isochrony in rhythmic sequences). This finding is particular relevant because it points to the possible separation of perception and action with regard to timing mechanisms, as previously observed in pitch processing17,22-25. Further dissociations were highlighted depending on the stimulus complexity2. Most poor synchronizers exhibited selective difficulties with complex stimuli (e.g., music or amplitude-modulated noise derived from music), while they still showed accurate and consistent synchronization with simple isochronous sequences; other poor synchronizers showed the opposite pattern. In summary, these results converge in indicating that there are a variety of phenotypes of timing disorders in the general population (as observed in other domains of musical processing such as pitch25,26), which require a sensitive set of tasks to be detected. Characterizing the patterns of rhythm disorders is particularly relevant to shed light on the specific mechanisms that are malfunctioning in the timing system.
The goal of the method illustrated here is to provide a set of tasks that can be used to uncover cases of beat deafness in the general population and detect different subtypes of timing disorders (e.g., affecting perceptual vs. sensorimotor timing or a particular class of rhythmic stimuli). Sensorimotor timing abilities have mostly been examined using finger tapping tasks with auditory material. Participants are asked to tap their index finger in synchrony with auditory stimuli, such as to a sequence of tones equally spaced in time or to music (i.e., in a synchronized or paced tapping task27-29). Another popular paradigm, which has been the source of considerable modeling efforts29-32, is the synchronization-continuation paradigm, in which the participant continues tapping at the rate provided by a metronome after the sound has stopped. Rhythm perception is studied with a variety of tasks ranging from duration discrimination, estimation, bisection (i.e., comparing durations to 'short' and 'long' standards), and detection of anisochrony (i.e., determining whether there is a deviant interval within an isochronous sequence) to the beat alignment task (i.e., detecting whether a metronome superimposed onto music is aligned with the beat)1,2,20,33,34. Most studies have focused on time perception, beat production or sensorimotor timing, which were tested in isolation. However, it is likely that such different tasks refer to somewhat different abilities (e.g., interval timing vs. beat-based timing, perceptual vs. sensorimotor timing) and do not reflect the functioning of the same timing mechanisms and the associated neuronal circuitry. This issue can be circumvented by using recently proposed batteries of tasks that assess both perceptual and sensorimotor timing abilities. These batteries allow researchers to obtain an exhaustive profile of an individual’s timing abilities. Examples of such batteries are the beat alignment test (BAT)34, the Battery for the Assessment of Auditory Sensorimotor Timing Abilities (BAASTA)35, and the Harvard Beat Assessment Test (H-BAT)36. These batteries consist of tapping tasks with a variety of rhythmic auditory stimuli ranging from music to isochronous sequences as well as perceptual tasks (e.g., duration discrimination, detection of the alignment of a metronome to the beat of music, and anisochrony detection). In all cases, the same set of musical excerpts was used in perceptual and sensorimotor tasks.
In this paper, we illustrate a set of tasks that are particularly efficient at revealing patterns of rhythm disorders in beat-deaf individuals and poor synchronizers, as shown in previous studies2. These tasks are part of a larger battery of tests, the BAASTA35. Sensorimotor timing abilities are tested by asking participants to tap their finger to the beat of simple and complex auditory stimuli (e.g., isochronous sequences, music, and rhythmic noise derived from musical stimuli)27,28. Perceptual timing is tested with an anisochrony detection task2,20,33,37. A set of isochronous tones is presented. In some cases, one of the tones (e.g., the penultimate) is presented sooner or later than expected based on the isochronous structure of the auditory sequence. Participants are asked to detect deviations from isochrony. The advantage of these sensorimotor and rhythm perception tasks is that they both involve sequences of stimuli (instead of single durations) and stimuli of different complexity. Thus, based on previous evidence, these tasks provide the optimal conditions to uncover different phenotypes of beat deafness and poor synchronization. Particular attention is paid to the technique adopted in the analysis of synchronization data. This technique is based on circular statistics, an approach that is particularly well-suited for examining inaccurate and inconsistent synchronization to the beat.
Protocol
1. Synchronization Tasks
- Preparation of Instruments:
- Connect a standard MIDI percussion instrument to the computer via a conventional MIDI interface. NOTE: Data acquisition is realized via a MIDI electronic percussion instrument. The device captures the exact timing of the finger taps during the motor synchronization tasks.
- Open the dedicated software for stimulus presentation and response recording. NOTE: The synchronization task is implemented using standard software for the presentation of audio material and recording of data from a digital MIDI musical instrument (with 1 msec precision).
- Sound Material and Procedure:
- From the software interface, select the pacing stimulus to be used in the synchronization task from among three choices (isochronous sequence, music, and amplitude-modulated noise obtained from the wave envelope of the musical stimulus). NOTE: The isochronous sequence is composed of 96 isochronously presented tones (duration = 30 msec). The musical stimulus is a computer-generated piano version of a fragment of the Radetzky March (Opus 228) by Johann Strauss that includes 96 beats (beat = quarter note). Excerpts of the three stimuli are provided as Additional Materials to this manuscript.
- Select the appropriate tempo for the selected pacing stimulus (450, 600, or 750 msec Inter-onset-interval (IOI) / Inter-beat-interval (IBI)) as indicated in the software interface. Ensure that the stimuli are delivered at a comfortable volume level over the headphones.
- Ask the participant to sit in a quiet room in front of the computer monitor.
- Ask the participant to tap on the MIDI percussion instrument using the index finger of her or his dominant hand in synchrony with the tones of the isochronous sequence or with the musical beats for more complex stimuli (music or noise). Instruct the participant to tap as regularly as possible, without changing the tapping rate, while synchronizing with the pacing stimulus.
- Start the stimulus presentation and recording of taps.
- End recording of taps after presenting the last tone or musical beat.
- Data Analysis: NOTE: Analyze the data from the synchronized tapping tasks using circular statistics38,39. This method is particularly well-suited for the analysis of synchronization data40,41; moreover, circular statistics are sensitive to individual differences in timing abilities and are therefore able to uncover cases of poor synchronization2,40. The analysis procedure outlined below is implemented using Matlab software (using the CircStat toolbox39).
- Transform the time of the taps relative to the pacing stimuli into angles on the unit circle (from 0-360°) following the procedure indicated by Berens39. 0° (which is equal to 360°) corresponds to the time of the occurrence of the pacing stimulus (i.e., the sounds or musical beats). Use the following formula to obtain the angle for each tap time: [angle(radians) = 2 × π × (time of the tap / IOI)]. Convert radians into degrees with the circ_rad2ang function39.
- Plot the angles obtained in the tapping trial as a distribution of dots on the unit circle. Do this using the circ_plot function39. Provide angles in radians as the argument for the function to display the plot (see example in Figure 1).
- For each tapping trial, use the angles (dots on the circle) to compute the mean resultant vector R 38,39,42 (see Figure 1). Use the circ_mean39 and circ_r39 functions, which allow for the computation of synchronization accuracy and consistency, respectively.
- Compute the synchronization accuracy (i.e., on average, how far from the pacing stimulus the participant taps in a synchronized tapping trial), which corresponds to the angle θ of vector R. Use the circ_mean function39. Provide angles in radians as the argument for the function.
- Submit the tapping data to the Rayleigh test43 to assess whether the distribution of the dots around the circle is random, using the circ_rtest function39. Provide angles in radians as the argument for the function. NOTE: In the Rayleigh test, reject the null hypothesis (i.e., circular uniformity, randomly distributed dots around the circle) if the R vector length is large enough (e.g., greater than 0.4), indicating that participants tapped at a given phase relationship with respect to the pacing stimulus above chance. Only when Rayleigh test is significant (i.e., when the distribution of dots around the circle is not random) synchronization accuracy can be properly interpreted.
- Compute the synchronization consistency (i.e., the variability in the discrepancy between the time of the taps and the pacing stimuli), which corresponds to the length of the vector R (from 0 to 1). Use the circ_r function39. Provide angles in radians as the argument for the function. NOTE: The consistency is 1 when all of the taps occur at exactly the same time interval before or after the pacing stimuli; the consistency is 0 when the taps are randomly distributed around the circle.
- Evaluation of Individual Results: NOTE: Compare the performance of a participant to a normative group or to a control group to uncover cases of poor synchronization accuracy or poor consistency. To perform this comparison, run a corrected t-test44 implemented in the singlims computer program (http://homepages.abdn.ac.uk/j.crawford/pages/dept/SingleCaseMethodsComputerPrograms.htm).
- Open the singlims computer program. Enter the mean and SD of the synchronization accuracy, and the sample size of the normative or control group. Provide the synchronization accuracy for the participant to be compared to the normative or control group. Click on the “Compute” button to obtain the results of the corrected t-test. NOTE: The participant performed significantly poorer than the normative or control group when the two-tailed probability of the corrected t-test is below 0.05.
- Enter the mean and SD of the synchronization consistency and sample size of the normative or control group. Provide the synchronization consistency for the participant who is to be compared to the normative or control group.
2. Rhythm Perception Tasks (Anisochrony Detection)
- Preparation of Instruments:
- Open the computer program used for implementing the anisochrony detection tasks. Ensure that the keys of the computer keyboard are properly set to record participants’ answers. NOTE: The rhythm perception tasks are implemented using standard software for running behavioral experiments (i.e., stimulus presentation and recording of behavioral responses).
- Sound Material and Procedure:
- Select the stimulus (either isochronous stimulus or music) as indicated by the software interface. Choose the appropriate tempo (450, 600, or 750 msec IOI/IBI) of the selected stimulus. Ensure that the stimuli are delivered over the headphones at a comfortable volume level. NOTE: Stimuli are based on the same auditory material used in the Synchronization tasks. Each stimulus includes only 8 isochronously presented tones or musical beats instead of 96. For each stimulus type, there is a “change” version (50% of the trials, n = 24) and a “no-change” version (50% of the trials, n = 24). In the change stimuli, the penultimate sound or musical beat occurs earlier or later than expected (by 8, 12, or 16% of the sequence IOI/IBI) based on the previous IOIs/IBIs. In the no-change stimulus, the IOIs/IBIs are completely isochronous.
- Instruct the participant to sit in a quiet room in front of the computer monitor, listen to the stimulus and then judge, after its presentation, whether a change in the interval between the stimuli or beats (i.e., anisochrony) is present or not. Encourage the participant to pay attention to the entire sequence.
- Start the stimulus presentation. Ask the participant to respond by pressing one of two keys on the computer keyboard (i.e., one key for “change” or the other key for “no-change” responses) after the presentation of the stimulus.
- Data Analysis: NOTE: Analyze the data obtained from the rhythm perception task by calculating the discriminability index (d’) at each level of change (at 8, 12, 16% of the IOI/IBI) and for each IOI/IBI. The higher the d’ value, the greater the sensitivity to anisochronies.
- Consider the responses (n = 48) yielded by each participant for a given stimulus, recorded in the output file by the software used to run the behavioral experiment. Count the number of responses when the anisochrony present in the stimulus has been correctly detected. Compute the Hits rate (i.e., number of Hits / number of change stimuli).
- Count the number of responses when the participant reported a change in the interval between stimuli or beats when there was no change. Compute the False-Alarm (FA) rate (i.e., number of FAs/number of no-change stimuli).
- Calculate the z-score for the Hits rate and FA rate, using the norminv Matlab function (z-score = norminv(Hits rate or FA rate)). Subtract the z-score for the FA rate from the z-score for the Hits rate to obtain d’.
- Evaluation of Individual Results: NOTE: Compare the performance of a participant to a normative or control group to uncover cases of poor rhythm perception. Regarding the results of the synchronization tasks, perform a corrected t-test using the singlims computer program.
- Open the singlims computer program. Enter the mean and SD of d’ and the sample size of the normative or control group. Provide the d’ value for the participant who is to be compared to the normative or control group. NOTE: The participant performed significantly poorer than the normative or control group when the two-tailed probability of the corrected t-test is below 0.05.
Representative Results
The tasks described above have been used with success to characterize the timing abilities of individuals without musical training2,34-36. In a recent representative study on beat-deafness2, a group of 99 non-musicians (university students) were screened using two simple synchronization tasks. Participants synchronized their finger tapping with an isochronous sequence and a musical excerpt at a comfortable tempo (with an IOI/IBI of 600 msec). Ten of the participants showed particularly poor synchronization with at least one of the two stimuli and were referred to as “poor synchronizers”. These participants showed synchronization accuracy that deviated by more than 2 SD from the mean of the screened group; synchronization consistency was lower than 2 SD from the mean of the group. They were compared to a group of 23 participants (controls) who were randomly selected among those students who did not exhibit poor synchronization on the screening tasks. Poor synchronizers and controls were submitted to thorough testing with the synchronization and rhythm perception tasks described here. The order of the tasks and stimuli was counterbalanced across participants.
Sequences of tapping times collected in the synchronization tasks served to compute the synchronization accuracy and consistency for poor synchronizers and controls with different pacing stimuli and at the different IOI/IBIs. Mean results for their accuracy and consistency are illustrated in Figure 2 and Figure 3, respectively. These data show that both poor synchronizers and controls significantly anticipate the pacing stimuli when tapping along with an isochronous sequence. This phenomenon, which is referred to as “mean negative asynchrony,” is well known in tapping studies27,45. Mean negative asynchrony tends to reduce or disappear with stimuli (e.g., music and noise) that are more complex than isochronously presented tones, an effect also reported in previous studies45. Note that poor synchronizers do not differ from controls in terms of accuracy. Thus, accuracy does not appear to be a measure that is sensitive enough to detect beat deafness or poor synchronization. The results were more revealing when considering synchronization consistency. Poor synchronizers were significantly less consistent than controls across all stimuli and IOIs/IBIs. This difference was more significant when participants tapped along with isochronous sequences and music compared with noise (across tempos). Therefore, synchronization consistency is very sensitive to synchronization deficits and thereby represents an ideal measure for uncovering and characterizing individual differences. Representative results from the same study obtained in the rhythm perception tasks are displayed in Figure 4. As seen, both poor synchronizers and controls were affected by the amount of change in the auditory sequence (i.e., greater discrepancies in the sequence are easier to detect) in both isochronous stimuli and music. The effect of the change was statistically significant and is more visible at faster tempos. However, on a group level, poor synchronizers did not perform worse than controls in the perceptual task.
The results obtained in these sensorimotor tasks (synchronization consistency) and in the rhythm perception tasks were used to uncover cases of poor synchronization. To illustrate the procedure used to identify these conditions, the data taken from the representative study were further analyzed to perform the evaluation of individual differences. In Table 1, data are presented for the 10 poor synchronizers who were identified in the screening tests. When participants performed significantly worse than controls on one of the tasks, as determined with corrected t-tests44, the values of their performance are presented in the Table. The cut-off scores to identify a participant as a poor synchronizer in terms of synchronization consistency were 0.92, 0.51, and 0.51 for isochronous sequences, music, and noise, respectively. The results obtained by the poor synchronizers in the rhythm perception tasks were also compared to controls’ performance. In the rhythm perception task with a metronome, the cut-off scores (d’) were 0.33, 1.38, and 1.84, for a 8%, 12%, and 16% change in duration (relative to the sequence IOI), respectively. With music, the cut-off scores were 1.52, 1.98, and 2.10 for the three changes.
This simple method used to analyze individual differences in the timing domain allows us to uncover the profiles of timing disorders (beat deafness or poor synchronization). Indeed, poor synchronization may or may not be accompanied by deficient rhythm perception. Moreover, individuals showing difficulties in synchronizing to the beat can perform more poorly with an auditory stimulus (e.g., music) than with the other stimuli (e.g., an isochronous sequence). The representative study reveals different profiles of impairment. For example, participants S2, S3, S8 and S9 showed poor synchronization across most of the pacing stimuli, as well as impaired rhythm perception. Impairments in both perceptual and sensorimotor timing was previously observed in studies on congenital amusia12,16. Participants S1 and S5 showed a different pattern. They performed similarly to controls in the rhythm perception task, with d′ values below the cutoff. Unimpaired perception in these two participants was confirmed in additional tasks, such as the MBEA2,15. However, S1 and S5 were poor synchronizers, particularly when tapping with complex stimuli such as music and amplitude-modulated noise. For example, S5′s performance was at chance when synchronizing taps to noise (i.e., the Rayleigh’s test was not significant) and just above chance with music (at chance with 750 msec IBI). Similar results were found for participants S6 and S10. Note that this dissociation between perceptual and sensorimotor timing cannot be accounted for by impaired motor control because the participants, in spite of their poor synchronization, were still able to tap at a spontaneous tempo, similar to controls. Finally, for some participants (e.g., S2, S5, and S6), poor synchronization, relative to the control group, can selectively concern only one type of stimuli (e.g., complex stimuli such as music or noise, as opposed to a metronome). In summary, different profiles of timing disorders can be uncovered with the aforementioned tasks. This is particularly relevant to shed light on the mechanisms behind different timing tasks, as well as to examine the interdependence of these mechanisms.
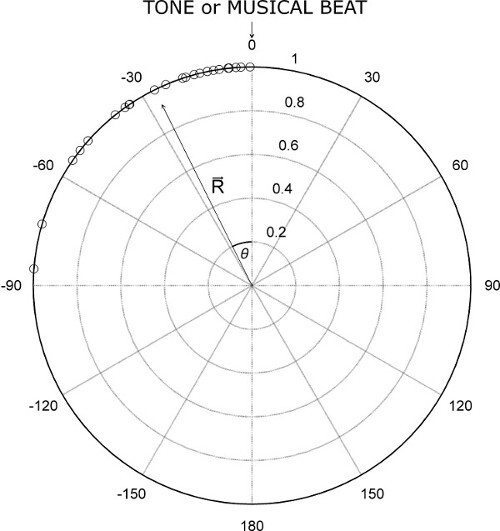 Figure 1: Example of the distribution of taps in a synchronization trial. The resultant vector R and its direction (angle theta, θ) are indicated. In the example, vector length = 0.95 and θ = -25°. (Adapted from Sowiński & Dalla Bella, 2013, with permission.)2
Please click here to view a larger version of this figure.
Figure 1: Example of the distribution of taps in a synchronization trial. The resultant vector R and its direction (angle theta, θ) are indicated. In the example, vector length = 0.95 and θ = -25°. (Adapted from Sowiński & Dalla Bella, 2013, with permission.)2
Please click here to view a larger version of this figure.
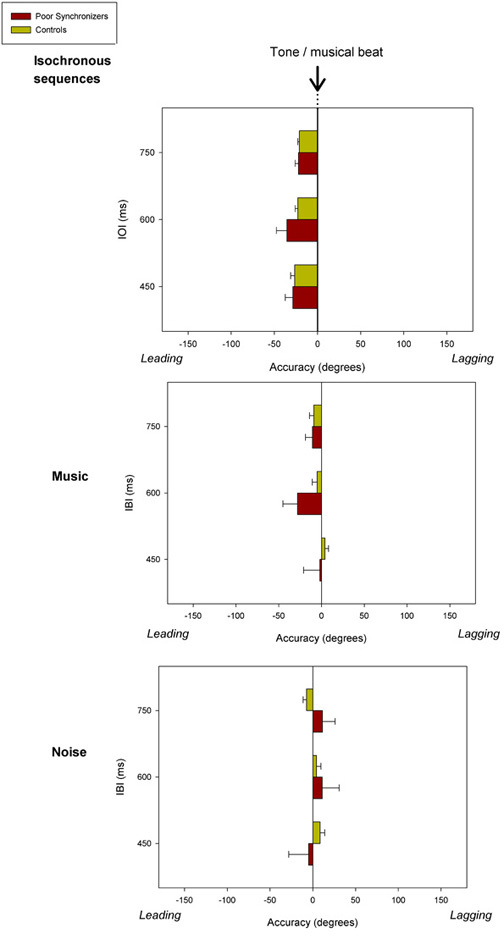 Figure 2: Synchronization accuracy for a group of poor synchronizers (n = 10) and controls (n = 23) with different pacing stimuli at different IOI/IBIs2. The occurrence of the pacing stimuli (e.g., tones or musical beats) corresponds to 0°. Negative angles indicate that, on average, participants’ taps precede the pacing stimuli (leading), whereas positive angles show that these taps occur after the stimuli (lagging). Error bars indicate the Standard Errors of the Mean (SEM). Please click here to view a larger version of this figure.
Figure 2: Synchronization accuracy for a group of poor synchronizers (n = 10) and controls (n = 23) with different pacing stimuli at different IOI/IBIs2. The occurrence of the pacing stimuli (e.g., tones or musical beats) corresponds to 0°. Negative angles indicate that, on average, participants’ taps precede the pacing stimuli (leading), whereas positive angles show that these taps occur after the stimuli (lagging). Error bars indicate the Standard Errors of the Mean (SEM). Please click here to view a larger version of this figure.
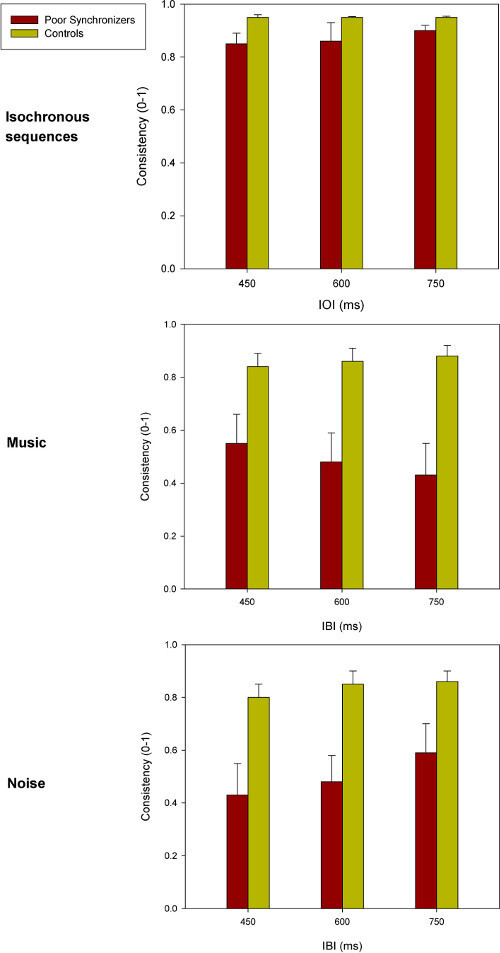 Figure 3: Synchronization consistency obtained in a previous study for a group of poor synchronizers (n = 10) and controls (n = 23) with different pacing stimuli at different IOI/IBIs2. Consistency ranges from 0 (no synchronization with a completely random distribution of the taps) to 1 (perfect consistency with taps that occur at exactly the same time interval before or after the pacing stimuli). Error bars indicate the SEM. Please click here to view a larger version of this figure.
Figure 3: Synchronization consistency obtained in a previous study for a group of poor synchronizers (n = 10) and controls (n = 23) with different pacing stimuli at different IOI/IBIs2. Consistency ranges from 0 (no synchronization with a completely random distribution of the taps) to 1 (perfect consistency with taps that occur at exactly the same time interval before or after the pacing stimuli). Error bars indicate the SEM. Please click here to view a larger version of this figure.
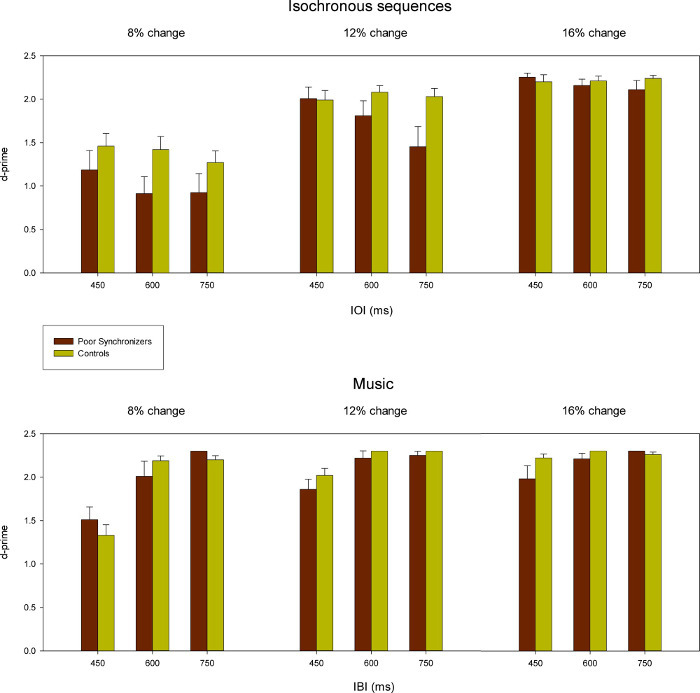 Figure 4: Results from the rhythm perception task (values of d’) obtained in a previous study for a group of poor synchronizers (n = 10) and controls (n = 23) with the isochronous sequence and with music at different IOI/IBIs. Error bars indicate the SEM. (Adapted from Sowiński & Dalla Bella, 2013, with permission.)2
Please click here to view a larger version of this figure.
Figure 4: Results from the rhythm perception task (values of d’) obtained in a previous study for a group of poor synchronizers (n = 10) and controls (n = 23) with the isochronous sequence and with music at different IOI/IBIs. Error bars indicate the SEM. (Adapted from Sowiński & Dalla Bella, 2013, with permission.)2
Please click here to view a larger version of this figure.
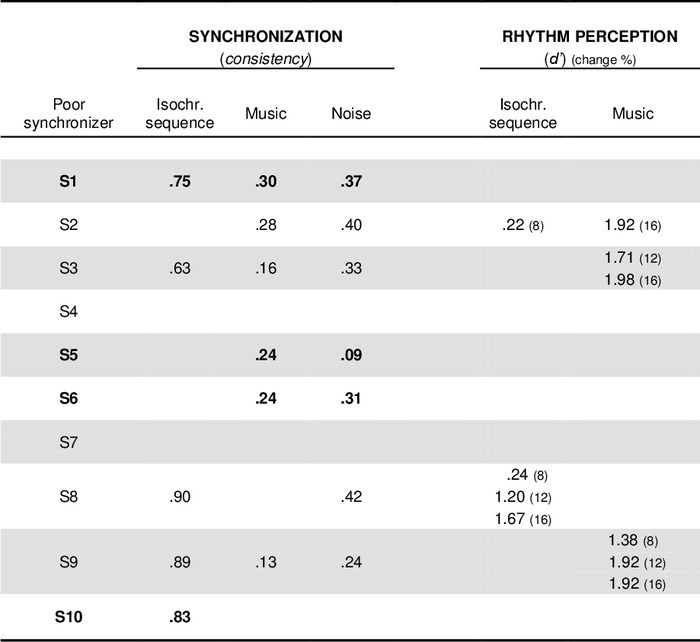 Table 1: Summary of the individual results obtained in the synchronization and rhythm perception tasks by a group of 10 poor synchronizers. Values on the different tests are reported only when participants performed significantly worse than controls. Participants who correctly perceived deviations from anisochrony in spite of their poor synchronization are indicated in bold. (Adapted from Sowiński & Dalla Bella, 2013, with permission.)2. Please click here to view a larger version of this table.
Table 1: Summary of the individual results obtained in the synchronization and rhythm perception tasks by a group of 10 poor synchronizers. Values on the different tests are reported only when participants performed significantly worse than controls. Participants who correctly perceived deviations from anisochrony in spite of their poor synchronization are indicated in bold. (Adapted from Sowiński & Dalla Bella, 2013, with permission.)2. Please click here to view a larger version of this table.
Discussion
The goal of the described method is to provide a set of tasks and analysis strategies to characterize the timing abilities of the majority of individuals and detect cases of beat deafness or poor synchronization. The critical steps of the protocol involve 1) the setup of the instruments used for stimulus presentation and collection of finger tapping data and subjects’ responses, 2) data collection using two sets of tasks (synchronization and rhythm perception), 3) analysis of synchronization data with circular statistics and rhythm perception data, and 4) evaluation of individual results. These steps can be easily carried out by trained experimenters. Data analysis is performed with Matlab software by implementing the steps described in our Protocol. A basic knowledge of circular statistics is required for the correct interpretation of the synchronization results.
The method has a few advantages compared to those in the existing literature1,27,46. First, timing is tested in tasks involving both perception and action as well as with comparable stimulus material. In most of the previous studies, sensorimotor synchronization and duration perception are typically studied independently using a variety of tasks1,27. However, there are indications that perception and action in time processing may dissociate in patients with brain damage8 or beat deafness2, as previously observed in pitch processing17,22-25. It is important to utilize a set of tasks capable of uncovering these dissociations without being biased by the choice of auditory materials. The tasks proposed in the methods illustrated here are successful in showing the dissociations between perception and action in time processing. However, we are aware of the fact that further confirmation of this dissociation would require the testing of perceptual and sensorimotor timing with a wider range of tasks, evaluating a variety of timing abilities. This objective can be achieved by using a battery of tests, such as the BAASTA35, as well as by including paced tapping and anisochrony detection tasks (using a maximum-likelihood procedure for computing detection thresholds) and the H-BAT36. Second, synchronization and perception tasks are performed with both simple and more complex auditory material; the latter includes either all of the elements of a musical piece (e.g., pitch and rhythmic structure) or solely its rhythmic features (i.e., amplitude-modulated noise). Variety in musical material can provide the optimal conditions for detecting impaired timing, which may be confined to metrical processing and beat extraction when processing complex rhythmic stimuli such as music. Finally, we illustrated that circular statistics are a valuable and relatively easy method that can be used for analyzing synchronization performance, as has been shown in previous studies2,40,41. This method has a few advantages, making it particularly well-suited to uncover and characterize individual differences in sensorimotor synchronization2,40. Circular statistics do not require a one-to-one correspondence between taps and pacing stimuli, a condition that is rarely met in participants showing poor synchronization. For example, beat-deaf individuals, children, and poor synchronizers tend to omit taps or produce more than one tap corresponding to the same pacing stimulus40. This makes the computation of synchronization accuracy impossible in many cases. By not requiring a one-to-one correspondence between taps and pacing stimuli, circular statistics overcome this difficulty so that all taps can be analyzed.
The representative results highlighted in this paper show that a set of behavioral tasks focusing on both sensorimotor synchronization with finger tapping and detecting the irregularity (anisochrony) in rhythmic sequences are sensitive enough to individual differences in perceptual and sensorimotor timing. These tasks and measures allow cases in which perceptual timing dissociates from sensorimotor timing to be discovered, as shown in a recent study from our laboratory2. We expect that the use of these tasks and methods (e.g., within extensive batteries of tests) for examining systematically perceptual and sensorimotor timing abilities can be successfully extended to populations of patients with brain damage47, neurodegenerative diseases (e.g., Parkinson’s disease)11,35, or developmental disorders (e.g., Attention Deficit Hyperactivity Disorder)48. A thorough assessment of perceptual and sensorimotor timing in these patient populations has the potential to pave the way for rehabilitation strategies when timing abilities seem to play a critical role (e.g., in the rehabilitation of gait in patients with Parkinson’s disease via auditory cueing)49,50.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This research was supported by an International Reintegration Grant (n. 14847) from the European Commission to SDB and a grant from Polish Ministry for Science and Education to JS.
References
- Grondin S. The Psychology of Time. Emerald, West Yorkshire: 2008. [Google Scholar]
- Sowiński J, Dalla Bella S. Poor synchronization to the beat may result from deficient auditory-motor mapping. Neuropsychologia. 2013;51(10):1952–1963. doi: 10.1016/j.neuropsychologia.2013.06.027. [DOI] [PubMed] [Google Scholar]
- Repp BH. Sensorimotor synchronization and perception of timing: Effects of music training and task experience. Hum. Mov. Sci. 2010;29(2):200–213. doi: 10.1016/j.humov.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Coull JT, Cheng R-K, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36(1):3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing AM. Voluntary timing and brain function: An information processing approach. Brain Cogn. 2002;48(1):7–30. doi: 10.1006/brcg.2001.1301. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RMC. The neural representation of time. Curr. Opin. Neurobiol. 2004;14(2):225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Watson SL, Grahn JA. Perspectives on rhythm processing in motor regions of the brain. Mus. Ther. Perspect. 2013;31(1):25–30. [Google Scholar]
- Fries W, Swihart AA. Disturbance of rhythm sense following right hemisphere damage. Neuropsychologia. 1990;28(12):1317–1323. doi: 10.1016/0028-3932(90)90047-r. [DOI] [PubMed] [Google Scholar]
- Schwartze M, Keller PE, Patel AD, Kotz SA. The impact of basal ganglia lesions on sensorimotor synchronization, spontaneous motor tempo, and the detection of tempo changes. Behav. Brain Res. 2011;216(2):685–691. doi: 10.1016/j.bbr.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Pressing JL, Wales RJ. Modelling rhythmic function in a musician post-stroke. Neuropsychologia. 2002;40(8):1494–1505. doi: 10.1016/s0028-3932(01)00198-1. [DOI] [PubMed] [Google Scholar]
- Allman MJ, Meck WH. Pathophysiological distortions in time perception and timed performance. Brain. 2012;135(3):656–677. doi: 10.1093/brain/awr210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Bella S, Peretz I. Congenital amusia interferes with the ability to synchronize with music. Ann. N. Y. Acad. Sci. 2003;999(1):166–169. doi: 10.1196/annals.1284.021. [DOI] [PubMed] [Google Scholar]
- Phillips-Silver J, et al. Born to dance but beat-deaf: a new form of congenital amusia. Neuropsychologia. 2011;49(5):961–969. doi: 10.1016/j.neuropsychologia.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Launay J, Grube M, Stewart L. Dysrhythmia: A specific congenital rhythm perception deficit. Front. Psychol. 2014;5:18. doi: 10.3389/fpsyg.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz I, Champod AS, Hyde KL. Varieties of musical disorders. The Montreal Battery of Evaluation of Amusia. Ann. N. Y. Acad. Sci. 2003;999(1):58–75. doi: 10.1196/annals.1284.006. [DOI] [PubMed] [Google Scholar]
- Ayotte J, Peretz I, Hyde KL. Congenital amusia: a group study of adults afflicted with a music-specific disorder. Brain. 2002;125(2):238–251. doi: 10.1093/brain/awf028. [DOI] [PubMed] [Google Scholar]
- Dalla Bella S, Giguère J-F, Peretz I. Singing proficiency in the general population. J. Acoust. Soc. Am. 2007;121(2):1182–1189. doi: 10.1121/1.2427111. [DOI] [PubMed] [Google Scholar]
- Peretz I. Musical disorders: from behavior to genes. Curr. Dir. Psychol. Sci. 2008;17(5):329–333. [Google Scholar]
- Peretz I, Hyde K. What is specific to music processing? Insights from congenital amusia. Trends in Cogn. Sci. 2003;7(8):362–367. doi: 10.1016/s1364-6613(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Peretz I. Brains that are out of tune but in time. Psychol. Sci. 2004;15(5):356–360. doi: 10.1111/j.0956-7976.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- Foxton JM, Nandy RK, Griffiths TD. Rhythm deficits in ‘tone deafness. Brain Cogn. 2006;62(1):24–29. doi: 10.1016/j.bandc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Dalla Bella S, Giguère J-F, Peretz I. Singing in congenital amusia. J. Acoust. Soc. Am. 2009;126(1):414–424. doi: 10.1121/1.3132504. [DOI] [PubMed] [Google Scholar]
- Loui P, Guenther F, Mathys C, Schlaug G. Action-perception mismatch in tone-deafness. Curr. Biol. 2008;18(8):R331–R332. doi: 10.1016/j.cub.2008.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths TD. Sensory systems: auditory action streams. Curr. Biol. 2008;18(9):R387–R388. doi: 10.1016/j.cub.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Dalla Bella S, Berkowska M, Sowiński J. Disorders of pitch production in tone deafness. Front. Psychol. 2011;2:164. doi: 10.3389/fpsyg.2011.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowska M, Dalla Bella S. Uncovering phenotypes of poor-pitch singing: the Sung Performance Battery (SPB) SPB). Front. Psychol. 2013;4(714) doi: 10.3389/fpsyg.2013.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repp BH. Sensorimotor synchronization: a review of the tapping literature. Psychon. Bull. Rev. 2005;12(6):969–992. doi: 10.3758/bf03206433. [DOI] [PubMed] [Google Scholar]
- Repp BH. Musical synchronization, and the brain. In: Altenmüller E, Kesselring J, Wiesendanger M, editors. Music, motorcontrol. Oxford University Press; 2006. pp. 55–76. [Google Scholar]
- Vorberg D, Wing A. Modeling variability and dependence in timing. In: Heuer H, Keele SW, editors. Handbook of perception and action. Vol. 2. Academic Press; 1996. pp. 181–162. [Google Scholar]
- Wing AM, Kristofferson AB. Response delays and the timing of discrete motor responses. Percept. Psychophys. 1973;14(1):5–12. [Google Scholar]
- Wing AM, Kristofferson AB. The timing of interresponse intervals. Percept. Psychophys. 1973;13(3):455–460. [Google Scholar]
- Ivry RB, Hazeltine RE. Perception and production of temporal intervals across a range of durations: Evidence for a common timing mechanism. J. Exp. Psychol. Hum. Percept. Perform. 1995;21(1):3–1037. doi: 10.1037//0096-1523.21.1.3. [DOI] [PubMed] [Google Scholar]
- Ehrlé N, Samson S. Auditory discrimination of anisochrony: influence of the tempo and musical backgrounds of listeners. Brain Cogn. 2005;58(1):133–147. doi: 10.1016/j.bandc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Iversen JR, Patel AD, et al. The Beat Alignment Test (BAT): Surveying beat processing abilities in the general population. In: Miyazaki K, editor. Proceedings of the 10th International Conference on Music Perception and Cognition (ICMPC10. Adelaide: Causal Productions; 2008. pp. 465–468. [Google Scholar]
- Benoit C-E, Dalla Bella S, et al. Musically cued gait-training improves both perceptual and motor timing in Parkinson's disease. Front. Hum. Neurosci. 2014;8:494. doi: 10.3389/fnhum.2014.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Schlaug G. The Harvard Beat Assessment Test (H-BAT): A battery for assessing beat perception and production and their dissociation. Front. Hum. Neurosci. 2013;7:771. doi: 10.3389/fnhum.2013.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze HH. The perception of temporal deviations in isochronic patterns. Percept. Psychophys. 1989;45(4):291–296. doi: 10.3758/bf03204943. [DOI] [PubMed] [Google Scholar]
- Fisher NI. Statistical analysis of circular data. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Berens P. CircStat: a Matlab Toolbox for circular statistics. J. Stat. Soft. 2009;31:1–21. [Google Scholar]
- Kirschner S, Tomasello M. Joint drumming: social context facilitates synchronization in preschool children. J. Exp. Child Psychol. 2009;102(3):299–314. doi: 10.1016/j.jecp.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Pecenka N, Keller PE. The role of temporal prediction abilities in interpersonal sensorimotor synchronization. Exp. Brain Res. 2011;211(3-4):505–515. doi: 10.1007/s00221-011-2616-0. [DOI] [PubMed] [Google Scholar]
- Mardia KV, Jupp PE. Directional statistics. New York: John Wiley; 1999. [Google Scholar]
- Wilkie D. Rayleigh test for randomness of circular data. Appl. Stat. 1983;32(3):311–312. [Google Scholar]
- Crawford JR, Garthwaite PH. Investigation of the single case in neuropsychology: Confidence limits on the abnormality of test scores and test score differences. Neuropsychologia. 2002;40(8):1196–1208. doi: 10.1016/s0028-3932(01)00224-x. [DOI] [PubMed] [Google Scholar]
- Aschersleben G. Temporal control of movements in sensorimotor synchronization. Brain Cogn. 2002;48(1):66–79. doi: 10.1006/brcg.2001.1304. [DOI] [PubMed] [Google Scholar]
- Repp BH, Su Y-H. Sensorimotor synchronization: A review of recent research (2006-2012) Psychon. Bull. Rev. 2013;20(3):403–452. doi: 10.3758/s13423-012-0371-2. [DOI] [PubMed] [Google Scholar]
- Stewart L, von Kriegstein K, Dalla Bella S, Warren JD, Griffiths TD. Disorders of musical cognition. In: Hallam S, Cross I, Thaut M, editors. Oxford Handbook of Music Psychology. Oxford University Press; 2009. pp. 184–196. [Google Scholar]
- Noreika V, Falter CM, Rubia K. Timing deficits in attention-deficit/hyperactivity disorder (ADHD): Evidence from neurocognitive and neuroimaging studies. Neuropsychologia. 2013;51(2):235–266. doi: 10.1016/j.neuropsychologia.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Lim I, et al. Effects of external rhythmical cueing on gait in patients with Parkinson's disease: a systematic review. Clin. Rehabil. 2005;19(7):695–713. doi: 10.1191/0269215505cr906oa. [DOI] [PubMed] [Google Scholar]
- Spaulding SJ, Barber B, et al. Cueing and gait improvement among people with Parkinson's disease: a meta-analysis. Arch. Phys. Med. Rehabil. 2012;94(3):562–570. doi: 10.1016/j.apmr.2012.10.026. [DOI] [PubMed] [Google Scholar]


