Abstract
Background
The essential role of the thalamus in neurocognitive processes has been well documented. In contrast, relatively little is known about its involvement in social cognitive processes such as recognition of emotion, mentalizing, or empathy.
The aim of the study
This study was designed to compare the performance of eight patients (five males, three females, mean age ± SD: 63.7±7.9 years) at early stage of unilateral thalamic lesions and eleven healthy controls (six males, five females, 49.6±12.2 years) in neurocognitive tests (CogState Battery: Groton Maze Learning Test, GML; Groton Maze Learning Test-Delayed Recall, GML-DR; Detection Task, DT; Identification Task, IT; One Card Learning Task, OCLT; One Back Task, OBT; Two Back Task, TBT; Set-Shifting Task, S-ST) and other well-known tests (Benton Visual Retention Test, BVRT; California Verbal Learning Test, CVLT; The Rey-Osterrieth Complex Figure Test, ROCF; Trail Making Test, TMT part A and B; Color – Word Stroop Task, CWST; Verbal Fluency Test, VFT), and social cognitive tasks (The Penn Emotion Recognition Test, ER40; Penn Emotion Discrimination Task, EmoDiff40; The Penn Emotional Acuity Test, PEAT40; Reading the Mind in the Eyes Test, revised version II; Toronto Alexithymia Scale, TAS-20).
Methods
Thalamic-damaged subjects were included if they experienced a single-episode ischemic stroke localized in right or left thalamus. The patients were examined at 3 weeks after the stroke onset. All were right handed. In addition, the following clinical scales were used: the Mini-Mental State Examination (MMSE), Spielberger State-Trait Anxiety Inventory (STAI), Beck Depression Inventory (BDI II). An inclusion criteria was a minimum score of 23/30 in MMSE.
Results
Compared with the healthy controls, patients revealed significantly lower scores in CVLT, GML-DR, and VFT. Furthermore, compared to healthy controls, patients showed significantly delayed recognition of “happiness” in EmoDiff40 and significantly worse performance on Reading the Mind in the Eyes Test, revised version II. Neuropsychological assessment demonstrated some statistically significant deficits in learning and remembering both verbal and visual material, long-term information storing, problem solving, and executive functions such as verbal fluency.
Conclusion
Patients at early stage of unilateral thalamic stroke showed both neurocognitive and social cognitive deficits. Further research is needed to increase understanding about diagnosis, early treatment, and prognosis of patients with thalamic lesions.
Keywords: social cognitive deficits, neurocognitive deficits, thalamic stroke, posterior, inferolateral, paramedian
Introduction
The thalamus is not only a major conduit for the bidirectional flow of neural signals between cortical and subcortical regions, but also links different cortical regions via transthalamic (ie, cortico–thalamocortical) pathways.1 The thalamus does not relay signals passively to cortex, but instead exerts complex and dynamic control of the information stream. Furthermore, it appears well equipped to play an active role in the dynamic processing and coordination of signals, both from the periphery and within the cortex. The thalamus is part of the limbic system, the region of the brain largely associated with emotions and is well recognized as the final relay station for perceptual data before it is passed on to the cerebral cortex. It receives input from diverse brain areas, including all of the senses except olfaction.2 Thus, pathology in the thalamus may induce structural as well as functional changes in regions with which it communicates via either mono- or polysynaptic pathways.
Despite the relatively low incidence of thalamic stroke (about 10% of all strokes3), the consequence of a short-term blood interruption in thalamus can result not only in motor impairment but also in cognitive, social, and emotional impairments. The solution is to offer the patients rehabilitation programs immediately, while still in hospital. Early cognitive therapy focuses on improving the gross neurocognitive system, including attention, memory, perceptual skills, visuospatial functions, and executive functions (including mental flexibility), to help the stroke survivors achieve the most independent level of functioning possible.
While a number of stroke studies have examined both neurocognitive and social cognitive functioning in subcortical lesions, mostly amygdale and basal ganglia, few have attempted to untangle the mysterious knot around the role of the thalamus in relation to emotional processing.4
Social cognition is defined as:
The ability to construct representations of the relation between oneself and others and to use those representations flexibly to guide social behaviour.5
This is thought to represent a specialized domain of cognition, which captures affect perception, social cue perception, theory of mind, empathy, and attribution style. Affect perception is the ability to infer emotional information presented either in visual or auditory form or in some combination (such as video clips). In other words, it is the ability to perceive how someone is feeling. Social cue perception refers to a person’s ability to ascertain social cues from behavior provided in a social context, and refers to a person’s comprehension of social rules. Theory of mind is the ability to simulate in one’s own mind the mental states of other people, including the ability to infer intentions, prospects, desires, and feelings of other people (“being in someone else’s shoes”). It also includes the ability to imagine yourself in different situations at the moment and the assessment of current thoughts and feelings, looking at each other from the outside.6–9 Empathy has many different definitions that encompass a broad range of emotional states, including caring for other people and having a desire to help them, experiencing emotions that match another person’s emotions, and discerning what another person is thinking or feeling.10 Attribution style, known as a personalizing bias, refers to an individual’s own perception and interpretation of facts and events.11 Neurocognition comprises the following processes: memory, attention, visuospatial functions, and executive functions including mental flexibility.
The thalamus has four major vascular territories: 1) tuberothalamic, 2) paramedian, 3) inferolateral, and 4) posterior choroidal. However, lesions within these territories are not selective for particular thalamic nuclei but involve these nuclei in different combinations. The consequences of thalamic lesions may differ depending on the vascular territory involved. In the literature, the most commonly reported impairments are in attention, executive functions, and memory and these can be attributed to damage of distinct thalamic nuclei. The main result of damage to the tuberothalamic territory supplied by tuberothalamic artery (or carotid artery) is impairment of short-term memory, new learning (both verbal and visual), autobiographical memory, and acalculia. These deficits are more prominent with left-sided lesions. Severe anterograde amnesia may be observed after left-sided infarction. Additionally, some authors describe thalamic aphasia caused by left-sided lesions localized in ventral lateral thalamic nucleus. In turn, right-sided damage in this area is manifested in the form of impaired visuospatial processes. It can also cause personality changes such as: disorientation of time and place, changes in the mood from euphoria to apathy, abulia, lack of spontaneity, and emotional unconcern.12,13
With regards to social cognitive and neurocognitive processes, the paramedian territory of the thalamus plays a crucial role, especially in those people with an absent tuberothalamic artery (the paramedian artery may supply that territory also). Unilateral thalamic infarction in the territory of the paramedian artery impairs arousal with decreased and fluctuating level of consciousness in the early stages, lasting for hours to days impairing learning and memory. The consequence of a left sided lesion is dynamic aphasia of Guberman and Stuss (hypophonia and dysprosody with frequent perseveration, markedly reduced verbal fluency with generally preserved syntactic structure with occasional paraphasic errors, and normal repetition). If the damage is on the right side, visuospatial deficits may be observed, including hemispatial neglect. In terms of personality changes, behavioral changes such as confusion, aggression, and apathy are recorded. Bilateral thalamic infarction in the territory of the paramedian artery may result in an acutely ill and severely impaired patient. In the early stages there is confusion, hypersomnolence, deep coma, “coma vigil” or akinetic mutism, eye movement abnormalities, severe memory impairment with perseveration, and confabulation. Anterograde and retrograde memory deficit and apathy can be severe and persistent.14,15
In the late stages inappropriate social behaviors, impulsive aggressive outbursts, emotional blunting, loss of initiative, and absence of spontaneous thoughts and mental activities (loss of psychic self-activation) are noticeable. There is disorientation in time (chronotaraxis), autobiographical memory impairment with relative sparing of knowledge of famous people and public events, and confabulations. Many authors describe the so-called “thalamic dementia”, in which the symptoms are similar to the thiamine-deficient Korsakoff syndrome (destruction of the medial dorsal thalamic nuclei along with the mammillary bodies). The phenomenon of transient global amnesia is also often described. This is thought to be related to transient ischemia in the paramedian thalamus.16
Much less is known about the effects of damage to inferolateral and posterior choroidal areas where neurological symptoms seem to dominate the clinical picture. Damage to the inferolateral territory causes contralateral hemisensory loss, hemiparesis, hemiataxia, postlesion pain syndrome (Dejerine–Roussy syndrome) (right hemisphere predominant), auditory consequences, and behavioral deficits.13,17
Damage to the posterior choroidal territory leads to visual field deficits (hemianopia, quadrantanopia), variable sensory loss, aphasia, memory impairment, dystonia, and hand tremor.13
Aim of the study
Our study was designed to examine social cognitive processes and neurocognitive functioning in eight acute patients with unilateral thalamic stroke assessed at 3 weeks after stroke onset and compare them with eleven healthy controls.
The aim of this study was to examine:
Group difference in social cognitive processes comprising emotion perception, mentalizing, and empathy to assess the severity level of these deficits as well as determine its relation to the age, years of education, current mood, anxiety level, and neurocognitive functioning;
Group difference in neurocognitive functioning based upon wide selection of tests used to assess its severity level as well as its relation to the age, years of education, current mood, anxiety level, and social cognitive functioning.
We hypothesized that the patients with unilateral thalamic lesions would demonstrate more impairment in both social cognitive and neurocognitive processes compared with healthy control group.
Participants
In this pilot study eight patients (five males, three females, mean age ± SD: 63.75±7.99 years) with unilateral thalamic infarction and eleven healthy volunteers (six males, five females, 49.63±12.21 years) participated after giving informed consent. Approval for the study was obtained from the ethics committee of the Institute of Psychiatry and Neurology in Warsaw, Poland. All participants were right-handed. The cognitive state of subjects was assessed by means of the Mini-Mental State Examination (MMSE).18 An inclusion criterion for all subjects was a minimum score of 23. None had a history of learning disability. Exclusion criteria for all subjects included: habitual drug or alcohol abuse, secondary neurological disorders (eg, epilepsy, dementia), and other psychiatric diagnoses. The subjects who had the difficulties with vision or hearing were also excluded. Additionally, people who were familiar with neuropsychological batteries and tests conducted in the study were also excluded to enhance sample cleanliness and the reliability of data analyses.
Five patients with right thalamic damage (R) and three patients with left thalamic lesions (L) were recruited from the neurology wards in Warsaw and surrounding districts. The subjects were included if they experienced a single episode of unilateral thalamic infarction. Thalamic lesions were localized in three main territories: posterior (two patients), inferolateral (three patients), and paramedian (three patients) (Table 1). Three out of eight patients (all with right-sided thalamus stroke) had motor deficits on the contralateral side to their lesions at the time of illness onset. The confirmation of the diagnosis was made by a neurologist blind to test scores, according to WHO criteria.19 All recruited patients at the time of the study assessments did not report elevated levels of pain and they were not on regular analgesic drugs. Magnetic resonance imaging (MRI) was used to confirm the unilateral nature of the lesions. We excluded the patients with transient ischemic attacks, subarachnoid hemorrhage, and previous cerebrovascular events causing a persistent neurological deficit. The patient group was assessed at 3 weeks post stroke onset.
Table 1.
Thalamic lesions location in the patient group
| N right | Thalamic lesions location | N left |
|---|---|---|
| 1 | Posterior | 1 |
| 2 | Inferolateral | 1 |
| 2 | Paramedian | 1 |
Notes: N represents the number of patients with thalamus damage to the right or left side.
Methods
For research purposes, the researchers used standardized neuropsychological measurements that are delivered via computerized software (CogState and The University of Pennsylvania Computerized Neuropsychological Test Battery) and individual tests.
Components of social cognition were evaluated using the following tests:
- Affect perception:
- Penn Emotion Recognition Test (ER40) is a measure of emotional recognition. Participants are shown a series of 40 faces, one at a time, and asked to determine what emotion the face is expressing. There are five answer choices: happy, sad, angry, frightened, and no emotion.
- Penn Emotion Discrimination Task (EmoDiff40) is a measure of emotion discrimination. Participants are shown 40 pairs of faces, one pair at a time. Each pair of faces consists of two pictures of the same person with or without a subtle, computer-generated difference in emotional expression. For each pair, the participant must decide which face expresses the given emotion (happy or sad) more intensely or whether they are equally emotional.20
- Penn Emotional Acuity Test 40 (PEAT40) is a measurement of emotion recognition and discrimination. The task presents 40 faces. The presentation takes place in two blocks, the first of which contains sad and neutral faces (sad–neutral block); the second, happy and neutral faces (happy–neutral block). Participants are asked to rate the emotional valence of the expression on each face on a 7-point scale: very sad, moderately sad, somewhat sad, neutral, somewhat happy, moderately happy, and very happy.
- Facial memory:
- Penn Facial Memory Test (PFMT) is a measure of facial memory. In the first part of the test participants are shown 20 faces that they will be asked to identify later, during both immediate and delayed recalls.
- Theory of mind/empathy:
- Reading the Mind in the Eyes Test (revised version II) is a measure of adult mentalizing, and an assessment of how well the participant can put themselves into the mind of another person and “tune in” to their mental state. In this test, the participant is presented with a series of 36 photographs of the eye region of unknown faces, and is asked to choose which of four words best describes what the person in the photo is thinking or feeling.21
- Toronto Alexithymia Scale (TAS-20) – a 20-item instrument that allows assessment of deficiency in understanding, processing, or describing emotions.22
Various neurocognitive functions were measured using well known tests/batteries:
Benton Visual Retention Test (BVRT) – visuospatial processing, visual memory;
California Verbal Learning Test (CVLT) – learning and remembering verbal material;
The Rey-Osterrieth Complex Figure Test (ROCF) – visuospatial abilities, memory, attention, planning, and working memory (executive functions);
Trail Making Test (TMT, part A and B) – test of visual attention and task switching;
Color – Word Stroop Task (CWST) – attention, executive functioning;
Verbal Fluency Test (VFT) – semantic memory, executive functioning;
CogState – Groton Maze Learning Test, GML; Groton Maze Learning Test-Delayed Recall, GML-DR; Detection Task, DT; Identification Task, IT; One Card Learning Task, OCLT; One Back Task, OBT; Two Back Task, TBT; Set-Shifting Task, S-ST – attention, learning and memory, and executive functioning/spatial problem solving.
In addition, the following clinical scales were used: the MMSE, Spielberger State-Trait Anxiety Inventory (STAI), and Beck Depression Inventory (BDI II).
Structural and functional magnetic resonance imaging (fMRI) investigations in the control and the experimental group were performed on a 1.5 T clinical imaging system (Philips Achieva Nova Dual; whole-body system, Philips Medical Systems, Warsaw, Poland). The study utilized primary fluid-attenuated inversion recovery, diffusion weighted imaging, and T1 sequences for diagnosis of thalamic lesions and the exclusion of other pathologies of the brain. The scans were read by a radiology specialist with several years of experience. The criteria, that were used to determine the presence of a unilateral lesions were hyperintense changes in diffusion weighted imaging images and a corresponding decrease in the apparent diffusion coefficient maps, usually also with visible outbreak in fluid-attenuated inversion recovery in this area.
Statistical analyses
Statistical analyses were performed using SPSS 22.0 statistical software for Microsoft Windows. Smirnov–Kolmogorov test was used to analyze data distribution; while due to the nature of findings and small sample size. The Mann–Whitney U-test was employed in data analyses. The Mann–Whitney U-test is the nonparametric test equivalent to the independent t-tests to compare differences between two independent sets of scores. Spearman correlation coefficients (rho) were calculated to search for potential relationship between the neuropsychological data which appeared significantly different compared to healthy controls and both demographic and clinical variables. All P-values lower than 0.05 were considered statistically significant.
Results
Subject groups were significantly different for age however independent samples Mann–Whitney U-test showed no statistically significant differences between groups in terms of MMSE scores as well as anxiety levels assessed by means of STAI (Table 2).
Table 2.
Demographic and clinical data for the two subject groups: patients (P) and healthy controls (HC)
| Variable | Measure | P (n=8) | HC (n=11) | Test statistics U (P-value) |
|---|---|---|---|---|
| Age | Years (X ± SD) | 63.75±7.99 | 49.63±12.21 | 0.012 |
| MMSE | 30 scorable responses | 27.62±2.97 | 29.09±0.94 | 0.272 |
| STAI state | (X ± SD) | 36.25±12.65 | 38.45±10.59 | 0.778 |
| STAI trait | (X ± SD) | 42.62±10.19 | 43.45±9.56 | 0.840 |
Notes: U – independent samples Mann–Whitney U-test. Asymptotic significances are displayed. The significance level is 0.05. Exact significance is displayed for this test. Data expressed as X ± SD; P – level of significance.
Abbreviations: MMSE, Mini-Mental State Examination; STAI, Spielberger State-Trait Anxiety Inventory; SD, standard deviation.
Mean BDI II scores in both subject groups appeared similar (patients: 10.75±6.81; healthy controls: 11.00±6.09), indicating no mood disorder (BDI II; range: 14–19, mild depression). Mean number of years of education for the patient group was 13.50±3.42 whereas mean number of years of education in the healthy volunteers was 16.81±2.85.
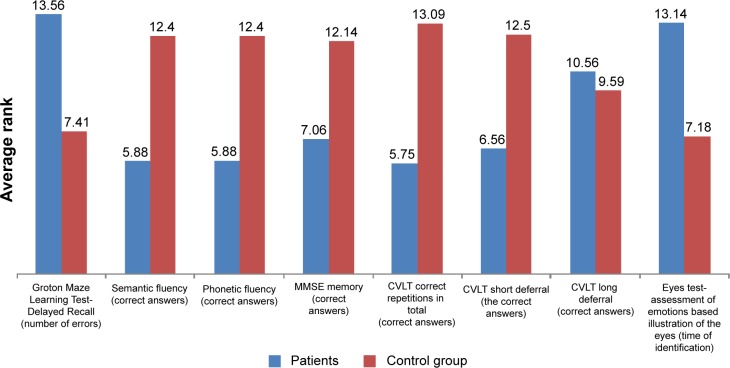
Statistical analysis showed a significant difference between groups in the number of errors made by the subjects in the GML-DR (U=15.50, P=0.01). The patients revealed difficulties in reconstructing the road in the maze after approximately a 30-minute break during which they performed other tasks. This may indicate weakened coding and extraction of visual–spatial information. Patients also needed more time to complete both Groton Maze tests. For other indicators of cognitive functioning, measured using the CogState battery, there were no differences between the groups (Figure 1). This result is likely due to a modest sample size – thalamic stroke is quite rare and difficult to study.
Figure 1.
Average test scores in control group and in patients with thalamic lesions.
Notes: In the graphs are given the value of the average rank results, which proved to be significant.
Abbreviations: CVLT, California Verbal Learning Test; MMSE, Mini-Mental State Examination.
As expected, we recorded statistically significant differences between groups in terms of semantic fluency (U=11, P=0.01), phonetic fluency (U=11, P=0.01), and tasks that required efficient extraction of information from lexical/semantic long-term memory and efficiency of action (executive functions) (Figure 1).
Analysis of short- and long-term auditory memory and assessment of the efficiency of learning verbal material, measured using the CVLT, revealed the existence of significant differences between the experimental group and the control group. The number of correct answers after a short delay (U=16.50, P=0.02), and after the long delay (U=16.50, P=0.02) indicates a reduced level of learning and verbal memory in patients after thalamic stroke (Figure 1). This may mean that short-term memory deficits were not compensated by the use of memory strategies, eg, semantic clustering of words. They cope better with recognition of words compared to casual reproduction. Moreover, patients had difficulty in remembering the three words after a short postponement of time in the MMSE (U=20.50, P=0.04), which can confirm the short-term memory and operational problems (Figure 1). The assessment of attention switching skills, measured by the CWST and TMT (both Part A and B), showed no significant differences between groups in terms of response time and number of errors.
The ability to recognize emotions in others, measured by the Reading the Mind in the Eyes Test, is worse in patients after thalamic stroke compared with the control group, thus suggesting their reduced ability to empathize (U=14.50, P=0.06) (Figure 1). In addition, the analysis revealed the presence of a significant difference between the study group and control group in Penn Emotion Discrimination Tasks (EmoDiff40), especially relating to the time taken to recognize the happy emotion: U=13; P=0.02. The time taken to identify happy emotions is significantly longer in patients with thalamic stroke, compared to respondents in the control group (Figure 2).
Figure 2.
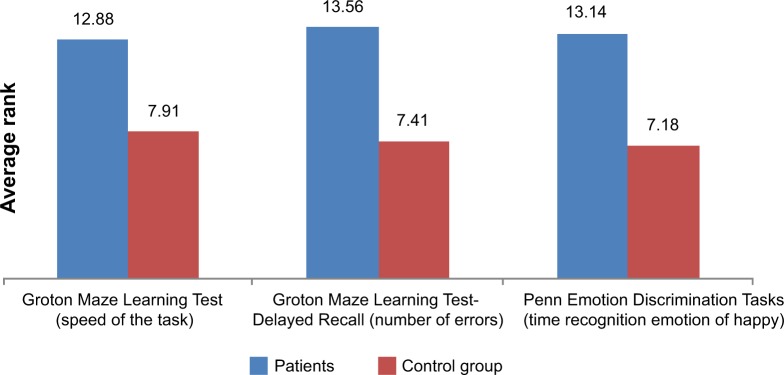
Comparison of average execution times: Groton Maze Learning Test and Penn Emotion Discrimination Tasks.
Notes: In the graphs are given the value of the average rank results, which proved to be significant.
There were significant Spearman correlations between age and semantic fluency (r=−0.82; P=0.044) and age and phonetic fluency (r=−0.84; P=0.036) in the patient group. Furthermore, semantic fluency score in patients correlated significantly with anxiety trait score (r=−0.82; P=0.020) and the results of anxiety state (r=0.92; P=0.008). No significant Spearman correlations between mean score of MMSE, years of education, and the neuropsychological tasks were found. Furthermore, current mood did not significantly influence the neuropsychological performance. Spearman correlations between the age, years of education, mean BDI II, STAI score, and the results of neuropsychological tasks did not reach significance in healthy controls.
Discussion
Our study partially confirms the results obtained by other authors indicating that the unilateral thalamic stroke can result in persistent deficits in several cognitive domains.12,13,17,23–25 Thalamic stroke patients came off much worse than healthy controls in memorizing both visual and verbal material measured by CVLT and GML-DR. The significantly weaker performance in memory tests and in VFTs may suggest deficits of memory and executive functions. However, the Spearman correlation results show that deficits in executive functions, measured by the VFTs, positively correlated with mean age of patient group.
Comparison of patients with a history of ischemic thalamic stroke with a history of short-term ischemic attack published by Liebermann et al23 showed no significant difference in clinical self-report questionnaires assessing memory, attention, executive functions, emotional status, and health-related quality of life. However, their detailed analyses showed that the patients with infarcts affecting the back thalamus in the right hemisphere showed significant emotional disturbances and elevated levels of anxiety compared with the patients with lesions localized in the front right thalamus. However, these conclusions were drawn on the basis of self-report data and further investigation is needed based upon more objective measures. In our study we did not observe such clear relationship in three patients with damage localized in inferolateral thalamus.
The results obtained by Cheung et al4 suggest that the consequences of unilateral damage localized in subcortical structures are impaired processes of facial affect recognition. Especially, the patients with lesions localized in basal ganglia performed worse than healthy controls in identifying facial expressions of anger, disgust, and fear. Other authors26 showed that these impairments could not be explained by comorbid cognitive deficits. In patients with damaged thalamus (six persons), impaired recognition of sadness was recorded whilst recognition of happy faces remained intact.4
In our study, the patients did not have problems with recognition of basic emotions, but they came off worse than healthy subjects in identifying more complex and veiled emotions in the Penn Battery. They performed worse in detecting some subtle differences in sad and happy facial expressions and their evaluation of emotion intensity appeared rather problematic. Furthermore, we found, that our patient group revealed the deficits in theory of mind/emotional empathy as measured by the Reading the Mind in the Eyes Test.
To the authors’ knowledge, no results were published so far on mentalizing processes in thalamus damage.
The strengths and limitations of the study
The thalamus is not a simple relay station therefore it requires further attention despite the challenging process of sample recruitment. To our knowledge, our pilot study is the first one aimed at exploring neurocognitive and social cognitive functioning in patients with acute one-sided infarct localized in the thalamus. Undoubtedly, the strength of the study is its administration by experienced clinical psychologists and the wide variety of standardized and cross-validated measures aimed at detection of specific deficits. Exclusion criteria for all subjects were enhanced by recruiting subjects naïve to neuropsychological measures used in the study. The outcome of this multidisciplinary project is the integration of up-to-date knowledge about the involvement of the thalamus in key emotional and cognitive processes in the context of treatment outcome and prognosis.
Our study only involves a limited number of cases due to the rarity of isolated unilateral thalamic strokes, limiting our statistical power. The extent to which our findings can be generalized certainly requires further investigation. Although neurocognitive deficits are often seen in the context of thalamic lesions, correlations between particular symptoms and particular nuclei cannot be tightly drawn because the lesions are rarely confined to a single thalamic nucleus. Nevertheless, the possible functional roles of the individual thalamic nuclei can be inferred from clinical–anatomic observations and from the reciprocal connections with behaviorally defined regions of the cerebral cortex. One of the weaknesses of our pilot study is an older patient group compared to healthy controls. Another potential limitation of the study is the lack of data pertaining to the premorbid emotional and cognitive functioning in the subjects, thus precluding us from comparing pre- and post-stroke functioning and limiting the value of our data. Recovery of functioning due to neuroplasticity could affect subject functioning in the tests, meaning our data may not be a realistic portrayal of postthalamic stroke functioning. Our results do not allow us to formulate the unequivocal hypothesis that impaired neurocognitive and social cognitive processes are the result of damage to the thalamus. It is recommended that further detailed research in this area, especially involving a larger patient group, be undertaken.
Implication for future research
The question of the severity and durability of neurocognitive and social cognitive deficits still remains open. The presented results are part of wider project in which, the researchers aim to show that combining the fMRI and lesion approaches can help reveal the source of functional modulatory influences between distant but interconnected brain regions. Further neuroimaging research is needed to find a solution to the nature of those deficits in relation to treatment provision and offering the patients rehabilitation program immediately while still in hospital. However, it is unlikely that interventions targeting only basic neurocognition will be sufficient to achieve optimal social cognitive functioning. Rapidly growing evidence indicates that impairments in the domain social cognition are important determinants of functional outcome and, for that reason, could be an even better target for stroke rehabilitation.
Acknowledgments
This research project is supported by National Centre of Science (NCN) grant (Poland) 2011/01/B/NZ5/02838 to KK, EW, and KS. Many thanks to Professor D Ryglewicz, Professor R Stefanski, and Dr Grzegorz Makowicz for their support throughout the project and their useful comments on the manuscript.
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Sherman SM, Guillery RW. Exploring the Thalamus and its Role in Cortical Function. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- 2.Oliveira JM, Amaral JR. Limbic system: the center of emotions. [Accessed, February 4, 2015];Brain and Mind: (E-Magazine on Neuroscience) 1998 5 Available from: http://www.cerebromente.org.br/n05/mente/limbic_i.htm. [Google Scholar]
- 3.Milandre L, Brosset C, Botti G, Khalil R. A study of 82 cerebral infarctions in the area of posterior cerebral arteries. Revue Neurologique. 1994;150(10):133–141. [PubMed] [Google Scholar]
- 4.Cheung CC, Lee MC, Yip JT, King KE, Li LS. The differential effects of thalamus and basal ganglia on facial emotion recognition. Brain Cogn. 2006;61:262–268. doi: 10.1016/j.bandc.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 6.Brüne M, Schaub D. Mental state attribution in schizophrenia: what distinguishes patients with “poor” from patients with “fair” mentalising skills? Eur Psychiatry. 2012;27(5):358–364. doi: 10.1016/j.eurpsy.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Lysaker PH, Gumley A, Dimaggio G. Metacognitive disturbances in persons with severe mental illness: theory, correlates with psychopathology and models of psychotherapy. Psychol Psychother. 2011;84(1):1–8. doi: 10.1111/j.2044-8341.2010.02007.x. [DOI] [PubMed] [Google Scholar]
- 8.Adolphs R. Is the human amygdala specialized for processing social information? Ann N Y Acad Sci. 2003;985:326–340. doi: 10.1111/j.1749-6632.2003.tb07091.x. [DOI] [PubMed] [Google Scholar]
- 9.Pickup GJ, Frith CD. Theory of mind impairments in schizophrenia: symptomatology, severity and specificity. Psychol Med. 2001;31(2):207–220. doi: 10.1017/s0033291701003385. [DOI] [PubMed] [Google Scholar]
- 10.Pijnenborg GH, Spikman JM, Jeronimus BF, Aleman A. Insight in schizophrenia: associations with empathy. Eur Arch Psychiatry Clin Neurosci. 2012;263(4):299–307. doi: 10.1007/s00406-012-0373-0. [DOI] [PubMed] [Google Scholar]
- 11.Annoni JM, Khateb A, Gramigna S, et al. Chronic cognitive impairment following laterothalamic infarcts: a study of 9 cases. Arch Neurol. 2003;60:1439–1443. doi: 10.1001/archneur.60.10.1439. [DOI] [PubMed] [Google Scholar]
- 12.Carrera E, Bogousslavsky J. The thalamus and behaviour: effects of anatomically distinct strokes. Neurology. 2006;66(12):1817–1823. doi: 10.1212/01.wnl.0000219679.95223.4c. [DOI] [PubMed] [Google Scholar]
- 13.Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34(9):2264–2278. doi: 10.1161/01.STR.0000087786.38997.9E. [DOI] [PubMed] [Google Scholar]
- 14.Caballero PEJ. Bilateral paramedian thalamic artery infarcts: report of 10 cases. J Stroke Cerebrovasc Dis. 2010;19(4):283–289. doi: 10.1016/j.jstrokecerebrovasdis.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Hermann DM, Siccoli M, Brugger P, et al. Evolution of neurological, neuropsychological and sleep-wake disturbances after paramedian thalamic stroke. Stroke. 2008;39(1):62–68. doi: 10.1161/STROKEAHA.107.494955. [DOI] [PubMed] [Google Scholar]
- 16.Carlesimo GA, Costa A, Serra L, Bozzali M, Fadda L, Caltagirone Vascular thalamic amnesia: a reappraisal. Neuropsychologia. 2011;49(5):777–789. doi: 10.1016/j.neuropsychologia.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 17.der Werf YD, Witter MP, Uylings HB, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38:613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Stroke 1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20(10):1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 20.Rojahn J, Kroeger TL, McElwain DC. Psychometric properties and preliminary norms of the Penn facial discrimination task in adults with mental retardation. J Develop Phys Disabil. 1995;7(4):285–301. [Google Scholar]
- 21.Baron-Cohen S, Jollife T, Mortimore C, Robertson M. Another advanced test of theory of mind: evidence from very high functioning adults with autism or Asperger syndrome. J Child Psychol Psychiatry. 1997;38:813–822. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 22.Bagby RM, Parker JDA, Taylor GJ. The twenty-item Toronto Alexithymia Scale-I. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 23.Liebermann D, Ostendorf F, Kopp UA, et al. Subjective cognitive-affective status following thalamic stroke. J Neurol. 2013;260:386–396. doi: 10.1007/s00415-012-6635-y. [DOI] [PubMed] [Google Scholar]
- 24.Liebermann D, Ploner CJ, Kraft A, Kopp UA, Ostendorf F. A dysexecutive syndrome of the medial thalamus. Cortex. 2013;49(1):40–49. doi: 10.1016/j.cortex.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Carlesimo GA, Costa A, Serra L, Bozzali M, Fadda L, Caltagirone C. Prospective memory in thalamic amnesia. Neuropsychologia. 2011;49(8):2199–2208. doi: 10.1016/j.neuropsychologia.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Yip JT, Leung KK, Li LS, Lee TM. The role of subcortical brain structures in emotion recognition. Brain Inj. 2004;18:1209–1217. doi: 10.1080/02699050410001719916. [DOI] [PubMed] [Google Scholar]