Abstract
Red/near-infrared light therapy (R/NIR-LT), delivered by laser or light emitting diode (LED), improves functional and morphological outcomes in a range of central nervous system injuries in vivo, possibly by reducing oxidative stress. However, effects of R/NIR-LT on oxidative stress have been shown to vary depending on wavelength or intensity of irradiation. Studies comparing treatment parameters are lacking, due to absence of commercially available devices that deliver multiple wavelengths or intensities, suitable for high through-put in vitro optimization studies. This protocol describes a technique for delivery of light at a range of wavelengths and intensities to optimize therapeutic doses required for a given injury model. We hypothesized that a method of delivering light, in which wavelength and intensity parameters could easily be altered, could facilitate determination of an optimal dose of R/NIR-LT for reducing reactive oxygen species (ROS) in vitro.
Non-coherent Xenon light was filtered through narrow-band interference filters to deliver varying wavelengths (center wavelengths of 440, 550, 670 and 810nm) and fluences (8.5 x 10-3 to 3.8 x 10-1 J/cm2) of light to cultured cells. Light output from the apparatus was calibrated to emit therapeutically relevant, equal quantal doses of light at each wavelength. Reactive species were detected in glutamate stressed cells treated with the light, using DCFH-DA and H2O2 sensitive fluorescent dyes.
We successfully delivered light at a range of physiologically and therapeutically relevant wavelengths and intensities, to cultured cells exposed to glutamate as a model of CNS injury. While the fluences of R/NIR-LT used in the current study did not exert an effect on ROS generated by the cultured cells, the method of light delivery is applicable to other systems including isolated mitochondria or more physiologically relevant organotypic slice culture models, and could be used to assess effects on a range of outcome measures of oxidative metabolism.
Keywords: Engineering, Issue 97, Red light therapy, reactive oxygen species, oxidative stress, photobiomodulation, optimization, irradiation
Introduction
Reactive oxygen species (ROS) are required for a range of signal transduction pathways and normal reactions of cellular metabolism, including those of neuroprotection 1. However, when endogenous antioxidant mechanism are unable to control the production of ROS, cells may succumb to oxidative stress 2,3. Following injury to the CNS, the associated increases in the presence of ROS and oxidative stress are thought to play a substantial role in the progression of damage 4,5. Despite the extensive number of strategies for attenuating oxidative stress that have been assessed, there are currently no completely effective, clinically relevant anti-oxidant strategies for attenuating ROS production and associated oxidative stress in clinical use following neurotrauma 6. Therefore the attenuation of oxidative stress remains an important goal for therapeutic intervention 7.
Improvements following R/NIR-LT have been reported in a wide range of injuries and diseases including reductions in cardial infarct size, renal and hepatic complications during diabetes, retinal degeneration, CNS injury and stroke 8, perhaps by reducing oxidative stress. With particular regard to CNS injury, preclinical studies of efficacy of 670nm light have shown good effects in models of retinal degeneration 9-11, spinal cord injury 12, neuronal death 13. Clinical trials have been conducted for dry age related macular degeneration and are currently underway for stroke 14, however the outcomes of these trials do not appear promising, perhaps due to a failure to employ effective treatment parameters 15. As such, R/NIR-LT has not been widely adopted as part of normal clinical practice in neurotrauma, despite being an easy to administer, non-invasive and relatively inexpensive treatment. Barriers to clinical translation include lack of a clearly understood mechanism of action and absence of a standardized effective treatment protocol 16,17. Current literature regarding light therapy reveals a plethora of variation in treatment parameters with respect to irradiation sources (LED or laser), wavelength (e.g., 630, 670, 780, 810, 830, 880, 904nm), total dose (joules of irradiation / unit area), duration (exposure time), timing (pre- or post- insult), treatment frequency and mode of delivery (pulse or continuous) 8. The variability in treatment parameters between studies makes comparison difficult and has contributed to skepticism regarding efficacy 16.
Therefore, optimization of R/NIR-LT is clearly required, with cell culture systems able to provide the high-throughput screening mechanism necessary to compare the multiple variables. However there are few commercially available illumination systems that can provide sufficient flexibility and control over wavelength and intensity to perform such optimization experiments. Commercially available LED devices are generally not able to deliver multiple wavelengths or intensities, resulting in investigators employing multiple LED devices from different manufacturers, which may vary not only in the intensity, but also the spectrum of wavelength of light emitted. We have addressed this issue by employing a broadband Xenon light source filtered through narrowband interference filters, thereby generating a range of wavelengths and fluences of light, allowing close, accurate control of the parameters of R/NIR-LT.
It is important to note that the therapeutic dose of treatment is defined by the number of photons interacting with the photoacceptor (chromophore), which, in the case of R/NIR-LT is postulated to be cytochrome c oxidase (COX) 18. Photon energy delivered varies with wavelength; meaning equal doses of energy at different wavelengths will be comprised of different numbers of photons. Therefore, the light emitted from the device was calibrated to emit an equal number of photons for each of the chosen wavelengths to be tested. We have developed a system that can be used to deliver R/NIR-LT at a range of wavelengths and intensities to cells in vitro and demonstrated the ability to measure the effects of the delivered R/NIR-LT on ROS production in cells subjected to glutamate stress.
Protocol
1. Optical Calibration: Measuring Light Output
To prepare the light delivery apparatus, connect a broadband light source (e.g., Xenon or tungsten lamp) to an appropriate power supply. Position a collimating lens in front of the light source to produce a collimated beam of light. Pass the light through a liquid heat filter to remove most of the heat from the light beam. Depending on the application, focus the collimated beam on to the entrance aperture of a liquid light guide, which provides for more flexible delivery of the light (e.g., into an incubator).
At the other end of the liquid light guide, position a second collimating lens and then a holder for the interference and neutral density filters. Check that this arrangement produces an evenly illuminated spot of light of the desired waveband and intensity. Note: The distance between the end of the light guide and the specimen plane may need to vary depending on the area that must be illuminated, but remember that as the distance from the end of the light guide increases, light intensity will decrease. In the present study, this distance is 14cm and produces a beam cross section that evenly illuminates a 3x3 well area on a 96-well plate.
Ensure that stray light from the lamp and associated optical components is unable to reach the specimen.
Turn on the water cooler for the liquid heat filter and ensure there is exchange of water through the filter jacket. Turn on the lamp power source and wait for at least 5 min for the broadband light source to stabilize. Note: The use of the water cooler is required to prevent over-heating of the device.
Select a narrowband interference filter (usually described by their center wavelength, peak transmittance and full width at half maximum (FWHM) bandwidth) to generate the desired waveband of illumination. Note: The narrowband interference filters used in the current study were 442 nm, 550 nm, 671 nm and 810 nm.
Measure the light produced by the lamp at the plane where the specimen is to be positioned during treatment. Measure light using a calibrated irradiance probe (cosine collector) connected to a suitable spectroradiometer, using propriety software according to manufacturer’s instructions.
Use neutral density filters to adjust the intensity of light until the desired output is obtained. Note: In the present study, the intensity of light passed by each interference filter is adjusted to give an equal quantal output at each of the four treatment wavebands. It is important to check the calibration regularly, as the lamp output is subject to change. Note: Following the set up of the apparatus, the cells are ready to be illuminated. Figure 1 is a representation of the light delivery apparatus used in the current study.
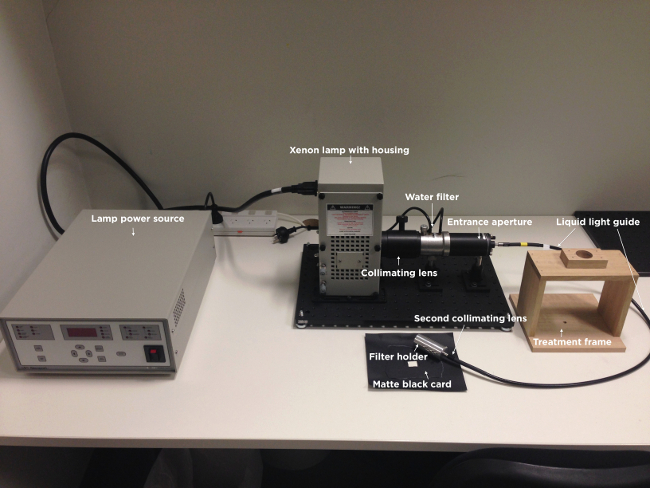
Figure 1. Image of the light delivery apparatus. Illustrated are the light power source, xenon lamp with housing, collimating lens, water filter, entrance aperture, liquid light guide, second collimating lens, filter holder, treatment frame and matte black card. Note that the narrowband wavelength and intensity filters are not shown.
2. Cell Preparation
The described R/NIR-LT delivery method can be applied to any cell type or in vitro model system; as such the following descriptions of cell culture are general, using well-established techniques to culture pheochromocytoma (PC12), Müller (rMC1) and primary mixed retinal cells.
Prior to cell seeding for light treatment and oxidative stress assay, culture immortalized cells in their respective growth media containing appropriate supplements (e.g., FBS, antibiotics) in T75 flasks until 70-80% confluent.
Detach immortalised cells from T75 flasks, using the method appropriate for the cell type to be tested e.g., trypsin. If necessary, before proceeding with the seeding of cells in 96-well assay plates, coat assay plate wells with 10µg/ml poly-L-lysine for 1h (e.g., for PC12 cells) or 10µg/ml poly-L-lysine for 1h followed by an O/N incubation with 10µg/ml laminin (e.g., for mixed retinal cells).
Centrifuge cells to collect as appropriate (e.g., 3 min at 405 x g for PC12 cells or 10 min at 218 x g for rMC1 cells), remove the supernatant and resuspend in 8 ml of appropriate cell culture media.
If mixed retinal cells are to be used, prepare from P0-5 neonatal rat pups by enzymatic digestion (papain) according to established procedures 19
Count the number of viable cells excluding 0.4% (w/v) trypan blue dye, using a haemocytometer.
Adjust cell density such that cells will be approximately 70-80% confluent after 24 or 48h in culture. (for PC12 cells the seeding density is 4.0 x 105 viable cells/ml, for rMC1 cells, it is 2.5 x 105 viable cells/ml and for mixed retinal cells it is 8 x 105 viable cells/ml). After adding cells to either clear or black 96-well plates (used for the H2O2 or DCFH-DA assay respectively), in 100 µl of appropriate growth media, allow plate to sit on a flat surface at 37°C, 5% CO2 (or whatever conditions are appropriate for the specific cell type) for at least 24 hr to allow cell adherence.
Culture cells in growth media in the appropriate 96 well trays until they are 70-80% confluent (24h for PC12 and rMC1 cells, 48h for mixed retinal cells).
3. Adding Glutamate Stressor to Cells
Prepare L-glutamic acid monosodium salt hydrate stressor concentrations of 0-10mM in appropriate full growth culture media.
Remove media from the cells and gently wash cells 3 times with PBS (PC12) or HBSS (rMC1 and mixed retinal cells). After washes, add glutamate-containing media to a total volume of 100 µl/well.
4. First Dosages of Light Treatment
- Immediately upon addition of glutamate or the stressor of choice, expose cells to light treatment at the desired wavelengths and intensities by placing under the prepared light delivery apparatus. Be sure to alter the quantal output of the light beam using the established combinations of neutral density filters depending on the wavelength being administered. Note: In the current experiments, expose cells to light treatment for 3 min maintain the temperature by resting the 96 well plates on a 37oC heat pad. Treatment time may be varied as required.
- Place a matte black card underneath the cells to prevent light reflecting into adjacent wells in the 96-well tray designated for other treatment parameters.
If treatment is desired for longer lengths of time, add 25mM Hepes buffer to maintain pH of the cell culture media or ideally, place the apparatus and treatment plates within an incubator with CO2 concentration regulated to 5% CO2, or conditions that are optimal for the specific cell type.
Following completion of the light treatment, place the cells on a level surface and incubate at 37°C and 5% CO2 (or whatever conditions are optimal for the cell type) for 24h. Note: Additional dosages of R/NIR-LT can be administered as described above, as desired.
5. Final Dosage of Light Treatment and Detection of ROS
Before performing the final round of light treatment, prepare the reagents to be used for ROS detection. Prepare 50mM citrate buffer (pH6.0), Triton X100, and H2O2 detection reagent working solution (1:2:97 ratio of 10mM H2O2 detection reagent stock solution; 10 U/ml horseradish peroxidase; 50mM sodium citrate (pH 6.0)). Prepare DCFH-DA at a final concentration of 100µM in appropriate media. Note: DCFH-DA reagent for PC12 cells is in RPMI media, DCFH-DA reagent for rMC1 cells is in DMEM media. Note that commercially available detection reagents have differential sensitivities to specific ROS and reagents should be chosen carefully to provide the information desired for an individual application.
Administer light treatment to the cells, as described in step 4.1-4.1.2. Ensure the cells are being treated with the desired fluences / wavelengths of light.
- Immediately following light treatment, remove the glutamate-containing media and wash twice with appropriate buffer solution (for PC12 cells, PBS is used and for rMC1 cells, HBSS is used). Perform the ROS assays on the cells as follows:
- H2O2 assay: add 45 µl of 50mM citrate buffer (pH 6.0) and 5 µl Triton X100 to each of the wells. Gently shake the cells on an orbital shaker for 30 sec and incubate at 37°C and 5% CO2 for 15 min. Add 50 µl of H2O2 detection working solution and incubate for 30 min at RT. Measure fluorescence using a plate reader with an excitation wavelength of 530nm and an emission wavelength of 480nm.
- DCF assay: add 100 µl of the 100 µM solution of DCFH-DA to each of the wells and incubate for 30min at 37°C and 5% CO2. Remove the media containing 100 µM DCFH-DA and wash with appropriate buffer solution (as described above) twice. Add 90 µl of buffer solution and 10 µl Triton X100 to each of the wells and gently shake on an orbital shaker for 30 sec before 15 min incubation at 37°C. Measure DCF derived fluorescence using a plate reader with an excitation wavelength of 480nm and an emission wavelength of 530nm.
- Express ROS values relative to the protein concentration of cells remaining in the wells, using a colorimetric kit to quantify protein concentration according to manufacturer’s instructions, with reference to a standard curve to calculate mg protein.
Representative Results
The output of light delivered at a wavelength of 670nm was calibrated using neutral density filters in order to irradiate cells with a range of fluences encompassing a dose of 670nm light previously shown to be beneficial in vivo (0.3 J/cm2) 20. As the number of neutral density filters in front of the light source increased, the intensity (W/m2) decreased, allowing less light to pass to the target area. Table 1 presents the calibration data of 670nm light generated from the light source fitted with a wavelength filter and includes the number of ND filters used and the intensity of light generated as a result, at described distances from the light output. Fluence, or dose of 670nm light (J/cm2), was calculated from the equation: [Dose (J/cm2) = (Light intensity (W/m2) / 10,000) x time (s)], where the time of treatment was 180s.
| Number of ND filters | Distance from light output (cm) | Intensity (W/m^2) | Dose (J/cm^2) |
| 0 | 10.5 | 20.11 | 0.38 |
| 0 | 14 | 10.55 | 0.19 |
| 1 | 14 | 4.91 | 0.075 |
| 2 | 14 | 2.28 | 0.041 |
| 3 | 14 | 1.03 | 0.018 |
| 4 | 14 | 0.47 | 0.0085 |
| 5 | 14 | 0.21 | 0.0038 |
| 6 | 14 | 0.094 | 0.00169 |
| 7 | 14 | 0.045 | 0.00081 |
| 8 | 14 | 0.021 | 0.000378 |
| 9 | 14 | 0.013 | 0.000234 |
Table 1: Output of the light delivery apparatus fitted with the 670nm wavelength filter.Number of ND filters refers to the number of neutral density filters fitted to the front of the light source output. Intensity (W/m2) refers to the intensity of the light as reported by the propriety software. Fluence, or dose was calculated by the equation[Dose (J/cm2) = (Light intensity (W/m2) / 10,000) x time (s)], where the time of exposure was 180s and distance from the light output was 10.5 or 14 cm.
Two clinically relevant quantal fluences of light were chosen to investigate differential effects of R/NIR-LT wavelength on production of ROS. A dose that can reach CNS tracts following transmission through overlying tissue using LED devices(i.e., 1.78 W/m2 at 670nm) 20 equated to 0.03 J/cm2 for a 3 min treatment, or 4.9 x 1014 photons/cm2/s. The light source equipped with filters to result in emission of 442, 550, 670 or 830nm was then calibrated using combinations of neutral density filters to emit equal quantal outputs (photons) for each wavelength as opposed to energy outputs (J/cm2), and the dosages (J/cm2) and intensities in W/m2 calculated (Table 2a). An additional higher dose that was within the recommended guidelines to stimulate cellular activity 21 was also used (1.29 x 1015 photons/cm2/s), and calibration conducted for each wavelength (Table 2b).
| Wavelength (λ) | Dose (J/cm^2) | Intensity (W/m^2) | Emission (Photons/cm^2/s) |
| 442 | 0.057 | 3.21 | 4.8 x 10^14 |
| 550 | 0.051 | 2.87 | 5.0 x 10^14 |
| 670 | 0.032 | 1.78 | 4.9 x 10^14 |
| 830 | 0.018 | 1.01 | 4.9 x 10^14 |
Table 2: Calibration of intensity of dosage delivered by equal numbers of photons of light at varying wavelengths. Intensity (W/m2), dosage for a 3 min treatment (J/cm2) and emission (photons/cm2/s) when output of xenon light emitting 442, 550, 670 and 830nm are calibrated to emit A) 4.9 x 1014 photons/cm2/s or B) 1.3 x 1015 photons/cm2/s at a distance of 14 cm from the light output.
In order to assess whether the light delivered by the apparatus was toxic to cells at the dosages used, we assessed the protein content remaining in PC12 cell culture wells following the ROS assays, using a colorimetric protein assay. There was no significant loss of protein at any of the higher output dosages of the light (P > 0.05), indicating that the dosage of light delivered was not causing cell death (Figure 2) and is appropriate to use for assessments of oxidative metabolism.
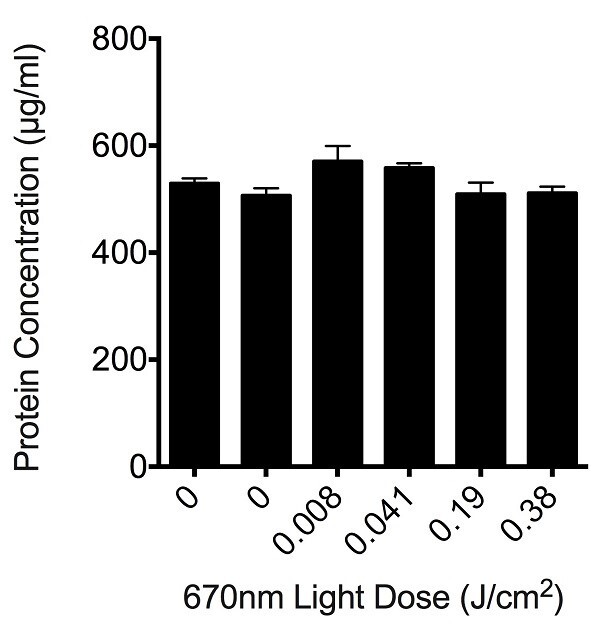
Figure 2: Effect of varying doses of light on the total protein concentration remaining in culture wells following R/NIR-LT and ROS assay. Histogram bars are the mean ± S.E.M protein concentrations in PC12 cell culture wells, 6 replicates / concentration, experiments were repeated 3 times. There were no statistically significant differences between control and any of the treatment groups as determined by analysis of variance (ANOVA), p > 0.05.
We initially assessed the effects of 670nm light, delivered at fluences ranging from 0.0085 to 0.38 J/cm2, as an example wavelength to assess suitability of the light delivery apparatus. No significant effect of 670nm light was observed at any of the fluences tested when assessing either H2O2 or DCF fluorescence in PC12, rMC1 or mixed retinal cells stressed with glutamate (Figure 3, P > 0.05). Similarly, there were no significant effects of varying wavelengths of R/NIR-LT delivered at 4.9 x 1014 photons/cm2/s or 1.3 x 1015 photons/cm2/s on ROS production, when assessing H2O2 or DCF fluorescence (P > 0.05, data not shown). Our ability to detect changes in reactive species is confirmed by an increase in fluorescence of the DCFH-DA reactive dye at 13.44 ± 0.67 mM glutamate to 22.10 ±2.10 at 10mM glutamate. While our data do not reveal positive effects of R/NIR-LT delivered using our light delivery apparatus on ROS production in the selected model system, neither were there negative effects as cells were not compromised, indicated by sustained protein content in culture wells following light therapy (Figure 2). As such, the described method provides a protocol for treating cells or mitochondria with a defined dosage of photons at a range of wavelengths and may be used to assess higher dosages and alternative outcome measures, which may enable optimization of R/NIR-LT parameters.
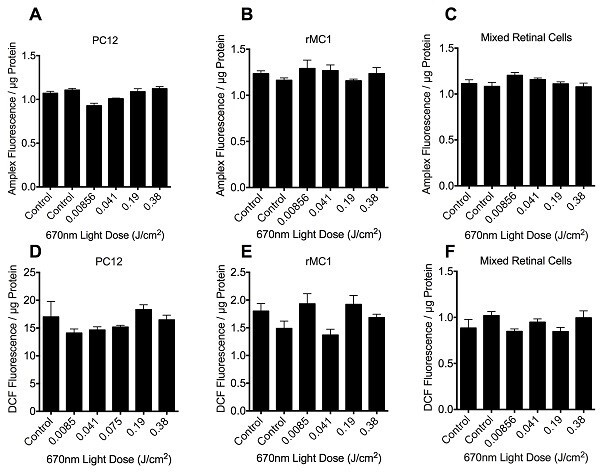
Figure 3:Quantification of H2O2 (A-C) and DCF (D-F) fluorescence in PC12 (A, D), rMC1 (B, E) or mixed retinal cell (C, F) cultures in the presence of 10mM glutamate stressor, following 670nm irradiation therapy at fluence doses ranging from 0 – 0.38 J/cm2. Histogram bars represent the mean arbitrary fluorescence units / µg protein ± SEM. There were no statistically significant differences between control and any of the treatment groups as determined by ANOVA, p > 0.05, 6 replicates per group, experiments were repeated 3 times.
Discussion
We have successfully adapted a precise and calibrated light delivery system to provide a mechanism for study of optimization of R/NIR-LT in vitro. Wavelength and intensity parameters of R/NIR-LT are able to be manipulated accurately and effectively using this system. We established that light treatment of the cells did not lead to cell death, although ROS were not reduced at the wavelengths and dosages delivered, in the cell types tested. The maximum intensities achieved by the current system at 670nm (20.11W/m2) are in excess of previously published measures of penetrance of irradiation into the rat brain, where 1.17 W/m2 reached the ventral surface of the optic nerve and 0.3W/m2 reached the ventral surface of the brain case. When delivered for 30 min, the dose received by cells of the optic nerve in vivo is therefore 0.054 – 0.31 J/cm2. The doses of 670nm light delivered in the current study (up to 0.38J/cm2) therefore encompass those received by cells in our previous in vivo studies.
The most widely accepted theory of R/NIR-LT efficacy is that of a photoacceptor based system 18, therefore it is imperative to ensure that cells treated with varying wavelengths receive equal quantal fluences of light (photons/cm2/s) 8. In addition, the exposure time used to deliver a particular fluence of light must be kept consistent between treatments, with only the intensity of the light changing to increase and decrease total fluence over time. Previous studies have demonstrated that changing the exposure time to deliver equal fluences results in differences in outcome measures and may act as a confounding factor, and hinder the identification of the optimal wavelength / fluence parameters required for a particular injury model 22,23. As such, we recommend the careful calibration of dosage at each wavelength tested, to ensure useful optimization of R/NIR-LT.
The described technique allows delivery of light to a range of in vitro model systems including organotypic slice cultures, which may result in more meaningful outcomes due to the preservation of cellular architecture and complex inter-cellular interactions. Variations on the injury model may require differences in treatment parameters of R/NIR-LT. This technique is limited by the maximum power output achievable with the described instrumentation. Higher power outputs may be required for some in vitro and most in vivo applications. If higher outputs of light are required, the apparatus may be altered to increase the intensity of light reaching the samples. Shortening the distance between the light termination and target cells will increase the intensity of light reaching the plate. Alternatively, the light may be reflected off a mirror and onto the cells as opposed to being filtered through a liquid light guide, however alternative wavelength and neutral density filters will be required to achieve this. Higher outputs using a more powerful light source would also be required if the method were to be adapted for delivery of R/NIR-LT in in vivo models of CNS injury, due to the increased dosages required to ensure effective penetrance of irradiation 8,20.
In conclusion, the current study describes a novel method of light delivery that provides a means of effectively altering intensity and wavelength parameters of light in R/NIR-LT studies. This methodology will be useful in optimization of R/NIR-LT employing a range of outcome measures and in vitro injury models.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by the Neurotrauma Research Program (Western Australia). This project is funded through the Road Trauma Trust Account, but does not reflect views or recommendations of the Road Safety Council.
References
- Gutterman DD. Mitochondria and reactive oxygen species an evolution in function. Circulation research. 2005;97:302–304. doi: 10.1161/01.RES.0000179773.18195.12. [DOI] [PubMed] [Google Scholar]
- Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. American Journal of Physiology-Cell Physiology. 2006;291:C1082–C1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radical Biology and Medicine. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacology & therapeutics. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Hall ED. Antioxidant therapies for acute spinal cord injury. Neurotherapeutics. 2011;8:152–167. doi: 10.1007/s13311-011-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, et al. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012;50:264–274. doi: 10.1038/sc.2011.111. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, et al. Red/near-infrared irradiation therapy for treatment of central nervous system injuries and disorders. Reviews in the Neurosciences. 2013;24:205–226. doi: 10.1515/revneuro-2012-0086. [DOI] [PubMed] [Google Scholar]
- Rutar M, Natoli R, Albarracin R, Valter K, Provis J. 670-nm light treatment reduces complement propagation following retinal degeneration. J Neuroinflammation. 2012;9:257. doi: 10.1186/1742-2094-9-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eells JT, et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proceedings of the National Academy of Sciences. 2003;100:3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum R, Powner MB, Hudson N, Hogg C, Jeffery G. Treatment with 670 nm light up regulates cytochrome C oxidase expression and reduces inflammation in an age-related macular degeneration model. PloS one. 2013;8:e57828. doi: 10.1371/journal.pone.0057828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes KR, et al. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers in surgery and medicine. 2005;36:171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- Liang HL, et al. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 2006;139:639–649. doi: 10.1016/j.neuroscience.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Zivin JA, et al. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009;40:1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. Transcranial near-infrared laser therapy applied to promote clinical recovery in acute and chronic neurodegenerative diseases. Expert review of medical devices. 2012;9:71–83. doi: 10.1586/erd.11.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, et al. The nuts and bolts of low-level laser (light) therapy. Annals of biomedical engineering. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, et al. Low-Level Laser Therapy for Closed-Head Traumatic Brain Injury in Mice: Effect of Different Wavelengths. Lasers in surgery and medicine. 2012;44:218–226. doi: 10.1002/lsm.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JT, et al. Role of low-level laser therapy in neurorehabilitation. PM&R. 2010;2:S292–S305. doi: 10.1016/j.pmrj.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, et al. Metallothionein-IIA promotes neurite growth via the megalin receptor. Experimental Brain Research. 2007;183:171–180. doi: 10.1007/s00221-007-1032-y. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, et al. Near infrared light reduces oxidative stress and preserves function in CNS tissue vulnerable to secondary degeneration following partial transection of the optic nerve. Journal of neurotrauma. 2010;27:2107–2119. doi: 10.1089/neu.2010.1426. [DOI] [PubMed] [Google Scholar]
- Ando T, et al. Low-level laser therapy for spinal cord injury in rats: effects of polarization. Journal of biomedical. 2013;18:098002. doi: 10.1117/1.JBO.18.9.098002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzafame RJ, et al. Reciprocity of exposure time and irradiance on energy density during photoradiation on wound healing in a murine pressure ulcer model. Lasers in surgery and medicine. 2007;39:534–542. doi: 10.1002/lsm.20519. [DOI] [PubMed] [Google Scholar]
- Castano AP, et al. Low-level laser therapy for zymosan induced arthritis in rats: Importance of illumination time. Lasers in surgery and medicine. 2007;39:543–550. doi: 10.1002/lsm.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]


