Abstract
Influenza infection is associated with about 36,000 deaths and more than 200,000 hospitalizations every year in the United States. The continuous emergence of new influenza virus strains due to mutation and re-assortment complicates the control of the virus and necessitates the permanent development of novel drugs and vaccines. The laboratory-based study of influenza requires a reliable and cost-effective method for the propagation of the virus. Here, a comprehensive protocol is provided for influenza A virus propagation in fertile chicken eggs, which consistently yields high titer viral stocks. In brief, serum pathogen-free (SPF) fertilized chicken eggs are incubated at 37 °C and 55-60% humidity for 10 – 11 days. Over this period, embryo development can be easily monitored using an egg candler. Virus inoculation is carried out by injection of virus stock into the allantoic cavity using a needle. After 2 days of incubation at 37 °C, the eggs are chilled for at least 4 hr at 4 °C. The eggshell above the air sac and the chorioallantoic membrane are then carefully opened, and the allantoic fluid containing the virus is harvested. The fluid is cleared from debris by centrifugation, aliquoted and transferred to -80 °C for long-term storage. The large amount (5-10 ml of virus-containing fluid per egg) and high virus titer which is usually achieved with this protocol has made the usage of eggs for virus preparation our favorable method, in particular for in vitro studies which require large quantities of virus in which high dosages of the same virus stock are needed.
Keywords: Infection, Issue 97, Influenza, A/PR/8/34, chicken eggs, allantoic fluid, virus growth, influenza virus propagation
Introduction
Influenza A continues to be a major threat to human health. It is a potentially devastating respiratory disease with a large global burden causing up to 500,000 deaths worldwide annually 1. Influenza viruses are in the family Orthomyxoviridae and carry 8 negative-sense single-stranded RNA in their genome 1,2. The high mutability (i.e., antigenic “drift”) of the viral genome prevents long-term immunity. Moreover, influenza is increasingly resistant to anti-viral drugs 3.
The 2009 H1N1 influenza pandemic highlighted all the challenging issues associated with influenza disease (pandemic strain, anti-viral resistance, delayed vaccine production). The vaccine formulation is determined annually by the World Health Organization for the most likely pathogenic influenza strains (one each for H1N1, H3N2 and influenza B) 4. Because this method is based on predicted influenza strains, pathogenic strains are occasionally incorrectly identified and vaccine efficacy drops dramatically. Moreover, the appearance of novel influenza strains can circumvent these preventative programs causing pandemic disease 2.
The creation of a universal influenza vaccine has been elusive 5. Therefore, research must continue into understanding the pathogenesis of lung injury. To facility laboratory research, several methods have been developed for viral isolation and propagation 6. Human influenza viruses can be amplified in a variety of mammalian cell substrates. However, generating high titer viruses in large quantities are best achieved in embryonated eggs. The following protocol describes a technique for influenza A virus propagation and storage from existing virus stocks.
Protocol
NOTE: General remarks: Perform all procedures involving manipulation of the egg under sterile conditions, and sterile technique should be used accordingly. Pre-clean all equipment with 70% ethanol before use. In general, influenza virus inoculation and harvest should be conducted in a BSL-2 laboratory. However, if this protocol is used to propagate much more pathogenic influenza viruses (e.g., pandemic and pre-pandemic strains, strains that pose a danger to poultry and livestock, highly pathogenic avian influenza strains, the 1918 H1N1 strain), higher biosafety levels and in some cases, biosecurity precautions and authorizations are required. The local Institutional Biosafety Committee should approve all research involving influenza virus.
1. Preparation of Eggs for Inoculation
Obtain serum pathogen-free (SPF) freshly fertilized avian eggs from a suitable vendor. Typically, chicken eggs are used. Eggs should be used immediately upon arrival.
Upon arrival place eggs in a humidified egg incubator with an automatic egg turner to rotate eggs regularly. If an automatic egg turner is not available, manually rotate eggs at least 2-3 times a day (make sure to wear gloves and keep everything as sterile as possible).
Incubate eggs at 37 °C and 55-60% humidity for 10-11 days.
2. Egg Candling
- Use an egg candler to examine the egg against a bright light in a dark room to check eggs for infertility by candling after about 7 or 8 days of incubation.
- Wipe the egg candler with 70% ethanol. Change gloves often and disinfect with 70% ethanol to minimize the risk of contamination by pathogens.
- Remove the eggs from the incubator and place them in empty egg cartons.
- Turn off the lights in the room.
- Hold the large end of each egg one at a time against the candler.
- Observe the egg to determine if it is fertile or infertile. NOTE: Thin blood vessels leading to a bean-shaped embryo should be clearly visible. Unfertilized eggs will appear as a small blood spot with a visible egg yolk.
Discard eggs that are unfertilized, and return the viable eggs to the incubator. Do not leave eggs outside of the incubator for more than 30 min. If the status of an egg cannot be confirmed, mark it, and observe it later.
3. Preparation of Virus Inoculum
Dilute influenza virus stock to about 103 – 104 pfu/ml in sterile PBS containing 10 mM HEPES (pH 7.4). Critical step: Dilute the virus stock immediately before use and keep the diluted virus on ice all the time.
4. Influenza Virus Inoculation via the Allantoic Route
On the day of virus inoculation (day 10 or 11), candle the eggs again and eliminate any dead embryos. Distinguish live embryos by looking for movement inside the egg.
Remove three eggs from the incubator, and arrange the eggs in a separate holder with the air sac up.
Mark the air space of the eggs with a permanent marker.
Wash the eggshell above the air space with 70% ethanol.
Put the eggs with the air space up in an egg carton and into a biosafety hood (BSL-2).
Use a sterile 18G needle to punch a small hole in the shell over the air sac of each egg. Be careful not to insert the needle too deeply to avoid stabbing the embryo or yolk.
Draw up the diluted influenza virus into a sterile 1 ml syringe and attach a 22G needle.
Carefully advance the syringe with needle at a 45° angle into the allantoic cavity.
Inject 0.2 ml of the diluted influenza virus.
Repeat the injection with the other two eggs, and then discard the syringe and needle.
Seal the hole with a drop of glue from a glue gun (or melted paraffin wax). Be careful that glue does not leak inside the egg.
Place the eggs back into the egg incubator with the air space pointed upward.
Repeat steps 4.2 – 4.12 until all the eggs have been inoculated.
5. Incubation Time
Incubate the infected eggs without turning at 37 °C and ~60% humidity.
Choose optimal incubation time depending on the influenza virus type. Incubate influenza A for 48 h; incubate influenza B and C for 72 hr.
6. Influenza Virus Harvest
After incubation period, chill the eggs at 4 °C for a minimum of 4 hr (or O/N) to kill the embryo and constrict the blood vessels to reduce the risk of contaminating the infected allantoic fluid with blood. Do this to prevent the red blood cells from absorbing the virus, which reduces the yield. Alternatively, chill eggs for 30 min at -20 °C; however this increases the risk of blood contamination.
After the eggs have been sufficiently chilled, transfer the eggs to a biosafety hood. Place the egg into a firm holder with the air sac facing up. Clean the egg surface with 70% ethanol.
Use sterile scissors to open the eggshell above the air sac. Remove the shell around the air sac while being careful not to destroy the chorioallantoic membrane.
Open the chorioallantoic membrane with sterile blunt forceps. Gently move aside the embryo and the yolk sac with a small spatula or spoon. Take care not to rupture the yolk.
Using a 1 ml Eppendorf pipette, carefully collect the allantoic fluid. If needed, tilt the egg a bit to the side to collect as much fluid as possible.
Combine the allantoic fluid from all the eggs into a 50 ml plastic conical tube. Keep tubes on ice at all times during virus harvest. One egg yields 5 – 10 ml of a slightly yellowish fluid.
Spin the virus-containing allantoic fluid at 1,000 x g for 10 min at 4 °C to pellet debris. Transfer the clear fluid into a new 50 ml tube kept on ice. Dispose eggs into a BSL-2 waste container.
7. Storage
Immediately aliquot the clarified allantoic fluid (e.g., 50, 100 or 500 ul aliquots) and snap-freeze in liquid nitrogen. Transfer frozen virus stocks to -80 °C freezer for long-term storage.
Representative Results
Multiple methods have been developed to titer influenza virus. One consideration when choosing an appropriate method is that some determine total particles regardless of viability (e.g., hemagglutinin assay) whereas others are based on infectivity, which will assess viable virions (e.g., plaque assay) 6. Here, the viral titer of allantoic fluid collected from chicken eggs inoculated with a mouse-adapted influenza virus (A/PR/8/34) was determined by plaque assay (Figure 1) and hemagglutination assay (Figure 2).
Influenza virus infection causes a cytopathic effect that result in plaques (circular zones of lysed cells) to form on a monolayer of cells. Plaques were visualized by staining the cells with crystal violet, and 65 plaques were counted in the well with the 10-12 dilution of virus (Figure 1). Because 500 L of diluted viral stock was placed on the cells, the final titer is 1.3 x 1014 PFU/ml.
The hemagglutination assay is a method to titer influenza virus bases on the ability of the virus to attach to the surface of red blood cells. A viral suspension will agglutinate the red blood cells, thus preventing them from settling out of suspension. By using serial dilutions of virus in a 96-well plate (either V- or round bottom) and adding a fixed amount of red blood cells, the viral titer can be determined. A clearly negative result was observed with the 1:210 dilution of virus in 50 µl (Figure 2). Therefore, the final titer is 2.1 x 104 HAU/ml.
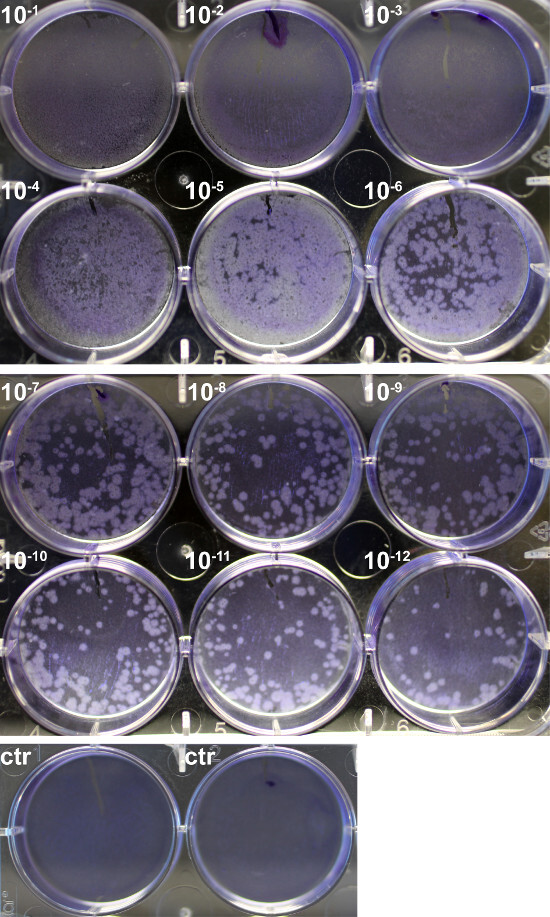 Figure 1. Quantification of PR8 Virus Titer by Plaque Assay. A monolayer of MDCK cells was infected with 10-fold serial dilutions of PR8 (10-1 – 10-12) for 1 hr, washed, and then overlaid with a 0.6% agarose solution. After incubation at 37 °C for 72 hr, agar was carefully removed, and cells were fixed and stained with 0.25% crystal violet. Plaque formation was compare to an uninfected control (ctr). Please click here to view a larger version of this figure.
Figure 1. Quantification of PR8 Virus Titer by Plaque Assay. A monolayer of MDCK cells was infected with 10-fold serial dilutions of PR8 (10-1 – 10-12) for 1 hr, washed, and then overlaid with a 0.6% agarose solution. After incubation at 37 °C for 72 hr, agar was carefully removed, and cells were fixed and stained with 0.25% crystal violet. Plaque formation was compare to an uninfected control (ctr). Please click here to view a larger version of this figure.
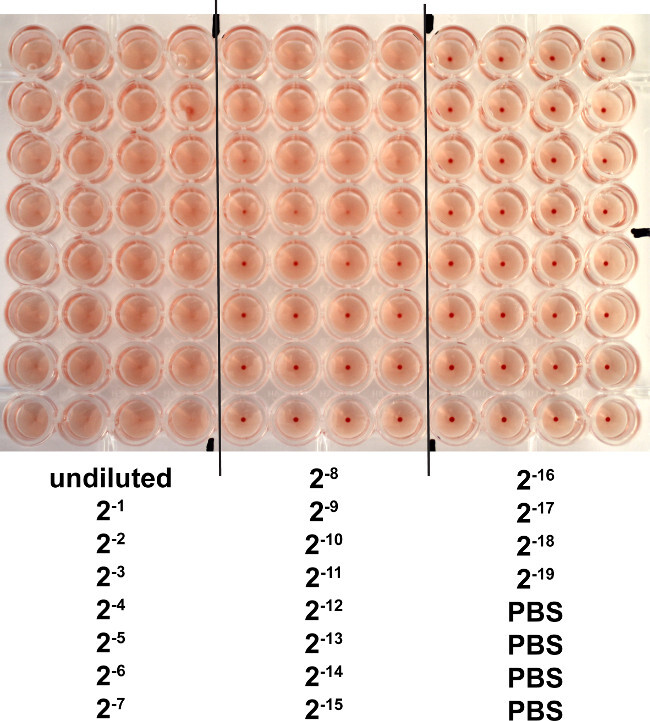 Figure 2. Hemagglutination (HA) Assay to Titer Egg-derived PR8 influenza Virus. PR8 virus was serially diluted 2-fold in PBS and mixed with 50 l of 0.5% turkey red blood cells. Dilutions were prepared in quadruplicates, and pictures were taken after 1 hr incubation at RT. Negative results appear as dots in the center of the wells whereas positive results form a uniform reddish color across the well. Please click here to view a larger version of this figure.
Figure 2. Hemagglutination (HA) Assay to Titer Egg-derived PR8 influenza Virus. PR8 virus was serially diluted 2-fold in PBS and mixed with 50 l of 0.5% turkey red blood cells. Dilutions were prepared in quadruplicates, and pictures were taken after 1 hr incubation at RT. Negative results appear as dots in the center of the wells whereas positive results form a uniform reddish color across the well. Please click here to view a larger version of this figure.
Discussion
Influenza causes a large global burden of disease, and work continues into understanding the pathogenesis of lung injury 7. To facilitated research in this deadly disease, various methods have been developed to propagate the influenza virus 6. Here, we describe a technique to produce influenza virus in chicken eggs. The advantage of this method is that it is highly reproducible and results in large quantities of high titer influenza virus stocks, which is often necessary for in vitro studies.
Most laboratories will be able to develop this protocol quickly and easily with minimal start-up costs. Although we recommend investing in an egg incubator with automatic turner, this protocol can be completed without such equipment, but will be more laborious. We also recommend using commercial research vendors for purchase of fertilized eggs to guarantee consistency and sterility of the eggs. The Office of Laboratory Animal Welfare (OLAW) interpretation of the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals states for the research use of live embryonated avian eggs that the PHS policy is only applicable after hatching. Therefore, most institutions will not require IACUC approval for this protocol. However, policies vary among institutions, and Institutional Biosafety Committee approval will be required before initiating this protocol.
When working with the virus, it is paramount to keep the virus at 4 C after harvesting the allantoic fluid from the infected embryonated egg. Each egg is expected to produce between 5-10 ml of allantoic fluid, and the titer depends on the strain of influenza virus. Our lab has routinely used this method to propagate a mouse-adapted H1N1 influenza strain (A/PR/8/34). This protocol can also be used for clinical samples, but in this situation, inoculation of the amniotic cavity may be preferable due to the presence of 2,6-linked sialic acids needed for binding within the amniotic lining 8,9. Another consideration is that this method may not be ideal for propagation avian influenza viruses (e.g., H5N1 strains) due to the pathogenicity to the embryo.
Various methods of titering the influenza virus have been developed all of which have their advantages and disadvantages. Indeed, viral quantification can be protein-based (e.g., hemagglutinin assay, ELISA), nucleic acid-based (e.g., quantitative PCR), or functional (e.g., viral plaque assay) 6. Non-functional assays will determine total viral titers regardless of if they are living or dead viral particles. Therefore, testing for the infectious activity of the influenza virus will measure living viruses and allow for comparable functional equivalence from lot to lot. The plaque-forming assay is a commonly used assay that can assess titers of live influenza virus, but can be slow and cumbersome to perform 10. In comparison, the hemagglutinin assay is simple and straightforward, but does not assess viability and determines all influenza particles.
Herein, we provide a relatively easy method to propagate high titer influenza virus. The de novo initiation of this protocol in a lab is straightforward, and the starting materials and equipment are relatively inexpensive.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The present study was supported by the NIH grants HL120947 (P.C.), HL103868 (P.C.), and the American Heart Association Grant-in-Aid (P.C.)
References
- Centers for Disease Control and Prevention (CDC) Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR. Morbidity and mortality weekly report. 2010;59(33):1057–1062. [PubMed] [Google Scholar]
- Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459(7249):931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey TM, Garcia-Sastre A, et al. Pathogenicity of Influenza Viruses with Genes from the 1918 Pandemic Virus: Functional Roles of Alveolar Macrophages and Neutrophils in Limiting Virus Replication and Mortality in Mice. J Virol. 2005;79(23):14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immu. 2006;7(5):449–455. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- Webster RG, Govorkova EA. Continuing challenges in influenza. Ann N Y Acad Sci. 2014;1323(1):115–139. doi: 10.1111/nyas.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szretter KJ, Balish AL, Katz JM. Current protocols in microbiology. Wiley; 2006. Unit 15G.1 Influenza: propagation, quantification, and storage. [DOI] [PubMed] [Google Scholar]
- Short KR, Kroeze EJBV, Fouchier RAM, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis. 2014;14(4):57–69. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- Ito T, Suzuki Y, et al. Differences in sialic acid-galactose linkages in the chicken egg amnion and allantois influence human influenza virus receptor specificity and variant selection. J Virol. 1997;71(4):3357–3362. doi: 10.1128/jvi.71.4.3357-3362.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JS, Nicolson C, Major D, Robertson EW, Wood JM. The role of amniotic passage in the egg-adaptation of human influenza virus is revealed by haemagglutinin sequence analyses. J Gen Virol. 1993;74(Pt 10):2047–2051. doi: 10.1099/0022-1317-74-10-2047. [DOI] [PubMed] [Google Scholar]
- Gaush CR, Smith TF. Replication and plaque assay of influenza virus in an established line of canine kidney cells) Appl Microbiol. 1968;16(4):588–594. doi: 10.1128/am.16.4.588-594.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


