Abstract
Endosomal acidification is critical for a wide range of processes, such as protein recycling and degradation, receptor desensitization, and neurotransmitter loading in synaptic vesicles. This acidification is described to be mediated by proton ATPases, coupled to ClC chloride transporters. Highly-conserved electroneutral protons transporters, the Na+/H+ exchangers (NHE) 6, 7 and 9 are also expressed in these compartments. Mutations in their genes have been linked with human cognitive and neurodegenerative diseases. Paradoxically, their roles remain elusive, as their intracellular localization has prevented detailed functional characterization. This manuscript shows a method to solve this problem. This consists of the selection of mutant cell lines, capable of surviving acute cytosolic acidification by retaining intracellular NHEs at the plasma membrane. It then depicts two complementary protocols to measure the ion selectivity and activity of these exchangers: (i) one based on intracellular pH measurements using fluorescence video microscopy, and (ii) one based on the fast kinetics of lithium uptake. Such protocols can be extrapolated to measure other non-electrogenic transporters. Furthermore, the selection procedure presented here generates cells with an intracellular retention defective phenotype. Therefore these cells will also express other vesicular membrane proteins at the plasma membrane. The experimental strategy depicted here may therefore constitute a potentially powerful tool to study other intracellular proteins that will be then expressed at the plasma membrane together with the vesicular Na+/H+ exchangers used for the selection.
Keywords: Cellular Biology, Issue 97, Intracellular compartments, Somatic cell genetics, Na+/H+ exchangers. Intracellular pH measurements. Fast kinetics of ion flux. Kinetic parameters.
Introduction
Most intracellular compartments display an acidic luminal pH, which is a key parameter for maturation, trafficking, recycling of proteins or hormones and neurotransmitters loading. It has been shown that the pH gradient between cytosol and vesicular content is generated by vacuolar H+ ATPases1, coupled to vesicular ClC chloride transporters2. Both in knock-out (KO) mice and human patients, the importance of these transporters has been highlighted by the heavy phenotypes caused by mutations in their genes3-6.
The members of the Sodium-Hydrogen exchangers SLC9A family, also termed NHEs for Na+/H+ exchangers, have been shown to be key effectors in intracellular pH and cell volume regulation, as well as in vectorial transport of acid-base equivalents across epithelia. Besides the plasma membrane NHEs, three highly conserved Na+/H+ exchangers, NHE 6, 7 and 9 are expressed in trans-Golgi network and in early endosomes7. Mutations in their genes have been linked with Angelman-like or Christianson Syndromes8-9, family-based autism10 and Attention Deficit Hyperactivity Disorder11-12. These exchangers have also been involved in neurodegenerative problems such as Alzheimer disease susceptibility13 and X-linked mental retardation contiguous genes syndromes14. Taken together, these studies highlight the importance of these intracellular NHEs in brain development and/or function.
The intracellular localization of these exchangers prevents accurate measurements of their ion selectivity, transport direction, kinetic parameters, and regulation. As is the case for all transporters expressed in intracellular compartments, it is extremely difficult to assess their biochemical activities and hence to fully understand their physiological roles and the mechanisms underlying their pathological implications. Based on the high cytosolic K+ concentration, the most-commonly accepted hypothesis was that they were working as K+ coupled proton efflux transporters. The existence of such a proton leak had been hypothesized, as it may counterbalance proton pumping by the V-ATPases in order to maintain a steady state vesicular pH. The aim of this visual article is (i) to demonstrate a method that allows the genetic selection of cell lines that express such vesicular transporters at their plasma membrane, and (ii) to show two independent approaches to measure the functions of these transporters.
Three decades ago, Pouysségur and Franchi have pioneered a genetic approach that enabled the molecular cloning and characterization of the members of the NHE family15. This was based on the toxicity of intracellular protons as a screening method. The first step was to obtain cell lines deficient in any Na+/H+ exchange expressed at the plasma membrane, using the reversibility of this transporter. Fibroblasts (CCL39 cell line) were preloaded with Na+ or Li+ and then placed in an acidic extracellular medium (pH 6.5) for 2 hr. This led to the death of cells expressing a functional Na+/H+ exchange and to the selection of antiporter-deficient cells (PS120 cell line)16. When cultivated in bicarbonate-free medium, these cells are very sensitive to acute intracellular acidification. Consequently, the expression of any functional proton efflux mechanism at the plasma membrane will be positively selected (see17) if such cells are submitted to acute intracellular acidifications. Such acidification techniques can be used to isolate cell lines with trafficking defects enabling the forced expression of WT intracellular NHEs at the plasma membrane.
As eukaryotic Na+/H+ exchangers are electroneutral, they are not measurable by the electrophysiological approaches that have been used with great success to measure channels. This manuscript therefore demonstrates how to measure the activity of this exchanger by intracellular pH measurements and rapid kinetics of lithium uptake. As the underlying concepts are the same, it is interesting to notice that many of the processes developed for the selection section are also used directly for functional measurements.
Interestingly, we have observed that the trafficking defect present in the cell lines selected using the approach described in this manuscript leads to a greater expression of other vesicular proteins at the plasma membrane such as the vesicular potassium channel TWIK118. This points out toward the selection of a general retention defect mechanism for vesicular transmembrane proteins. Hence this selection procedure and the cells that it generates may constitute a promising tool for the scientific community working on the membrane proteins of intracellular compartments. As well the measurement techniques presented here might be applicable for studying other non-electrogenic transporters.
Protocol
1. H+ Killing Selection
- Cell lines
- Stably transfect NHE-deficient cells (e.g., the CCL39-derived PS120 cell line16) using any combination of mammalian expression vector and transfection method that will produce efficient transfection yields and selection in a fibroblast cell line. NOTE: For many years Calcium Phosphate precipitation19 was used with good transfection yields. This has been replaced more recently by commercial reagents such as Lipofectamine2000, the transfections being performed following the manufacturer’s instructions.
- Solutions
- Prepare three sterile solutions described as below. Sterilize these solutions by filtration (0.22 µm).
- Prepare a NH4+ Loading solution (pH 7.4) comprised of 50 mM NH4Cl, 70 mM choline chloride, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose and 15 mM MOPS at pH 7.4.
- Prepare a rinse Solution (pH 7.0) comprised of 120 mM choline chloride, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose and 15 mM HEPES at pH 7.0.
- Prepare a Recovery solution (pH 7.4) comprised of 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose and 15 mM HEPES at pH 7.4.
Before starting the selection, obtain a cell culture incubator that can be used at 37 °C with no additional CO2 from external tank (termed CO2-“free” incubator), as 5% CO2 will result in buffering that may impair the acidification. Amplify the cell line expressing the NHE of interest up to about 2 x 108 cells (typically about 20 confluent 100 mm plates).
- H+ killing procedure:
- Incubate the cells expressing the intracellular NHE of interest for 1 hour in the Loading Solution, at 37 °C in the CO2-free incubator.
- Aspirate the loading solution and then rinse them twice in the above described-rinse solution. Perform this step efficiently to eliminate the residual extracellular NH4Cl that would impair the creation of a steep NH4+ gradient that will generate the acidification.
- Eliminate the rinse medium by aspiration and incubate the cells for one hour in recovery solution again at 37 °C in the CO2 free incubator.
- Replace the recovery medium with regular culture medium (typically DMEM with 7.5% FCS) and grow the cells in standard culture conditions (37 °C in a humidified atmosphere of 5% CO2 and 95% air).
- Repeat this cycle of selection twice a week until stable clones emerge. NOTE: Take special care concerning the cell culture and selection conditions. Months of work can be ruined by any contamination that is more prone to occur after a long period of culture and repeated cell manipulations.
- Here, choose whether to amplify the clones and characterize them individually as further described in sections 2-3, or pool them together to generate a cellular population before characterization.
Apply occasional selection (about once every two weeks) to retain the acidification-resistant cells by counter-selecting those that may have reverted to the parental acid-sensitive phenotype (Figure 1B).
2. Intracellular pH Measurements
2.1) Fluorescence pH Imaging
- Use the ratiometric pH-sensitive fluorescent dye BCECF/AM, which is pH sensitive when excited at 490 nm and possesses a 445 nm isosbestic point, following manufacturer’s protocols.
- Alternatively, use other pH–sensitive probes, following the manufacturer’s protocols.
- Use an imaging set consisting of an inverted microscope coupled to a high sensitivity video camera. Equip this set with 450 nm and 490 nm narrow band interference filters paired with appropriate quartz neutral-density filters for excitation.
- If possible, use the appropriate set of filters and illumination conditions to setup comparable fluorescence values for λ1 and λ2 so that the ratio approaches 1. Also avoid basal fluorescence values that are set either saturating or too low, both for λ1 and/or λ2. This may yield important artifacts as then the ratio may not been detectable although intracellular pH is changing. NOTE: This system must enable rapid perfusion, time-lapse excitation at the two above-mentioned wavelengths and acquisition at the proper emission wavelength (535 nm for BCECF). Equip the system with software enabling automatic data acquisition and storage.
2.2) Measurement of NHE Forward Activity (Extrusion of Protons from the Cytosol)
Acidify cells by a 1 hr incubation in the NH4+ Loading solution (see step 1.4) in the absence of CO2.
Add BCECF-AM (5 µM final concentration) to the solution for the last five minutes and then rinse it with NH4+ loading solution to eliminate the extracellular probe.
Mount the cells on the microscope and take several images to record a stable baseline. Perfuse the rinse solution (see above). This results in a drop of fluorescence corresponding to the acidification.
After pH stabilization, perfuse any of the solutions given below to measure pH recovery rates mediated by Li+/H+, Na+/H+ or K+/H+ exchange. Each solution contains a potential coupling cation to be tested for H+ exchange. If one of those is transported, this will result in a fluorescence increase at 495 nm that will correspond to a raise in intracellular pH: 120 mM LiCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose and 15 mM HEPES at pH 7.4 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose and 15 mM HEPES at pH 7.4 125 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose and 15 mM HEPES at pH 7.4
2.3) Measurement of NHE Reverse Activity
NOTE: The principle here is to invert the transmembrane ionic gradients.
Prior to measurement, load the cells with the intracellular cation of interest (see below).
Incubate them for 5 min with BCECF/AM (see step 2.2.2); rinse them, mount them on the video microscope.
Perfuse the loading solution and take several images to record a stable baseline.
After the baseline stabilization, image the pH variations when cells are perfused with an extracellular acidic medium containing 120 mM choline chloride, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose and 15 mM MES at pH 6.5.
For LiCl loading, incubate cells for 2 hr in a solution composed of 120 mM LiCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose and 15 mM HEPES at pH 7.4. Intracellular lithium measured by atomic absorption spectroscopy yields a value of about 60 mM.
For Na+ loading, incubate cells for two hours in a solution composed of 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM glucose and 15 mM HEPES at pH 7.4 in the presence of 1 mM ouabain to block the Na/K ATPase. In these conditions, intracellular Na+ concentration is in the range of 40 mM. NOTE: K+ loading is not necessary as cytosolic K+ concentration is in the range of 140 mM and an outwardly-directed K+ gradient is therefore easy to achieve.
2.4) Data Treatment and Calibration:
NOTE: This step applies both to the forward and reverse measurements.
At the end of each experiment, perfuse cells with a solution of 140 mM KCl, 20 mM HEPES and 5 µM nigericin adjusted to pH values between 6.5 and 7.4.
Collect data as fluorescence measurements from the images (grey levels representing intensity levels) and export under text format and treat as explained below using a spreadsheet program.
Calculate intracellular pH values for each individual cell or region of interest using the following equation: pH = pKa + (Log (R-Rmin)/(Rmax-R))x Fmin(λ2)/Fmax(λ2)
Use the Fmin(λ2)/Fmax(λ2) ratio if there is probe bleaching or leakage, resulting in a decrease in the 450 nm fluorescence throughout the experiments. This is however very rarely observed in the conditions used in the described experiments.
- As the calibration data are present at the end of each pH measurement, check whether the corresponding calculated values differ from the pH values used for calibration. If it is the case, then adjust all experimentally-determined pH values to the calibration using the following procedure:
- Calculate the following quotient (Q, the difference between the measured values/Difference between the calibration values). Multiply the whole dataset by Q. Make the final adjustment by addition or subtraction so that the calculated values will equal the corresponding calibration values.
3. Measurements of Initial Rates of NHE7 by Fast Li+ Uptake
Seed cells on multi well plates (6 to 24-well) and acidify them using either the NH4+ loading technique described above or alternately the nigericin/bovine serum albumin (BSA) acidification described in20.
Rinse plates twice rapidly using the above-described rinse solution and then incubate the cells in uptake solutions containing 1-10 mM lithium (slightly below Km values) and the desired concentrations of the cations and/or inhibitors of interest for the desired amount of time.
Maintain short and consistent uptake durations (typically one minute or below) to ensure that transport operates in initial rates conditions. Also make sure that all the solutions used for uptake are isotonic.
At the end of the uptake time, carefully eliminate the uptake medium and wash the cells four times with ice-cold phosphate buffered saline (PBS). Perform these washes as fast as possible (less than 10 sec for the four of them is ideal) to prevent lithium efflux.
Lyse the cells in 25% Nitric acid. Apply 250 µl per well of 25% Nitric acid and let it sit for at least 1 h, then scrap each well with the end of the pipette tip and transfer the whole well suspension in a new microcentrifuge tube.
Transfer the lysate in 1.5 ml microcentrifuge tubes, centrifuge them for 5 minutes at 15,000 x g (room temperature) to remove the cellular debris.
Measure the lithium content of the supernatants by atomic absorption spectroscopy21. NOTE: Different companies provide atomic absorption spectrometers, which have to be equipped with a Lithium hollow cathode lamp. Follow the manufacturer’s instructions as these machines are very precise but also quite delicate.
Representative Results
Selection:
The H+ killing selection is based on the diffusion of the ammonium weak base, as depicted in Figure 1A. The effect of weak bases and acids diffusion on intracellular pH has been pioneered by Walter Boron and collaborators22. The elegant idea to use this phenomenon to produce a lethal acidification for positive genetic selection was then developed by Jacques Pouysségur17. Under such a protocol, the intracellular pH drops to about 5.5 after the rinse step. Cells which do not express a functional NHE at the plasma membrane cannot recover a neutral pH value and show a typical acidified aspect with a flat shape and a granular cytosol (Figure 1B). After repeated selection cycles (about two H+ killing procedures/week), cells that fully and stably survive this acidification (Figure 1C) emerge, at a frequency of about 10-7. This will result in the formation of individual cellular clones that can be either characterized individually or pooled to obtain cellular populations if desired. Standard experiments such as cell surface biotinylation or RNA silencing can be conducted to control that the protein expressed at the plasma membrane is indeed an intracellular NHE such as NHE7 (see for example Milosavljevic et al.18). As well, RT-PCR and subsequent sequencing should be performed to check for eventual mutations in the sequence of the intracellular NHEs expressed in the selected cell line.
Functional Characterization:
Fluorescence Videomicroscopy:
The activity and ion selectivity of the intracellular NHEs expressed at the plasma membrane can be characterized by measuring the changes in intracellular pH induced by these NHEs in various conditions. For this a videomicroscopy set equipped with illumination and acquisition settings to image a ratiometric probe such as BCECF/AM is schematized in Figure 2A. Figure 2B and 2C show the typical variations in fluorescence values directly obtained for excitations at 490 and 450 nm, following a NH4+ loading acidification. Figure 2D shows the pH curve obtained from these fluorescence values following data treatment described in 2.4).
Lithium Uptake:
Together with hydrogen and sodium, lithium comprises part of the first column of the periodic table and has been shown to be an efficient coupling cation for Na+/H+ exchangers. It is possible to take advantage of this information to setup fast kinetics of lithium uptake, in order to measure in details the functional parameters of Na+/H+ exchangers. As this cation is absent from cell cytoplasm, its accumulation upon cytosolic acidification will quantitatively reflect the activity of the plasma membrane NHE. Moreover, nanomolar to picomolar concentrations of lithium can be measured with great accuracy using atomic absorption spectrometry. Thus, this approach is very powerful when combined with the selection of plasma membrane expressed vesicular exchangers. Figure 3 illustrates the measurement protocol described in 3) of the protocols section. Cells, preferably seeded on multiple-well plates (6 to 24) are acidified as previously described. The kinetic starts with the incubation of the cells in medium containing lithium, and the other cations or inhibitors of interest (Figure 3A).
To stop the uptake, cells are then submitted to four rapid rinses in ice-cold PBS. Operating rapidly removes any extracellular lithium while ensuring that no significant lithium efflux occurs (Figure 3B). Lithium concentration in each well is then measured using atomic absorption spectroscopy (Figure 3C), and initial rates of Lithium uptake, which directly yield the activity of the exchanger at steady-state are obtained as slope measurements of the lithium accumulation time course (Figure 3D).
As for enzyme kinetics, dose-response of initial rates can be used to derive Km values for substrates (Figure 4A) of Ki values for inhibitors. An interesting feature of transporters is that different coupling cations will compete for transport. This yields the possibility of measuring the Km for sodium by competition with lithium uptake (Figure 4B).
 Figure 1: Selection of cells expressing an intracellular Na+/H+ exchanger at their plasma membrane. (A) Strategy for the three steps H+ killing selection of cells expressing a functional non-mutated and non-tagged intracellular NHE at the plasma membrane. (B) Phase contrast microscopy image of PS120 parental cells submitted to an acid load. Scale bar: 25 µm (C) Phase contrast microscopy image of PS120 cells selected for the expression of a vesicular exchanger at the plasma membrane, following an acid load. Scale bar: 25 µm Please click here to view a larger version of this figure.
Figure 1: Selection of cells expressing an intracellular Na+/H+ exchanger at their plasma membrane. (A) Strategy for the three steps H+ killing selection of cells expressing a functional non-mutated and non-tagged intracellular NHE at the plasma membrane. (B) Phase contrast microscopy image of PS120 parental cells submitted to an acid load. Scale bar: 25 µm (C) Phase contrast microscopy image of PS120 cells selected for the expression of a vesicular exchanger at the plasma membrane, following an acid load. Scale bar: 25 µm Please click here to view a larger version of this figure.
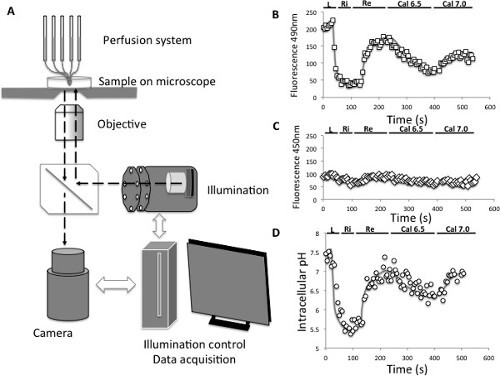 Figure 2: Intracellular pH measurements. (A) Principle of pH videomicroscopy measurement. (B) Evolution of the emitted fluorescence (595 nm) of the BCECF pH probe following an excitation at 490 nm. L: acid load, Ri; Rinse, Re: recovery, Cal6.5: calibration at an intracellular pH of 6.5, Cal7.0: Calibration at an intracellular pH of 7.0 (C) Evolution of the emitted fluorescence (595 nm) of the BCECF pH probe following an excitation at 450 nm. L: acid load, Ri; Rinse, Re: recovery, Cal6.5: calibration at an intracellular pH of 6.5, Cal7.0: Calibration at an intracellular pH of 7.0 (D) Evolution of the intracellular pH computed from the 490/450 nm fluorescence ratios of the BCECF pH probe and from the calibration points. L: acid load, Ri; Rinse, Re: recovery, Cal6.5: calibration at an intracellular pH of 6.5, Cal7.0: Calibration at an intracellular pH of 7.0 Please click here to view a larger version of this figure.
Figure 2: Intracellular pH measurements. (A) Principle of pH videomicroscopy measurement. (B) Evolution of the emitted fluorescence (595 nm) of the BCECF pH probe following an excitation at 490 nm. L: acid load, Ri; Rinse, Re: recovery, Cal6.5: calibration at an intracellular pH of 6.5, Cal7.0: Calibration at an intracellular pH of 7.0 (C) Evolution of the emitted fluorescence (595 nm) of the BCECF pH probe following an excitation at 450 nm. L: acid load, Ri; Rinse, Re: recovery, Cal6.5: calibration at an intracellular pH of 6.5, Cal7.0: Calibration at an intracellular pH of 7.0 (D) Evolution of the intracellular pH computed from the 490/450 nm fluorescence ratios of the BCECF pH probe and from the calibration points. L: acid load, Ri; Rinse, Re: recovery, Cal6.5: calibration at an intracellular pH of 6.5, Cal7.0: Calibration at an intracellular pH of 7.0 Please click here to view a larger version of this figure.
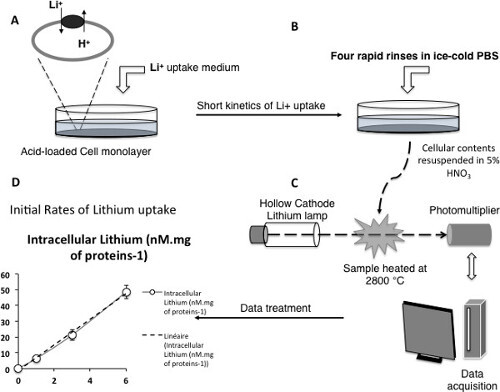 Figure 3: NHE activity measured by initial rates of Li+ uptake. (A-C) Scheme depicting the different critical steps of the measurement technique (D) Example of a measured time course of lithium uptake for the vesicular NHE7 exchanger expressed at the plasma membrane. Note the linearity of the kinetic within the experimental conditions, ensuring initial rates measurement. Please click here to view a larger version of this figure.
Figure 3: NHE activity measured by initial rates of Li+ uptake. (A-C) Scheme depicting the different critical steps of the measurement technique (D) Example of a measured time course of lithium uptake for the vesicular NHE7 exchanger expressed at the plasma membrane. Note the linearity of the kinetic within the experimental conditions, ensuring initial rates measurement. Please click here to view a larger version of this figure.
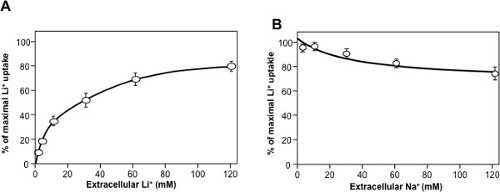 Figure 4: Typical Dose-response curves for NHE7 (original data from18). Initial rates of Lithium uptake were measured using 1-minute kinetics. (A) Dose-response curve for extracellular lithium. (B) Competition of different concentrations of extracellular sodium on 1mM extracellular lithium uptake. Please click here to view a larger version of this figure.
Figure 4: Typical Dose-response curves for NHE7 (original data from18). Initial rates of Lithium uptake were measured using 1-minute kinetics. (A) Dose-response curve for extracellular lithium. (B) Competition of different concentrations of extracellular sodium on 1mM extracellular lithium uptake. Please click here to view a larger version of this figure.
Discussion
This protocol describes how to select cells expressing intracellular Na+/H+ exchangers at the plasma membrane without altering their primary sequence by site-directed mutagenesis. These exchangers can now be characterized.
This method is based on the cellular toxicity of intracellular protons to select cell lines that will express vesicular Na+/H+ exchangers at the plasma membrane. It might be possible, in principle, to use other cations that may be suspected to be transported, such as Li+, K+, or Cs+. However, this adds an additional level of uncertainty. If the selection does not yield any positive clone, it will then be difficult to assess whether the tested cation is not transported or the exchanger is not at the plasma membrane. For these reasons, selections have been performed using Na+ as a coupling cation as this is the extracellular substrate found in highest abundance in physiological conditions. This led to the selection of variants expressing NHE718, as well as NHE6 and NHE9 at the plasma membrane (Poët and Counillon, unpublished results).
This plasma membrane expression enables the measurement of the transporters functional parameters by methods that could so far only be used for plasma membrane transporters. Of course it is not possible to exclude that such a change in membrane environment may affect the functional features of the vesicular NHEs. However, this strategy might be worth using for at least three reasons: (i) the intracellular expression of NHE6, 7 and 9 precludes any detailed functional measurement, (ii) membrane environments and protein or lipid partners have mostly been shown to affect subtle regulatory mechanisms of the other NHEs and not their very fundamental features of these transporters (affinities for coupling cations, selectivity, directionality, pharmacological features). So it is very likely that those will remain unchanged by the membrane expression of the vesicular NHEs. (iii) Knowing the functional mechanisms and pharmacological profiles of a plasma membrane-expressed vesicular mechanism, it is then possible to make vesicular pH measurements to address those possible discrepancies between plasma membrane and vesicular expression.
Another potential limitation of this technique is that the strategy described here may lead to the selection of cells expressing other H+ extruding mechanism than the transfected vesicular Na+/H+ exchanger (such as a plasma membrane NHE, or a H+ ATPase for example). Although it was never observed in the laboratory, it is therefore crucial to use classical cell surface labeling coupled to silencing to actually verify that the NHE of interest is indeed expressed at the plasma membrane and mediates the Na+ or Li+ -dependent proton efflux (see for example Milosavljevic et al.18).
To characterize the activity of these transporters, two protocols, respectively based on intracellular pH variations and ion fluxes are described. The first method requires the use of pH-sensitive fluorescent probes and an adequate fluorescence video microscopy system. This method, which is used by many groups in the field, has been developed in the 1980s, first using fluorimeters to measure signals form cells in suspension23, then using digital acquisition to measure individual cells by coupling fluorescence excitation and acquisition to videomicroscopy24. The latter technique has now become very fast and easy, due to the high performance of cameras, illumination and computer systems. Interestingly, companies usually provide very informative technical sheets together with their fluorescent probes. These pH variations provide important information to determine the nature of the extracellular cations coupled with proton efflux. In this respect, it is important to notice that the duration of the ammonium preincubation has to be carefully adjusted depending on the cells used for the measurements. CCL39-derived fibroblasts, support very well a one hour loading with 50 mM NH4Cl. This results in a very strong acidification that enables NHE full activation and produces a strong signal for measurements (Vmax conditions). However, other cells like cardiomyocytes or primary renal cells would not support such an important ammonium loading. It is worth performing several preliminary trials with different incubation times or NH4Cl concentrations. The reason for such differences in sensitivity to NH4Cl are not clear. They may be related to the possible expression of ammonium transport systems25,26. Similarly the sodium-free solution that is used for rinsing the loading solution may not be tolerated by cells, such as cardiomyocytes for example. Although initial velocities will be less precisely determined, it is necessary to perfuse directly with the recovery solution after ammonium loading.
In addition, intracellular pH measurements also provide a straightforward way to investigate the reversible properties of these exchangers as the reverse mode will produce an acidification that will be easily detected by a decrease in the BCECF fluorescence level that can be expressed as a pH values following calibration. The limitations of these experiments is lack of an accurate quantification of transport activities, as the experiments measure pH variations, that operate on a logarithmic scale and are limited by intracellular buffering capacity. The latter can be measured and pH variations multiplied by buffering capacity yield ion fluxes. However, this process combines several experimental errors (on pH measurements, buffering capacity measurements, and calibration) and is therefore not highly quantitative. Hence to access the enzymatic constants of the plasma membrane expressed transporter, rapid ion flux techniques are much more straightforward and accurate.
Because intracellular contents are in the millimolar range, detecting NHE-mediated changes in cytosolic sodium is not possible. Henceforward, for years such uptake techniques were based on the use of radioisotopes such as 22Na+. This manuscript describes a non-radioactive method for which 22Na+ is replaced by lithium, based on the rationale that this cation which is also used by NHEs, is absent from cell cytosol and can be measured with great accuracy using atomic absorption spectrometry21. Once the appropriate conditions (time and lithium concentration) to measure linear uptakes (i.e., being in initial rates conditions) are determined, affinities for the other potential coupling cations and/or inhibitors can be accurately measured as shown in Figures 3 and 4. A last potential limitation is that the lithium uptake method can of course only function if the exchanger of interest significantly transports this cation. If it is not the case, the experimenter will have to rely either on the above-mentioned intracellular pH measurements or will have to use radioactive sodium uptake.
Such a set of experiments have led recently led to the observation that the NHE7 intracellular Na+/H+ exchanger, which was initially believed to be a H+/K+ transporter which would alkalinize vesicles was in fact an endosomal acidification mechanism coupled to sodium18 . This has deep implications for intracellular compartments as so far this role was thought to be devoted to the V-H+ ATPases. As each member of the NHE family appears to display a very specific kinetic signature owing to its physiological role, it is tempting to hypothesize that the characterization of NHE6 and NHE9 using the methods described in this article will reveal novel and unexpected features that will highlight their physiological functions.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors are deeply indebted to all the members of the scientific community working on pH and ion transport, who have originated and improved the measurements described here. They particularly thank Dr. Jacques Pouysségur who originated the H+-killing selection technique used here. They acknowledge the University of Nice-Sophia Antipolis, the CNRS, the ANR (JCJC SVSE1 NHEint) and the ICST Labex for support.
References
- Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol. 2008;20(4):415–426. doi: 10.1016/j.ceb.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol. 2007;578(3):633–640. doi: 10.1113/jphysiol.2006.124719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornak U, et al. Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet. 2000;9(13):2059–2063. doi: 10.1093/hmg/9.13.2059. [DOI] [PubMed] [Google Scholar]
- Gunther W, Piwon N, Jentsch TJ. The ClC-5 chloride channel knock-out mouse - an animal model for Dent's disease. Pflugers Arch. 2003;445(4):456–462. [Google Scholar]
- Kasper D, et al. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. Embo J. 2005;24(5):1079–1091. doi: 10.1038/sj.emboj.7600576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poet M, et al. Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc Natl Acad Sci U S A. 2006;103(37):13854–13859. doi: 10.1073/pnas.0606137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem. 2005;280(2):1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- Gilfillan GD, et al. SLC9A6 mutations cause X-linked mental retardation, microcephaly, epilepsy, and ataxia, a phenotype mimicking Angelman syndrome. Am J Hum Genet. 2008;82(4):1003–1010. doi: 10.1016/j.ajhg.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot C, et al. Novel mutation in SLC9A6 gene in a patient with Christianson syndrome and retinitis pigmentosum. Brain & Development. 2013;35(2):172–176. doi: 10.1016/j.braindev.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Morrow EM, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321(5886):218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky-Su J, et al. Genome-wide association scan of the time to onset of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1355–1358. doi: 10.1002/ajmg.b.30869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke B, Neale BM, Faraone SV. Genome-wide association studies in ADHD. Hum Genet. 2009;126(1):13–50. doi: 10.1007/s00439-009-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, et al. A large scale multivariate parallel ICA method reveals novel imaging-genetic relationships for Alzheimer's disease in the ADNI cohort. Neuroimage. 2012;60(3):1608–1621. doi: 10.1016/j.neuroimage.2011.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, et al. A microdeletion in Xp11.3 accounts for co-segregation of retinitis pigmentosa and mental retardation in a large kindred. Am J Med Genet A. 2006;140(4):349–357. doi: 10.1002/ajmg.a.31080. [DOI] [PubMed] [Google Scholar]
- Sardet C, Franchi A, Pouysségur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989;56(2):271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Sardet C, Franchi A, L'Allemain G, Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984;81(15):4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi A, Cragoe E, Pouysségur J. Isolation and properties of fibroblast mutants overexpressing an altered Na+/H+ antiporter. J Biol Chem. 1986;261(31):14614–14620. [PubMed] [Google Scholar]
- Milosavljevic N, et al. The Intracellular Na+/H+ Exchanger NHE7 effects a Na+ coupled, but not K+ coupled proton-loading mechanism in endocytosis. Cell Reports. 2014;7(3):1–8. doi: 10.1016/j.celrep.2014.03.054. [DOI] [PubMed] [Google Scholar]
- Wigler M, et al. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Lacroix J, Poët M, Maherel C, Counillon L. A mechanism for the activation of the Na/H exchanger NHE-1 by cytoplasmic acidification and mitogens. EMBO Reports. 2004;5(1):91–96. doi: 10.1038/sj.embor.7400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosavljevic N, et al. Nongenomic Effects of Cisplatin: Acute Inhibition of Mechanosensitive Transporters and Channels without Actin Remodeling. Cancer Res. 2010;70(19):7514–7522. doi: 10.1158/0008-5472.CAN-10-1253. [DOI] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso AM, Tsien RY, Machen TE. Na+-H+ exchange in gastric glands as measured with a cytoplasmic-trapped, fluorescent pH indicator. Proc Natl Acad Sci U S A. 1984;81(23):7436–7440. doi: 10.1073/pnas.81.23.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso AM, Tsien RY, Machen TE. Digital image processing of intracellular pH in gastric oxyntic and chief cells. Nature. 1987;325:447–450. doi: 10.1038/325447a0. [DOI] [PubMed] [Google Scholar]
- Quentin F, et al. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol. 2003;14(3):545–554. doi: 10.1097/01.asn.0000050413.43662.55. [DOI] [PubMed] [Google Scholar]
- Geyer RR, Musa-Aziz R, Enkavi G, Mahinthichaichan P, Tajkhorshid E, Boron WF. Movement of NH3 through the human urea transporter B: a new gas channel. Am J Physiol Renal Physiol. 2013;304(12):F1447–F1457. doi: 10.1152/ajprenal.00609.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


