Abstract
EPA Method 1615 was developed with a goal of providing a standard method for measuring enteroviruses and noroviruses in environmental and drinking waters. The standardized sampling component of the method concentrates viruses that may be present in water by passage of a minimum specified volume of water through an electropositive cartridge filter. The minimum specified volumes for surface and finished/ground water are 300 L and 1,500 L, respectively. A major method limitation is the tendency for the filters to clog before meeting the sample volume requirement. Studies using two different, but equivalent, cartridge filter options showed that filter clogging was a problem with 10% of the samples with one of the filter types compared to 6% with the other filter type. Clogging tends to increase with turbidity, but cannot be predicted based on turbidity measurements only. From a cost standpoint one of the filter options is preferable over the other, but the water quality and experience with the water system to be sampled should be taken into consideration in making filter selections.
Keywords: Environmental Sciences, Issue 97, enteric virus, environmental microbiology, water, virus occurrence, electropositive cartridge filters, sample collection
Introduction
Human enteric viruses replicate within the gastrointestinal tract and are spread through the fecal-oral route. These viruses are often found in sewage in high concentrations 1-3. They can persist in sewage effluents 4,5, and in surface 6,7, ground 8-10, and treated drinking 11 waters. When present, the concentration of virus in environmental waters in the U.S. typically is too low for direct measurement 12,13. This requires that viruses be concentrated from large volumes of water. During the Information Collection Rule (ICR) monitoring conducted by the U.S. Environmental Protection Agency (USEPA) 14, the virus concentrations of positive samples in the source water of large utilities nationwide ranged from 0.009 to 19.7 most probable number of infectious units (MPN)/L. Median and mean concentrations of positive samples were 0.03 and 0.17 MPN/L for source waters from flowing streams, 0.01 to 0.07 MPN/L for those from lakes and reservoirs, and 0.04 to 0.74 MPN/L for those using groundwaters 11 (data from the ICR Aux1 18 month access database dated 4/25/2000). Virus concentrations of positive samples from a USEPA study of national groundwaters ranged from 0.009 to 2.12 infectious units/L with median and mean concentrations of 0.13 and 0.29 infectious units/L 8. The concentration of virus in positive groundwater samples was higher than those in flowing streams. Most of the facilities using groundwater in these studies obtained water from aquifers located in karst regions. These, along with those located in limestone and crystalline (fractured bedrock) settings are likely to have higher virus concentrations than in other settings 8,15,16. USEPA virus methods specify sampling volumes of 200 L (ICR) to 300 L (Method 1615) of surface water and 1,000 L (ICR) to 1,500 L (Method 1615) of groundwater 17,18. However, even with the use of large sample volumes, most surface and ground water samples are negative for virus 8,11,19,20.
Viruses present in surface waters pose a potential health risk to consumers of drinking water. The Surface Water Treatment Rule requires all treatment plants using surface water to reduce virus concentrations by at least 4-log. Even with a 4-log reduction, infectious virus concentrations in source water as small as 0.0044 MPN/L could lead to one infection per day assuming such average exposure and treatment conditions and the dose response parameters for rotavirus 11,21. The risk from virus in untreated groundwaters could be even greater due to the lack of treatment and viral occurrence. Borchardt and colleagues estimate that up to 22% of acute gastroenteritis in adults and 63% in children less than five years could be due to virus in drinking water in communities using untreated groundwaters 19.
USEPA Method 1615 was developed to detect enterovirus and norovirus during the Unregulated Contaminant Monitoring Regulation's third monitoring cycle (UCMR3) 22 as a national follow up to the findings of Borchardt and colleagues 19,23. The USEPA method was designed primarily for measuring virus in systems using untreated groundwaters, but was written more generally to include other water matrix types. The new method is a hybrid preserving many components from the previous virus method used during the ICR 17, the addition of molecular procedures based upon the method of Borchardt et al. 19,23, and additional primer sets for norovirus 24. The purpose of this paper is to describe the sampling procedure and the steps needed to maintain the integrity of the sample during collection and shipment. An evaluation of the overall method is described in Cashdollar et al. 25. This protocol covers simple field collection of surface and ground waters where a pump and prefilter are not necessary and where adjustments are not required for pH or the presence of a disinfectant in the water to be sampled. The more complex sampling requirements are described in Fout et al. 17,18.
Protocol
1. Preliminary Procedures
Assemble a standard filter apparatus as shown in Figure 1 consisting of an intake module, a cartridge housing module, and a discharge module as described in Supplemental Materials.
- Calibrate the flow meter/totalizer at the flow rates used for sampling before the first use. Check the calibration after the first use and then after one month of use. If the flow rate calibration has changed after either the first use or after the first month of use, determine the frequency for calibration checks as described in Supplemental Materials.
- Connect a discharge module to an intake module and then connect the intake module to a tap. Set the flow meter/totalizer to read the flow rate. Turn on the tap completely and then adjust the flow rate to 10 L/min using the bronze globe valve. Once the flow rate is stable at 10 L/min, reset the flow meter/totalizer to read total volume.
- Place the outlet of the discharge module into a 4 L or larger graduated cylinder and simultaneously reset the totalizer to zero. Measure the totalizer reading at the 4-L mark and the time required to reach the 4 L mark on the cylinder. Change the flow meter/totalizer setting back to flow rate. NOTE: If the flow rate had dropped to below 9.95 L or increased to more than 10.05 L after completing Step 1.2.2, wait until the flow rate is stable and repeat Step 1.2.2. A correctly calibrated meter will reach the 4 L mark on the graduated cylinder at 24 ± 1 sec with a totalizer reading of 4.0 ± 0.04 L.
- If the totalizer reading is less than 3.96 or more than 4.04 L, calculate a correction factor needed to adjust for the observed difference. For example, if the flow meter/totalizer reads 3.9 L at the 4 L mark on the graduated cylinder, the correction factor would be 1.026 (4 ÷ 3.9). Thus if the desired volume of a field sample were 300 L, the volume collected according the reading on the flow meter/totalizer should be 300 ÷ 1.026 = 292.4 L.
- Sterilize the standard filter apparatus and prepare it for sample collection.
- Sterilize the intake and cartridge housing modules with 0.525% sodium hypochlorite for at least 30 min before each use by either completely immersing the modules in the solutions or by circulating the sodium hypochlorite though the modules using a pump. Rinse the modules with sterile dH2O and then dechlorinate in a solution containing 50 ml of 1 M sodium thiosulfate per liter of sterile reagent grade water for 5 min. Cover the filter sampling apparatus module ends with sterile aluminum foil.
- Add an electropositive cartridge filter to the cartridge housing module aseptically. NOTE: Take care to ensure that cartridge filter is properly seated in the housings. Properly seated filters will not leak and the filter will not move within the housing when shaken. The gaskets on a properly seated filter will have visible indentations showing where the filter contacts them. The gaskets should always be checked for indentations immediately after the filter is removed from the housing.
- Record a unique sample number on a Sample Data Sheet (see Supplemental Materials for an example) and then take or ship the filter sampling apparatus and the Sample Data Sheet to the individual who will be collecting the field sample, along with any necessary instructions concerning sample collection (e.g., sample volume, sampling location, etc.).
2. Preparation for Sample Collection at the Sampling Site
Wash hands upon arrival at the collection site and don surgical gloves to minimize the possibility of sample contamination. Do not collect samples if experiencing intestinal or respiratory symptoms. Change gloves frequently, and especially after touching water taps and other items that may be contaminated.
Record relevant sample tracking information on a Sample Data Sheet. Relevant information include site and sample identification, sampler’s name, and equipment models and serial numbers.
Turn on the water tap for 2–3 min to clear any debris that has settled in the line. If necessary, use a garden hose or tubing to reach a drain during this step.
If the sampling apparatus modules are connected, disconnect them and protect the open ends of the cartridge housing module with sterile foil. Connect the discharge module to the intake module and then connect both to the water tap. Connect a garden hose on the end of the discharge module and place the free end in the 1 L polypropylene container.
- Turn on the tap and pass at least 75 L of the water to be sampled through the apparatus.
- Measure and record the water quality parameters during the flush period on water flowing into the polypropylene container—chlorine residual, pH, temperature, and turbidity.
- After turning off the water at the tap, disconnect the discharge module from the intake module, and then connect the cartridge housing module to the outlet of the intake module and the discharge module to the outlet of the cartridge housing module. Reset the totalizer reading to zero.
3. Field Sample Collection
- Record the initial totalizer reading along with the date and time and then slowly open the water tap.
- Ensure that the cartridge housing is in an upright position and that it completely fills with water. Some housings have a vent button which can be pressed to expel air from the housing during the filling process.
- Open the tap completely. Adjust the flow rate to 10 L/min using the bronze globe valve and then record the initial flow rate.
Pass 300 L of surface water or 1,500 to 1,800 L of groundwater through the apparatus using the totalizer readings (as adjusted, if necessary). If the filter clogs before the required volume is reached and more than half the volume has been collected, use a second filter housing module to collect the remaining sample, if one is available.
Observe the flow rate as the sample volume approaches the end of the sampling period and then turn off the flow of water at the sample tap. Record the final flow rate, date, time of day, the final totalizer reading, and the total sample volume.
- Disconnect the filter sampling apparatus from the tap. Disconnect the cartridge housing module from the other modules.
- Turn the filter housing(s) upside down and allow excess water to flow out until no more water will drain from both the inlet and outlet ports.
- Turn the housing upright and cover the quick connects on each end with sterile aluminum foil. Place the housing into one or more sealable plastic bags.
- Drain the water from the intake and discharge modules. Place the modules into one or more sealable plastic bags.
4. Shipment of Field Samples
Place the filter sampling apparatus modules into an insulated storage and transport cooler.
Add frozen ice packs or double-bagged ice cubes to the storage and transport cooler to ensure that the sample remains between 1-10 °C during shipment. Two large or 6-8 small ice packs should be sufficient.
Place a temperature-recording device in the insulated storage and transport chest in a location without contact to ice packs or ice bags. NOTE: The temperature-recording device is optional if the analytical laboratory will be using a visual infrared thermometer to measure the temperature of the cartridge housing immediately upon arrival and the opening of the container.
Fill any void space with packing material and then place the Sample Data Sheet, protected in a sealable plastic bag, on top. Close the insulated storage and transport chest and tape it to prevent any leakage of water.
Transport or ship the sample to the analytical laboratory. Ensure that the sample arrives at the analytical laboratory no later than 24 hr after the start of field sample collection (i.e., the time recorded at step 3.1).
Representative Results
Filter clogging is a major potential problem that can be encountered with Method 1615. Clogging decreases the flow rate of water through the filter. In some cases this can be overcome by opening the globe valve to allow greater flow. In other cases the filter will become completely clogged before the required volume has passed through it. Table 1 shows the percentage of samples with reduced volumes as a function of filter type, water type, and turbidity. Clogging occurred with both groundwater and surface water samples, with the aluminum oxide nanofiber-based filter outperforming the quaternary amine-based filter for groundwater samples while the reverse was the case for surface water samples. Overall, 6% of samples collected using the quaternary amine-based filter were deficient in volume while 10% of samples collected with the aluminum oxide nanofiber-based filters failed to meet the required minimum specified volume due to clogging. In general, clogging increased with turbidity, but a number of different components contribute to turbidity and some of these do not lead to clogging. For example, 43% of all clogging events occurring during the ICR study were confined to two river systems (data not shown). During the ICR the minimum target volume for surface water was 200 L. The average volume collected during the study was 217 ± 32 L with a median volume of 208 L (data from the ICR Aux1 18 month access database dated 4/25/2000). Thus, the full volume can be collected from many waters even with high turbidities.
The ICR required samplers to use a prefilter when turbidities were over 75 NTU, but even with a prefilter, 34% of the samples collected in waters with turbidities over 75 NTU clogged. Method 1615 recommends the use of a prefilter with waters over 50 NTU; however, since the publication of Method 1615, several different prefilter options in addition to that listed in the method have been tested. None of these resulted in a significant improvement in sample volume (data not shown).
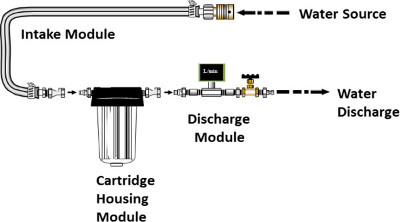 Figure 1. Standard Filter Apparatus. The standard filter apparatus consists of intake, filter housing, and discharge modules (see Supplemental Materials for a description of each module). Please click here to view a larger version of this figure.
Figure 1. Standard Filter Apparatus. The standard filter apparatus consists of intake, filter housing, and discharge modules (see Supplemental Materials for a description of each module). Please click here to view a larger version of this figure.
| Total Samples a | Number of Deficient Samples b | Percent Deficient Samples | Turbidity (NTU) |
| Groundwater using NanoCeram Filters c | |||
| 113 | 1 | 1 | < 20 |
| Surface Water using NanoCeram Filters c | |||
| 83 | 12 | 14 | < 20 d |
| 8 | 4 | 50 | 20 - < 50 |
| 6 | 5 | 83 | ≥ 50 d |
| Total NanoCeram c | |||
| 210 | 22 | 10 | ≥ 0 |
| Groundwater using 1MDS Filters c | |||
| 374 | 75 | 20 | < 20 |
| Surface Water using 1MDS Filters c | |||
| 2,693 | 27 | 1 e | < 20 e |
| 505 | 36 | 7 f | 20 - < 50 f |
| 122 | 19 | 16 e | 50 - < 75 g |
| 175 | 60 | 34 e,f | ≥ 75 e,f,g |
| Total 1MDS c | |||
| 3,869 | 217 | 6 | ≥ 0 |
Table 1. Filter Capacity. a Samples are from the following studies from which volume and turbidity data are available: 1) USEPA’s ICR study (data from the ICR Aux1 18 month access database dated 4/25/2000), 2) USEPA groundwater study 8, 3) USEPA Lawrence and Lowell, MA study (unpublished data), 4) USEPA Mississippi River study (unpublished data), 5) USEPA drinking water treatment plant study (unpublished data), 6) USEPA Method 1615 evaluation study 25, and 7) the first three months of the UCMR3 monitoring (unpublished data). b Samples where the filter clogged before the minimum volume of water specified for each study was collected. This was 200 L for surface waters and 1,500 L for groundwaters, but was 200 L for source waters that were groundwater under the USEPA ICR and 1,893 L for the USEPA groundwater study. c The ability to collect the full minimum specified volume is significantly different for samples collected using NanoCeram and 1MDS filters (P = 0.002) and between surface waters and groundwaters both overall (P < 0.001) and for each filter type (P < 0.001) using the Mann-Whitney Rank Sum test. d – g Groups with the same superscript value are significantly different (P < 0.05) according to the Kruskal-Wallis One Way Analysis of Variance on Ranks test and Dunn’s Pairwise Multiple Comparison Procedure. For all statistical tests the dependent variable is the volume passed through the filter as a fraction of the minimum specified volume, but with a maximum value of 1.0.
Discussion
Different filter types for concentrating viruses from environmental waters have been used over the years 26. Current methods employ ultrafilters 27, electronegative filters 13,28,29, glass wool filters 23, and electropositive filters 30. Electronegative filters were widely used for many years, but a requirement for the addition of salt and the adjustment of the water pH in the field prior to or during sampling limits their usefulness 13. The most practical filter choice for field sampling is electropositive filters. These filters allow the sampling of large volumes of water at high flow rates and without any conditioning of the water. Glass wool filters are the least expensive option, but have slower flow rates than electropositive filters and are not commercially available. Ultrafilters provide the highest virus recoveries over a large range of water quality, but the equipment required for sampling is not readily field portable and the time required to collect samples is much longer 27. Methods recently have been developed that use preconditioned electronegative filters to avoid the need for adjustment in the field, but these may not be applicable for collecting large sample volumes 28,29.
EPA Method 1615 uses electropositive cartridge filters which obtain their positive charge from either aluminum oxide nanofibers or quaternary amines. The advantages of the former over the latter is that it is less expensive and efficiently collects virus from waters over a wider range of natural pH values 30,31; however, each cartridge, as well as the glass wool filters used by Borchardt and colleagues give similar recoveries of enterovirus and norovirus from water 23,31,32 (Cashdollar, unpublished data). Cartridge filters are placed into a simple sampling apparatus that is designed to simplify the collection of samples and reduce contamination during sampling.
Standard methods are invaluable when large studies are conducted using multiple analytical laboratories. EPA Method 1615 provides standard procedures and guidance to minimize the two major sample collection issues that can affect data collected during these studies—false positive results stemming from contamination during sample collection or from inadequately disinfected apparatus components, and the clogging of filter pores by components in the water being sampled.
Just as enteric viruses can be spread person-to-person due to inadequate hygiene, viruses can be introduced into samples from poorly washed hands or hands with contaminated gloves 33. It is essential that samplers understand the potential routes of contamination and use aseptic technique during sampling. Samplers should understand that gloves are primarily used to protect the sampler from exposure and not to protect the apparatus from contamination. Hands should be washed before the start of sampling and care must be taken during the donning of gloves to prevent hand to glove contamination. Samplers with gastroenteritis or respiratory symptoms must not collect samples, as they could be shedding enteroviruses or noroviruses in high levels.
Second, care must be taken to prevent carryover of virus from previous sampling events. To minimize this potential source of contamination, the sampling apparatus in Method 1615 was modified from that of the ICR by not including a pressure regulator and pressure meter between the inlet and the cartridge housing module. These components were removed because the pressures observed during sampling events were always below the maximum housing rating (e.g., 125 psi for 5-inch cartridge housings) and because they were difficult to disinfect. The latter problem was demonstrated through the use of equipment blank controls in studies subsequent to the ICR 6,20. The degree to which it affected the ICR data is unknown, but was likely small; there were only two false positive negative performance evaluation samples during the study (data not shown). To further reduce the possibility of carryover contamination, it also is recommended that the inlet module tubing be replaced after each sampling event. It is especially important to ensure adequate disinfection if the apparatus was used for a quality or performance control that was seeded with virus. Prior to performing disinfection, the concentration of free chlorine should be measured for any loss during storage. In addition to the changes in the configuration of the apparatus described above, it is essential that regular equipment blanks be run to demonstrate that disinfection is effective. Method 1615 mandates that equipment blanks be performed using apparatuses that have been disinfected after being used for virus-seeded controls, thereby simplifying the procedure by eliminating the need to pass a virus-seeded solution through the apparatus prior to disinfection. The concentration of disinfectant was increased to 0.525% hypochlorite for Method 1615, as this concentration is required both to inactivate any viable viruses on the equipment and to degrade nucleic acids. Therefore, equipment blanks must be analyzed using both cell culture and qPCR assays.
Both types of electropositive filters were subject to clogging during the studies reported in Table 1. An unknown number of samples with reduced volumes may have been due to sampling errors, such as a misreading of the totalizer or an intentional early stop of sampling to meet another deadline, especially for waters with turbidity readings less than 20 NTU. The degree of clogging is dependent both upon the filter type and water quality parameters. Prefilters provide some measure of improvement, but if used, should be processed and analyzed separately from the electropositive filter. The current recommendation for the UCMR3 is that the sample be collected without prefilters using two aluminum oxide nanofiber-based filters if at least half the volume can be collected using the first filter.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors thank numerous EPA personnel whose contributions made the monitoring conducted during the ICR and UCMR3 possible, the following lead investigators of other EPA studies reported: Daniel Dahling, Alfred Dufour, Andrey Egorov, Susan Glassmeyer, Asja Korajkic, Richard Lieberman, Robert Safferman, and Tim Wade; and Shannon Griffin and Michael Ware for critically reviewing this manuscript. The authors thank the Indian Hill Water Works for the use of one of their pump houses to demonstrate sample collection. Although this work was reviewed by USEPA and approved for publication, it may not necessarily reflect official Agency policy. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- Katayama H, et al. One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res. 2008;42(6-7):1441–1448. doi: 10.1016/j.watres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- La Rosa G, Pourshaban M, Iaconelli M, Muscillo M. Quantitative real-time PCR of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy. Annali Dell Istituto Superiore Di Sanita. 2010;46(3):266–273. doi: 10.4415/ANN_10_03_07. [DOI] [PubMed] [Google Scholar]
- Sedmak G, Bina D, MacDonald J. Assessment of an enterovirus sewage surveillance system by comparison of clinical isolates with sewage isolates from milwaukee, wisconsin, collected august 1994 to december 2002. Appl. Environ. Microbiol. 1994;69(12):7181–7187. doi: 10.1128/AEM.69.12.7181-7187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H, Lodder W, van der Poel W, Vennema H, de Roda Husman AM. Genetic diversity of noroviruses in raw and treated sewage water. Res Microbiol. 2005;156(4):532–540. doi: 10.1016/j.resmic.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Haramoto E, et al. Seasonal profiles of human noroviruses and indicator bacteria in a wastewater treatment plant in Tokyo, Japan. Water Sci Technol. 2006;54(11-12):301–308. doi: 10.2166/wst.2006.888. [DOI] [PubMed] [Google Scholar]
- Denis-Mize K, Fout GS, Dahling DR, Francy DS. Detection of human enteric viruses in stream water with RT-PCR and cell culture. J Water Health. 2004;2(1):37–47. [PubMed] [Google Scholar]
- Hamza IA, Jurzik L, Stang A, Sure K, Uberla K, Wilhelm M. Detection of human viruses in rivers of a densly-populated area in Germany using a virus adsorption elution method optimized for PCR analyses. Water Res. 2009;43(10):2657–2668. doi: 10.1016/j.watres.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Lieberman RJ, et al. Microbial monitoring of vulnerable public groundwater supplies. Denver, CO: American Water Works Association; 2002. [Google Scholar]
- Parshionikar SU, et al. Waterborne outbreak of gastroenteritis associated with a norovirus. Appl. Environ. Microbiol. 2003;69(9):5263–5268. doi: 10.1128/AEM.69.9.5263-5268.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J, Bell D, Simmons GC, Rivera-Aban M, Wolf S, Greening GE. Gastroenteritis outbreak caused by waterborne norovirus at a New Zealand ski resort. Appl. Environ. Microbiol. 2007;73(24):7853–7857. doi: 10.1128/AEM.00718-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S, Regli S, Chen J. Virus occurrence and health risks in drinking water. In: Maguire MJ, McLain JL, Odolensky A, editors. Information Collection Rule Data Analysis. AWWA Research Foundation and American Water Works Association; 2002. pp. 437–462. [Google Scholar]
- Sobsey MD, Wallis C, Henderson M, Melnick JL. Concentration of Enteroviruses from Large Volumes of Water. Appl Microbiol. 1973;26(4):529–534. doi: 10.1128/am.26.4.529-534.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WF, Akin EW, Benton WH, Metcalf TG. Virus in water. II. Evaluation of membrane cartridge filters for recovering low multiplicities of poliovirus from water. Appl Microbiol. 1972;23(5):880–888. doi: 10.1128/am.23.5.880-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. 40 CFR Part 141 National Primary Drinking Water Regulations: Monitoring Requirements for Public Drinking Water Supplies; Final Rule. Federal Register. 1996;61(94):141. [Google Scholar]
- Pang L. Microbial removal rates in subsurface media estimated from published studies of field experiments and large intact soil cores. J Environ Qual. 2009;38(4):1531–1559. doi: 10.2134/jeq2008.0379. [DOI] [PubMed] [Google Scholar]
- Johnson TB, et al. Ground Water. 1. Vol. 49. Tennessee, USA: 2011. Viruses and Bacteria in Karst and Fractured Rock Aquifers in East; pp. 98–110. [DOI] [PubMed] [Google Scholar]
- Fout GS, Schaefer FW, 3rd, Messer JW, Dahling DR, Stetler RE. ICR Microbial Laboratory Manual. Environmental Protection Agency; 1996. [Google Scholar]
- Fout GS, et al. Method 1615: Measurement of enterovirus and norovirus occurrence in water by culture and RT-qPCR. U.S. Environmental Protection Agency; 2012. pp. 1–91. [Google Scholar]
- Borchardt MA, Spencer SK, Kieke BA, Lambertini E, Loge FJ. Viruses in nondisinfected drinking water from municipal wells and community incidence of acute gastrointestinal illness. Environ Health Perspect. 2012;120(9):1272–1279. doi: 10.1289/ehp.1104499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francy DS, Bushon RN, Stopar J, Luzano EJ, Fout GS. Scientific Investigations Report 2004-5219, U.S. Geological Survey. Environmental factors and chemical and microbiological water-quality constituents related to the presence of enteric viruses in ground water from small public water supplies in southeastern Michigan. 2004. pp. 1–54.
- Regli S, Rose JB, Haas CN, Gerba CP. Modeling the Risk from Giardia and Viruses in Drinking-Water. Journal American Water Works Association. 1991;83(11):76–84. [Google Scholar]
- USEPA. Parts 141 and 142 Revisions to the Unregulated Contaminant Monitoring Regulation (UCMR3) for Public Water Systems; Final Rule. Federal Register. 2012;77(85):26072–26101. [Google Scholar]
- Lambertini E, Spencer SK, Bertz PD, Loge FJ, Kieke BA, Borchardt MA. Concentration of enteroviruses, adenoviruses, and noroviruses from drinking water by use of glass wool filters. Appl. Environ. Microbiol. 2008;74(10):2990–2996. doi: 10.1128/AEM.02246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butot S, Le Guyader FS, Krol J, Putallaz T, Amoroso R, Sanchez G. Evaluation of various real-time RT-PCR assays for the detection and quantitation of human norovirus. J Virol Methods. 2010;167(1):90–94. doi: 10.1016/j.jviromet.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Cashdollar JL, et al. Development and Evaluation of EPA Method 1615 for Detection of Enterovirus and Norovirus in Water. Appl. Environ. Microbiol. 2013;79(1):215–223. doi: 10.1128/AEM.02270-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashdollar JL, Wymer L. Methods for primary concentration of viruses from water samples: a review and meta-analysis of recent studies. J Appl Microbiol. 2013;115(1):1–11. doi: 10.1111/jam.12143. [DOI] [PubMed] [Google Scholar]
- Rhodes ER, Hamilton DW, See MJ, Wymer L. Evaluation of hollow-fiber ultrafiltration primary concentration of pathogens and secondary concentration of viruses from water. J Virol Methods. 2011;176(1-2):38–45. doi: 10.1016/j.jviromet.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Katayama H, Shimasaki A, Ohgaki S. Development of a virus concentration method and its application to detection of enterovirus and norwalk virus from coastal seawater. Appl. Environ. Microbiol. 2002;68(3):1033–1039. doi: 10.1128/AEM.68.3.1033-1039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E, Katayama H, Utagawa E, Ohgaki S. Recovery of human norovirus from water by virus concentration methods. J Virol Methods. 2009;160(1-2):206–209. doi: 10.1016/j.jviromet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Sobsey MD, Jones BL. Concentration of poliovirus from tap water using positively charged microporous filters. Appl. Environ. Microbiol. 1979;37(3):588–595. doi: 10.1128/aem.37.3.588-595.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim MR, Rhodes ER, Brinkman N, Wymer L, Fout GS. New electropositive filter for concentrating enteroviruses and noroviruses from large volumes of water. Appl. Environ. Microbiol. 2009;75(8):2393–2399. doi: 10.1128/AEM.00922-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko G, et al. Evaluation of electropositive filtration for recovering norovirus in water. J Water Health. 2011;9(1):27–36. doi: 10.2166/wh.2010.190. [DOI] [PubMed] [Google Scholar]
- Liu P, et al. Laboratory Evidence of Norwalk Virus Contamination Levels on the Hands of Infected Individuals. Appl. Environ. Microbiol. 2013;79(24):7875–7881. doi: 10.1128/AEM.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


