Abstract
Area F5c is a monkey premotor area housing mirror neurons which responds more strongly to grasping observation when the actor is visible than when only the actor's hand is visible. Here we used this characteristic fMRI signature of F5c in seven imaging experiments – one in macaque monkeys and six in humans – to identify the human homologue of monkey F5c. By presenting the two grasping actions (actor, hand) and varying the low level visual characteristics, we localized a putative human homologue of area F5c (phF5c) in the inferior part of precentral sulcus, bilaterally. In contrast to monkey F5c, phF5c is asymmetric, with a right-sided bias, and is activated more strongly during the observation of the later stages of grasping when the hand is close to the object. The latter characteristic might be related to the emergence, in humans, of the capacity to precisely copy motor acts performed by others, and thus imitation.
Keywords: Premotor cortex, Non-human primates, Functional imaging, Action observation, Grasping, Inter-hemispheric asymmetry
Highlights
-
•
We use parallel fMRI to identify the human homologue of macaque F5c.
-
•
In premotor cortex only F5c reacts more to observing grasping with the actor visible.
-
•
Two bilateral inferior precentral sulcus sites respond similarly for many stimuli.
-
•
The human homologues of F5c are asymmetric and require fixation near the target.
Introduction
Ventral premotor area F5 is involved in planning actions, using visual and other sensory inputs. Traditionally, it is thought to house two types of visuomotor neurons: canonical neurons responsive to the presentation of 3D objects (Murata et al., 1997) and mirror neurons active during the execution of specific motor acts and during the observation of similar motor acts performed by other individuals (Gallese et al., 1996). Area F5 comprises three distinct cytoarchitectonic sectors: F5p, F5a and F5c (Belmalih et al., 2009). It was generally accepted (for review see Rizzolatti et al., 2014) that canonical and mirror neurons were prevalent in areas F5p and F5c respectively. A recent study, using multi-electrode arrays to record from a large sample of neurons, showed, however, that mirror and canonical neurons are located in both F5c and F5p, with only a tendency for mirror neurons to be more frequent in F5c than F5p (Bonini et al., 2014). These electrophysiological findings are consistent with fMRI data showing that F5c, F5a, and to a lesser degree F5p, are all activated during grasping observation (Nelissen et al., 2005). The latter study also revealed that, area F5c, unlike F5a and F5p, is more strongly activated when the stimuli show a person grasping an object rather than merely the hand grasping it. On the basis of these findings and the connection of F5c with PFG, an inferior parietal lobule area involved in understanding motor intention (Fogassi et al., 2005), it has been suggested that area F5c is involved in understanding the goal of and the intention behind an observed motor act.
Can one functionally localize the different sectors of area F5 – and F5c in particular – in humans? Many imaging studies have demonstrated that, as in the monkey, human ventral premotor cortex becomes active during action observation (Aziz-Zadeh et al., 2006; Buccino et al., 2001; Caspers et al., 2010; Gazzola et al., 2007; Grafton et al., 1996; Grosbras et al., 2012; Iacoboni et al., 1999; Jastorff et al., 2010; Molenberghs et al., 2012; Rizzolatti et al., 1996b; Van Overwalle and Baetens, 2009). While it is likely that the activated regions included the homologue of F5c, they did not identify this homologue. Recently Neubert et al. (2014) used diffusion tensor imaging (DTI) in humans and monkeys in an attempt to localize the human homologue of F5 based on its connections. The degree to which DTI allows following cortical fibers into the cortical mantle, however, is presently unclear (Van Essen et al., 2014). Hence, some caution is required when interpreting such comparative DTI studies. Neubert et al. (2014) subdivided human PMv into two parts, 6v and 6r, which they tentatively identified as the homologues of monkey F5c and F5a, leaving F4 and F5p unaccounted for.
Here we adopt a different strategy for identifying the homologue of F5c, using monkey fMRI as the link between monkey single neuron studies and human fMRI studies (Orban, 2002). This strategy, used previously to locate areas housing gradient-selective neurons encoding 3D shape, in human cortex (Orban, 2011; Vanduffel et al., 2014), consists of two steps. The first is to define an fMRI paradigm that uniquely activates a given monkey cortical region, using a property of the neurons housed in that area, in this case the overall responsiveness of F5c neurons to observed actions. The second step uses the same paradigm in humans, looking for activated cortical regions. We applied this strategy to F5c, using videos similar to those of Nelissen et al. (2005) and examined human frontal cortex for the interaction between the factors action and person, an fMRI characteristic of monkey F5c. The series of fMRI experiments reported here revealed bilateral interaction sites in human ventral premotor cortex (PMv), which we propose as putative homologue of area F5c (phF5c).
Material and methods
Subjects
The seven fMRI experiments (four main and three supplementary experiments) used 54 human volunteers and 3 monkeys. Eighteen right-handed volunteers (13 females) with a mean age of 24.2 ± 3.6 years (range 18 to 30 years) participated in the Leuven component of the study (Experiments 1 & S1). Thirty six right handed volunteers (14 females) with a mean age of 22.9 ± 3.5 years (range 19 to 31 years) participated in the Parma component of the study (Experiments 3–4, S2–3). No participant reported a history of neurological disease or was taking psycho- or vasoactive medication. Participants were informed about the experimental procedures and provided written informed consent. The study design was approved by the local Ethics Committee of Biomedical Research at KU Leuven and the Provincia of Parma and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
One female (M13) and two male (M17, M18) rhesus monkeys (Macaca mulatta, 3–5 kg, 3–5 years of age) also participated in the Leuven component of the study (Experiment 2). All animal care and experimental procedures met the national and European guidelines and were approved by the Ethical committee of the KU Leuven.
Visual stimuli
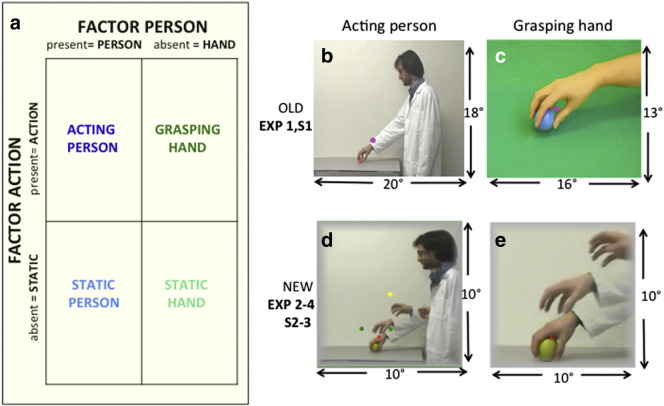
All video clips show human actors performing grasping. The study of Nelissen et al. (2005) has shown that these evoke strong MR responses in monkey premotor cortex. Furthermore, Jastorff et al. (2012b) explicitly compared video clips portraying monkey and human full-body actions such as running or climbing, and found little advantage to using monkey actions in investigating monkey STS (see their Fig. 8). Electrophysiological studies of premotor cortex (Rizzolatti et al., 1996a; Caggiano et al., 2014) also support the view that both human and monkey actions drive premotor mirror neurons with only a slight advantage for monkey actions, although a detailed quantitative comparison is still lacking. The experiments followed a factorial 2 × 2 design with person (present, absent) and action (present, absent) as factors (Fig. 1a), and used either ‘old’ (Experiments 1, S1) or ‘new’ stimuli (Experiments 2–4, S1, S2, S3). They all included a fixation baseline condition (fixation point on empty screen).
Fig. 1.
Factorial design (a) and the two types of action video in old (b, c) and new (d, e) action videos: acting person (b, d) and grasping hand (c, e). Static frames of the four videos are shown: frames near the middle of the video in a, c, and multiple frames showing the pre-shaping of the hand in d, e. Sizes were 18 × 20° in b, 13 × 16° in c, and 10 × 10° in d, e. The colored dots in d indicate the positions of the fixation point (red in the actual experiments) relative to the target object: red dot: Experiment 2: (monkey); right green dot: Experiments 1 & S1, both green dots: Experiments 3–4 and part of Experiment S3, yellow dot: Experiment S2. In a the factorial design shown is for the new stimuli; for the old stimuli several control conditions were used: 3 in Experiment 1 and 2 in Experiment S1 (Table 1). For calculation of the interaction the different controls were averaged, so that in practice the design remained 2 × 2.
The old stimuli
(Figs. 1b, c) are exactly those used by Nelissen et al. (2005). They consisted of video clips showing a hand (and forearm) grasping objects (‘grasping hand’, 13 by 16° in size) and video clips showing a full view of a person grasping objects with the hand (‘acting person’, 18 by 20° in size). Four different ‘grasping hand’ video sequences were used: a male or female hand grasping and picking up a candy (precision grip) or a ball (whole hand grasp). One action cycle (grasping and picking up) lasted 3.3 s, with 7 randomly selected cycles presented in a block. Six different ‘acting person’ video sequences were presented in random order in a block: a man or woman grasping and picking up an apple (whole hand grasp), or a peanut or a piece of carrot (precision grip). For the ‘actor’ video sequences, one cycle (grasping and picking up) lasted 6 s and therefore 4 randomly selected cycles were presented in a block. The longer duration of the action cycle for the ‘acting person’ videos was due to the larger number of static frames at the beginning and end of the videos. The duration of the hand movement period was reasonably similar in the two movies: averaging 2.1 s in the ‘grasping hand’ videos and 2.7 s in the ‘acting person’ videos. Frame rate was 20/s. Two types of control stimuli were used: first, static single frames of the action videos, one from the middle of the video sequence when the hand is about to grasp the object and one from the end of the video sequence when the object is grasped and picked up and second, scrambled videos produced by phase scrambling each frame of the video sequences. Static stimuli were refreshed by showing a frame from another sequence every 3.3 s or every 6 s in ‘acting hand’ and ‘acting person’ videos respectively. The fixation condition used a white and green background, matched to that in the videos, for the ‘acting person’ and ‘grasping hand’, respectively. These stimuli, even if imperfect (see below), were taken from Nelissen et al. (2005), since exactly the same stimuli had to be used for both species.
The new stimuli
(Figs. 1d, e) were all derived from the person acting videos in Nelissen et al.'s (2005) study, but corrected the imperfections. Old hand and person videos differed in actor, type of grip, color, size, duration and position of the fixation target. These differences were eliminated, but size of hand and upper limb remained different. The person acting video was simply cropped to 10 × 10° and its duration reduced to 3 to 6 s, depending on the frame rate (ranging from 14 to 30 fr/s). The grasping-hand video was taken from the person-acting video, cropped to 10 × 10° and shortened to the same duration. The cropping avoided the arm appearing to be floating in space in the hand conditions. The sizes of the hand and head were reduced in the new compared to the old stimuli (Fig. S1). However in relative terms, relative to the frame, they increased, which may create the impression that the action is closer to the observer. The difference in the size of the hand in the person and hand videos was only slightly reduced in the new stimuli (2.85° vs 3.4°). In the new stimuli the grips shown in the two types of videos (person and hand) were identical. The fixation point was presented in the same position (with respect to the object) in all new videos (Figs. 1d, e). Finally, because all control conditions for the old stimuli yielded similar weak activation levels in both species, only one static control condition (middle frame) was retained. This allowed us to test acting-person and grasping-hand videos within the same runs. It is worth noting that, since the analysis concentrated on the interaction between factors person and action, lower order motion was automatically controlled by the grasping hand condition, in which the upper limb movement equals that in the person acting condition.
Scanning and analysis procedures
Experiment 1
This experiment is the human counterpart of Nelissen et al. (2005), looking for a human premotor region showing an interaction between person and action. Thirteen human subjects lay in a supine position and viewed the screen trough a mirror tilted at 45°. They were instructed to maintain fixation during scanning on red square in (0.2° × 0.2°) the center of the screen. The fixation target is shown as a purple dot in Figs. 1b, c. The eye position was monitored at 60 Hz during all fMRI scanning sessions using the ASL 5000/LRO eye tracker system positioned at the back of the magnet (Applied Science Laboratories, Bedford, MA, USA) to track pupil position and corneal reflection. The ‘old’ visual stimuli, controlled by in-house software (video frame rate 20 fr/s), were projected onto a transparent screen in front of the subject using a Barco (Kortrijk, Belgium) liquid crystal display projector (1024 × 768 pixels; 60 Hz). Optical path length between the eyes and the stimulus measured 36 cm.
The MRI images of human subjects in Leuven were acquired in a 3-T Intera MR scanner (Philips, Best, Netherlands), using a 6 element SENSE head coil (MRI Devices Corp., Waukesha, WI). A functional time series consisted of 120 whole brain BOLD weighted field-echo echoplanar images (FE-EPI) with TR/TE = 3000/30 ms, field of view = 200 mm, acquisition matrix = 80 × 80 (reconstructed to 128 × 128), SENSE reduction factor = 2, slice thickness = 2.5 mm, interslice gap = 0.3 mm, acquiring 50 horizontal slices covering the entire brain. Sixteen time series (runs) were acquired per subject divided over two sessions. In a single session, 4 runs of the grasping hand movies and their control conditions were interleaved with 4 runs of the person acting movies. Runs included 24 s blocks corresponding to the five conditions, which were repeated twice for a total duration of 360 s. The order of conditions within runs was counterbalanced within and across subjects. At the end of each scan session a 3D high resolution T1-weighted anatomical image (TR/TE = 9.68/4.6 ms, TI = 1100 ms, field of view = 250 mm, matrix = 256 × 256, slice thickness = 1.2 mm, 182 slices, SENSE factor = 2) was acquired.
Human data were analyzed with the Statistical Parameter Mapping package, SPM2 and SPM 8 (Wellcome Department of Cognitive Neurology, London, UK), implemented in Matlab (The Mathworks Inc.). Results were very similar for the two versions of SPM. For each subject, motion correction was performed by realignment of all the functional EPI volumes to the first volume of the first time series and a mean image of the realigned volumes was created. This mean image was co-registered to the anatomical T1-weighted image. Both the anatomical and the mean EPI image were warped to a standard reference system (Talairach and Tournoux, 1988), by normalizing both to their respective template images (Montreal Neurological Institute, MNI). Subsequently, the derived normalization parameters from the mean EPI image, were applied to all EPI volumes, which were subsampled to a voxel size of 2 × 2 × 2 mm and smoothed with a Gaussian kernel of 5 mm FWHM.
Statistical analysis was performed using the General Linear Model (K.J. Friston et al., 1995; K.J.J. Friston et al., 1995) by modeling each condition using a delayed boxcar function and by convolving this with the hemodynamic response function. Global signal intensity was normalized and an appropriate high-pass temporal filter (2 times the total duration of all different conditions in a run) was applied to remove low frequency drifts independent of stimuli-induced signal changes. The first level contrasts for the different action types (hand action and actor action) compared to their three respective controls were calculated for each individual subject. These contrast images were subjected to a second level, random-effects ANOVA analysis. The interactions were investigated at this second level by subtracting hand action minus its averaged controls from actor action minus its averaged controls. These interactions were masked inclusively with the single effect of actor action compared to its controls (at p < 0.05 uncorrected) to favor voxels in which the interactions are due to a strong response to person acting conditions. Thresholds were set at p < 0.001 uncorrected for the interaction effects. This threshold is adequate since two variances are summed in interactions, and has been used systematically in our previous studies (Abdollahi et al., 2013; Georgieva et al., 2009; Jastorff and Orban, 2009; Jastorff et al., 2012b; Peeters et al., 2009).
Activity profiles plot, for any given ROI, the MR signal changes relative to fixation, using actual values, in percent of fixation activity. The profiles were calculated from the single subject data obtained by averaging all the voxels in the ROI. The first two data points within each block were not taken into account to compensate for the hemodynamic delay of the BOLD response. In all profiles the standard error (SE) of the mean was determined across the different subjects. The ROIs were defined by the interaction contrast, using all voxels reaching p < 0.001 uncorrected level.
The fMRI data were mapped onto the human PALS atlas (Van Essen, 2005) surface in SPM-Talairach space, for both right and left hemispheres using a volume to surface tool in Caret (Van Essen et al., 2001). Anatomical landmarks were taken from the Caret database and local maxima were inserted as nodes on these flattened cortical maps in Caret. The ROIs used by Jastorff et al. (2010) to tile the premotor action were defined on the flatmap and transferred from that study.
Experiment S1
This experiment tests whether the interaction between action and person in human premotor cortex is similar for old and new stimuli. It was similar to Experiment 1, except that both new and old stimuli with only the middle static control condition were used. The runs with old and new stimuli were interleaved. Given the small number of subjects (n = 5) only a fixed-effect analysis (SPM2) was carried out and the ROI was defined as the 27 voxels surrounding the local maximum (LM) of the interaction.
Experiment 2
This experiment repeats the experiment of Nelissen et al. (2005) with the new stimuli and tests the interaction between person and action for all monkey motor cortical areas. Details of the surgical procedures, training, eye position monitoring, scanning procedures and statistical analysis of fMRI data have been described previously (Ekstrom et al., 2008; Nelissen et al., 2005; Vanduffel et al., 2001) and will only be described briefly. The monkey subjects (n = 3) sat in a sphinx position in a plastic monkey chair directly facing a LCD screen (1024 × 768 pixels, 60 Hz, also controlled by in-house software). Video frame rate was 30 fr/s. Monkeys were required to maintain fixation in a 2 × 2° window centered on a small red dot (0.3° × 0.3°) positioned in the middle of the screen (indicated in Fig. 1e as a red dot). Eye position was monitored at 120 Hz through pupil position and corneal reflection (Iscan, Burlington, Mass, USA). The monkeys were rewarded (fruit juice) for fixating the red dot for long periods of time (up to several minutes).
Functional images were acquired with a 3 T full body scanner (TIM Trio Siemens) using a gradient-echo T2* weighted echo-planar imaging sequence (40 horizontal slices, TR 2 s, TE 17 ms, 1.25 × 1.25 × 1.25 mm isotropic voxels) with a custom built eight-channel phased-array receive coil, and a saddle shaped, radial transmit only coil (Kolster et al., 2009). Before each monkey scanning session, a contrast agent, monocrystalline iron oxide nanoparticle (Mion, Sinerem Laboratoire Guerbet) was injected into the femoral/saphenous vein (6–11 mg/kg). Use of the contrast agent improved the contrast to noise ratio (Leite et al., 2002; Vanduffel et al., 2001) and enhanced the spatial selectivity of the MR signal changes (Zhao et al., 2006) compared to blood oxygenation level-dependent (BOLD) measurements. Whereas BOLD measurements depend on cerebral blood volume (CBV), blood flow, and oxygen extraction, MION measurements depend only on CBV (Mandeville and Marota, 1999). Accordingly we have inverted the polarity of all signal change values to account for the difference between MION and BOLD activation maps. Six to eight runs were collected in a single session. Runs included 7 conditions (four in the factorial design, fixation, and 2 other conditions not analyzed), shown in 20 s blocks repeated once. Fifteen orders of conditions within runs were presented in pseudorandom order to each subject.
Monkey fMRI data were analyzed using SPM5 and Match software. Only runs in which the monkeys fixated > 90% of the time were analyzed. Spatial preprocessing consisted of realignment and rigid co-registration with a template anatomy (M12, Ekstrom et al., 2008). To compensate for the echo-planar distortions in the images as well as inter-individual anatomical differences, the functional images were warped to the template anatomy using nonrigid matching software (Chefd'hotel et al., 2002). The functional volumes were then resliced to 1 mm3 isotropic and smoothed with an isotropic Gaussian filter (FWHH at 1.5 mm). Group analyses (fixed effects) were performed with an equal number of volumes per monkey and the level of significance was set at p < 0.05 corrected (family wise error, FWE) for multiple comparisons. In the ROI analyses the main effects and interaction were directly tested by t tests corrected for the number of ROIs (18).
Activity profiles plot the MR signal changes relative to fixation for a given ROI, either actual values in percent of fixation activity or fitted values (Marsbar), in percent of the average signal in the run, with similar results. The profiles were calculated from individual run data obtained by averaging all the voxels in the ROI. The first two data points within each block were not taken into account to compensate for the hemodynamic delay of the MION response. In all profiles the standard error (SE) of the mean was determined across the different runs. The motor ROIs were defined by anatomical criteria (cytoarchitectonics, see Belmalih et al., 2009; Luppino et al., 2000; Nelissen et al., 2005).
Experiment 3
This experiment tests the interaction between person and action in human premotor cortex with the new stimuli and with the fixation point left or right of the object. Eighteen participants lay supine in the bore of the scanner. The ‘new’ visual stimuli were presented by means of a head mounted display (up to 85 Hz refresh rate) with a resolution of 800 horizontal pixels × 600 vertical pixels (Resonance Technology, Inc., Northridge, CA) in each eye. The display was controlled by an ATI Radeon 2400 DX dual output video card (AMD, Sun Valley, CA). The presentation of the stimuli was controlled by E-Prime software (Psychology Software Tools, Inc., Sharpsburg, PA). Sound-attenuating headphones were used to muffle scanner noise and to give instructions to the subjects. To reduce the amount of head motion during scanning, the subjects' head was padded with PolyScanTM vinyl-coated cushions. Subjects were instructed to fixate a small red square (0.2° by 0.2°) in the center of the display. The fixation target was located in two positions relative to the video, indicated by green dots in Fig. 1d. Throughout the scanning session, eye movements (right eye) were recorded with an infrared eye tracking system (60 Hz, Resonance Technology Inc., Northridge, CA).
The MRI images were acquired using a 3 T MR scanner (GE Discovery MR750, Milwaukee, IL) with an 8 channel receiver coil, located at the University Hospital of the University of Parma. Functional images were acquired using gradient-echoplanar imaging with the following parameters: 49 horizontal slices (2.5 mm slice thickness; 0.25 mm gap), repetition time (TR) = 3 s, time of echo (TE) = 30 ms, flip angle = 90°, 96 × 96 matrix with FOV 240 (2.5 × 2.5 mm in plane resolution), and ASSET factor of 2. The 49 slices contained in each volume encompassed the entire brain from the cerebellum to the vertex. A 3D high-resolution T1-weighted IR-prepared fast SPGR (Bravo) image covering the entire brain was acquired in one of the scanning sessions and used for anatomical reference. Its acquisition parameters were as follows: TE/TR 3.7/9.2 ms; inversion time 650 ms, flip-angle 12°, acceleration factor (ARC) 2; 186 sagittal slices acquired with 1 × 1 × 1 mm3 resolution. All runs included 5 conditions shown in 24 s blocks repeated twice for a total duration of 360 s. In this experiment, duration of the videos was extended to 6 s to have equal numbers (n = 2) of whole hand and precision grips in each block, at the cost of reducing frame rate to 14 fr/s. Subjects participated in a single 90 min scanning session, in which 10 runs were collected. Ten orders of conditions within runs were presented in pseudorandom order across subjects.
fMRI data were analyzed with the Statistical Parameter Mapping package, SPM 8 (Wellcome Department of Cognitive Neurology, London, UK), implemented in Matlab (The Mathworks Inc.). For each subject, motion correction was performed by realignment of all the functional EPI volumes to the first volume of the first time series and a mean image of the realigned volumes was created. This mean image was co-registered to the anatomical T1-weighted image. Both the anatomical and the mean EPI image were warped to a standard reference system (Talairach and Tournoux, 1988), by normalizing each to its respective template image (Montreal Neurological Institute, MNI). Subsequently, the derived normalization parameters from the mean EPI image, were applied to all EPI volumes, which were subsampled to a voxel size of 2 × 2 × 2 mm and smoothed with a Gaussian kernel of 6 mm FWHM.
Statistical analysis was performed using the General Linear Model (K.J. Friston et al., 1995; K.J.J. Friston et al., 1995), by modeling each condition using a delayed boxcar function and by convolving this with the hemodynamic response function. Global signal intensity was normalized and an appropriate high-pass temporal filter (2 times the total duration of all different conditions in a run) was applied to remove low frequency drifts independent of stimuli-induced signal changes. The first level interactions between action and person were calculated for each individual subject. These contrast images were subjected to a second level, random-effects ANOVA analysis. These interactions were masked inclusively with the single effect of actor action compared to its controls (at p < 0.05 uncorrected) to favor voxels in which the interactions are due to strong response in person-acting conditions. Thresholds were set at p < 0.001 uncorrected for the interaction effects. This threshold is adequate since two variances are summed in interactions (see above).
Activity profiles plot the MR signal changes relative to fixation, using fitted values (Marsbar), in percent of the average signal in the run. The profiles were calculated from the single subject data obtained by averaging all the voxels in the ROI. The first 5 data points within each block were not taken into account to compensate for the hemodynamic delay of the BOLD response. In all profiles the standard error (SE) of the mean was determined across the different subjects. The ROIs were defined by the whole brain interaction contrast, using all voxels reaching p < 0.001 uncorrected (Table 1). The human fMRI data were mapped onto the human PALS atlas (Van Essen, 2005) surface in SPM-Talairach space, for both right and left hemispheres using a volume to surface tool in Caret (Van Essen et al., 2001) as described for Experiment 1.
Table 1.
Features of the experiments.
| Humans |
Monkeys |
||||||
|---|---|---|---|---|---|---|---|
| Experiment | 1 | S1 | 3 | 4 | S2 | S3 | 2 |
| Site & Scanner | Leuven Philips | Leuven Philips | Parma GE | Parma GE | Parma GE | Parma GE | Leuven Siemens |
| Subjects | 13 | 5 | 18 | 12 | 7 | 9 | 3 |
| Stimuli | Old | Old/New | New | New | New | New | New |
| Frame rate | 20 | 20 | 14 | 14, 20, 28 | 14 | 14 | 30 |
| Controls | 2 Static & Scramble | Sta-mid | Sta-mid | Sta-mid | Sta-mid | Sta-mid | Sta-mid |
| Fixation pt (Fig. 1) | To right of object: purple | To right of object: purple | To left and right of object: green | To left and right of object: green | To right and up: yellow | To left or right (green)/no | To the right of object: red |
| Analysis | Wh Brain Rnd Eff p < 0.001 uncorr | Wh Brain Fix Eff p < 0.001 uncorr | Wh Brain Rnd Eff p < 0.001 uncorr | Wh Brain Rnd Eff SV Corr p < 0.05 | ROI from Exp 3 p < 0.05 corr mu co | Wh Brain Rnd Eff SV Corr p < 0.05 | ROIs from anat p < 0.05 corr mu co |
| Activity profiles | % Fix all signif voxels | % Fix 27 voxels | % Avg all signif voxels | % Avg 8 most sig voxels | % Avg all voxels | % Avg 8 most sig voxels | Both all voxels |
Abbreviations: sta: static; mid: middle frame of video; Scramble: scrambled; pt: point; Wh Brain: whole brain; Rnd Eff: random effects, Fix Eff: fixed effects, SV Corr: small volume correction; corr mu co: correction for multiple comparisons; Fix: fixation; Avg: average, sig or signif: significant.
The rows list species, experiment number, the scanner type and site, number of subjects, type of stimuli (old /new), frame rate, control conditions, location of fixation target, type of analysis performed (whole brain or ROI, fixed or random effects) and statistical threshold, the voxels on which profiles are calculated and the reference condition for profiles (fixation of average of all conditions).
Experiment 4
Experiment 4 tests the interaction between person and action in human premotor cortex with the new stimuli for 3 different frame rates. It was similar to Experiment 3, except that three frame rates 14 f/s, 20 f/s and 28 f/s were compared. The 12 subjects participated in two sessions in each of which 9 runs were collected, yielding 6 runs for each rate. Sets of 6 runs with a given rate were presented in pseudorandom order across the two sessions (middle set spread over the sessions) to groups of two subjects. The random effects analysis used a small volume correction with the right phF5c site obtained in Experiment 3 (109 voxels centered on 48, 0, 48) as a priori ROI. A symmetrical ROI (109 voxels centered − 48, 0, 48) was used for the LH. The activity profiles were calculated as for Experiment 3 but using only the 8 most significant voxels centered on the local maximum (LM) of the interaction in the ROI. The profiles were the basis for calculating the differential activation (action minus static control) for acting person and grasping hand.
Experiment S2
This experiment tests the interaction between person and action in human premotor cortex for the new stimuli but with the fixation point removed from the target object. It was similar to Experiment 3, except that the fixation target was located 2° higher (yellow dot in Fig. 1d) than the right position used in Experiment 3. The 7 subjects participated in a single session in which 9 runs were collected. The analysis was restricted to the right phF5c ROI as defined in Experiment 3 (109 voxels centered on 48, 0, 48), in which these subjects had also participated. Results of the present experiment were compared to those runs of Experiment 3 in which the fixation target was on the right side of the object (right green dot in Fig. 1d).
Experiment S3
This experiment tests the interaction between person and action in human premotor cortex for the new stimuli with and without control of fixation. It was also similar to Experiment 3, except that in half the runs no fixation target was provided and subjects were instructed to pay attention to the video rather than to fixate a target. The nine subjects participated in 4 sessions, in each of which 6 runs were collected, 3 with fixation and 3 without fixation, yielding a total of 12 runs with and 12 without fixation. In five subjects, the first and the third sessions started with fixation runs followed by no-fixation runs, with the opposite order in the two other sessions. In the other four subjects the order was reversed. The random effects analysis used a small volume correction with the same right a priori ROI as in Experiment 4. Activity profiles were also computed as in Experiment 4.
Results
All experiments followed a factorial 2 × 2 design with person and action as factors (Fig. 1a), their interaction characterizing F5c and hence phF5c. All runs included a fixation baseline condition to evaluate the visual nature of the responses in the experimental conditions. Seven experiments were performed, 4 main and 3 supplementary experiments (Table 1), forming two fMRI cycles in which the same paradigm was applied first in monkeys and then humans. Experiment 1 completed the first cycle initiated by Nelissen et al.'s (2005) study, using in humans the very same stimuli – here referred to as “old” stimuli – as in Nelissen et al. (2005) (Figs. 1b–c). The second cycle was initiated with Experiment 2 using monkeys and an improved set of stimuli (Figs. 1d–e, “new stimuli”). The new stimuli were validated by comparing human activations obtained using “old” and “new stimuli” in Supplementary experiment 1. Experiments 3 & 4 using human volunteers and the new stimuli completed the second cycle. Further analyses of Experiments 3 &4, as well as Supplementary experiments 2 & 3 investigated the influence of fixation position.
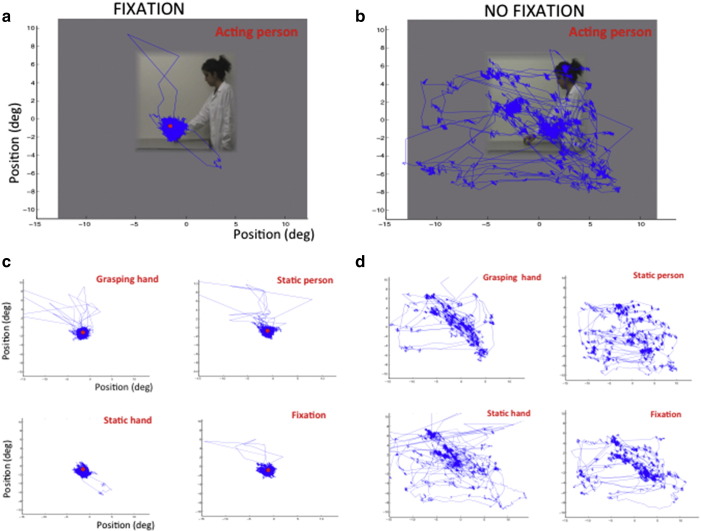
In all but some specific runs of Supplementary experiment 3, subjects (humans or monkeys) had to fixate a red point located in the center of the screen, indicated in Fig. 1. Eye recordings indicated that human subjects fixated well (Figs. 2a, c) and averaged 6–14 saccades/min in the various experiments (Table 2). Monkeys made between 6 and 9 saccades/min in Experiment 2. One-way repeated measures ANOVAs testing for differences in number of saccades between conditions revealed no significant differences in any of the human experiments (groups of subjects) or in Experiment 2 (individual subjects, Table 2).
Fig. 2.
Experiment S3: Eye position of a representative subject during single runs with a fixation point (left position) (a, c) and without (b, d). Eye position is plotted for the 72 s (3 blocks) of each experimental condition and superimposed on a frame of the person-acting conditions (a, b) but not the other conditions (c, d). Although many more saccades were made when the fixation point was absent (b), the number of saccades did not differ significantly between conditions for the group of subjects: F4,4 = 0.08, p > 0.90.
Table 2.
Eye movement statistics.
| Average number of saccades | One-way ANOVA | |
|---|---|---|
| Human groupsa | ||
| Experiment 1 | 9/min | F4,8 = 0.3, ns (hand runs) F4,8 = 0.44, ns (person runs) |
| Experiment S1b | 14/min | |
| Experiment 3 | 6/min | F4,13 = 0.93, ns |
| Experiment 4 | 9/min | F4,7 = 0.2, ns |
| Experiment S2 | 3/min | F4,2 = 0.18, ns |
| Experiment S3 (fixation runs) | 8/min | F4,4 = 0.12, ns |
| Monkey subjects (Experiment 2)c | ||
| M1 | 6/min | F4,1 = 0.61, ns |
| M2 | 9/min | F4,1 = 1.19, ns |
| M3 | 7/min | F4,1 = 2.34, ns |
One-way ANOVA over subjects.
Too few subjects to perform ANOVA.
One-way ANOVA over runs.
Completion of the first cycle: Human counterpart (Experiment 1) of previous monkey experiments
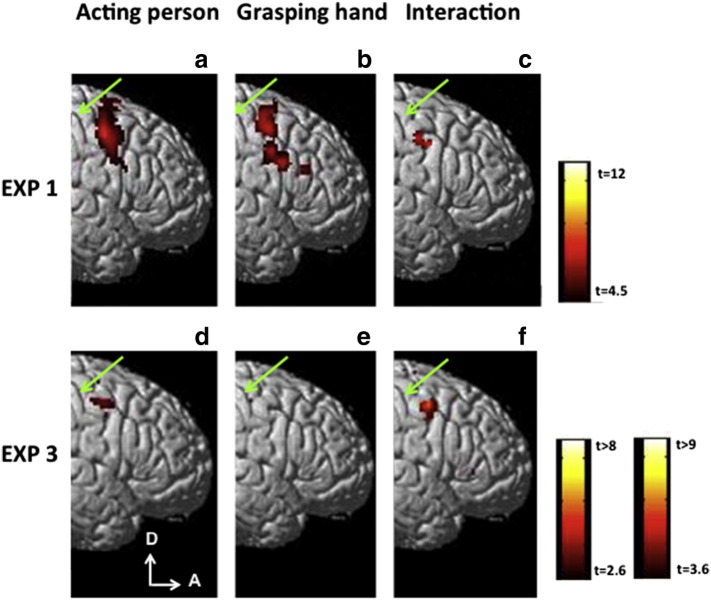
In Experiment 1 volunteers viewed the following visual stimuli: grasping hand, acting person, static frames taken from the middle and the end of the action videos, and scrambled action stimuli, the same as those used by Nelissen et al. (2005). Contrasting observation of the acting person with its three controls (Fig. 3a) yielded activation in the right precentral sulcus (PCS) and adjoining precentral gyrus (PCG). This activation extended medio-laterally from the junction of PCS with the superior frontal sulcus (SFS) to that with the inferior frontal sulcus (IFS). The same contrast for the grasping hand revealed a similar precentral activation (Fig. 3b) but with a central gap. At this position (40, − 4, 44) the interaction (Fig. 3c) tested at the random-effects level proved significant (t = 4.81, p < 0.001 unc). While this activation did not reach FWE corrected significance at the single voxel level (p < 0.345), it did reach this threshold at the cluster level (p < 0.03).
Fig. 3.
Statistical parametric maps (SPMs) showing in color the voxels activated in the subtraction acting person vs controls (a, d) grasping hand vs controls (b, e) and the interaction i.e., the difference between the previous subtractions (c, f) obtained in Experiment 1 (a–c) and Experiment 3 (d–f) rendered on the lateral view of right frontal cortex. Thresholds: p < 0.001 uncorrected in a–c and f and p < 0.01 uncorrected in d, e. Top color scale applies to a–c, the lower left to d–e and that on lower right to f. Green arrows indicate central sulcus. The weaker activation in d, e likely reflects the lower frame rate in Experiment 3 relative to 1 (see Fig. 8).
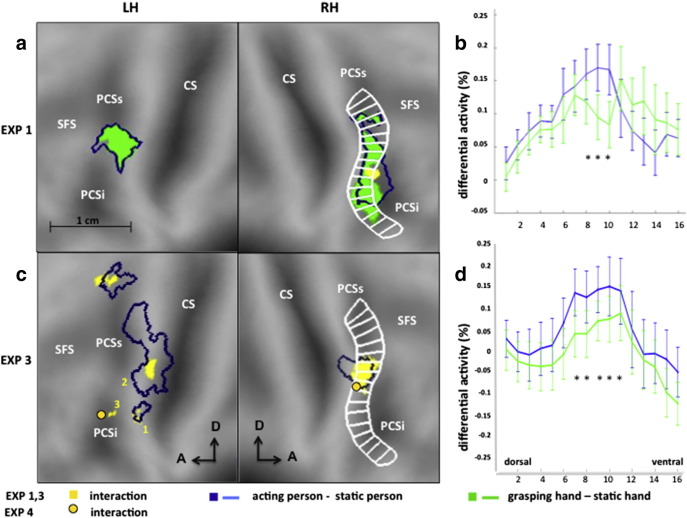
The precentral sulcus (PCS) is subdivided into two parts (Germann et al., 2005) by a horizontal ridge at the level of the middle frontal gyrus (MFG): superior (PCSs) and inferior (PCSi) parts, connecting to the superior frontal sulcus (SFS) and inferior frontal sulcus (IFS), respectively (Fig. 4a). The right interaction region (yellow) is located at the junction of the posterior PCSi bank and the lower bank of the ridge. Above and below this interaction region, additional activations were present during the observation of both the grasping hand (green) and the acting person (blue outline). In the left hemisphere (LH) only a dorsal region was activated by both the acting person and grasping hand (Fig. 4a). Even lowering the threshold failed to reveal any interaction in the left hemisphere.
Fig. 4.
a, c: Statistical parametric maps (SPMs) showing in color the voxels activated in the subtraction acting person vs controls (blue outline), grasping hand vs controls (green), and the interaction, i.e. the difference between the previous subtractions (yellow) obtained in Experiment 1 (a) and Experiment 3 (c), shown on the flatmaps of the anterior portions of left (LH) and right (RH) hemispheres. Thresholds: p < 0.001 uncorrected in a and interaction in c, p < 0.01 uncorrected blue outline in c. Local maxima in c: 1: LH1: − 44, − 10, 56, 12 voxels, 2: LH2: − 56, 6, 38, 27 voxels; 3: LH3: − 32, − 10, 40, 29 voxels; 4: RH : 48, 0, 48, 109 voxels; notice that LH3 is located in the posterior branch of PCSi which folds behind the PCG in 3D; yellow dots encircled by black: local maxima of interaction in Experiment 4 averaging over frame rate (uncorrected: − 40, 0, 36, t = 4.77, p < 0.001 and 46, − 4, 44, t = 3.32, p < 0.003); b, d: differential activity (as % of fixation baseline) evoked by acting person vs controls (blue) and grasping hand vs controls (green) obtained in Experiment 1 (b) and Experiment 3 (d), plotted from dorsal to ventral for the miniROIs, shown in white in RH of a, c. Vertical bars SE. The difference between differential activity for acting person and grasping hand was significant in bins 8–10 in b (paired t tests p < 0.05) and bins 7–11 in d (p < 0.05 in post hoc t-tests following interaction in a 2 × 17 two way ANOVA, F16, 17 = 2.25, p < 0.005).
The activity profile for the interaction site (75 voxels, Fig. 5a; see also Fig. S2) plotting the % MR signal change, relative to fixation baseline, in the four conditions pertaining to the acting person (blue bars) and the grasping hand (green bars) closely corresponds to that of monkey F5c (Nelissen et al., 2005). This indicates that the interaction reflects a robust response to observing the acting person rather than a strong static hand control response, as expected from the masking procedure (see Material and methods).
Fig. 5.
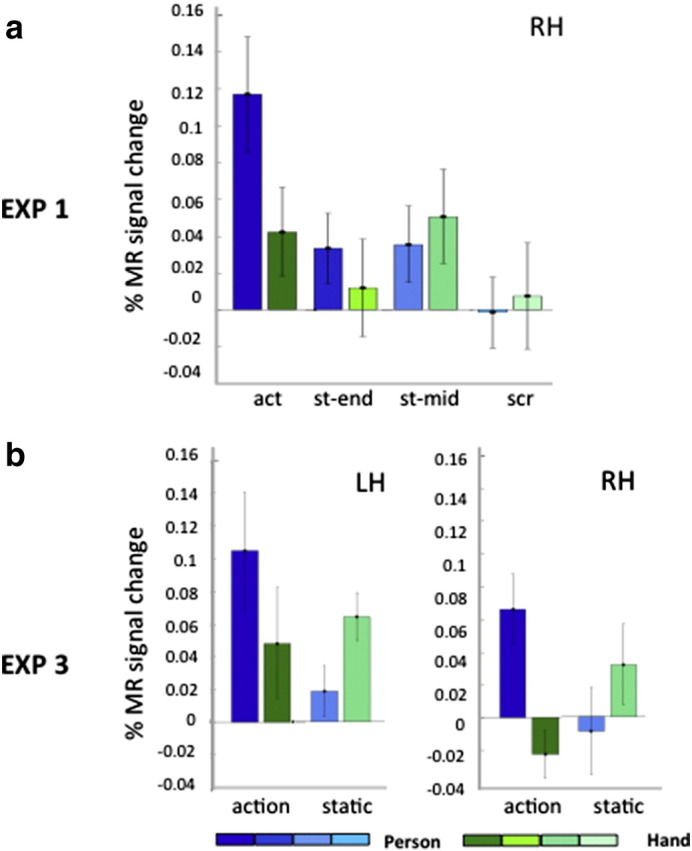
Activity profiles of left and right phF5c obtained in Experiment 1 (a) and Experiment 3 (b). Blue bars: observing person acting and controls; green bars: observing hand grasping and controls. Vertical bars: SE across subjects. In a: only right phF5c is shown: 75 voxels defined by the whole brain interaction, in b left and right phF5c: 29 and 109 voxels defined by whole brain interaction. In a observing acting person was significantly different (correcting for 3 comparisons) from 3 controls (paired t tests all t > 3.8, p < 0.002) but observing grasping hand was not (all t < 2.05, p > 0.06); in b: for statistics see Table 1. Activity profiles were obtained with SPM 8. Similar results were obtained for the right PCSi interaction region (a) when analyzed with SPM 2: see Fig. S1.
Second cycle: a) Monkey experiment with new visual stimuli (Experiment 2)
Experiment 1, completing the first monkey–human cycle, validated our strategy of comparing fMRI activations in humans and monkeys using similar stimuli. The two types of action videos in Experiment 1, however, differed in lower-order visual aspects (e.g., different color, size) and in the position of the fixation point. The results could therefore, in principle, be due to these factors. Hence, the stimuli were redesigned by creating the hand-grasping video (Fig. 1c) from the acting-person video in such a way to minimize visual differences (see Material and methods).
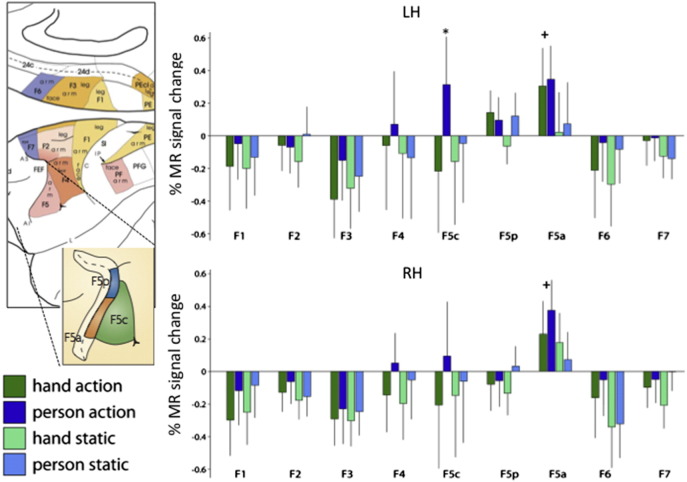
Experiment 2 was carried out in three monkeys using these new stimuli. The results are summarized by the group activity profiles of all monkey motor cortical areas, plotting the % MR signal change relative to the average activity in the run, in the four experimental conditions (Fig. 6). T tests revealed significant interaction between person and action in a single area: left F5c (t = 3.61, p < 0.005 corrected for number of ROIs). This interaction was significant in each of the three monkey subjects (t = 2.3, 2.8, and 2.74 in subjects 1, 2 and 3 with p < 0.01, p < 0.003 and p < 0.004 respectively). Given the location of the fixation point (Fig. 1e, red dot), this left lateralization might suggest that monkey F5c becomes active during observation of the hand-shaping phase of grasping, before the hand contacts the object, i.e., when the hand is in the right visual hemifield. Indeed, even if we consider the size of the fixation window, sensitivity to the actual grasping (hand on object) should yield a right-sided or bilateral activation. The left F5c profile indicates that its activation by the acting person exceeded that of all three other conditions, with the main effect of action being non-significant (for statistics, see legend in Fig. 6). Contrasting the acting person and fixation conditions confirmed that the acting person evoked a significant visual response in left F5c. In contrast to the interaction, the action main effect reached significance in the F5a ROIs bilaterally (see legend in Fig. 6).
Fig. 6.
Activity profiles of the motor and premotor areas in left (a) and right (b) hemisphere of the monkey: F1, F2, F3, F4, F5c, F5p, F5a, F6 and F7 obtained in Experiment 2 (group of 3 monkeys). Activity is expressed in % of average activity, but very similar results were obtained using the actual values expressed in % of fixation (see Material and methods). Same color code as in Fig. 5. Insets (left) indicate locations of areas (taken from Rizzolatti and Luppino, 2001; Rizzolatti and Sinigaglia, 2010). Vertical bars indicate SE (over 18 runs). The main effect of action reached significance (corrected for multiple comparisons 2 × 9) in F5a bilaterally (left: t = 4.14, p < 0.0005 and right t = 3.16, p < 0.02), but not in left F5c (t = 2.73, p > 0.05). The interaction proved significant only in left F5c (t = 3.61, p < 0.005). The response of left F5c to the person acting observation was significantly larger than the response to any of the three other conditions (all t > 4.1, p < 0.0005). The response of left F5c to the person acting observation also differed significantly from fixation baseline (t = 4.07, p < 0.0005). Note that the absence of activation for person grasping in F5p and F1 is consistent with recent reports (Kraskov et al., 2009; Vigneswaran et al., 2013) indicating that in these areas about equal proportions of neurons are activated and suppressed by observing grasping.
Second cycle: b) Human experiments with the new visual stimuli (Experiments S1, 3 & 4)
Experiment S1
To validate the new stimuli, we compared in Experiment S1 activations due to old and new stimuli, with identical fixation point for all new stimuli (Fig. 1d, right green dot, as in Experiment 1). The results showed a significant interaction between person and action in right PMv, in similar sites for both the old (52, − 4, 40) and new stimuli (52, − 6, 42). Activity profiles were similar for the two stimulus types (Fig. S3), but the static hand condition evoked a stronger response in the case of the new stimuli. This finding probably reflects low-level visual differences between old and new stimuli (see Material and methods) and underscores the sensitivity of the technique.
Experiment 3
Human Experiments 1 and S1 yielded interaction sites located in the right hemisphere (RH), while the interaction was limited to the left hemisphere in the monkey (Experiment 2). This discrepancy might indicate that the human putative F5c region, rather than being more sensitive to hand-shaping than to actual grasping, as in monkey F5c, is actually more sensitive to the actual grasping (hand on object), that took place in the left visual field in these experiments (Fig. 1d). To test this possibility, we positioned the fixation point in half of the runs of Experiment 3 to the right of the object, as in Experiments 1 & S1, and in the other half in a symmetrical position to the left of the object (Fig. 1d, green dots).
The analysis of Experiment 3, pooling the results of both fixation positions, yielded a pattern of results in the right hemisphere similar to those of Experiment 1. The observation of acting person (Fig. 3d) evoked an activation in PMv, which was weaker (reaching only t = 2.6, p < 0.01 uncorrected) and more restricted than in Experiment 1, possibly due to the smaller stimuli or the lower frame rate (see below). The observation of grasping hand evoked a very weak response, below the threshold of t = 2.6 (Fig. 3e). An interaction, however, was present at the dorso-caudal edge of the PCSi (Fig. 3f) in a location (48, 0, 48) similar to that of Experiment 1 and with a similar significance level (t = 3.6, p < 0.001). While this activation did not reach FWE corrected significance at the single voxel level (p < 0.128), it did reach this threshold at the cluster level (p < 0.02). Also the size of the interaction site (109 voxels) was rather similar to that in Experiment 1 (75 voxels). Its 436 mm2 is about 9 times the area of cytoarchitectonic macaque F5c, coinciding with the 9-fold increase in total cortical surface in humans compared to monkeys (Van Essen et al., 2012). Experiment 3 thus independently replicated the findings of Experiment 1.
The flat-map projection confirmed that the location of the right premotor interaction was similar to that found in Experiment 1 (Figs. 4a, c). To further document similarities in the interactions, we plotted the differential activations, i.e., action relative to static control, for acting-person and grasping-hand conditions in the mini-ROIs of Jastorff et al. (2010), (white outlines in Figs. 4a, c), sampling the activity along the PCS and PCG. In both experiments (Figs. 4b, d) the curves peaked at the same positions, showing significant dissociations between the two curves in bins 8–10 for Experiment 1 and bins 7–11 for Experiment 3. Thus the right interaction sites overlap in the two experiments.
Pooling the two fixation point positions also yielded three interaction sites reaching p < 0.001 uncorrected in left premotor cortex (Arabic numbers in Fig. 4c), plus another site dorsal to SFS. Two sites (sites 1 and 2) were located in the precentral gyrus, with site 1 corresponding to the dorsal left hemisphere (LH) activation in Experiment 1. A third site (site 3) was located in the left PCSi in a slightly more ventral position than its right counterpart. Only this latter site reached significance (− 40, 2, 40, t = 4.09, p < 0.05) with a small volume correction using a ROI symmetrical to that in RH, indicating a location symmetrical to the right interaction site.
To assess the status of possible homologues of F5c among the four PMv interaction sites, we exploited the four statistically validated functional properties of F5c as revealed by Experiment 2, listed in Table 3. To examine these properties, we subjected the activity profiles of these four sites (Fig. 4) to a 2 × 2 ANOVA, yielding the expected interaction, but allowing us to test the main effect of action and to perform critical post-hoc t-tests. Also, we used a paired t-test to compare the acting person and baseline fixation conditions. The results showed that two of the four interaction sites share the characteristics of F5c (Table 3). In the right interaction site (Fig. 5b) the static hand condition reached about half the activation level evoked by acting person, yet t-tests showed that the latter condition evoked significantly more activity than the other 3 factorial conditions, or the fixation baseline. Since this site showed no main effect of action, it met all four criteria derived from Experiment 2. In the left hemisphere, only the PCSi site (site 3 in Fig. 4c) met these four criteria (Table 3, Fig. 5b). The lower PCG site (LH2) failed in the criterion comparing observing person grasping to the other factorial conditions, as it was equally active in the static hand and acting person conditions. It also failed the last criterion, as the visual response to person grasping was not significant. The upper PCG site (LH1) had a relatively strong response to the grasping hand, even if the acting person condition significantly exceeded each of the three other factorial conditions. Unlike monkey F5c, however, it showed a main effect of action, failing the third criterion. The location of this LH1 site was indeed similar to the dorsal left hemisphere region which was activated in the two single contrasts in Experiment 1 (Fig. 4a). Experiment 3 thus yielded in addition to the right interaction site, a single left-sided interaction site (site 3, 29 voxels) matching F5c in statistical characteristics (Fig. 5b). This left site was located in a position matching that of the right, since it reached corrected significance using the right site as an a priori ROI. The left site was not only smaller than its right counterpart, it was also less significant as it failed to reach FWE significance at the cluster level (p > .65). We refer to these two sites as left and right phF5c. Single-subject analysis confirmed that the right phF5c was present in all individual subjects in approximately the same cortical location (Fig. 7).
Table 3.
Statistical analysis using 4 criteria for 4 interaction sites in Experiment 3: RH = 109 voxels, LH3 = 29 voxels, LH1 = 12 voxels, LH2 = 27 voxels.
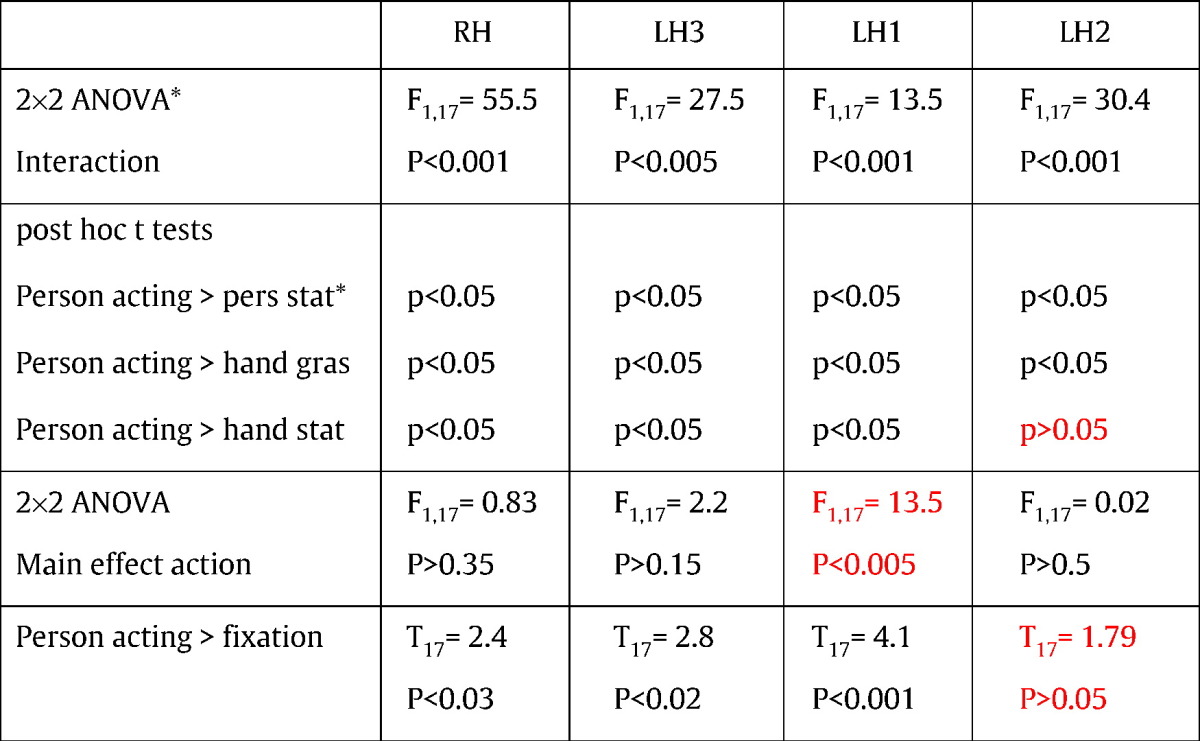
Numbers in red: failure to meet a criterion.
Asterisks: tests expected to be significant from the subtractions defining the sites.
Abbreviations: pers: person, stat: static, gras: grasping.
Fig. 7.
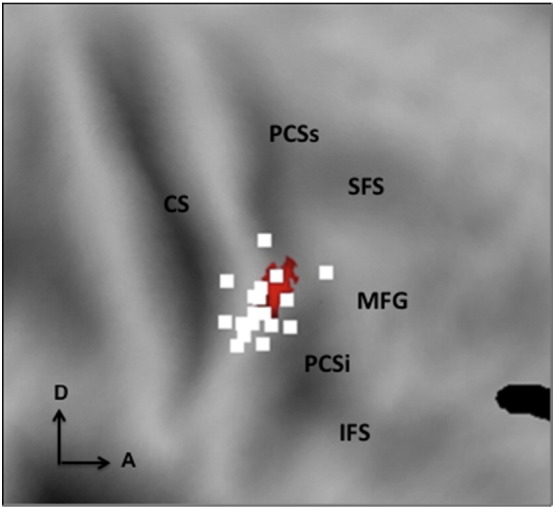
Experiment 3: Local maxima (white squares, p > 0.01 uncorrected) of the interaction person-action in 18 individual subjects, superimposed on the group interaction site (colored voxels, p < 0.001 unc) in right PCSi, shown on flat map.
Experiment 4
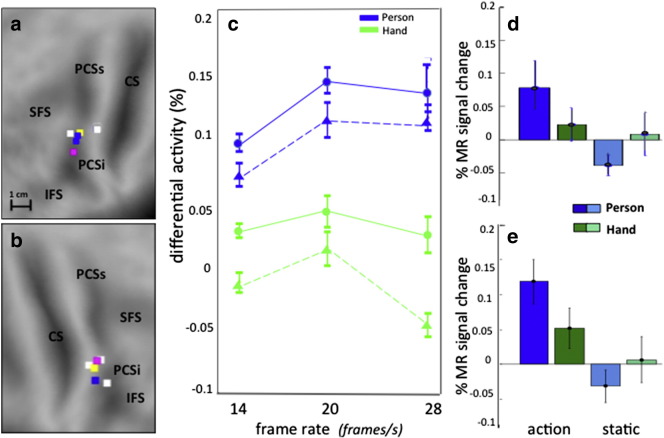
Experiment 3 employed the new stimuli exactly as in monkey Experiment 2, except for a single parameter, frame rate which was 30 and 14 Fr/s in Experiments 2 and 3 respectively. Slow frame rates correspond to slow grasping actions, which might influence activation levels. Hence in Experiment 4 the interaction between person and action was compared at 14, 20 and 28 f/s, keeping other stimulus aspects constant and using the two fixation positions of Experiment 3. As in Experiment 3 we pooled data from the two fixation positions. We used a small volume correction (SVC) with the right phF5c (from Experiment 3, center 48, 0, 48) and a symmetrical ROI for LH (center − 48, 0, 48) as a priori ROIs.
A random effects analysis yielded significant (after SVC) local maxima (LM) for each frame rate in LH (− 52, 0, 50, t = 2.4, p < 0.02; − 40, − 2, 46, t = 2.1, p < 0.04; and − 44, 0, 46, t = 2.9, p < 0.008, Fig. 8a), and RH (50, − 4, 48, t = 2.4, p < 0.02; 44, − 2, 48, t = 3.7, p < 0.002 and 46, 0, 40, t = 2.8, p < .008; Fig. 8b), although not all LM remained significant after correction for 3 tests on a single a priori ROI. The number of significant voxels was much greater for 20 fr/s in RH (46 voxels compared to 4 and 3 voxels at 14 and 28 fr/s) and for 28 fr/s in LH (9 voxels compared to 5 and 2 voxels at 14 and 20 fr/s). The number of voxels reaching significance also confirmed that the interaction was stronger on the right than the left. The differential activation, relative to static control, for acting-person and grasping-hand conditions increases with frame rate, more so for acting-person than grasping-hand (Fig. 8c). The interaction (distance between differential activations of person and hand) is relatively invariant for frame rate in both hemispheres, with a broad optimum at 20/s (Figs. 8d, e). Experiment 4 shows that, if anything, the interaction is stronger at faster frame rates than that used in Experiment 3, explaining the weaker activation in Experiment 3 compared to 1 (Fig. 3). It also generalizes the results of the previous experiment with respect to the identification of left and right phF5c to another low-level visual feature: motion speed.
Fig. 8.
Experiment 4: a–b: flatmaps showing locations of the local maxima of the interaction in left (a) and right (b) PMv; c: plot of differential activation (in % of average activity) for person acting (blue) and hand grasping (green) as a function of frame rate in left (dashed lines) and right (solid lines) phF5c; d–e: activity profile for 20 fr/s in left (d) and right (e) phF5c. Vertical bars in c–e: SE across subjects. Colored squares in a: yellow center of the a priori ROI (− 48, 0, 48) white: LM at 14 fr/s: − 52, 0, 50, t = 2.4, p < 0.02; − 44, 2, 50, t = 2.1, p < 0.03; pink: LM at 20 fr/s: − 40, − 2 36, t = 2, p < 0.04; blue : LM at 28 fr/s − 44, 0, 46, t = 2.9, p < 0.008; − 48, 2, 50, t = 2.5, p < 0.01; colored squares in b: yellow: center of the ROI (48, 0, 48); white LM at 14 fr/s: 50, − 4, 48, t = 2;4, p < 0.02; 38, − 2, 36 t = 2.1, p < 0.03; 42, 2, 52, t = 2.0, p < 0.04; pink: LM at 20 fr/s: 44, − 2, 48, t = 3.7, p < 0.003; blue: LM at 28 fr/s: 46, 0, 40, t = 2.8, p < 0.008. The plots of c–e are based on the 8 most significant voxels after SVC with 1.3 < t < 2.4 (left) and 0.85 < t < 3 (right) for 14 fr/s, 1.3 < t < 2 (left) and 2.6 < t < 3.7 (right) for 20 fr/s, 2.3 < t < 2.9 (left) and 1.6 < t < 2.8 (right) for 28 fr/s.
The analysis of human data in both cycles (Experiments 1, 3, 4) concentrated on the frontal lobe, which is where the homologue of a premotor cortex region should be expected. For completeness it is worth mentioning that all whole brain analyses of these experiments yielded additional interaction sites predominantly in the left hemisphere. These are only briefly mentioned as this topic is outside the scope of the paper. They included several parietal sites, hMT + region (overlapping with the EBA) and early visual cortex (EVC) in the LH, with one site in right posterior STS. Experiment 4 revealed, however, that most of these sites vanish at the fastest frame rate (and correspondingly shorter duration) where only left visual sites – the four areas of the MT cluster with extension into LO1 (Abdollahi et al., 2014) – remained active, corresponding with the position of the body of the person in the right visual field. The same was observed in the monkey (Experiment 2 using fast frame rate) in which only a left occipital activation site was observed straddling V4 and MT.
Second cycle: c) Control experiments and analyses in humans with new stimuli: manipulation of fixation position (Experiments 3 & 4, S2, S3)
Experiments 3 & 4
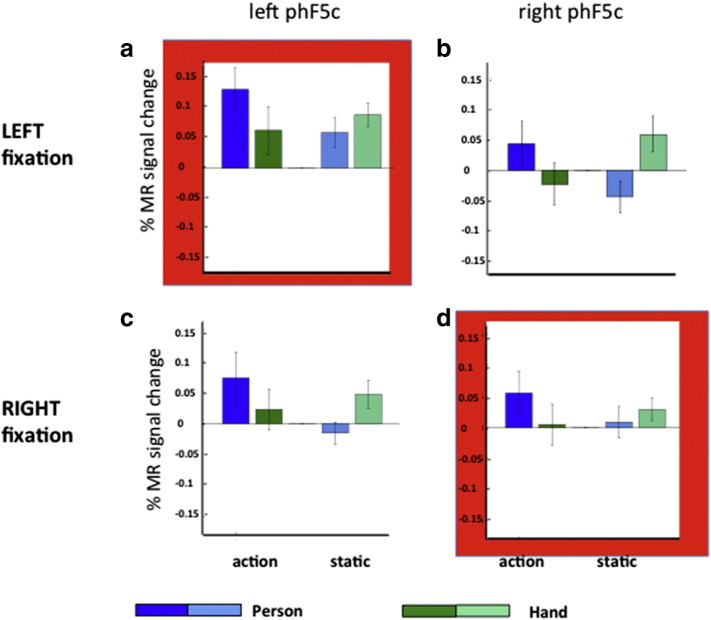
Thus far, we have presented interactions between action and person in Experiments 3 & 4 by pooling the data obtained with the two fixation points. However, if phF5c is sensitive to the actual grasping, the activity of the phF5c profiles should depend on the fixation point position. Hence we computed the profiles of left and right phF5c analyzing the runs for the two fixation point positions separately (Fig. 9). A three-way ANOVAs with fixation side, action and person as factors revealed a significant 3-way interaction (F1,17 = 17.02, p < 0.001) for the right site, but not the left (F1,17 = 0.04, p > 0.80). Thus the interaction between action and person, characteristic of phF5c, depends more on fixation position in the right hemisphere than in the left.
Fig. 9.
Experiment 3: Activity profiles of left (a, c) and right (b, d) phF5c plotted separately for runs with fixation to the left (a, b) and right of target (c, d). Red outlines indicate regions contralateral to the target, i.e., where the actual grasping takes place in the contralateral hemifield. Vertical bars indicate SE across subjects. The three-way interaction between factors person, action, and fixation position was significant for right phF5c (F1,17 = 17.02, p < 0.001), but not left phF5c (F1,17 = 0.04, p > 0.8). The two-way interaction between action and person factors was significant in all four panels: F1,17 = 6.2, p < 0.025 (a), F1,17 = 35, p < 0.001 (b), F1,17 = 11.3, p < 0.005 (c) and F1,17 = 10.4, p < 0.005 (d), but the response to static hand was strongest in panel B.
In Experiment 4 after averaging over all frame rates, a random effects analysis with SVC yielded, consistent with the above analysis (see Experiment 4), 12 significant voxels in right ROI (LM 46, − 4, 46, t = 2.5, p < 0.02) but only 4 voxels in left ROI (LM − 50, 2 50, t = 1.97, p < 0.03). Separating the runs with right and left fixation targets yielded 13 and 3 significant voxels respectively in the right ROI. Thus as in Experiment 3, right phF5c showed more interaction for the right fixation position, i.e., when the actual grasping took place in the left visual field.
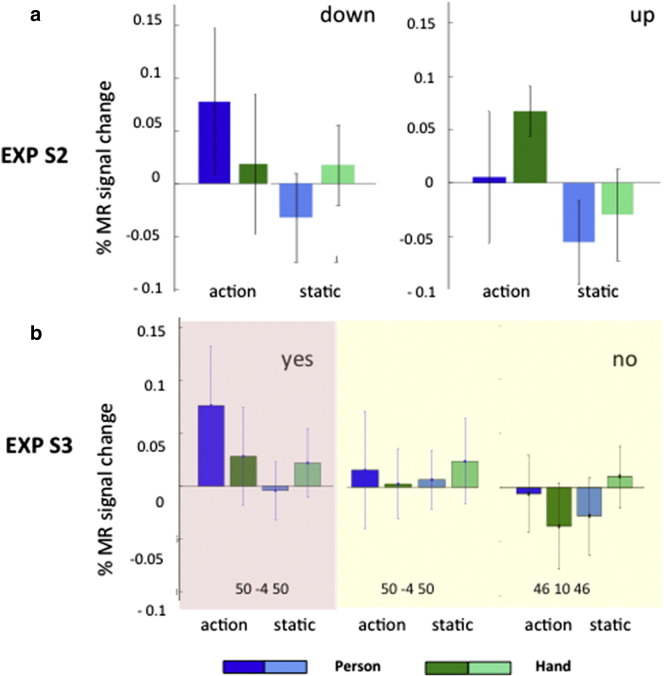
Experiment S2
To confirm the dependence of right phF5c on fixation position, we moved the fixation point 2° higher (Fig. 1d) in Experiment S2. The change in fixation point position had a clear effect on right phF5c, with the interaction vanishing when the fixation point was raised (Fig. 10a). The 3-way interaction (action × person × fixation position) showed a trend towards significance (F1,6 = 2.7, p = 0.1).
Fig. 10.
a: Experiment S2: Activity profiles of right phF5c (109 voxels from Experiment 3) in 7 subjects common to Experiments 3 (only runs with right fixation) and S2. Same color code as in Fig. 5. The interaction proved significant for fixation down (Experiment 3, F1,6 = 25, p < 0.005, left), but not for fixation up (Experiment S2, F1,6 = 0.2, p > 0.6, right). b: Experiment S3. Activity profiles of 8 most significant voxels after SVC in the a priori ROI derived from Experiment 3, for the fixation runs (left, ‘yes’, orange hatching) and no-fixation runs (middle & right, ‘no’, yellow hatching). For the fixation runs the LM (50, − 4, 50, t = 2.25, p < 0.03) was located within 5 mm of the LM in Experiment 4, for non-fixation runs the LM (46, 10, 46, t = 2.09, p < 0.04) was located at more than 10 mm from the LM in Experiment 4. The visual response to the person acting was significant in the fixation runs (‘yes’, paired t test with fixation t8 = 2.5, p < 0.04), but not the non-fixation runs (‘no’, t8 = 0.27, p > 07 for 50, − 4, 50, and t8 = 0.15 p > 0.7 for 46, 10, 46). Vertical bars indicate SE across subjects.
Experiment S3
The strong influence of fixation position on hpF5c responses raised the question of how active F5c would be if no fixation point were to be presented and subjects were required only to look at the videos. To answer this question, in Experiment S3, we compared the activity of right hpF5c when subjects fixated, as in all other experiments (Table 2), and when the fixation point was absent. The number of saccades differed markedly in the two conditions. The subjects made 8 saccades/min when instructed to fixate, compared to 64 saccades/min with the fixation point absent and subjects were merely asked to inspect the videos. This difference in oculomotor behavior (Fig. 2) had a clear effect on right phF5c. Using a random effects analysis and a small volume correction with the same a priori ROI as used in Experiment 4, we obtained 8 voxels showing a significant interaction in the runs with fixation compared to a single voxel in the no-fixation runs. Furthermore the LM in the fixation runs (50, − 4, 50) was very close to the local maxima obtained in Experiments 3 and 4, while the single significant voxel in the no-fixation runs was located more anteriorly (46, 10, 46). Plotting the profiles for the two maxima (Fig. 10b) shows that while there is a clear visual response to person acting in the fixation runs (acting person vs fixation t8 = 2.5, p < 0.04), there is none in the no-fixation conditions (50, − 4, 50, t8 = 0.27, p > 0.6; 46, 10, 46, t8 = 0.15, p > 0.7). Hence, an interaction between the factors action and person reflecting the visual response to person acting was present when the volunteers maintained fixation, but not when they simply watched the videos. The eye recordings (Fig. 2) indicate that subjects rarely fixated the hand grasping the target in those conditions. This confirms and amplifies the results of Experiments 3,4 and S2, indicating that, at least in right hpF5c, the mirror mechanism is activated by action stimuli in central vision, while its activation is much reduced for actions in peripheral vision.
Discussion
Our results show that a sector of human PMv, which is larger in the right hemisphere, becomes active during the observation of grasping actions, but only if the person performing these actions is visible. In the monkey this property characterizes a specific sector of PMv: area F5c.
Interpretation and limitations of the study
Videos showing an acting person or grasping hand differ in a number of visual properties. Hence rather than reflecting the presence of the acting person, the response specificity of monkey F5c and phF5 may be explained by some low-level visual property. The new stimuli used in Experiments 2–4, S2–3 represented an attempt to minimize such differences and to test whether the “acting person” effect persists, despite changes in visual stimulus characteristics. The results observed in three monkeys and over fifty human subjects, combined with those of Nelissen et al. (2005) showed that the cortical activation pattern remained similar for a range of frame rates and for the “old” or “new” videos. In particular, Experiments 1 and 3 independently yielded interactions in virtually identical ventral premotor regions and mutually support each other, at least with respect to the identification of right phF5c. In these experiments we used an uncorrected significance level of p < 0.001. This level is appropriate for interactions, which are differences of differences and for which the variances therefore sum. This is supported by the specific activity profiles obtained in earlier studies using this threshold (Abdollahi et al., 2013; Georgieva et al., 2009; Jastorff et al., 2012b; Peeters et al., 2009). Furthermore, in Experiments 1 and 3 the right phF5c reached FWE corrected significance at the cluster level.
With the new stimuli the left and right phF5c sites shared all four characteristics of the monkey F5c profile, which was unique with respect to other monkey premotor areas. The strength of the argument lies in the identical fMRI responses to stimuli that rely on properties of neurons present in the monkey area: in this case the visual responses of premotor neurons to observed actions (i.e., the presence of neurons with mirror properties). It is not at all necessary that the human MR responses capture the mirror neurons' properties in detail; they should capture only the monkey MR responses' details. The assumption underlying our strategy is simply the responsiveness of a fraction of F5c neurons to observed actions, i.e., the mere presence of mirror neurons in F5c, which is well established (Kilner and Lemon, 2013; Rizzolatti et al., 2014 for review). Indeed the visual fMRI responses of F5c (and phF5c) are unlikely to reflect the canonical neurons, the other type of visuo-motor neurons in F5, as the object, target of the action, was present in the control conditions of the interaction as well as in the action videos, and the F5c activation lateralization depended on the lateralization of the action and not the object in the video. Our results need not to imply that mirror neurons respond differentially to person acting and grasping hand. While this is a possibility for F5c mirror neurons, or at least some sizeable fraction thereof, it is unlikely to be a general property of mirror neurons, given the present fMRI results and those of Nelissen et al. (2005) indicating that F5a responds equally well to acting person and grasping hand videos. Further work in the monkey, preferably combining single cell recordings and fMRI, is required to solve these issues. Also, one should not necessarily derive from our results that observed actions are processed in the same way in F5c of both species (see Differences between phF5c and F5c).
While the size of the right phF5c corresponds to what one might expect given the nine-fold increase of the overall cortical surface of humans relative to monkeys (Van Essen et al., 2012), the left phF5c was found to have only twice the area of monkey F5c. More work is needed to determine the actual extent of phF5c, which may have been underestimated in our experiments, partially because it may appear smaller at low frame rate. Furthermore, we used only grasping actions whereas many other motor acts are known to be coded in both monkey and human ventral premotor cortex (see Rizzolatti et al., 2014).
PhF5c and F5c share the property that an acting person has to be visible in the videos in order to be activated. When we observe another person acting in the daily life, the acting person is generally visible. It is only when an action is seen from nearby or when the agent is partially occluded that only the hand is visible. A person acting on an object is ethologically the natural stimulus. It is possible therefore, that this is the original evolutionary stimulus for mirror neurons, which attracts the viewer's attention and, given that the observed agent makes an intended action, triggers processes attempting to understand the agent's intention. If this interpretation is correct, one may speculate that area F5a, which responds to both the acting person and grasping hand, is an evolutionarily more “advanced” area that has achieved invariance over observational distance and, by focusing attention on the acting fingers, provides further information on the agent's future acts according to hand shape/object affordance relationship.
Supporting evidence
The present experiments provide compelling evidence for the homology of phF5c with F5c. However, this evidence rests on a single functional stimulus characteristic, the presence of the actor in the scene, even if repeatedly tested and generalized over several low-level features. Additional support for the homology between F5c and phF5c is provided by studies showing that phF5c shares some functional properties with F5c and has similar topological relationships (Orban et al., 2004). Fig. S4 shows the data from Jastorff et al. (2010). These data indicate that the miniROIs 8–11 in the RH are activated more strongly by observation of mouth and hand actions than of foot actions. This finding fits well with the extensive representation of the hand and mouth found in F5c (see Rizzolatti et al., 2014). As far as the topological relationships are concerned, it has been suggested that the boundary between human PMd and PMv lies just ventral to the junction of SFS and PCS – Z = 50 in Talairach coordinates (Rizzolatti and Arbib, 1998; Rizzolatti et al., 2002) – a position confirmed by DTI connectivity studies (Schubotz et al., 2010; Tomassini et al., 2007). Hence the location of phF5c, although rather dorsal, is well inside human PMv.
In the monkey, PMv includes F5a, F5p and F4, in addition to F5c. It is premature to postulate homologues of these other PMv subdivisions, except possibly for F5a. Indeed, a ventral premotor disparity-sensitive region, showing an interaction between surface and stereo, tracking the presence of higher order disparity neurons, and documented in monkey F5a (Joly et al., 2009; Theys et al., 2012), is located below right phF5c (black arrow in Fig. S4, Georgieva et al., 2009), just as F5a neighbors F5c in the monkey, suggesting that topological relationships are retained in the two species. This relative position of phF5c and possible homologue of F5a, is very similar to the locations of 6r and 6v provided by Neubert et al. (2014). The main difference is that the functional definition of the F5c and F5a homologues is more restricted than the connectivity based definition of these homologues, leaving room for the homologues of F4 and F5p. The present results are also consistent with earlier reports showing that human PMv is not a single area (Amunts et al., 2010; Schubotz et al., 2010). Further work is needed to clarify the exact relationship of human PMv parcels to monkey subdivisions of PMv.
Differences between phF5c and F5c
The present results also document two differences between phF5c and monkey F5c.
First, phF5c is activated by observing the end-point of grasping, when the hand is about to contact the object, while monkey F5c is activated by observing the earlier hand-shaping stage of grasping. This is consistent with the results of Maranesi et al. (2013) and Umiltà et al. (2001) indicating that the firing of mirror neurons in the monkey anticipates the action, provided the monkey can predict the goal of the evolving motor act under observation. However, since we studied only average responses of phF5c, we cannot exclude the possibility that some fraction of its voxels behave like their monkey counterparts. In addition, phF5c, particularly in the right hemisphere, seems sensitive to observing grasping only within a few degrees of the fixation point (Experiments 1, S1, 3, 4, S2), which may seem to contradict the requirement of the actor's presence within the video. However, the latter requirement may reflect a facilitatory surround mechanism, while the excitatory center of the putative receptive field is sensitive to the hand enclosing the object. Center-surround interactions are well established in the visual system (Orban, 2008), and have been observed in parietal cortex (LIP, Falkner et al., 2010), yet it is fair to say such mechanisms have yet to be described in premotor cortical areas. If the centers of the putative receptive fields, sensitive to the contact between the grasping hand and object, were small and concentrated in the central visual field, only grasping near the fixation point would be effective. The recent results of Maranesi et al. (2013) have indeed indicated that the discharge of more than half of the recorded F5 mirror neurons (38/71) was gaze dependent, being significantly stronger in trials in which the monkey looked at the action compared with those in which it did not look. Thus central vision seems be specialized not only to see the detail of objects but also of actions, even if underlying mechanisms might be very different.
Whatever the mechanism, the exquisite sensitivity of phF5c to central vision, as well as its sensitivity for the actual grasping, which is to say contact with the object, match the oculomotor behavior of human subjects executing or observing manipulative actions described by Flanagan and Johansson (2003). These authors showed that individuals observing manipulative actions shift their gaze to the forthcoming contact point and stay on this target until the action is accomplished, just as the actors themselves do. The oculomotor behavior observed in the no-fixation runs of Supplementary experiment 3 seems at first glance to contradict the observations of Flanagan and Johansson (2003). There are however many differences between the two studies, both in stimuli and instructions. In the present study brief actions were presented repeatedly in the center of a head-mounted display, while in Flanagan's study subjects freely viewed long action sequences occupying varying locations against a black background.
This combination of sensitivity for central stimulation and for the actual grasping might also explain the lack of activation of phF5c in the no-fixation runs of Experiment S3. Indeed actual grasping occurs in the videos only 24 times per run (4 times per block, 6 blocks). If this relatively infrequent stimulus is in addition infrequently fixated, the number of stimuli driving phF5c will be insufficient to evoke a statistically significant response from phF5c, as was indeed observed. Thus, the no-fixation condition should not be taken as evidence that phF5c is not functioning in active vision, rather its activity simply could not be captured in the no-fixation runs by fMRI, which relies on averaging and thus requires frequent fixation of actual grasping (hand on object). Finally it is worth noting that the sensitivity of phF5c for central vision and actual grasping may represent the neural basis of imitation, a function of human mirror neurons (see Rizzolatti et al., 2014), which is poorly developed, if even present (see Ferrari et al., 2006) in the monkey. Imitation requires exactly this knowledge: a precise description of the object and of the action executed upon it.
PhF5c also differs from monkey F5c with regard to hemispheric asymmetries in size and response strength. If the smaller left phF5c houses fewer neurons, and these have to cover the same area of the visual field as those in right phF5c, their visual receptive fields, or at least their excitatory centers (see above), must necessarily be larger. Hence the left phF5c should be less sensitive to the positions of actions in the visual field than its right counterpart, as was indeed observed in Experiment 3 & 4. On the other hand, the right phF5c with its smaller RFs (or their excitatory centers) should be sensitive to slower speeds, as suggested by Experiment 4. Indeed animal studies indicate that smaller RFs are responsive to slower speeds (Orban et al., 1986).
One possible explanation for the small size of left phF5c is the proximity of the speech areas. The increase in cortical space devoted to speech might have decreased that related to action understanding and imitation in the left hemisphere. The presence in the vicinity of left phF5c (Fig. S5) of a small area activated by intelligible speech (Joly et al., 2012) may be taken as a suggestion that part of F5c devoted to observation of mouth actions has transformed into a speech-processing region, consistent with the lack of responses to the observation of manipulative mouth actions in left PMv (Jastorff et al., 2010). A possible alternative explanation lies in the 2D presentation of the action videos. Indeed preliminary results (Jastorff et al., 2012a) suggest that 3D presentation may enhance activity in left ventral premotor cortex. It is worth adding that others have also reported asymmetries between left and right PMv for observation of hand movements (Caspers et al., 2010; Grosbras et al., 2012). Ehrsson et al. (2000) reported stronger activation of right PMv than the left during the execution of precision grips compared to power grips.
Conclusions
Whatever the exact functions of right and left phF5c, the present results provides novel, compelling functional evidence for homology between monkey F5c and a subregion of human ventral premotor cortex. While further confirmation will be welcomed, this identification will open a path for new studies further characterizing further the properties of phF5c as well as exploring the homology between the sectors of F5 and the various areas of human PMv. The experiments were not intended to localize mirror neurons in the human brain, only to identify the homologue of one of the cortical areas housing such neurons in monkeys. Yet the similarity in MR responses of phF5c and F5c and the presence of mirror neurons in F5c strongly suggest that mirror neurons are present also in phF5c, something that has so far proved difficult to establish (Dinstein, 2008; Kilner et al., 2009; Lingnau et al., 2009; Oosterhof et al., 2012). Only direct electrophysiological recordings targeting phF5c can provide a definite answer. Finally, the experiments underscore the value of non-human primates as model of the human brain, as it allows making areal predictions for the human cortex.
Acknowledgments
Supported by IUAP (6/24 and 7/11), EF/PF (10/008), FWO G0593.09, Hercules Foundation, and advanced ERC grants Parietalaction and Cogsystems.
Appendix A. Supplementary data
Supplementary material.
References
- Abdollahi R.O., Jastorff J., Orban G.A. Common and segregated processing of observed actions in human SPL. Cereb. Cortex. 2013;23:2734–2753. doi: 10.1093/cercor/bhs264. [DOI] [PubMed] [Google Scholar]
- Abdollahi R.O., Kolster H., Glasser M.F., Robinson E.C., Coalson T.S., Dierker D., Jenkinson M., Van Essen D.C., Orban G.A. Correspondences between retinotopic areas and myelin maps in human visual cortex. Neuroimage. 2014;99:509–524. doi: 10.1016/j.neuroimage.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K., Lenzen M., Friederici A.D., Schleicher A., Morosan P., Palomero-Gallagher N., Zilles K. Broca's region: novel organizational principles and multiple receptor mapping. PLoS Biol. 2010;8(9):e1000489. doi: 10.1371/journal.pbio.1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Zadeh L., Koski L., Zaidel E., Mazziotta J., Iacoboni M. Lateralization of the human mirror neuron system. J. Neurosci. 2006;26:2964–2970. doi: 10.1523/JNEUROSCI.2921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmalih A., Borra E., Contini M., Gerbella M., Rozzi S., Luppino G. Multimodal architectonic subdivision of the rostral part (area F5) of the macaque ventral premotor cortex. J. Comp. Neurol. 2009;512:183–217. doi: 10.1002/cne.21892. [DOI] [PubMed] [Google Scholar]
- Bonini L., Maranesi M., Livi A., Fogassi L., Rizzolatti G. Space-dependent representation of objects and other's action in monkey ventral premotor grasping neurons. J. Neurosci. 2014;34:4108–4119. doi: 10.1523/JNEUROSCI.4187-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G., Binkofski F., Fink G.R., Fadiga L., Fogassi L., Gallese V., Seitz R.J., Zilles K., Rizzolatti G., Freund H.J. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Caggiano V., Giese M., Thier P., Casile A. Encoding of point of view during action observation in the local field potentials of macaque area F5. Eur. J. Neurosci. 2014:1–11. doi: 10.1111/ejn.12793. [DOI] [PubMed] [Google Scholar]
- Caspers S., Zilles K., Laird A.R., Eickhoff S.B. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefd'hotel C., Hermosillo G., Faugeras O. Flows of diffeomorphisms for multimodal image registration. Proc. IEEE Int. Symp. Biomed. Imaging. 2002:753–756. [Google Scholar]
- Dinstein I. Human cortex: reflections of mirror neurons. Curr. Biol. 2008;18:956–959. doi: 10.1016/j.cub.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Ehrsson H.H., Fagergren A., Jonsson T., Westling G., Johansson R.S., Forssberg H. Cortical activity in precision- versus power-grip tasks: an fMRI study. J. Neurophysiol. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Ekstrom L.B., Roelfsema P.R., Arsenault J.T., Bonmassar G., Vanduffel W. Bottom-up dependent gating of frontal signals in early visual cortex. Science. 2008;321:414–417. doi: 10.1126/science.1153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner A.L., Krishna B.S., Goldberg M.E. Surround suppression sharpens the priority map in the lateral intraparietal area. J. Neurosci. 2010;30:12787–12797. doi: 10.1523/JNEUROSCI.2327-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari P.F., Visalberghi E., Paukner A., Fogassi L., Ruggiero A., Suomi S.J. Neonatal imitation in rhesus macaques. PLoS Biol. 2006;4:1501–1508. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J.R., Johansson R.S. Action plans used in action observation. Nature. 2003;424:769–771. doi: 10.1038/nature01861. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Ferrari P.F., Gesierich B., Rozzi S., Chersi F., Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Ashburner J., Frith C.D., Poline J., Heather J.D., Frackowiak R.S.J. Spatial registration and normalization of images. Hum. Brain Mapp. 1995;3:165–189. [Google Scholar]
- Friston K.J.J., Holmes A.P., Worsley K.J., Poline J.P., Frith C.D., Frackowiak R.S.J., Holmes A.P., Worsley K.J., Poline J.-P., Frith C.D., Frackowiak R.S.J. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1995;2:189–210. [Google Scholar]
- Gallese V., Fadiga L., Fogassi L., Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gazzola V., Rizzolatti G., Wicker B., Keysers C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage. 2007;35:1674–1684. doi: 10.1016/j.neuroimage.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Georgieva S., Peeters R., Kolster H., Todd J.T., Orban G.A. The processing of three-dimensional shape from disparity in the human brain. J. Neurosci. 2009;29:727–742. doi: 10.1523/JNEUROSCI.4753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann J., Robbins S., Halsband U., Petrides M. Precentral sulcal complex of the human brain: morphology and statistical probability maps. J. Comp. Neurol. 2005;493:334–356. doi: 10.1002/cne.20820. [DOI] [PubMed] [Google Scholar]
- Grafton S.T., Arbib M.A., Fadiga L., Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp. Brain Res. 1996;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Grosbras M.H., Beaton S., Eickhoff S.B. Brain regions involved in human movement perception: a quantitative voxel-based meta-analysis. Hum. Brain Mapp. 2012;33:431–454. doi: 10.1002/hbm.21222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M., Woods R.P., Brass M., Bekkering H., Mazziotta J.C., Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jastorff J., Orban G.A. Human functional magnetic resonance imaging reveals separation and integration of shape and motion cues in biological motion processing. J. Neurosci. 2009;29:7315–7329. doi: 10.1523/JNEUROSCI.4870-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastorff J., Begliomini C., Fabbri-Destro M., Rizzolatti G., Orban G.A. Coding observed motor acts: different organizational principles in the parietal and premotor cortex of humans. J. Neurophysiol. 2010;104:128–140. doi: 10.1152/jn.00254.2010. [DOI] [PubMed] [Google Scholar]
- Jastorff J., Abdollahi R., Orban G.A. Stereopsis enhances the fronto-parietal processing of biological motion. Soc. Neurosci. 2012;127:101. [Google Scholar]
- Jastorff J., Popivanov I.D., Vogels R., Vanduffel W., Orban G.A. Integration of shape and motion cues in biological motion processing in the monkey STS. Neuroimage. 2012;60:911–921. doi: 10.1016/j.neuroimage.2011.12.087. [DOI] [PubMed] [Google Scholar]
- Joly O., Vanduffel W., Orban G.A. The monkey ventral premotor cortex processes 3D shape from disparity. Neuroimage. 2009;47:262–272. doi: 10.1016/j.neuroimage.2009.04.043. [DOI] [PubMed] [Google Scholar]
- Joly O., Pallier C., Ramus F., Pressnitzer D., Vanduffel W., Orban G.A. Processing of vocalizations in humans and monkeys: a comparative fMRI study. Neuroimage. 2012;62:1376–1389. doi: 10.1016/j.neuroimage.2012.05.070. [DOI] [PubMed] [Google Scholar]
- Kilner J.M., Lemon R.N. What we know currently about mirror neurons. Curr. Biol. 2013;23:1057–1062. doi: 10.1016/j.cub.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner J.M., Neal A., Weiskopf N., Friston K.J., Frith C.D. Evidence of mirror neurons in human inferior frontal gyrus. J. Neurosci. 2009;29:10153–10159. doi: 10.1523/JNEUROSCI.2668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolster H., Mandeville J.B., Arsenault J.T., Ekstrom L.B., Wald L.L., Vanduffel W. Visual field map clusters in macaque extrastriate visual cortex. J. Neurosci. 2009;29:7031–7039. doi: 10.1523/JNEUROSCI.0518-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraskov A., Dancause N., Quallo M.M., Shepherd S., Lemon R.N. Corticospinal neurons in macaque ventral premotor cortex with mirror properties: a potential mechanism for action suppression? Neuron. 2009;64:922–930. doi: 10.1016/j.neuron.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite F.P., Tsao D., Vanduffel W., Fize D., Sasaki Y., Wald L.L., Dale A.M., Kwong K.K., Orban G.A., Rosen B.R., Tootell R.B.H., Mandeville J.B. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. Neuroimage. 2002;16:283–294. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- Lingnau A., Gesierich B., Caramazza A. Asymmetric fMRI adaptation reveals no evidence for mirror neurons in humans. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9925–9930. doi: 10.1073/pnas.0902262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G., Luppino G., Rizzolatti G., Rizzolatti G. The organization of the frontal motor cortex. News Physiol. Sci. 2000;15:219–224. doi: 10.1152/physiologyonline.2000.15.5.219. [DOI] [PubMed] [Google Scholar]
- Mandeville J.B., Marota J.J. Vascular filters of functional MRI: spatial localization using BOLD and CBV contrast. Magn. Reson. Med. 1999;42:591–598. doi: 10.1002/(sici)1522-2594(199909)42:3<591::aid-mrm23>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Maranesi M., Ugolotti Serventi F., Bruni S., Bimbi M., Fogassi L., Bonini L. Monkey gaze behaviour during action observation and its relationship to mirror neuron activity. Eur. J. Neurosci. 2013;38:3721–3730. doi: 10.1111/ejn.12376. [DOI] [PubMed] [Google Scholar]
- Molenberghs P., Cunnington R., Mattingley J.B. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev. 2012;36:341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Murata A., Fadiga L., Fogassi L., Gallese V., Raos V., Rizzolatti G. Object representation in the ventral premotor cortex (area F5) of the monkey. J. Neurophysiol. 1997;78:2226–2230. doi: 10.1152/jn.1997.78.4.2226. [DOI] [PubMed] [Google Scholar]
- Nelissen K., Luppino G., Vanduffel W., Rizzolatti G., Orban G.A. Observing others: multiple action representation in the frontal lobe. Science. 2005;310:332–336. doi: 10.1126/science.1115593. [DOI] [PubMed] [Google Scholar]
- Neubert F.X., Mars R.B., Thomas A.G., Sallet J., Rushworth M.F.S. Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron. 2014;81:700–713. doi: 10.1016/j.neuron.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Oosterhof N.N., Tipper S.P., Downing P.E. Viewpoint (in)dependence of action representations: an MVPA study. J. Cogn. Neurosci. 2012;24:975–989. doi: 10.1162/jocn_a_00195. [DOI] [PubMed] [Google Scholar]
- Orban G. a. Functional MRI in the awake monkey: the missing link. J. Cogn. Neurosci. 2002;14:965–969. doi: 10.1162/089892902760191171. [DOI] [PubMed] [Google Scholar]
- Orban G.A. Higher order visual processing in macaque extrastriate cortex. Physiol. Rev. 2008;88:59–89. doi: 10.1152/physrev.00008.2007. [DOI] [PubMed] [Google Scholar]
- Orban G.A. The extraction of 3D shape in the visual system of human and nonhuman primates. Annu. Rev. Neurosci. 2011;34:361–388. doi: 10.1146/annurev-neuro-061010-113819. [DOI] [PubMed] [Google Scholar]
- Orban G.A., Kennedy H., Bullier J. Velocity sensitivity and direction selectivity of neurons in areas V1 and V2 of the monkey: influence of eccentricity. J. Neurophysiol. 1986;56:462–480. doi: 10.1152/jn.1986.56.2.462. [DOI] [PubMed] [Google Scholar]
- Orban G.A., Van Essen D., Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn. Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Peeters R., Simone L., Nelissen K., Fabbri-Destro M., Vanduffel W., Rizzolatti G., Orban G.A. The representation of tool use in humans and monkeys: common and uniquely human features. J. Neurosci. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Arbib M.A. Language within our grasp. Trends Neurosci. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat. Rev. Neurosci. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fadiga L., Gallese V., Fogassi L. Premotor cortex and the recognition of motor actions. Cogn. Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fadiga L., Matelli M., Bettinardi V., Paulesu E., Perani D., Fazio F. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp. Brain Res. 1996;111:246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fogassi L., Gallese V. Motor and cognitive functions of the ventral premotor cortex. Curr. Opin. Neurobiol. 2002;12:149–154. doi: 10.1016/s0959-4388(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Cattaneo L., Fabbri-Destro M., Rozzi S. Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol. Rev. 2014;94:655–706. doi: 10.1152/physrev.00009.2013. [DOI] [PubMed] [Google Scholar]
- Schubotz R.I., Anwander A., Knösche T.R., von Cramon D.Y., Tittgemeyer M. Anatomical and functional parcellation of the human lateral premotor cortex. Neuroimage. 2010;50:396–408. doi: 10.1016/j.neuroimage.2009.12.069. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Neuropsychologia. 1988 [Google Scholar]
- Theys T., Pani P., van Loon J., Goffin J., Janssen P. Selectivity for three-dimensional shape and grasping-related activity in the macaque ventral premotor cortex. J. Neurosci. 2012;32:12038–12050. doi: 10.1523/JNEUROSCI.1790-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini V., Jbabdi S., Klein J.C., Behrens T.E.J., Pozzilli C., Matthews P.M., Rushworth M.F.S., Johansen-Berg H. Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. J. Neurosci. 2007;27:10259–10269. doi: 10.1523/JNEUROSCI.2144-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umiltà M.A., Kohler E., Gallese V., Fogassi L., Fadiga L., Keysers C., Rizzolatti G. I know what you are doing: a neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C., Drury H.A., Dickson J., Harwell J., Hanlon D., Anderson C.H. An integrated software suite for surface-based analyses of cerebral cortex. J. Am. Med. Inform. Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C., Glasser M.F., Dierker D.L., Harwell J., Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb. Cortex. 2012;22:2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C., Jbabdi S., Sotiropoulos S.N., Chen C., Dikranian K., Coalson T., Harwell J., Behrens T.E.J., Glasser M. Mapping connections in humans and non-human primates: aspirations and challenges for diffusion imaging. In: Johansen-Berg H., Behrens T.E., editors. Chapter 16 in Diffusion MRI: From Quantitative Measurement to In Vivo Neuroanatomy. Academic Press; 2014. [Google Scholar]
- Van Overwalle F., Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vanduffel W., Fize D., Mandeville J.B., Nelissen K., Van Hecke P., Rosen B.R., Tootell R.B.H., Orban G.A. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Vanduffel W., Zhu Q., Orban G.A. Monkey cortex through fMRI glasses. Neuron. 2014;83:533–550. doi: 10.1016/j.neuron.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran G., Philipp R., Lemon R.N., Kraskov A. M1 corticospinal mirror neurons and their role in movement suppression during action observation. Curr. Biol. 2013;23:236–243. doi: 10.1016/j.cub.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Wang P., Hendrich K., Ugurbil K., Kim S.G. Cortical layer-dependent BOLD and CBV responses measured by spin–echo and gradient–echo fMRI: insights into hemodynamic regulation. Neuroimage. 2006;30:1149–1160. doi: 10.1016/j.neuroimage.2005.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.