Highlights
-
•
Nitric oxide release profiles were characterised for commonly used donors.
-
•
Released NO differs greatly between donors and depends on storage conditions.
-
•
High release donors (NOC-5, PAPA NONOate) decay quickly.
-
•
SNP and GSNO show greater stability releasing consistent lower NO levels.
-
•
This comprehensive characterisation provides knowledge to define NO concentrations released in vitro.
Abbreviations: GSNO, S-nitrosoglutathione; MNTB, medial nucleus of the trapezoid body; NOC-5, 3-(aminopropyl)-1-hydroxy-3-isopropyl-2-oxo-1-triazene; PAPA NONOate, 3-(2-hydroxy-2-nitroso-1-propylhydrazino)-1-propanamine; PKG, protein kinase G; SNAP, S-nitroso-N-acetylpenicillamine; SNP, sodium nitroprusside; WPI, World Precision Instruments Ltd.
Keywords: Nitric oxide concentration, NO donor, Stock solution, Release profile, Apollo-1000, NO sensitive electrode
Abstract
Background
Nitric oxide (NO) is a vital signalling molecule in a variety of tissues including the neuronal, vascular and reproductive system. However, its high diffusibility and inactivation make characterisation of nitrergic signalling difficult. The use of NO donors is essential to characterise downstream signalling pathways but knowledge of donor release capacities is lacking, thus making comparisons of donor responses difficult.
New method
This study characterises NO profiles of commonly used NO donors. Donors were stored under defined conditions and temporal release profiles detected to allow determination of released NO concentrations.
Results
Using NO-sensitive microsensors we assessed release profiles of NO donors following different storage times and conditions. We found that donors such as NOC-5 and PAPA-NONOate decayed substantially within days, whereas SNP and GSNO showed greater stability releasing consistent levels of NO over days. In all donors tested, the amount of released NO differs between frozen and unfrozen stocks.
Comparison with existing method(s)
Fluorescent and amperometric approaches to measure NO concentrations yield a wide range of levels. However, due to a lack of characterisation of the release profiles, inconsistent effects on NO signalling have been widely documented. Our systematic assessment of release profiles of a range of NO donors therefore provides new essential data allowing for improved and defined investigations of nitrergic signalling.
Conclusions
This is the first systematic comparison of temporal release profiles of different NO donors allowing researchers to compare conditions across different studies and the use of defined NO levels by choosing specific donors and concentrations.
1. Introduction
Nitric oxide (NO) is a fundamental and conserved signalling molecule across different species and plays important roles in a myriad of physiological processes within the cardiovascular, nervous, reproductive and other systems (Ignarro et al., 1987; Knowles and Moncada, 1992; Bogdan, 2001; Garthwaite, 2008). Physiologically, NO signals are generated by neuronal NO synthase (nNOS) or endothelial NO synthase (eNOS) and additionally by the immune system-relevant inducible NO synthase (iNOS). In target cells, through specialised receptors possessing an intrinsic soluble guanylyl cyclase (sGC), NO accumulation results in cyclic guanosine monophosphate (cGMP) production which leads to activation of downstream signalling molecules such as protein kinase G (PKG). Furthermore, higher levels of NO production results in post-translational modifications of proteins through either S-nitrosylation of thiol groups or via generation of peroxynitrite leading to tyrosine nitration of proteins (Knott and Bossy-Wetzel, 2009). Unlike conventional neurotransmitters, NO is not constrained by cellular membranes and diffuses in three dimensions from its source of production (Garthwaite and Boulton, 1995). Particularly in the nervous system, the concentration gradient associated with this diffusion is important in signalling mechanisms such as regulation of plasticity (Hardingham and Fox, 2006; Hardingham et al., 2013) and development (Bradley et al., 2010; Jay et al., 2014). Among the many unknowns are the exact levels of NO generated by different sources (i.e. neuronal or endothelial), how far it diffuses in active concentrations, the molecular targets other than sGC-coupled receptors and how it is inactivated, particularly in many neurodegenerative and cardiovascular diseases where enhanced NO levels are reported (Naseem, 2005; Nakamura and Lipton, 2008, 2009; Steinert et al., 2010a; Wolin et al., 2010).
Various attempts have been made to determine levels of produced NO. Generally, fluorescent probes such as diamino-fluorescein (DAF)- or dichloro-fluorescin (DCF)-related compounds (fluorescein framework) (Gunasekar et al., 1995; Kasim et al., 2001) or recently developed diamino-rhodamine probes (DAR, rhodamine based chromophore) have been used to characterise NO production and concentration profiles (Takata et al., 2005; Ye et al., 2008; Steinert et al., 2010b). Although these fluorescent probes allow some spatial and temporal characterisation, they do not directly react with NO. Instead a change in fluorescence occurs when non-specific oxidation of the fluorophore leads to the transformation of an amino group to an NH radical which then binds NO. Therefore a simple change in the redox state could lead to changes in oxidation of the fluorophore and thus availability of radical species available for NO binding resuls in problematic quantification of fluorescent signals (Wardman, 2007; Hall and Garthwaite, 2009). However, recent studies using a fluorescent cGMP biosensor, δ-FlincG (Nausch et al., 2008), reported an improved and physiologically relevant way of measuring NO profiles in neurons (Wood et al., 2011).
The use of electrodes provides additional tools to detect direct temporal changes in NO profiles (Finnerty et al., 2012; Jensen et al., 2013). Nevertheless, detection of NO levels yielded values that span over 4 orders of magnitude, from the low picomolar to the micromolar range, with a similar variability observed in different tissues (Hall and Garthwaite, 2009). This is largely a consequence of the selectivity and sensitivity of the recording sensor surface or inaccurate calibration (Bedioui and Villeneuve, 2003).
Investigations of nitrergic signalling also require the use of NO-releasing compounds. In countless publications various NO donors, at various concentrations, have been applied leading to not always reproducible and even controversial findings. The main drawback of using NO donors is the unknown release capacity of each donor which depends on the concentration applied and the environment. In order to identify profiles of NO release, we measured NO concentrations of various donors at different concentration in standard phosphate buffered saline (PBS, pH 7.4) over time using NO sensing electrodes. The NO microsensor chosen for this study (NOPF100 NO microsensor; WPI) possess a multi-layered selective coating that eradicates non-specific detection of other species related to NO research such as arginine, ascorbic acid, cysteine, dopamine, nitrate, nitrite, N2, O2 among others and shows reliable NO measurements (Hurst and Clark, 2003). Therefore our data provide a systematic and comprehensive comparison of NO release by different donors in standard in vitro conditions, which provides important insight to study nitrergic signalling and allows a better evaluation of reported nitrergic signalling outcomes.
2. Materials and methods
2.1. Nitric oxide donors
During this study the following NO donors were used: 3-(aminopropyl)-1-hydroxy-3-isopropyl-2-oxo-1-triazene (NOC-5, 5–20 μM, Santa Cruz Biotechnology, Inc. and Enzo Life Sciences), S-nitrosoglutathione (GSNO, 100–300 μM, Santa Cruz Biotechnology, Inc.), propylamine propylamine NONOate (PAPA NONOate, 5–20 μM, Santa Cruz Biotechnology, Inc. and Enzo Life Sciences), S-nitroso-N-acetylpenicillamine (SNAP, 5–20 μM, Life Technologies) and sodium nitroprusside dehydrate (SNP, 100–300 μM, Sigma). NO concentrations are expressed as mean ± SEM and displayed in figures as box and whisker plots to indicate median value and interquartile range.
2.2. Apollo 1000 Free Radical Analyser
NO levels were captured using the Apollo 1000 Free Radical Analyser and the NOPF100 NO microsensor (WPI). Data were recorded using Labscribe v3 (WPI) and analysed in Prism v6 (GraphPad) software. Before use, microsensors were polarised by immersing in copper (II) sulphate solution (0.1 M CuSO4, Merck Millipore) under continuous stirring. This provides a potential difference between the recording electrode relative to the reference electrode, which is amplified and recorded when NO is oxidised on the probe membrane. Polarising also provides a reduction in background current. The poise voltage on the Apollo 1000 was set to 865 mV, the current range was set at 10 nA and data were sampled at 10 Hz. The microsensor was left undisturbed for 2 h until a stable baseline was reached.
2.3. Calibration
As the microsensors measure very small voltage changes following oxidation of NO on the sensor, they are very sensitive to external noise, temperature fluctuation and drift and probe sensitivity can change significantly over time (Simonsen et al., 1999). Calibration following polarisation was conducted on a daily basis before and after measurements. The method chosen for calibration involves using the NO donor SNAP and CuSO4, as recommended in the user manual provided by WPI. The probe was placed in a beaker containing 20 ml CuSO4 (0.1 M) with a stirring bar to ensure constant mixing of solution. Once a stable baseline voltage was reached, 2, 4, 8, 16 and 32 μl of 100 μM SNAP solution containing 44 μM ethylenediaminetetraacetic acid (EDTA, EMD Millipore Chemicals) was subsequently added. Upon addition of each volume of SNAP the voltage increased rapidly before reaching a plateau, before plateau decay the next volume of SNAP was added. Each addition of SNAP resulted in released NO as calculated: the conversion efficiency of SNAP to NO in CuSO4 is 0.6 (60%), therefore for every mole of SNAP, 0.6 mole of NO is liberated. From the known amount of NO released from SNAP a calibration curve of voltage response vs. NO concentration was constructed.
2.4. NO donor release profiles
The microsensor probe was inserted into PBS solution (pH 7.4), under constant stirring, and the voltage response was allowed to settle over a period of 5–10 min. 10 mM GSNO stock was made in PBS (pH 7.4) with limited exposure to light and oxygen. Once the baseline voltage was stable, 100 μM of GSNO was added to the PBS and the voltage response was recorded for 30 min. After this time NO was scavenged from the solution using 100 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (c-PTIO, Santa Cruz Biotechnology, Inc.). This caused a reduction in the voltage response to around baseline values confirming specificity of the recorded signal. The experiment was then repeated on the same day by adding 200 μM and 300 μM GSNO to fresh PBS each time. In between experiments the GSNO stock was kept at 4 °C, 15 min before each experiment the GSNO was brought out of the fridge to room temperature. The experiments were then repeated on 3 subsequent days using the same GSNO stock. Aliquots of GSNO stock were also frozen and re-tested 4 weeks later to assess effects of frozen storage on NO release. This procedure was then repeated using the following NO donors and concentrations: NOC-5 (5, 10 and 20 μM), PAPA NONOate (5 μM, 10 μM and 20 μM), SNAP (5 μM, 10 μM and 20 μM) and SNP (100 μM, 200 μM and 300 μM). The concentration of NOC-5, PAPA NONOate and SNAP had to be reduced in comparison to GSNO and SNP as the higher concentrations led to a voltage response that exceeded the 10 nA range and were outside the optimal sensitivity range of the sensor. Frozen stocks were not tested for SNP or SNAP. All experiments were conducted at standard room temperature which ranged from 21 to 24 °C and each donor has been tested on 4 different three-day periods to give mean release values.
3. Results
3.1. NO microsensor calibration
We have utilised the microsensors for studying the release of NO from a selection of widely used donors in vitro over a time period of three days in a standard lab environment to characterise the nature of NO release from stock preparations.
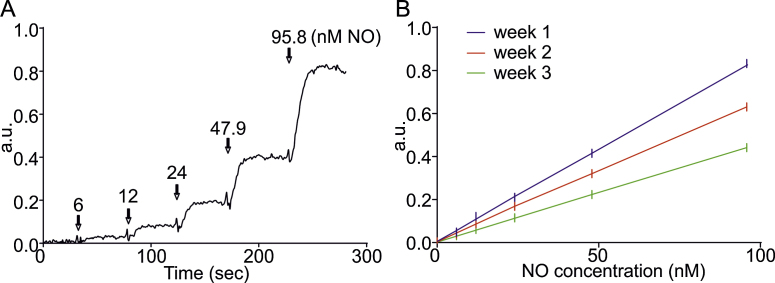
The NOPF100 microsensor was calibrated using increasing concentrations of SNAP diluted in CuSO4, as recommended in the user manual provided by WPI. The output of the microsensor correlated linearly with the concentration of NO liberated from each SNAP application and a calibration curve was constructed (Fig. 1A and B). However, as stated previously, the probes are extremely sensitive to external factors, which is also demonstrated in this study. Fig. 1B shows the average calibration curve taken twice daily for a week on three different weeks showing a significant variation over that time. Therefore we would advise calibration of NO probes daily before use and after experimentation to avoid variation and to provide accurate determination of NO release during experimentation.
Fig. 1.
SNAP calibration of NO microsensors. (A) Graph depicting sequential additions of increasing concentrations of SNAP in 0.1 M copper sulphate and the equivalent concentration of NO produced in nM. Arrows indicate time points of SNAP addition. (B) Graph showing the mean calibration curves constructed from twice daily SNAP calibrations over three different weeks.
3.2. Release by SNP
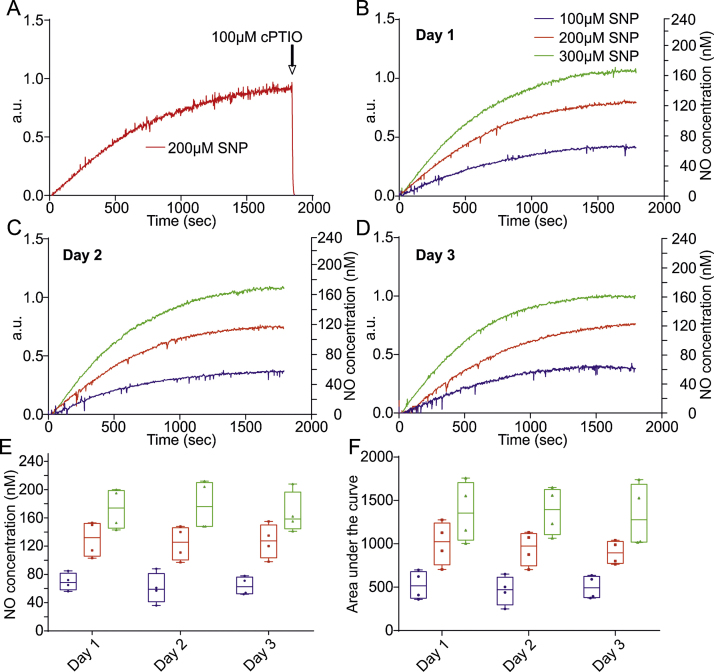
The NO donor SNP is an iron nitrosyl compound that can be stored for years at room temperature if kept protected from light in a dry environment. To characterise the NO release from SNP we recorded NO levels over a period of three consecutive days from freshly made SNP stock. In order to establish that the response captured by the microsensor is specific to NO we added 100 μM of the NO scavenger cPTIO after each 30 min recording period. Addition of cPTIO resulted in an instant decrease in the response back to baseline indicative of a specific response to NO (Fig. 2A). Although cPTIO scavenging was conducted after every experiment to ensure accurate NO detection and measurement, it is not shown in all figures for simplicity. Addition of 100 μM SNP led to a steady rise followed by a plateau at ∼25 min (Fig. 2B). At 200 μM and 300 μM SNP the rise before the plateau is steeper with both plateauing at ∼25 min (Fig. 2B). Over a consecutive three day period, SNP stocks are remarkably stable if kept in the fridge and protected from light (Fig. 2C and D). Plateau concentrations of NO released from 100 μM SNP are 70 ± 6 nM on day one, 61 ± 11 nM on day two and 64 ± 6 nM on day three. 200 μM SNP results in NO release values of 130 ± 13 nM on day one, 124 ± 12 nM on day two and 127 ± 12 nM on day three. NO release from 300 μM SNP is 173 ± 14 nM day one, 178 ± 17 nM day two and 167 ± 15 nM day three (Fig. 2E, Table 1). To compare the total exposure of NO we calculated the area under the curve (AUC, Fig. 2F) which accounts for NO release as well as degradation which is particularly important for non-linear kinetics of the curve. The total amount of NO released over the 30 min recording period is consistent over the three consecutive days at all concentrations, demonstrating a high degree of stock stability over time.
Fig. 2.
Temporal release of NO from SNP. (A) Representative example of the NO release profile from 200 μM SNP, demonstrating the application of the NO scavenger cPTIO (100 μM) at the end of all recordings to confirm microsensor specificity. Arrow depicts addition of cPTIO to recording solution. (B) Average release profile of NO from 100, 200 and 300 μM fresh SNP over a 30 min recording period. (C) Average profile of NO release over 30 min from two day old stock at 100, 200 and 300 μM. (D) Average release profiles of NO yield over 30 min from three day old SNP stock at 100, 200 and 300 μM. (E) Box and whisker plots showing the plateau concentration range of NO released from one day, two day and three day old stock at 100, 200 and 300 μM. (F) Box and whisker plots depicting the area under the curve for each recording using one day, two day and three day old SNP at 100, 200 and 300 μM.
Table 1.
Release profiles of NO donors at varying concentrations over a 30 min time course. NO liberated during this period is recorded from fresh stock (day 1) and then for the following two consecutive days. NO release is also recorded for stock that has been frozen for a period of 4 weeks. All NO values depict plateau concentrations.
| NO donor | Concentration (μM) | Day 1 (nM NO) | Day 2 (nM NO) | Day 3 (nM NO) | Frozen stock (nM NO) |
|---|---|---|---|---|---|
| SNP | 100 | 70 ± 6 | 61 ± 11 | 64 ± 6 | n/a |
| 200 | 130 ± 13 | 124 ± 12 | 127 ± 12 | n/a | |
| 300 | 173 ± 14 | 178 ± 17 | 167 ± 15 | n/a | |
| GSNO | 100 | 145 ± 21 | 145 ± 13 | 136 ± 18 | 105 ± 6 |
| 200 | 283 ± 29 | 279 ± 26 | 262 ± 28 | 193 ± 6 | |
| 300 | 381 ± 32 | 384 ± 39 | 359 ± 38 | 279 ± 13 | |
| NOC-5 | 5 | 330 ± 37 | 131 ± 24 | 86 ± 30 | 103 ± 9 |
| 10 | 480 ± 49 | 217 ± 38 | 141 ± 48 | 179 ± 8 | |
| 20 | 657 ± 84 | 352 ± 68 | 230 ± 76 | 290 ± 20 | |
| PAPA NONOate | 5 | 292 ± 41 | 74 ± 18 | 30 ± 7 | 55 ± 5 |
| 10 | 403 ± 51 | 123 ± 24 | 51 ± 13 | 89 ± 6 | |
| 20 | 562 ± 91 | 201 ± 31 | 93 ± 24 | 158 ± 16 | |
3.3. Release by PAPA NONOate
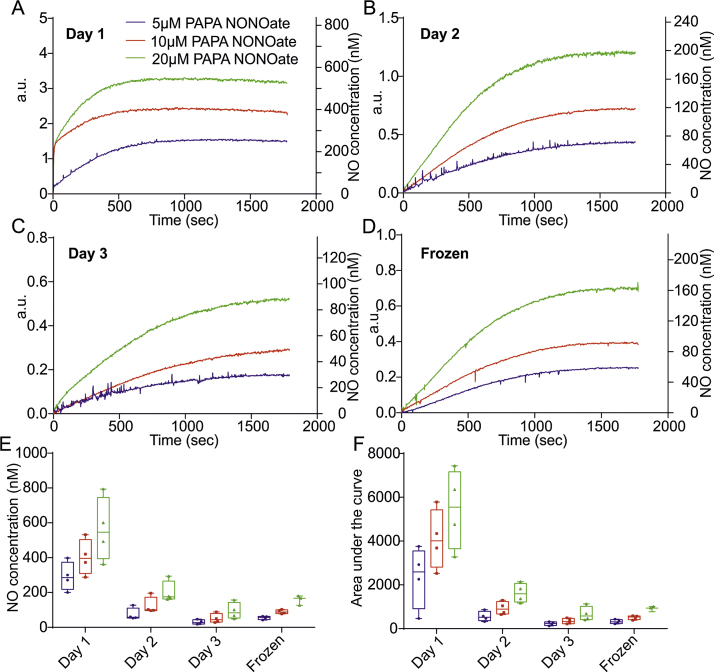
PAPA NONOate is a diazeniumdiolate compound that spontaneously decomposes in solution with first order kinetics, yielding two moles of NO per mole of PAPA NONOate (Feelisch, 1998; Scatena et al., 2010). NO release from this donor is unaffected by biological reactants but is dependent on pH, temperature and acid catalysed reactions. Owing to these characteristics this donor is a popular choice for reliable generation of NO. Here we characterise NO concentrations released from a stock solution of PAPA NONOate in PBS over a period of three consecutive days. On day one NO release from the donor is rapid with a steep rise in NO concentration upon application and reaching a plateau at ∼17 min at 5 μM and ∼11 min at both 10 and 20 μM (Fig. 3A). NO release on day two slows and reduces with NO release plateauing at ∼25 min at 5 μM and 10 μM and ∼23 min at 20 μM (Fig. 3B). Day three results in a further decrease in NO release at all concentrations, with a much slower release profile plateauing at ∼26 min (Fig. 3C). Frozen stock for four weeks showed a reduced release response to around 70% of the NO released from fresh stock at equivalent donor concentrations (Fig. 3A and D). Plateau concentrations of NO from day one are 292 ± 41 nM at 5 μM, 403 ± 51 nM at 10 μM and 562 ± 91 nM at 20 μM. Day two shows over a 50% fall in released NO (5 μM: 74 ± 18 nM, 10 μM: 123 ± 24 nM and 20 μM: 201 ± 31 nM) and day three release is down to less than 20% of day one (5 μM: 30 ± 7 nM, 10 μM: 51 ± 13 nM and 20 μM: 93 ± 24 nM). Frozen stock liberates plateau concentrations at 5 μM: 55 ± 5 nM, 10 μM: 89 ± 6 nM and 20 μM: 158 ± 16 nM (Fig. 3E, Table 1). Total amounts of NO released over the recording period are shown using AUC and it is demonstrated that total NO release is decreased at days 2, 3 as well as in the frozen stock at 4 weeks (Fig. 3F).
Fig. 3.
Temporal release of NO from PAPA NONOate. (A) Average NO release profiles from fresh PAPA NONOate at 5, 10 and 20 μM over a 30 min recording period. (B) Average release profile of NO yield from two day old stock at 5, 10 and 20 μM over 30 min. (C) Average profiles of NO release over 30 min from three day old stock at 5, 10 and 20 μM. (D) Average NO release profiles of 4 week old frozen stock at 5, 10 and 20 μM over a 30 min recording period. (E) Box and whisker plots depicting the plateau concentration range of NO recorded on day one, two and three and from frozen stock. (F) Box and whisker plots showing the range of the area under the curve using the same stock over 3 consecutive days and frozen stock.
3.4. Release by NOC-5
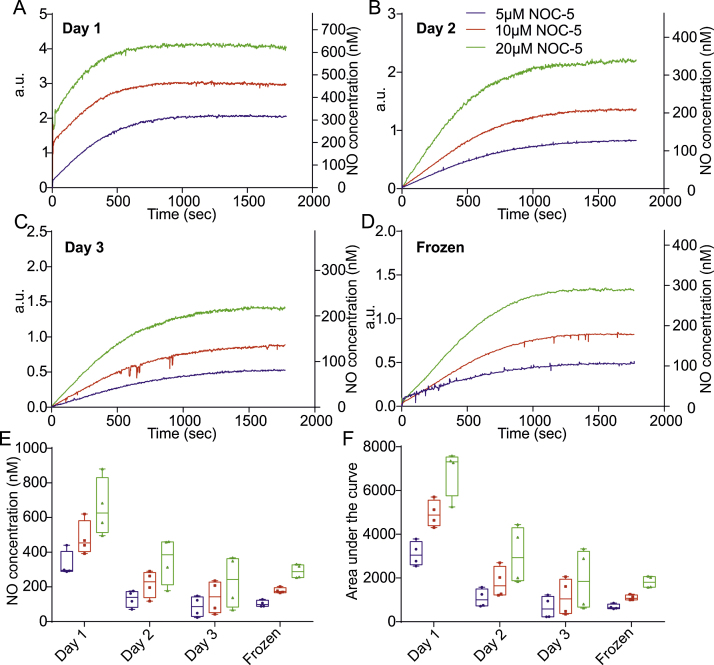
NOC-5 is a diazeniumdiolate compound that belongs to the same family of NO donors as PAPA NONOate. As such, this donor possesses all of the favourable qualities of PAPA NONOate with the addition of a slightly longer half-life of 93 min at 22 °C, pH 7.4 compared to 77 min for PAPA NONOate. Accordingly, NO release from NOC-5 stock over three consecutive days resulted in a greater NO release over 30 min. In a similar manner to PAPA NONOate, all concentrations of fresh NOC-5 in PBS led to a rapid increase in NO release on day one. A steady NO release plateau was reached at ∼17 min at 5 μM, ∼14 min at 10 μM and ∼12 min at 20 μM (Fig. 4A). On days two and three NO release was slower resulting in shallower curves and longer plateau times of ∼25 and ∼26 min (Fig. 4B and C). Frozen stock liberated less NO compared to fresh stocks (Fig. 4D). NO release was greatest on day one with 5 μM yielding a plateau concentration of 330 ± 37 nM, 10 μM: 480 ± 49 nM and 20 μM: 657 ± 84 nM NO. By day two the amount of NO released at plateau has decreased by half (5 μM: 131 ± 24 nM, 10 μM: 217 ± 38 nM and 20 μM: 352 ± 68 nM) and furthermore by day three (5 μM: 86 ± 30 nM, 10 μM: 141 ± 48 nM and 20 μM: 230 ± 76 nM). Frozen stock gave plateau concentrations of NO that range between that seen at day two and three in fresh stock (5 μM: 103 ± 9 nM, 10 μM: 179 ± 8 nM, 20 μM: 290 ± 20 nM) (Fig. 4D, Table 1). Total NO release over the 30 min (AUC) recording period decreased with concentration and time (Fig. 4F).
Fig. 4.
Temporal release of NO from NOC-5. (A) Average release profile of NO yield from fresh NOC-5 stock at 5, 10 and 20 μM over 30 min. (B) Average NO release profiles on day two at 5, 10 and 20 μM over a 30 min recording period. (C) Average profiles of NO release over 30 min from three day old stock at 5, 10 and 20 μM. (D) Average NO release profiles of 4 week old frozen NOC-5 stock at 5, 10 and 20 μM over a 30 min recording period. (E) Box and whisker plots showing the plateau concentration range of NO recorded using NOC-5 stock on day one, two and three and frozen stock. (F) Box and whisker plots depicting the area under the release curve using the same stock over 3 consecutive days and frozen stock.
3.5. Release by GSNO
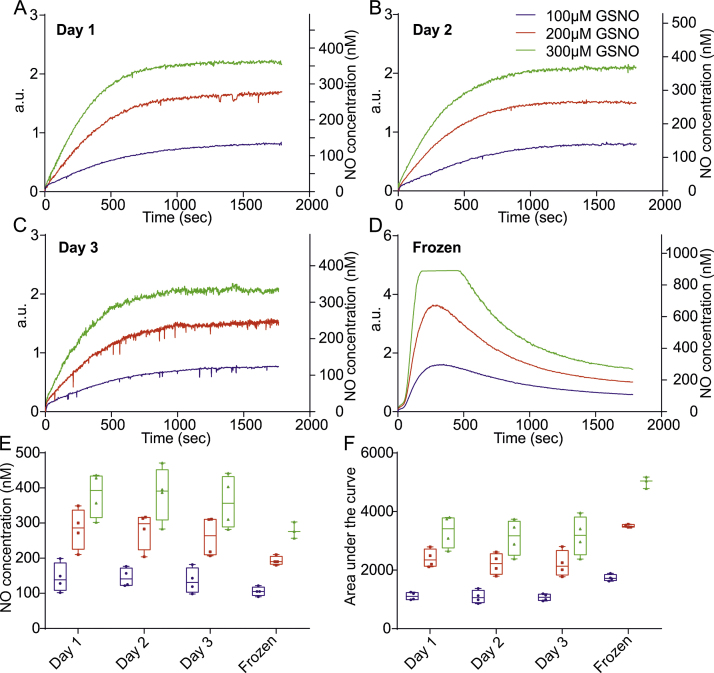
GSNO is a S-nitrosothiol which is synthesised through the S-nitrosation of primary and secondary thiols (Feelisch, 1998). S-nitrosothiols are catalysed by thiols, light and transition metals, and additionally, their stability in solution depends on temperature, pH and oxygen pressure. Here we sought to characterise the NO release from fresh GSNO stock over a three day period and stock frozen for four weeks. On day one NO release resulted in a steady increase to plateau at ∼24 min at 100 μM, ∼17 min at 200 μM and ∼16 min at 300 μM (Fig. 5A). NO release on day one to three was similar with plateaus reached by ∼24, ∼19 and ∼20 min for 100, 200 and 300 μM, respectively (Fig. 5A–C). Stock frozen for a month produced different NO release curves compared to the fresh stock. There is a rapid release of NO which increases with increased concentration; the response then decays over the 30 min recording period without reaching a plateau (Fig. 5D). Plateau concentrations of NO over the three consecutive days from fresh stock are remarkably stable with very little decay. The NO concentration from 100 μM GSNO is 145 ± 21 nM on day one, 145 ± 13 nM on day two and 136 ± 18 nM on day three. 200 μM GSNO liberates 283 ± 29 nM, 279 ± 26 nM and 262 ± 28 nM NO for day one, two and three, respectively. Finally, 300 μM GSNO resulted in NO plateau concentrations of 381 ± 32 nM, 384 ± 39 nM and 359 ± 38 nM for day one, two and three, respectively. Frozen stocks have lower plateau values of NO release (100 μM: 105 ± 6 nM, 200 μM: 193 ± 6 nM, 300 μM: 279 ± 13 nM) (Fig. 5E, Table 1). Total amount of NO released during the recording period is very stable across the subsequent days, however, the frozen stock showed a bell shape NO release curve. This resulted in a larger total amount of NO released (AUC) in comparison to fresh stock (Fig. 5F).
Fig. 5.
Temporal release of NO from GSNO. (A) Average profiles of NO release over 30 min from fresh GSNO stock at 100, 200 and 300 μM. (B) Average release profile of NO yield from two day old stock at 100, 200 and 300 μM over 30 min. (C) Average NO release profiles from three day old GSNO at 100, 200 and 300 μM over a 30 min recording period. (D) Average NO release profiles of 4 week old frozen stock at 100, 200 and 300 μM over a 30 min recording period, note the saturation of the signal at 300 μM. (E) Box and whisker plots displaying the plateau concentration range of NO recorded using GSNO stock on day one, two and three and frozen stock. (F) Box and whisker plots depicting the area under the curve using GSNO stock over 3 consecutive days and frozen stock, note that values for 300 μM frozen stock are a slight underestimation due to saturation of the signal.
3.6. Release by SNAP
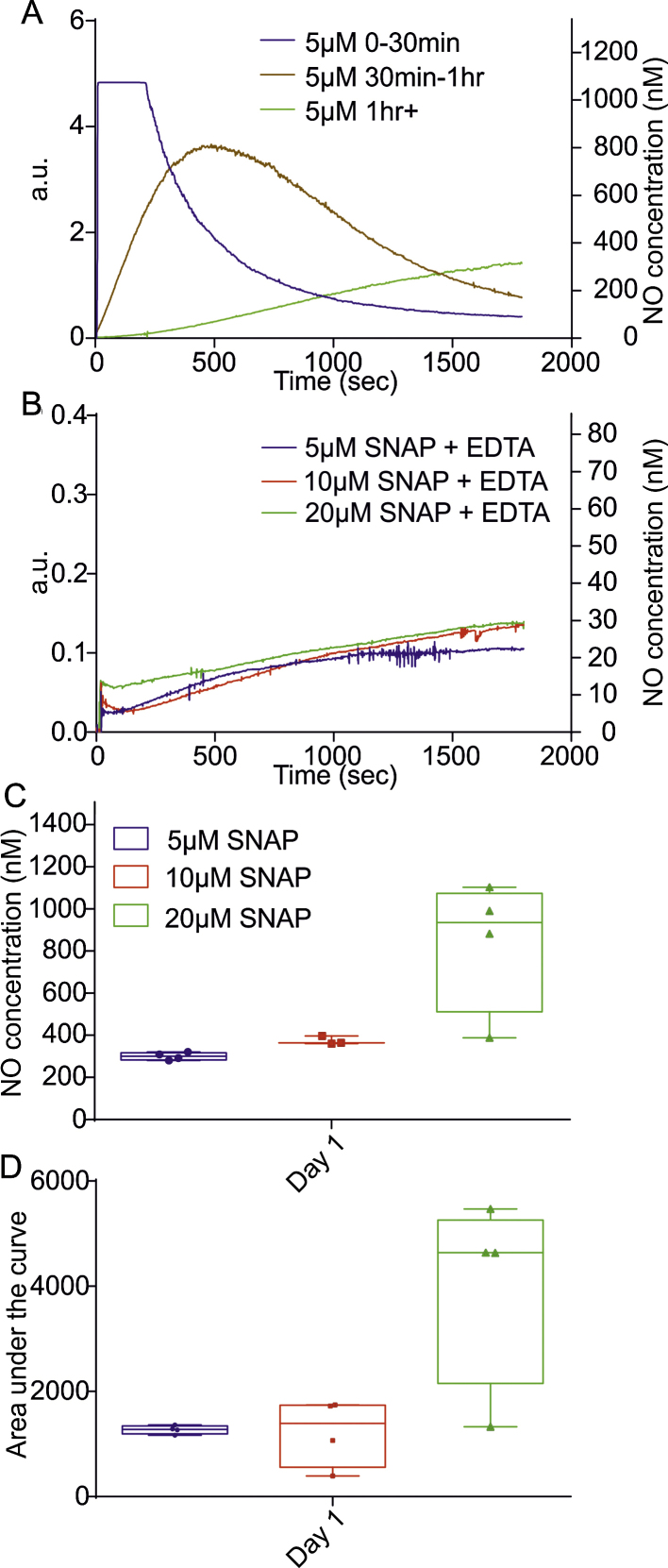
SNAP is an S-nitrosothiol that is obtained through the S-nitrosation of tertiary thiols. SNAP releases NO by catalytic activity of various physical, chemical and enzymatic factors that include temperature, light, transition metals, thiols and superoxide. Here we characterised the release of NO from fresh stock over a one day period only due to the presence of an uncontrolled variability in NO release following longer time periods of storage. Fresh stock that was diluted in PBS immediately after it was made up has a different release profile and saturated the used sensitivity range in comparison to release tested 30 min later. The release profile changed once more and settles when recorded >1 h after making stock solutions (Fig. 6A). This characteristic was unique to SNAP and not seen in any other donor. As copper is a potent catalyst of SNAP decomposition and copper is present as a contaminant in varying levels in water used to prepare buffer solutions (Dicks and Williams, 1996), we next sought to chelate any free copper ions using EDTA in both the fresh stock solution (40 μM) and recording PBS solutions (200 nM). Addition of EDTA resulted in a dramatic reduction in NO release from SNAP at all concentrations due to removal of catalysing transition metal ions (Mcaninly et al., 1993) (5 μM: 21 ± 9 nM, 10 μM: 29 ± 15 nM and 20 μM: 27 ± 6 nM NO) (Fig. 6B). However, when copper ions were re-introduced to the PBS solution (100 μM CuSO4) the NO release from 20 μM SNAP rose rapidly to NO concentrations that were above the measurable constraints of the probe (not shown). As plateau levels of NO release were not reached during the 30 min recording period (after >1 h of making fresh SNAP stock) the NO concentration given is the final value at the end of the 30 min recording period in PBS with no EDTA (300 ± 9 nM at 5 μM, 374 ± 12 nM NO at 10 μM and 841 ± 158 nM NO at 20 μM donor, Fig. 6C). The total amount of NO released (AUC) following >1 h after making up the stock increased with donor concentration as expected (Fig. 6D). These results indicate the importance of controlling copper levels if using SNAP as an NO donor.
Fig. 6.
Temporal release of NO from SNAP. (A) Graph depicting the varying NO release profiles from the same concentration of SNAP (5 μM) at three consecutive time points: 0–30 min, 30 min–1 h and 1 h+, note the signal saturation at recordings 0–30 min. (B) Average release profiles of NO yield from fresh SNAP stock at 5, 10 and 20 μM in the presence of the copper chelator EDTA. (C) Box and whisker plots displaying the NO concentration range at plateau after 30 min recording time from fresh SNAP stock (1 h+) in PBS with no EDTA at 5, 10 and 20 μM. (D) Box and whisker plots demonstrating the area under the curve from fresh SNAP stock (1 h+) with no EDTA at 5, 10 and 20 μM.
4. Discussion
In order to characterise nitrergic signalling, many researchers use exogenous NO releasing compounds to activate this pathway. However, several studies using this approach resulted in controversial and contradictory results. As shown in many studies, the levels of NO are highly important in order to determine their downstream signalling, with low levels being responsible for the specific activation of sGC, whereas higher levels can lead to the generation of peroxynitrite with subsequent irreversible formation of 3-nitrotyrosine residues of various proteins. Furthermore, NO can also nitrosylate thiol groups of cysteine residues leading to S-nitrosylation of respective proteins. Based on this information, it is crucial to identify the exact concentrations of NO in any system studied, however, for the total number of publications regarding NO signalling only a small percentage attempted to decipher its levels (Bedioui and Villeneuve, 2003). In the present approach, we have systematically compared the release profiles of various NO donors using the Apollo 1000 Free Radical Analyser and NOPF100 NO microsensor. Our data suggest that even equal concentrations of different donors release substantially different amounts of NO over time. Furthermore, we found that the release profile of a given donor changes depending whether and how long it has been stored as a frozen or unfrozen aliquot. Therefore, care must be taken with how long and under what conditions donors are stored. The variations in NO release observed from multiple NO donors in this study and their stability is largely due to their differing chemical compositions and the variety of reactants that induce NO release. Reactants include thiols, light, oxygen, pH, transition metals, acid catalysis and reducing conditions. SNP, which is an iron nitrosyl, is extremely photosensitive and has been widely documented to release NO from irradiation with light but caution is to be taken with this donor as cyanide is a toxic by-product of NO liberation (Arnold et al., 1984). NO release from SNP is also enhanced by thiols and reducing agents and its decomposition is accelerated by oxygen, however, we found in this study that SNP was remarkably stable in stock when protected from light and stored at 4 °C. Furthermore, the amount of NO released, although lower compared to other donors studied, was consistent over three subsequent days and only to be matched in terms of stability by GSNO. GSNO and SNAP are S-nitrosothiols, which are photosensitive molecules, and their stability in solution is dependent upon many factors such as temperature, pH, nucleophiles, oxygen pressure, redox-active species, transition metals and enzymes such as superoxide dismutase and dehydrogenases (Feelisch, 1998; Scatena et al., 2010). Of particular importance in this study is the decomposition of these donors, specifically SNAP, through catalysis by Cu+ ions present in buffer solutions (Mcaninly et al., 1993). Therefore careful control of the metal content of buffer solutions is needed during experimentation to ensure reproducible NO release rates from SNAP. The final two donors studied were PAPA NONOate and NOC-5, both of these donors are diazeniumdiolates and are known as ‘true’ NO donors due to their spontaneous decomposition in solution following first order kinetics. Furthermore, NO release by PAPA NONOate and NOC-5 is not influenced by biological reactants such as thiols and is only dependant on pH, temperature and acid catalysed reactions (Scatena et al., 2010). As such, NO is released at predictable levels making these donors a popular choice. In this study we show that PAPA NONOate and NOC-5 release high levels of NO on day one. Specifically, fresh stock yields double the amount of NO compared to GSNO and 5 times more NO than SNP at just 1/10th of the donor concentration. The only donor that released more NO from fresh stock is SNAP but this is in solution containing free Cu+, so therefore the amount of NO released is dependent on the Cu+ concentration and is subsequently variable unless Cu+ levels are controlled for. This study also demonstrates the stability of NO donor stocks over a period of three consecutive days. SNP and GSNO show great stability but PAPA NONOate and NOC-5 decay substantially over this period with less than 25% NO being released by day three. Moreover, stocks can be frozen for up to four weeks but it is important to consider that during this time some NO will decompose, as shown in PAPA NONOate and NOC-5 where NO release was 20% and 30% of the fresh stock, respectively. Frozen stocks were not tested for SNAP due to increased variability with time and SNP as it has a robust stability at room temperature in crystalline form. As such, this study provides an overview of NO release and stock stability of some of the most commonly used NO donors to aid experimenters in choosing the right donor for their needs. In previous studies there has been wide documentation of contradictory findings regarding NO signalling and this is largely through using different donors. Even when using different NO donors at the same concentration, the yield of NO release can vary greatly (as stated above, Table 1) and therefore lead to non-comparative results. Evidence of this can be seen in studies characterising NO-dependent changes in neuronal excitability. Here, application of 100 μM of a NO donor (NOC-7), which, when extrapolated from our data for the similar donor NOC-5 (Fig. 4) corresponds to NO release of several micromoles, induced transient increases of excitability (Artinian et al., 2010). In comparison, another study has used 100 μM SNP (Steinert et al., 2008), which according to our study releases ∼70 nM of NO (Fig. 2), results in reduced neuronal excitability via voltage-gated potassium channel modulation. Similar effects leading to reduced neuronal excitability, via NO-mediated Kv3 and Kv2 modulation, were produced by enhanced synaptic activity which most likely leads to the synthesis of lower range levels of NO (Steinert et al., 2011). On the other hand, inhibition of M-currents by higher concentrations of SNAP (0.1–1 mM; generating several micromoles of NO) enhances neuronal excitability (Ooi et al., 2013), illustrating the multitude of NO donor- and concentration-dependent modulatory effects.
Although differences between studies could be due to the use of different model systems, an inhibitory and enhancing effect of NO has been reported for the large calcium activated potassium channel (BK). Here, NO donor application (100 μM NOC-7, corresponding to micromolar levels of NO release, as shown for the similar donor NOC-5, Fig. 4) suppressed BK currents in B5 neurons of the mollusk Helisoma Trivolvis (Artinian et al., 2010; Zhong et al., 2013), whereas NO generated by endogenous nNOS activity had an augmenting effect on BK channel activity in rabbit carotid body glomus cells (Li et al., 2010). This data suggests that higher levels of NO have negative impacts, whereas low and physiologically activated NO release enhances channel activities further suggesting a concentration dependency of NO signalling. Clear evidence for this concentration-dependent difference in nitrergic effects has also been demonstrated in one study where 1 mM SNP suppresses and 10 μM SNP enhances neuronal excitability of rat substantia gelatinosa neurons of the spinal cord (Park et al., 2014). Another informative study assessed the effects of two different concentrations of SNAP (20 and 100 μM) and found a bell-shaped curve of NO-dependent activation of delayed rectifier potassium currents (Han et al., 2006) illustrating the importance of defined NO levels.
Additional studies have also shown that the NO donor SNAP (100 μM), which, when extrapolated from our study releases several hundreds to thousands of nanomoles NO, attenuated P/Q-type voltage-gated calcium channels in rat cortical neurons (Petzold et al., 2005), whereas 100 μM SNP (equivalent to ∼70 nM NO) augmented P/Q-type channels in principle neurons of the mouse MNTB (Tozer et al., 2012) and endogenous (low level) NO was necessary for calcium channel activity (Artinian et al., 2012), again reiterating that it is crucial to define applied NO concentrations when comparing studies. Furthermore, higher levels of NO generated by 500 μM DETA NONOate induced L-type calcium channel S-nitrosylation thereby enhancing currents in rat hippocampal neurons (Jian et al., 2007). Whereas, L-type calcium channels were suppressed by 500 μM SNAP in a cGMP-dependent manner in rat DRG neurons (Yoshimura et al., 2001). Collectively, this differential effect of NO (low level endogenous, medium level released by 100 μM SNP or high level released by ≥100 μM SNAP/500 μM DETA NONOate) could account for a great variation of observed nitrergic effects. According to our measurements, SNP released roughly 4–5 times less NO relative to SNAP (at 1/10th of the SNP concentration) resulting in dramatic differences in NO exposure in any study, potentially contributing to inconsistent results and thereby making direct comparisons across data sets difficult.
Our study therefore has identified the release profiles of commonly used NO donors in vitro. It is well established that NO has a variety of cellular effects and depending on its concentration, it can activate the sGC/PKG pathway or induce S-nitrosylation and 3-nitrotyrosination. It is essential for any study to exactly determine which NO concentration has been applied. This current data presents a comprehensive and comparative in vitro study to allow researchers to choose and identify donors in order to apply known concentrations of NO thereby minimising experimentally variable conditions. We illustrate crucial differences between the NO release levels of a variety of donors over a standard time period at equivalent concentrations, thus providing the end user with the appropriate information to pick the most relevant donor for their experimental design.
Acknowledgement
This work was supported by the Medical Research Council UK.
References
- Arnold W.P., Longnecker D.E., Epstein R.M. Photodegradation of sodium nitroprusside: biologic activity and cyanide release. Anesthesiology. 1984;61:254–260. doi: 10.1097/00000542-198409000-00004. [DOI] [PubMed] [Google Scholar]
- Artinian L., Zhong L., Yang H., Rehder V. Nitric oxide as intracellular modulator: internal production of NO increases neuronal excitability via modulation of several ionic conductances. Eur J Neurosci. 2012;36:3333–3343. doi: 10.1111/j.1460-9568.2012.08260.x. [DOI] [PubMed] [Google Scholar]
- Artinian L., Tornieri K., Zhong L., Baro D., Rehder V. Nitric oxide acts as a volume transmitter to modulate electrical properties of spontaneously firing neurons via apamin-sensitive potassium channels. J Neurosci: Off J Soc Neurosci. 2010;30:1699–1711. doi: 10.1523/JNEUROSCI.4511-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedioui F., Villeneuve N. Electrochemical nitric oxide sensors for biological samples – principle, selected examples and applications. Electroanalysis. 2003;15:5–18. [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Bradley S., Tossell K., Lockley R., McDearmid J.R. Nitric oxide synthase regulates morphogenesis of zebrafish spinal cord motoneurons. J Neurosci: Off J Soc Neurosci. 2010;30:16818–16831. doi: 10.1523/JNEUROSCI.4456-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicks A.P., Williams D.L. Generation of nitric oxide from S-nitrosothiols using protein-bound Cu2+ sources. Chem Biol. 1996;3:655–659. doi: 10.1016/s1074-5521(96)90133-7. [DOI] [PubMed] [Google Scholar]
- Feelisch M. The use of nitric oxide donors in pharmacological studies. Naunyn-Schmiedeberg's Arch Pharmacol. 1998;358:113–122. doi: 10.1007/pl00005231. [DOI] [PubMed] [Google Scholar]
- Finnerty N.J., O’Riordan S.L., Palsson E., Lowry J.P. Brain nitric oxide: regional characterisation of a real-time microelectrochemical sensor. J Neurosci Methods. 2012;209:13–21. doi: 10.1016/j.jneumeth.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J., Boulton C.L. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Gunasekar P.G., Kanthasamy A.G., Borowitz J.L., Isom G.E. Monitoring intracellular nitric oxide formation by dichlorofluorescin in neuronal cells. J Neurosci Methods. 1995;61:15–21. doi: 10.1016/0165-0270(95)00018-p. [DOI] [PubMed] [Google Scholar]
- Hall C.N., Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide: Biol Chem/Off J Nitric Oxide Soc. 2009;21:92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han N.L., Ye J.S., Yu A.C., Sheu F.S. Differential mechanisms underlying the modulation of delayed-rectifier K+ channel in mouse neocortical neurons by nitric oxide. J Neurophysiol. 2006;95:2167–2178. doi: 10.1152/jn.01185.2004. [DOI] [PubMed] [Google Scholar]
- Hardingham N., Fox K. The role of nitric oxide and GluR1 in presynaptic and postsynaptic components of neocortical potentiation. J Neurosci: Off J Soc Neurosci. 2006;26:7395–7404. doi: 10.1523/JNEUROSCI.0652-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham N., Dachtler J., Fox K. The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front Cell Neurosci. 2013;7:190. doi: 10.3389/fncel.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst R.D., Clark J.B. The utility of the nitric oxide electrochemical sensor in biomedical research. Sensors. 2003;3:321–329. [Google Scholar]
- Ignarro L.J., Buga G.M., Wood K.S., Byrns R.E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay M., Bradley S., McDearmid J.R. Effects of nitric oxide on neuromuscular properties of developing zebrafish embryos. PLoS ONE. 2014;9:e86930. doi: 10.1371/journal.pone.0086930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen G.C., Zheng Z., Meyerhoff M.E. Amperometric nitric oxide sensors with enhanced selectivity over carbon monoxide via platinum oxide formation under alkaline conditions. Anal Chem. 2013;85:10057–10061. doi: 10.1021/ac402633t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian K., Chen M., Cao X., Zhu X.H., Fung M.L., Gao T.M. Nitric oxide modulation of voltage-gated calcium current by S-nitrosylation and cGMP pathway in cultured rat hippocampal neurons. Biochem Biophys Res Commun. 2007;359:481–485. doi: 10.1016/j.bbrc.2007.05.113. [DOI] [PubMed] [Google Scholar]
- Kasim N., Branton R.L., Clarke D.J. Neuronal nitric oxide synthase immunohistochemistry and 4,5-diaminofluorescein diacetate: tools for nitric oxide research. J Neurosci Methods. 2001;112:1–8. doi: 10.1016/s0165-0270(01)00387-9. [DOI] [PubMed] [Google Scholar]
- Knott A.B., Bossy-Wetzel E. Nitric oxide in health and disease of the nervous system. Antioxid Redox Signal. 2009;11:541–554. doi: 10.1089/ars.2008.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R.G., Moncada S. Nitric oxide as a signal in blood vessels. Trends Biochem Sci. 1992;17:399–402. doi: 10.1016/0968-0004(92)90008-w. [DOI] [PubMed] [Google Scholar]
- Li Y.L., Zheng H., Ding Y., Schultz H.D. Expression of neuronal nitric oxide synthase in rabbit carotid body glomus cells regulates large-conductance Ca2+-activated potassium currents. J Neurophysiol. 2010;103:3027–3033. doi: 10.1152/jn.01138.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcaninly J., Williams D.L.H., Askew S.C., Butler A.R., Russell C. Metal-ion catalysis in nitrosothiol (Rsno) decomposition. J Chem Soc Chem Comm. 1993:1758–1759. [Google Scholar]
- Nakamura T., Lipton S.A. Emerging roles of S-nitrosylation in protein misfolding and neurodegenerative diseases. Antioxid Redox Signal. 2008;10:87–101. doi: 10.1089/ars.2007.1858. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Lipton S.A. Cell death: protein misfolding and neurodegenerative diseases. Apoptosis: Int J Progr Cell Death. 2009;14:455–468. doi: 10.1007/s10495-008-0301-y. [DOI] [PubMed] [Google Scholar]
- Naseem K.M. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Nausch L.W., Ledoux J., Bonev A.D., Nelson M.T., Dostmann W.R. Differential patterning of cGMP in vascular smooth muscle cells revealed by single GFP-linked biosensors. Proc Natl Acad Sci U S A. 2008;105:365–370. doi: 10.1073/pnas.0710387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi L., Gigout S., Pettinger L., Gamper N. Triple cysteine module within M-type K+ channels mediates reciprocal channel modulation by nitric oxide and reactive oxygen species. J Neurosci: Off J Soc Neurosci. 2013;33:6041–6046. doi: 10.1523/JNEUROSCI.4275-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A.R., Lee H.I., Semjid D., Kim do K., Chun S.W. Dual effect of exogenous nitric oxide on neuronal excitability in rat substantia gelatinosa neurons. Neural Plasticity. 2014;2014:628531. doi: 10.1155/2014/628531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold G.C., Scheibe F., Braun J.S., Freyer D., Priller J., Dirnagl U. Nitric oxide modulates calcium entry through P/Q-type calcium channels and N-methyl-d-aspartate receptors in rat cortical neurons. Brain Res. 2005;1063:9–14. doi: 10.1016/j.brainres.2005.09.048. [DOI] [PubMed] [Google Scholar]
- Scatena R., Bottoni P., Pontoglio A., Giardina B. Pharmacological modulation of nitric oxide release: new pharmacological perspectives, potential benefits and risks. Curr Med Chem. 2010;17:61–73. doi: 10.2174/092986710789957841. [DOI] [PubMed] [Google Scholar]
- Simonsen U., Wadsworth R.M., Buus N.H., Mulvany M.J. In vitro simultaneous measurements of relaxation and nitric oxide concentration in rat superior mesenteric artery. J Physiol. 1999;516(Pt 1):271–282. doi: 10.1111/j.1469-7793.1999.271aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert J.R., Chernova T., Forsythe I.D. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist: Rev J Bringing Neurobiol Neurol Psychiatr. 2010;16:435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- Steinert J.R., Postlethwaite M., Jordan M.D., Chernova T., Robinson S.W., Forsythe I.D. NMDAR-mediated EPSCs are maintained and accelerate in time course during maturation of mouse and rat auditory brainstem in vitro. J Physiol. 2010;588:447–463. doi: 10.1113/jphysiol.2009.184317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert J.R., Robinson S.W., Tong H., Haustein M.D., Kopp-Scheinpflug C., Forsythe I.D. Nitric oxide is an activity-dependent regulator of target neuron intrinsic excitability. Neuron. 2011;71:291–305. doi: 10.1016/j.neuron.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert J.R., Kopp-Scheinpflug C., Baker C., Challiss R.A., Mistry R., Haustein M.D. Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron. 2008;60:642–656. doi: 10.1016/j.neuron.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Takata N., Harada T., Rose J.A., Kawato S. Spatiotemporal analysis of NO production upon NMDA and tetanic stimulation of the hippocampus. Hippocampus. 2005;15:427–440. doi: 10.1002/hipo.20064. [DOI] [PubMed] [Google Scholar]
- Tozer A.J., Forsythe I.D., Steinert J.R. Nitric oxide signalling augments neuronal voltage-gated L-type (Ca(v)1) and P/q-type (Ca(v)2.1) channels in the mouse medial nucleus of the trapezoid body. PLoS ONE. 2012;7:e32256. doi: 10.1371/journal.pone.0032256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Wolin M.S., Gupte S.A., Mingone C.J., Neo B.H., Gao Q., Ahmad M. Redox regulation of responses to hypoxia and NO-cGMP signaling in pulmonary vascular pathophysiology. Ann N Y Acad Sci. 2010;1203:126–132. doi: 10.1111/j.1749-6632.2010.05557.x. [DOI] [PubMed] [Google Scholar]
- Wood K.C., Batchelor A.M., Bartus K., Harris K.L., Garthwaite G., Vernon J. Picomolar nitric oxide signals from central neurons recorded using ultrasensitive detector cells. J Biol Chem. 2011;286:43172–43181. doi: 10.1074/jbc.M111.289777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., Rubakhin S.S., Sweedler J.V. Simultaneous nitric oxide and dehydroascorbic acid imaging by combining diaminofluoresceins and diaminorhodamines. J Neurosci Methods. 2008;168:373–382. doi: 10.1016/j.jneumeth.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N., Seki S., de Groat W.C. Nitric oxide modulates Ca(2+) channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol. 2001;86:304–311. doi: 10.1152/jn.2001.86.1.304. [DOI] [PubMed] [Google Scholar]
- Zhong L.R., Estes S., Artinian L., Rehder V. Nitric oxide regulates neuronal activity via calcium-activated potassium channels. PLoS ONE. 2013;8:e78727. doi: 10.1371/journal.pone.0078727. [DOI] [PMC free article] [PubMed] [Google Scholar]