Abstract
Emerging evidence implied that chronic stress has been exerting detrimental impact on immune system functions in both humans and animals. Toll-like receptors (TLRs) have been shown to play an essential role in modulating immune responses and cell survival. We have recently shown that TLR9 deficiency protects against lymphocyte apoptosis induced by chronic stress. However, the exact role of TLR9 in stress-mediated change of macrophage function remains unclear. The results of the current study showed that when BALB/c mice were treated with restraint stress (12 h daily for 2 days), the number of macrophages recruited to the peritoneal cavity was obviously increased. Results also demonstrated that the sustained effects of stress elevated cytokine IL-1β, TNF-α and IL-10 production yet diminished IFN-γ production from macrophage, which led to apoptotic cell death. However, TLR9 deficiency prevented the chronic stress-mediated accumulation of macrophages. In addition, knocking out TLR9 significantly abolished the chronic stress-induced imbalance of cytokine levels and apoptosis in macrophage. TLR9 deficiency was also found to reverse elevation of plasma IL-1β, IL-10 and IL-17 levels and decrease of plasma IFN-γ level under the condition of chronic stress. These results indicated that TLR9-mediated macrophage responses were required for chronic stress-induced immunosuppression. Further exploration showed that TLR9 deficiency prevented the increment of p38 MAPK phosphorylation and reduction of Akt/Gsk-3β phosphorylation; TLR9 deficiency also attenuated the release of mitochondrial cytochrome c into cytoplasm, caused upregulation of Bcl-2/Bax protein ratio, downregulation of cleavage of caspase-3 and PARP, as well as decreased TUNEL-positive cells in macrophage of stressed mice. Collectively, our studies demonstrated that deficiency of TLR9 maintained macrophage function by modulating macrophage accumulation and attenuating macrophage apoptosis, thus preventing immunosuppression in restraint-stressed mice.
Introduction
Experimental studies and clinical observations have indicated that stress serves as an important risk factor in the etiology of infectious and autoimmune diseases [1, 2]. Both acute and chronic stress has been found to have dramatic impacts on the immunological parameters in both humans and animals. Data suggested a positive effect of acute stress on the immune system, while chronic stress frequently leads to immunosuppression [3]. These effects at least partly depend on the function of and apoptotic cell death of immune cells. Numerous studies have revealed that chronic stress leads to a decrease of thymocytes and splenocytes by a mechanism associated with stress-induced lymphocyte apoptosis [4, 5]. As one of the most important immune cells, macrophages might express inducible nitric oxide synthase (iNOS), H2O2, tumor necrosis factor (TNF)-α and interleukin (IL)-1, which participate in the macrophage-induced suppression of immune responses[6] [7]. However, it remains unknown whether macrophages are involved with the immune suppression due to chronic stress. Recent study reveals that stressful life events are associated with altered levels of macrophages in rat models of prostate and breast cancers[8], we hypothesize that chronic stress plays immunosuppressive function partially by inducing macrophage responses. Here, we employed the physical restraint stress mouse model to examine the relationship between chronic stress and macrophage, and to explore the effect of chronic stress on macrophage apoptosis and the possible molecular mechanism.
Macrophages play important roles in regulating immunity by virtue of their ability to secrete a multitude of proinflammatory cytokines and chemokines. Many studies have shown that toll-like receptors (TLRs) modulate the activation of macrophages by pathogens. Among the subsets of TLRs, several pattern recognition receptors have previously been implicated in the chronic stress-induced immune response, including TLR2 and TLR4, as well as the downstream the phosphoinositide 3-kinase (PI3K)/Akt signaling [5, 9]. Previous studies have indicated an involvement of TLR9 in the development of innate immune responses, but the precise role of TLR9 and the underlying mechanisms in the macrophage response after chronic stress exposure is still poorly documented. Recent studies reported that stressed mice showed increased intestinal permeability which resulted in bacterial translocation to the peritoneal cavity [10]. These peritoneal bacteria are a major source of CpG DNA, which can trigger the activation of macrophage TLR9 and cause immune response[11]. Recent studies from us and others have revealed that activation of TLR9 signaling triggers activation of pro-apoptotic signaling pathways, and cause cell apoptosis in various system [11–13].
TLR9 stimulation activates PI3K/Akt and mitogen-activated protein kinase (MAPK) signaling pathway[14]. Akt is an important cellular factor which exerts critical roles in regulating many cellular functions, such as cellular activation, inflammatory response, and apoptosis[15]. GSK-3β is a constitutively active enzyme that is inactivated by Akt which regulates cell survival and apoptosis [16]. MAPKs are associated with some important aspects of immune responses [17]. Among MAPK families, p38 MAPK is easily activated by stress signals [18]. Earlier studies established that activation of p38 MAPK and down-regulation of Akt kinase led to leucocytes apoptosis by the disruption of Bcl-2, caspase activation and subsequent apoptotic features[19].
A distinctive feature of activated macrophages is their capacity to rapidly generate TNF-α in response to diverse stimuli. In addition to producing TNF-α, activated macrophages secrete the cytokine IL-10 which contributes to the down-regulation of IFN-γand consequently, in the apoptosis process of macrophage, highlighting the importance of macrophage in innate and adaptive immune responses[20]. This report investigates mechanisms by which TLR9 inhibition suppresses chronic stress-induced imbalance of cytokines production. We demonstrated that TNF-α, IL-1βand IL-10 production, as well as p38 activation, cleaved caspase-3 and cleaved poly ADP-ribose polymerase (PARP) induced by chronic stress were impaired in macrophages from TLR9-deficient mice. We also showed that TLR9 deficiency did restore chronic stress-impaired IFN-γproduction, Akt/GSK-3β phosphorylation and Bcl-2/Bax ratio in macrophage.
Materials and Methods
Experimental animals
Breeding pairs of TLR9 knockout (not a functional knockout) mice on a BALB/c background were kindly provided by Dr. Shizuo Akira (Osaka University, Osaka, Japan) via Dr. Dennis Klinman (National Cancer Institute, Frederick, MD). Wild type BALB/c male mice were purchased from the Harlan (Indianapolis, Indiana) and all mice were kept in the Division of Laboratory Animal Resources at East Tennessee State University (ETSU), a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All animals were maintained in a specific pathogen-free room under controlled conditions at the room temperature (23±1℃) with a 12-h light-dark cycle. All experiments were adhered to the animal use protocol approved by the ETSU Committee on Animal Care.
Experimental model of restraint stress
All mice (male, weight 23~25g) were healthy and six to eight-week-old. The protocol used to establish chronic physical restraint model was proved to be effective in our laboratory as well as others [21]. Briefly, wild type mice and TLR knockout mice were randomly divided into 2 groups, 7 in each group, respectively. Each individual mouse of stress group was placed in a 50-ml polypropylene conical centrifuge tube (Corning, NY). The tubes were arranged with multiple punctures for ventilation. Mice were restricted horizontally in the tubes for 12 h (from21:00 to next day 9:00) followed by a 12 h rest (from 9:00 to 21:00). The stressed mice were kept next to each other. The stressed mice were provided with food and water during the rest period in an ordinary cage. Food and water were provided to control littermates in their original cage only during the 12 h rest. The cages were transparent, well-ventilated and only contained food, water and bedding materials during the rest period. Observations in our laboratory showed that during restraint, the mice did not suffer from any physical suppression or pain. After 2 cycles, mice were humanely killed by cervical dislocation for the subsequent experiments.
Isolation of peritoneal macrophages
After the two cycles of stress finished, mice were humanely killed by cervical dislocation, and peritoneal macrophages were collecting by injecting 5 ml of phosphate-buffered saline (PBS) into the peritoneal cavity. The cell suspension was cultured with RPMI-1640 containing 10% fetal bovine serum (FBS) for 60 min at 37°C in to allow the macrophages to adhere as described by Mantovani [22]. After being washed in PBS, the non-adherent cells were removed. The purity of macrophages was >95%.
Determination of apoptosis by TUNEL assay
TUNEL assay was performed according to our previous study [23]. Apoptotic nuclear DNA fragments were investigated using the In Situ Cell Death Detection kit (Roche Diagnostic, Indianapolis, IN). Briefly, macrophages (5 × 105 cells) from wild type and TLR9 knockout mice were fixed in 4% formaldehyde/PBS for 20 min at 37°C,permeabilized in 0.1% sodium citrate solution containing 0.1% Triton X-100, for 10 min, after that, the sections were incubated with 50 μL of TUNEL reaction mixture for 60 min at 37°C. After convert-AP incubation, 50 μL of substrate solution was placed on the slices. Finally, sections were conterstained with haematoxylin. Slices were observed under a light microscope using a 40× objective.
Western blot analysis
Western blotting was performed as described previously[1, 20]. Briefly, the cellular proteins were fractionated by 10% SDS-PAGE gel and electroblotted onto Hybond ECL membranes (Amersham Pharmacia, NJ). After being blocked with nonfat milk, the membranes were blotted overnight at 4°C with following primary antibody [anti-TLR9, anti-phospho-p38, anti-p38, anti-phospho-Akt, anti-Akt, anti-cleaved-caspase-3, anti-caspase-3, anti-PARP, anti-Bcl-2, anti-Bax, anti-phospho-GSK-3β, anti- GSK-3β, anti-GAPDH (Cell Signaling Technology, Beverly, MA)][20]. Next day, after incubation with HRP-conjugated secondary antibodies (Cell Signaling Technology, Inc.), membranes were then developed with the Super Signal West Dura Extended Duration substrate (Pierce Biotechnology, Rockford, IL). The bands were quantified by densitometry using a Bio-Image Analysis System (Bio-Rad).
Enzyme linked immunosorbent assay (ELISA) for cytokines
Equal amounts of peritoneal macrophages (5×105 cells/ mL) were planted in 96-well plates. The supernatants were harvested after 24 h of incubation. The concentration of cytokines in the supernatants was detected by ELISA kits (R&D Systems, Minneapolis, MN) according to our previous studies [5].
Statistical analysis
Data were expressed as mean ± S.E.M. Statistical analysis were performed using one-way analysis of variance (ANOVA) followed by Bonferroni tests to examine whether differences among groups existed. A P value < 0.05 was accepted as significant.
Results
TLR9 is required for chronic stress-induced macrophages accumulation
Recent evidence showed that TLR9 is largely expressed on macrophages, however, the exact role of TLR9 in modulating macrophage function is not known yet [24]. Data suggests that chronic stress increases TLR9 expression in peritoneal macrophage (Fig 1A). We therefore asked whether TLR9 is involved in stress-mediated changes of macrophage function. Since macrophages in peritoneal cavity of chronic stress-induced mice, irrespective of their location, can significantly contribute to inflammation and immune response by producing cytokines and free oxygen radicals [25] [26], it is important to assess the total number of macrophages accumulated in peritoneal cavity. Therefore, we decided to examine the pattern of total macrophages increase after stress treatment in the peritoneal cavity of TLR9 knockout and wild type mice. We observed a robust accumulation of macrophages in peritoneal cavity 2 days after stress challenge, representing a > 2-fold increase over baseline number of cells; no significant change in number of peritoneal macrophages was observed after stress challenge compared with that in control group in TLR 9 knockout mice (Fig 1B). Therefore, TLR9 knockout mice lose their sensitivity to chronic stress-induced accumulation of macrophages, supporting a critical role of TLR9 in stress-induced immune response.
Fig 1. A deficiency of TLR9 blocks chronic stress-induced accumulation of macrophages in peritoneal cavity TLR9 knockout mice or wild type BALB/c mice aged 6 to 8 weeks were subjected to a 12 h physical restraint daily.
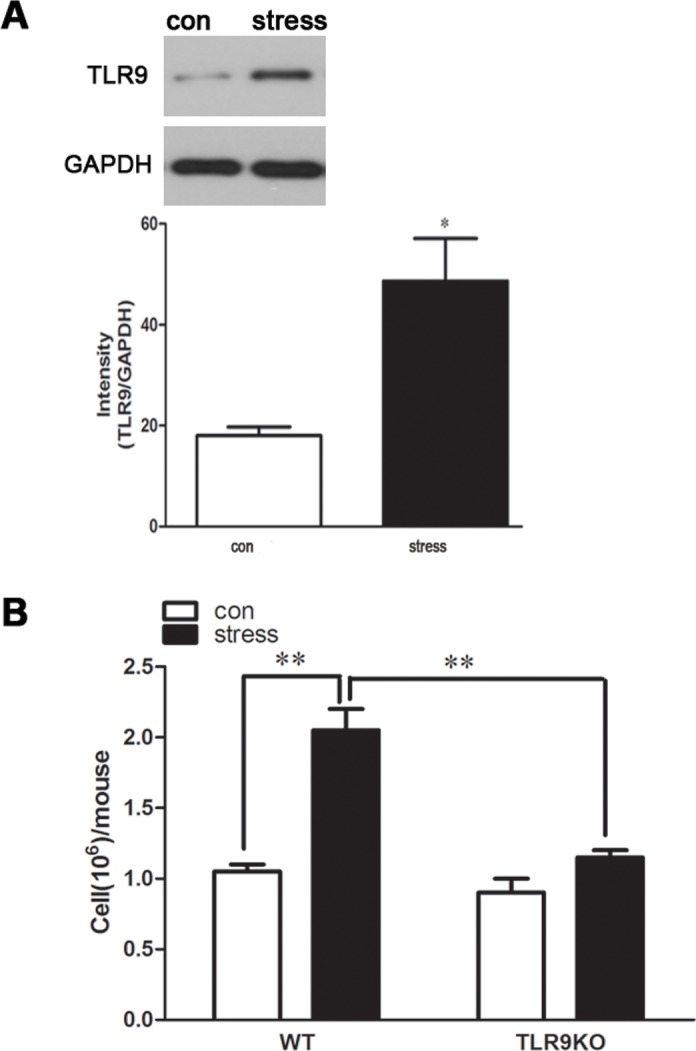
After 2 d stress, mice were sacrificed by cervical dislocation, and the peritoneal macrophages were harvested and the counts were performed. For TLR9 protein expression evaluating, the macrophages were harvested and cultured for 24 hours. The expression of TLR9 was analyzed by Western blot. Means and SEs were calculated from 7 mice per group. * p < 0.05, ** p< 0.01 compared with indicated groups.
Macrophages from TLR9 knockout mice display impaired changes of chronic stress-induced cytokine levels
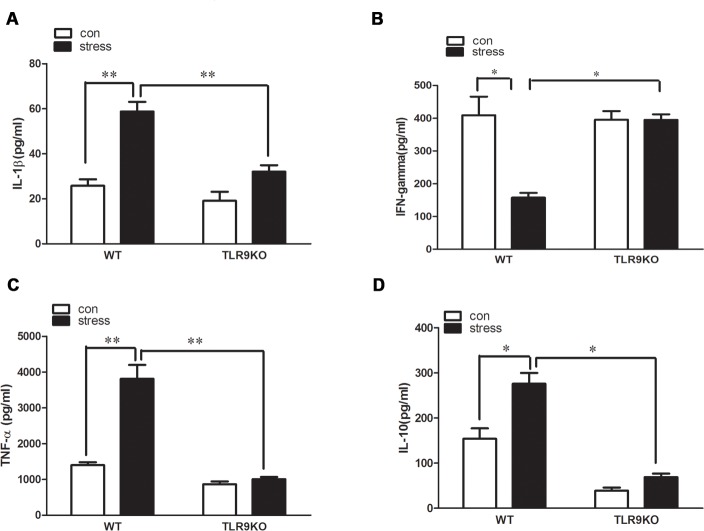
In response to a large range of stimulation, macrophages secrete powerful biological substances, such as TNF-αand interleukins. This secretion results in inflammation. To investigate whether the diminished accumulation observed in the TLR9 knockout mice was secondary to altered macrophage function, we have detected generation of major pro-inflammatory cytokines by macrophages. Wild type and TLR9 knockout mice were subjected to stress as described previously, peritoneal macrophages were harvested and cultured for 24 hours. Our data showed that IL-1β, TNF-α, IL-10 were significantly overproduced in supernatants of macrophages of stressed wild type mice, increasing by 2.2-, 3.1- and 1.8-fold, compared to that of control wild type mice, respectively. However, IL-1β, TNF-α, IL-10 levels of stressed TLR9 knockout mice displayed no distinctive change compared to control TLR9 knockout mice and (Fig 2A, 2C and 2D). Chronic stress significantly inhibited IFN-γ production in supernatant of macrophages from stressed wild type mice by 2.5-fold than that from control wild type mice, but no change in IFN-γ expression level in supernatant of macrophages was observed after chronic stress challenge in TLR9 knockout mice (Fig 2B).
Fig 2. A deficiency of TLR9 decreases chronic stress-induced changes of pro-inflammatory cytokine levels by macrophages TLR9 knockout mice or wild type BALB/c mice aged 6 to 8 weeks were subjected to a 12 h physical restraint daily.
After 2 d stress, mice were sacrificed by cervical dislocation, and the macrophages were harvested, purified and cultured (5 × 105 cells/well) on culture plates for 24 hours. IL-1β, TNF-α, IL-10 and IFN-γ levels were measured in supernatants of macrophages by ELISA kit. Means and SEs were calculated from 7 mice per group. * p < 0.05, ** p< 0.01 compared with indicated groups.
TLR9 deficiency blocks chronic stress-induced changes of pro-inflammatory cytokines in serum
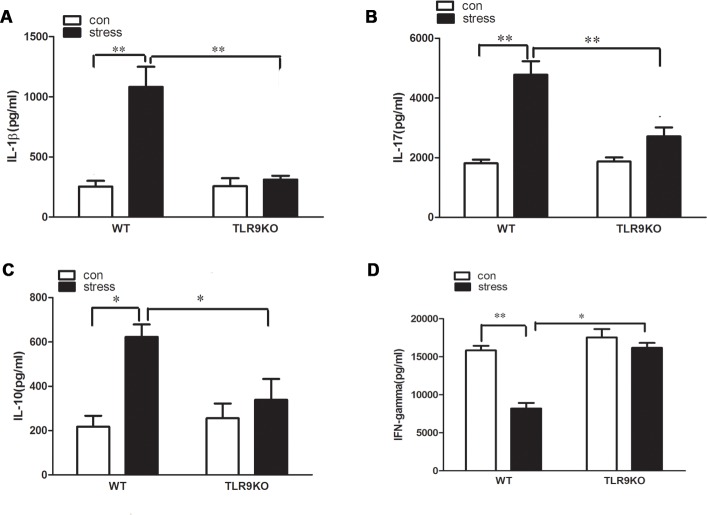
It is known that excessive production of plasma proinflammatory cytokines in response to chronic stress can promote the development of immune suppression. We next assessed the expression of IL-1β, IL-10, IL-17 and IFN-γ in the serum of wild type and TLR9 knockout mice challenged with chronic stress. Our data showed that expression level of IL-1β, IL-10, IL-17 in the serum of stressed wild type mice increased by 3.3-, 2.9- and 2.8-fold, compared to that of control wild type mice, respectively. However, the expression level of IL-1β, IL-10, IL-17 did not differ between the control TLR9 knockout mice and stressed TLR9 knockout mice (Fig 3A, 3B and 3C). Chronic stress significantly inhibited IFN-γ production in the serum from stressed wild type mice by 2.1-fold than serum from control wild type mice, but chronic stress failed to induce the change in TLR9 kncokout mice (Fig 3D).
Fig 3. A deficiency of TLR9 suppressed change of cytokine levels caused by chronic stress.
TLR9 knockout mice or wild type BALB/c mice aged 6 to 8 weeks were subjected to a 12 h physical restraint daily. After 2 d stress, mice were sacrificed by cervical dislocation, and the serum were harvested and the levels of IL-1β, IL-10, IL-17 and IFN-γ in serum were examined by ELISA kit. Means and SEs were calculated from 7 mice per group. * p < 0.05, ** p< 0.01 compared with indicated groups.
TLR9 deficiency blocks chronic stress-induced macrophage apoptosis
Our recent study showed that chronic stress induces lympocyte apoptosis [2]. We also reported that chronic stress promotes cell apoptosis through TLR9 [12]. To determine whether TLR9 is associated with stress-induced macrophages apoptosis, wild type and TLR9 knockout mice were subjected to stress as described previously, peritoneal macrophages were then harvested and cultured for 24 hours and TUNEL assay was performed to detect cell apoptosis. We found that a large amount of wild type macrophages were undergoing apoptosis after restraint stress, whereas only a few apoptotic cells were detected in the TLR9 deficient macrophages following stress challenge (Fig 4). Therefore, stress-induced macrophage apoptosis requires TLR9.
Fig 4. A deficiency of TLR9 is resistant to stress-induced macrophage apoptosis TLR9 knockout mice or wild type BALB/c mice aged 6 to 8 weeks were subjected to a 12 h physical restraint daily.
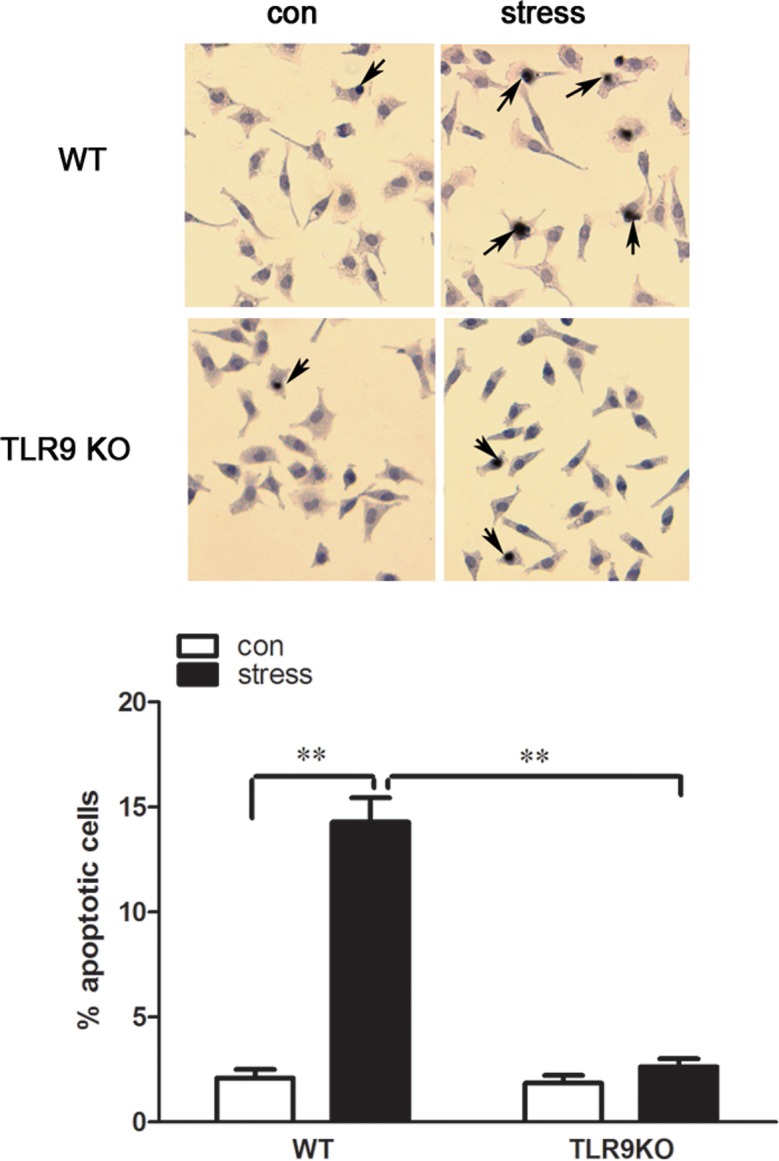
After 2 d stress, mice were sacrificed by cervical dislocation, and the macrophages were harvested, purified and cultured (5 × 105 cells/well) on culture plates for 24 hours. Apoptotic cells (dark brown color cells) were determined by TUNEL assay. Photographs of representative TUNEL-stained cells are shown at the top. Magnification 200×. The bar graph shows the percentage of apoptotic cells. Means and SEs were calculated from 7 mice per group. * p < 0.05, ** p< 0.01 compared with indicated groups.
TLR9 deficiency attenuates stress-induced activation of caspase-3 and PARP and alteration of Bcl-2/Bax ratio
The levels of major apoptosis-related proteins were detected to further assess the mechanisms underlying cellular changes observed in mice after stress challenge. We found that the levels of cleaved caspase-3 and cleaved PARP, two well-known characteristics of apoptosis, were remarkably increased in macrophages of wild type mice following stress treatment, whereas the increases of cleaved caspase-3 and cleaved PARP were attenuated markedly in TLR9 deficient macrophages (Fig 5A). As protein expressions of Bcl-2 and Bax are involved in the chronic stress-induced apoptotic pathway [27], we examined the ratio of Bcl-2 and Bax in macrophages to elucidate the mechanism of stress-induced apoptosis. Stress challenge markedly decreased the ratio of Bcl-2/Bax in wild type macrophages; moreover, the expressions of Bcl-2 and Bax in the macrophages were not altered in TLR9 deficient mice. Our data suggested that Bcl-2 family participate in TLR9-mediated macrophage signaling after stress treatment (Fig 5B).
Fig 5. TLR9 deficency inhibits stress-induced change in caspase-3 and PARP activation and ratio of Bcl-2/Bax TLR9 knockout mice or wild type BALB/c mice aged 6 to 8 weeks were subjected to a 12 h physical restraint daily.
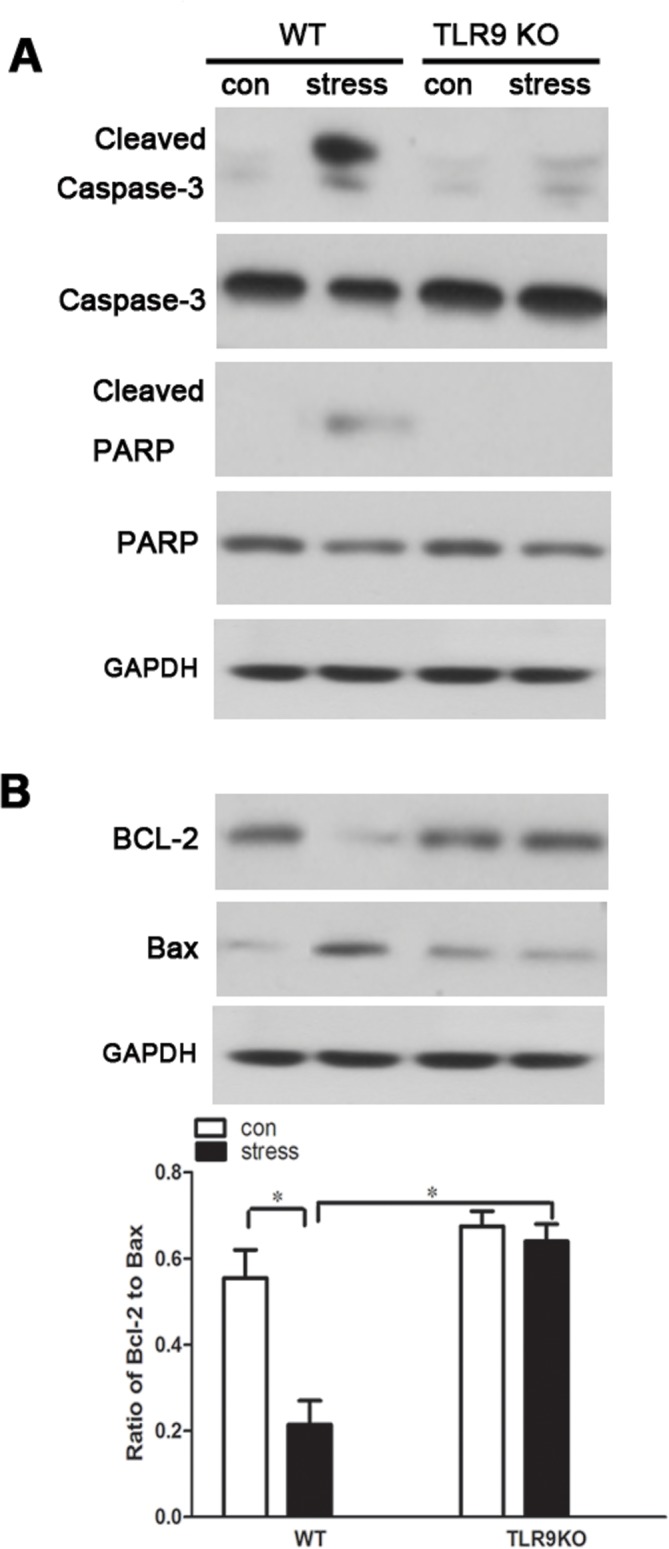
After 2 d stress, mice were sacrificed by cervical dislocation, and the macrophages were harvested, purified and cultured (5 × 105 cells/well) on culture plates for 24 hours. The expression of cleaved caspase-3 and cleaved PARP (A), and Bcl-2/Bax (B) was analyzed by Western blot. Means and SEs were calculated from 7 mice per group. * p < 0.05, ** p< 0.01 compared with indicated groups.
TLR9 deficiency blocks chronic stress-induced changes of apoptosis related pathways
Accumulating evidence indicates that p38 MAPK participates as a modulator in Bcl-2/Bax-mediated apoptosis in neuroblastoma cells [28] [29]. It also has been demonstrated that activated Akt alters the ratio of Bcl-2 and Bax and exhibits an anti-apoptotic role in various cells [30]. Additionally, recent studies have revealed cross-talk between TLR signaling and the Akt/GSK-3β or p38 MAPK signaling pathway [13] [31]. To examine whether chronic stress activates p38 MAPK and Akt/GSK-3β signaling in TLR9-mediated signaling, the levels of phosphorylated p38 (phospho-p38), phospho-Akt and phospho-GSK-3β in macrophages following stress treatment were examined by western blot analysis. The results of the present study confirmed that chronic stress promoted p38 phosphorylation in wild type macrophage. Moreover, stress-induced p38 MAPK activation was reversed in TLR9 knockout macrophages suggesting that stress markedly increases the level of phospho-p38 through TLR9 (Fig 6). We also found that the increasing levels of phospho-Akt and phospho-GSK-3β were significantly abolished by chronic stress in wild type macrophages but not in TLR9 deficient macrophages, demonstrating that chronic stress decreases the activation of phospho-Akt/phospho-GSK-3β signaling in a TLR9-dependent manner (Fig 6).
Fig 6. TLR9 deficiency attenuates chronic stress-induced changes of apoptosis related pathways TLR9 knockout mice or wild type BALB/c mice aged 6 to 8 weeks were subjected to a 12 h physical restraint daily.
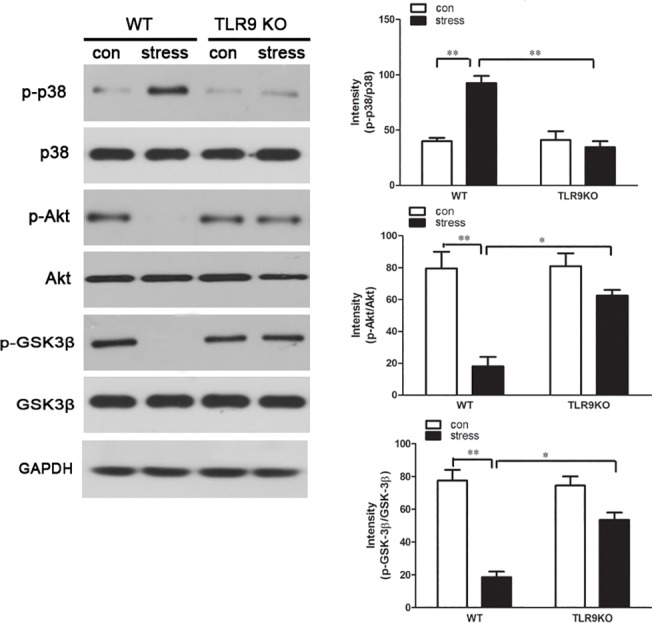
After 2 d stress, mice were sacrificed by cervical dislocation, and the macrophages were harvested, purified and cultured (5 × 105 cells/well) on culture plates for 24 hours. The expression of total and phospho-p38, total and phospho-Akt, total and phospho-GSK-3β were analyzed by Western blot. Means and SEs were calculated from 7 mice per group. * p < 0.05, ** p< 0.01 compared with indicated groups.
Discussion
The knowledge on pattern recognition receptors (PRRs) recognition and activation of an efficient immune response against chronic stress has progressively increased, mainly in regards to TLR2, TLR4 [5, 27]. Several lines of evidence suggest that TLR9 seems to participate in chronic stress-mediated immune suppression, however, the involvement of TLR9 in functional change of macrophages following chronic stress treatment has not yet been addressed. Our data presented herein clearly revealed that chronic stress might act through TLR9 to generate macrophage inflammation and apoptosis, further supporting that TLR9 plays a role in immune response.
In response to multiple waves of pathogenic stimuli, inflammatory mediators including TNF-α, IFN-γ, IL-1β, IL-6 and IL-10 may be liberated by macrophages. These molecules with diverse physiological effects might play critical roles in the recruitment and apoptosis of macrophages. Indeed, in the present study, we showed that chronic stress induced series inflammatory response characterized by recruitment of macrophages into the peritoneum; generation of pro-inflammatory mediators from macrophages, such as IL-1β and TNF-α and induction of apoptosis. Interestingly, TLR9 deficiency markedly diminished these inflammatory responses induced by chronic stress.
Our previous study indicates that chronic stress causes an imbalance in the Th1 and Th2 responses [32]. Increase of IL-1βand TNF-αsecretion in blood and brain turns out to be a common feature of diverse models of stress [33], whereas the decrease of other cytokines like IFN-γ and IL-10 seems to be more controversial [34–36]. IL-17, an important cytokine produced by Th17 cells, is able to indirectly induce the recruitment of macrophages and neutrophils during inflammation[37]. In fact we showed in this study that chronic stress could induce a dramatic increase of IL-1β, IL-10 and IL-17 production and a significant decrease of IFN-γsynthesis.In contrast, the production of these cytokines was almost unaffected in TLR9 deficient mice under chronic stress influence. These results corroborate a previous report showing that malaria in TLR9 knockout mice significantly diminishes changes of Th1 and Th2 cytokines as compared to control wild type mice [38], indicating that chronic stress leads to immune suppression in a TLR9-dependent manner.
We next attempted to investigate the mechanistic pathway by which TLR9 modulates immune responses. During the process of apoptosis several targets have been identified as characteristic of cell death, including the potential decreases in mitochondrial membrane [25]. Apoptotic stimulation causes the change in mitochondrial membrane potential and the release of cytochrome C into the cytoplasm, activating caspase-9, triggering activation of other caspase members, including caspase-7 and caspase-3, to initiate a caspase cascade, which leads to apoptosis [39, 40]. Furthermore, PARP acts as the main cleavage targets of caspase-3 [41]. For these reason, we detected the expression of cleaved-caspase-3 and cleaved-PARP. In our current study, we demonstrated that chronic stress dramatically upregulated cleavage of caspase-3 and PARP in wild type macrophages. This agrees with our previous report on chronic stress-induced apoptosis accompanied by caspase-3 activation in splenocytes [27]. We also found that the elevated activation of caspase-3 as well as PAPR was significantly blocked to almost control level in TLR9 deficient macrophages. In addition, apoptosis is commonly associated with an imbalance between pro- and anti-apoptotic members of the Bcl-2 family. In the current study, the ratio of Bcl-2/Bax was markedly reduced in chronic stress-induced macrophage, indicating that cells were undergoing apoptosis, however, TLR9 deficiency elevated the Bcl-2/Bax ratio remarkably in macrophage, further confirming that TLR9-deficient macrophage was resistant to chronic stress-induced apoptosis.
Accumulating evidence suggests that p38 MAPK and Akt signaling pathways acts to regulate the cell cycle progression and proliferation [42] [43]. The data of current study indicated that the p38 signaling pathway was significantly activated by chronic stress treatment in wild type macrophages, indicating that p38 was involved in stress-induced macrophage apoptosis. In contract, stress-induced p38 activation was suppressed in TLR9 knockout macrophages, suggesting that stress promoted p38 phosphorylation through TLR9. Consistently, a recent study showed that activation of p38 MAPK signaling pathway by morphine induced apoptosis in a TLR9 dependent manner [31]. As recent evidence implicates, there is a cross-talk between TLR signaling and the Akt/GSK-3β signaling pathway. In previous studies we documented that TLR2 was required for chronic stress-induced apoptosis via PI3K/Akt/GSK-3β signaling cascade in lymphocytes [9]. Additionally, the PI3K/Akt signaling cascade may participate in TLR4-mediated immune responses as an endogenous negative feedback regulator [5]. Considering that Akt/GSK-3β has an important role on immune cells activation in a TLR dependent manner, we then addressed the question of whether this signaling pathway was associated with chronic stress-induced macrophage apoptosis. As expected, no phosphorylation was detected in macrophages when wild type mice were subjected to chronic stress. In contract, phosphorylation of Akt and GSK-3β was restored to the normal level in TLR9 deficient macrophages, suggesting that in TLR9 deficient mice, the higher level of phosphor-Akt and phosphor-GSK-3β prevents macrophages from stress-induced inflammation and apoptosis. Collectively, our data implicates that TLR9 participates in chronic stress-induced immune response via mediating apoptosis-related signaling pathways and proteins.
In summary, our data demonstrated that the chronic stress plays immunosuppressive function partially by inducing macrophage responses and was characterized by a vigorous TLR 9-mediated accumulation of macrophages and release of cytokine, resulting in alteration of macrophage cell signaling and immunosuppression. Theses cytokines might further participate in the apoptosis of macrophages. Bcl-2 family and caspase-3; p38 MAPK and Akt/GSK-3β signaling take part in TLR9-mediated chronic stress-induced apoptosis in macrophage.
Acknowledgments
This work was supported in part by research grant NIGMS94740 from NIH to D. Yin. The authors wish to express their appreciation to Dr. Shizuo Akira, Osaka University, Osaka, Japan and Dr. Dennis Klinman, National Cancer Institute, Frederick of MD, for providing breeding pairs of TLR9 knockout mice.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported in part by research grant NIGMS94740 from NIH to D. Yin.
References
- 1. Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–25. Epub 2004/10/07. 10.1016/S1470-2045(04)01597-9 . [DOI] [PubMed] [Google Scholar]
- 2. Shi Y, Devadas S, Greeneltch KM, Yin D, Allan Mufson R, Zhou JN. Stressed to death: implication of lymphocyte apoptosis for psychoneuroimmunology. Brain, behavior, and immunity. 2003;17 Suppl 1:S18–26. Epub 2003/03/05. . [DOI] [PubMed] [Google Scholar]
- 3. Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain, behavior, and immunity. 1997;11(4):286–306. Epub 1998/03/26. 10.1006/brbi.1997.0508 . [DOI] [PubMed] [Google Scholar]
- 4. Dominguez-Gerpe L, Rey-Mendez M. Alterations induced by chronic stress in lymphocyte subsets of blood and primary and secondary immune organs of mice. BMC immunology. 2001;2:7 Epub 2001/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y, Miao J, Hanley G, Stuart C, Sun X, Chen T, et al. Chronic restraint stress promotes immune suppression through toll-like receptor 4-mediated phosphoinositide 3-kinase signaling. Journal of neuroimmunology. 2008;204(1–2):13–9. Epub 2008/09/26. 10.1016/j.jneuroim.2008.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riquelme P, Tomiuk S, Kammler A, Fandrich F, Schlitt HJ, Geissler EK, et al. IFN-gamma-induced iNOS expression in mouse regulatory macrophages prolongs allograft survival in fully immunocompetent recipients. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21(2):409–22. Epub 2012/08/30. 10.1038/mt.2012.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palermo-Neto J, de Oliveira Massoco C, Robespierre de Souza W. Effects of physical and psychological stressors on behavior, macrophage activity, and Ehrlich tumor growth. Brain, behavior, and immunity. 2003;17(1):43–54. Epub 2003/03/05. . [DOI] [PubMed] [Google Scholar]
- 8. Sarkar DK, Murugan S, Zhang C, Boyadjieva N. Regulation of cancer progression by beta-endorphin neuron. Cancer research. 2012;72(4):836–40. Epub 2012/01/31. 10.1158/0008-5472.CAN-11-3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li H, Chen L, Zhang Y, Lesage G, Wu Y, Hanley G, et al. Chronic stress promotes lymphocyte reduction through TLR2 mediated PI3K signaling in a beta-arrestin 2 dependent manner. Journal of neuroimmunology. 2011;233(1–2):73–9. Epub 2010/12/25. 10.1016/j.jneuroim.2010.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ponferrada A, Caso JR, Alou L, Colon A, Sevillano D, Moro MA, et al. The role of PPARgamma on restoration of colonic homeostasis after experimental stress-induced inflammation and dysfunction. Gastroenterology. 2007;132(5):1791–803. Epub 2007/05/09. 10.1053/j.gastro.2007.02.032 . [DOI] [PubMed] [Google Scholar]
- 11. Liang X, Moseman EA, Farrar MA, Bachanova V, Weisdorf DJ, Blazar BR, et al. Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells. Blood. 2010;115(24):5041–52. Epub 2010/03/27. 10.1182/blood-2009-03-213363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li H, Zhao J, Chen M, Tan Y, Yang X, Caudle Y, et al. Toll-Like Receptor 9 Is Required for Chronic Stress-Induced Immune Suppression. Neuroimmunomodulation. 2013;21(1):1–7. Epub 2013/10/02. 10.1159/000354610 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen L, Shi W, Li H, Sun X, Fan X, Lesage G, et al. Critical role of toll-like receptor 9 in morphine and Mycobacterium tuberculosis-Induced apoptosis in mice. PloS one. 2010;5(2):e9205 Epub 2010/02/23. 10.1371/journal.pone.0009205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beutler B. Innate immunity: an overview. Molecular immunology. 2004;40(12):845–59. Epub 2003/12/31. . [DOI] [PubMed] [Google Scholar]
- 15. Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, et al. Role of translocation in the activation and function of protein kinase B. The Journal of biological chemistry. 1997;272(50):31515–24. Epub 1998/02/12. . [DOI] [PubMed] [Google Scholar]
- 16. Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nature immunology. 2005;6(8):777–84. Epub 2005/07/12. 10.1038/ni1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annual review of immunology. 2002;20:55–72. Epub 2002/02/28. 10.1146/annurev.immunol.20.091301.131133 . [DOI] [PubMed] [Google Scholar]
- 18. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–2. Epub 2002/12/10. 10.1126/science.1072682 . [DOI] [PubMed] [Google Scholar]
- 19. Moon DO, Kim MO, Lee JD, Choi YH, Lee MK, Kim GY. Molecular mechanisms of ZD1839 (Iressa)-induced apoptosis in human leukemic U937 cells. Acta pharmacologica Sinica. 2007;28(8):1205–14. Epub 2007/07/21. 10.1111/j.1745-7254.2007.00615.x . [DOI] [PubMed] [Google Scholar]
- 20. Ropert C, Almeida IC, Closel M, Travassos LR, Ferguson MA, Cohen P, et al. Requirement of mitogen-activated protein kinases and I kappa B phosphorylation for induction of proinflammatory cytokines synthesis by macrophages indicates functional similarity of receptors triggered by glycosylphosphatidylinositol anchors from parasitic protozoa and bacterial lipopolysaccharide. J Immunol. 2001;166(5):3423–31. Epub 2001/02/24. . [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Foster R, Sun X, Yin Q, Li Y, Hanley G, et al. Restraint stress induces lymphocyte reduction through p53 and PI3K/NF-kappaB pathways. Journal of neuroimmunology. 2008;200(1–2):71–6. Epub 2008/07/26. 10.1016/j.jneuroim.2008.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Current protocols in immunology / edited by John E Coligan [et al]. 2008;Chapter 14:Unit 14 1. Epub 2008/11/20. 10.1002/0471142735.im1401s83 [DOI] [PMC free article] [PubMed]
- 23. Li H, Sun X, LeSage G, Zhang Y, Liang Z, Chen J, et al. beta-arrestin 2 regulates Toll-like receptor 4-mediated apoptotic signalling through glycogen synthase kinase-3beta. Immunology. 2010;130(4):556–63. Epub 2010/05/26. 10.1111/j.1365-2567.2010.03256.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thirunavukkarasu S, de Silva K, Whittington RJ, Plain KM. In vivo and in vitro expression pattern of Toll-like receptors in Mycobacterium avium subspecies paratuberculosis infection. Veterinary immunology and immunopathology. 2013;156(1–2):20–31. Epub 2013/09/24. 10.1016/j.vetimm.2013.08.008 . [DOI] [PubMed] [Google Scholar]
- 25. Maus U, Huwe J, Ermert L, Ermert M, Seeger W, Lohmeyer J. Molecular pathways of monocyte emigration into the alveolar air space of intact mice. American journal of respiratory and critical care medicine. 2002;165(1):95–100. Epub 2002/01/10. 10.1164/ajrccm.165.1.2106148 . [DOI] [PubMed] [Google Scholar]
- 26. Elias JA, Schreiber AD, Gustilo K, Chien P, Rossman MD, Lammie PJ, et al. Differential interleukin 1 elaboration by unfractionated and density fractionated human alveolar macrophages and blood monocytes: relationship to cell maturity. J Immunol. 1985;135(5):3198–204. Epub 1985/11/01. . [PubMed] [Google Scholar]
- 27. Hu D, Denney J, Liang M, Javer A, Yang X, Zhu R, et al. Stimulatory Toll-like receptor 2 suppresses restraint stress-induced immune suppression. Cellular immunology. 2013;283(1–2):18–24. Epub 2013/07/16. 10.1016/j.cellimm.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang CW, Tsai WH, Chuang WJ, Lin YS, Wu JJ, Liu CC, et al. Procaspase 8 and Bax are up-regulated by distinct pathways in Streptococcal pyrogenic exotoxin B-induced apoptosis. The Journal of biological chemistry. 2009;284(48):33195–205. Epub 2009/10/06. 10.1074/jbc.M109.020586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singhal PC, Bhaskaran M, Patel J, Patel K, Kasinath BS, Duraisamy S, et al. Role of p38 mitogen-activated protein kinase phosphorylation and Fas-Fas ligand interaction in morphine-induced macrophage apoptosis. J Immunol. 2002;168(8):4025–33. Epub 2002/04/09. . [DOI] [PubMed] [Google Scholar]
- 30. Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, et al. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. The Journal of biological chemistry. 2000;275(15):10761–6. Epub 2001/02/07. . [DOI] [PubMed] [Google Scholar]
- 31. He L, Li H, Chen L, Miao J, Jiang Y, Zhang Y, et al. Toll-like receptor 9 is required for opioid-induced microglia apoptosis. PloS one. 2011;6(4):e18190 Epub 2011/05/12. 10.1371/journal.pone.0018190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Y, Woodruff M, Miao J, Hanley G, Stuart C, Zeng X, et al. Toll-like receptor 4 mediates chronic restraint stress-induced immune suppression. Journal of neuroimmunology. 2008;194(1–2):115–22. Epub 2008/01/15. 10.1016/j.jneuroim.2007.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maes M. Psychological stress and the inflammatory response system. Clin Sci (Lond). 2001;101(2):193–4. Epub 2001/07/28. . [PubMed] [Google Scholar]
- 34. Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, et al. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PloS one. 2013;8(3):e58488 Epub 2013/03/23. 10.1371/journal.pone.0058488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu D, Wan L, Chen M, Caudle Y, LeSage G, Li Q, et al. Essential role of IL-10/STAT3 in chronic stress-induced immune suppression. Brain, behavior, and immunity. 2014;36:118–27. Epub 2014/02/12. 10.1016/j.bbi.2013.10.016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kour K, Bani S. Augmentation of immune response by chicoric acid through the modulation of CD28/CTLA-4 and Th1 pathway in chronically stressed mice. Neuropharmacology. 2011;60(6):852–60. Epub 2011/01/29. 10.1016/j.neuropharm.2011.01.001 . [DOI] [PubMed] [Google Scholar]
- 37. Shan M, Yuan X, Song LZ, Roberts L, Zarinkamar N, Seryshev A, et al. Cigarette smoke induction of osteopontin (SPP1) mediates T(H)17 inflammation in human and experimental emphysema. Science translational medicine. 2012;4(117):117ra9 Epub 2012/01/21. 10.1126/scitranslmed.3003041 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gowda NM, Wu X, Gowda DC. TLR9 and MyD88 are crucial for the development of protective immunity to malaria. J Immunol. 2012;188(10):5073–85. Epub 2012/04/21. 10.4049/jimmunol.1102143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. The Journal of biological chemistry. 1999;274(8):5053–60. Epub 1999/02/13. . [DOI] [PubMed] [Google Scholar]
- 40. Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. The EMBO journal. 1998;17(8):2215–23. Epub 1998/05/26. 10.1093/emboj/17.8.2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376(6535):37–43. Epub 1995/07/06. 10.1038/376037a0 . [DOI] [PubMed] [Google Scholar]
- 42. Cheng M, Sexl V, Sherr CJ, Roussel MF. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1). Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):1091–6. Epub 1998/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nilsson EM, Brokken LJ, Harkonen PL. Fibroblast growth factor 8 increases breast cancer cell growth by promoting cell cycle progression and by protecting against cell death. Experimental cell research. 2010;316(5):800–12. Epub 2009/12/08. 10.1016/j.yexcr.2009.11.019 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.