Abstract
Cortical atrophy and brain vascular disease are both associated with dementia, but there are only limited pathological data on the association of brain vascular disease with cortical atrophy. We studied pathological material from the Rush Memory and Aging Project (MAP, N = 445). Cortical and hippocampal atrophy, and atherosclerosis at the circle of Willis (large vessel disease, LVD) and arteriolosclerosis (small vessel disease, SVD) were rated by neuropathologists unaware of this study’s hypothesis. Quantitative measures of Alzheimer’s disease (AD) pathology, specifically neuronal neurofibrillary tangles (NFT) and amyloid–beta (Aβ) burden, were also obtained. Chronic micro and macroscopic infarcts were noted. In ordinal logistic regression models that included age at death, sex, apoE genotype, statin-use, Aβ and NFT, more severe LVD was significantly associated with more severe cortical and hippocampal atrophy. The odds ratio for the association of the most severe LVD (compared to the least) with cortical atrophy was 2.7 (CI: 1.5–4.7) p = 0.001; for hippocampal atrophy the odds ratio was 2.8 (CI: 1.5–5.2), p = 0.001. The association of SVD with atrophy did not follow a consistent pattern. Neither macroscopic infarcts nor microscopic infarcts were associated with cortical or hippocampal atrophy (p’s > 0.15). Tangle density was associated with cortical (p = 0.014) and hippocampal atrophy (p < 0.001). In contrast, amyloid burden was associated with less cortical (p = 0.02) or hippocampal (p = 0.002) atrophy. In this large autopsy study LVD was associated with cortical and hippocampal atrophy. The relationship between SVD and atrophy requires further study.
Keywords: Alzheimer’s disease, amyloid, brain aging, cerebrovascular disease, cortical atrophy, hippocampal atrophy
INTRODUCTION
Cortical atrophy is common in aging and in neurodegenerative disease [1–4], yet there is little known about the pathogenesis of the volume loss. Two obvious candidates to be driving forces underlying this volume loss are Alzheimer’s disease and vascular disease. Vascular disease often coexists with Alzheimer’s disease pathology in the aging brain yet, few previous pathological studies [5] have investigated the association of both AD and vascular markers with cortical and hippocampal atrophy. Jagust et al. [5] studied 93 demented subjects and found that composite measures of AD and of vascular disease, but not most measures of infarcts, were significantly negatively associated with gray matter volume. Their study suggests that brain vascular disease can lead to cortical atrophy by a mechanism that is independent of infarcts and of AD [6–7]. Because atrophy has been associated with dementia, albeit imperfectly, if the association between vessel disease and atrophy were confirmed in other post mortem studies, it could have important implications for the evaluation and treatment of dementia. For example, one could then hypothesize that treatment of small and/or large vessel disease might have beneficial effects on the risk and course of dementia and could lead to large studies investigating such a hypothesis.
The Rush Memory and Aging project provided data on 445 subjects with postmortem ratings of cortical atrophy and small and large vessel disease that we used to determine the associations between these markers.
MATERIALS AND METHODS
Cohort
Participants included 436 deceased and autopsied subjects from the Rush Memory and Aging Project (MAP) who had a complete data with all variables used in the analyses as of November 2012. Details of recruitment and participant evaluation for the MAP [8] have been described previously. MAP participants were from 40 retirement communities and senior subsidized housing residences across northeastern Illinois. Criteria for enrollment included no known dementia, signing an Anatomical Gift Act agreeing to brain donation, and willingness to undergo yearly medical, neurological, and neuropsychological evaluations. From November 1997 through November 2012, 1588 persons enrolled in the study and completed baselines. During this interval, 588 participants had died, autopsy rate was 79.1%.
Pathology
Brains were removed in a standardized manner, weighed (grams), and one hemisphere was immersion fixed in 4% paraformaldehyde for at least 72 hours; and the other frozen. Cerebral and cerebellar hemispheres were cut into 1 cm slabs and all slabs and the brainstem were digitally photographed. Both fixed brain and the photographs were reviewed by an expert neuropathologist to assess atrophy.
Measurement of cortical atrophy was based on width of the gyri and of sulcal spaces and rated as ‘0’ (none), ‘1’ (possible), ‘2’ (mild), ‘3’ (mild to moderate), ‘4’ (moderate), ‘5’ (moderate to severe), and ‘6’ (severe). Both hemispheres and all four lobes were visualized and an overall grade of atrophy was rendered by considering the severity of the narrowing of the width of the gyri and enlargement of sulci in all lobes. No direct quantitative measurements of gyri or sulci were performed for this study.
The distribution of cortical ratings was ‘0’ (0.2%), ‘1’ (1.3%), ‘2’ (21.5%), ‘3’ (18.5%), ‘4’ (34.3%), ‘5’ (17.1%), and ‘6’ (7%). For this analysis, and because there were few brains thought to have no atrophy or possible atrophy, the groups ‘0’ to ‘2’ groups were combined into a single group yielding 5 cortical atrophy groups from none/mild (0) to severe (4) for analysis.
Measurement of hippocampal atrophy was based on the size of the hippocampus itself and also rated as ‘0’ (none) to ‘6’ (severe). The score was based on visualization of the size of the hippocampus rather than enlargement of the temporal horn of the lateral ventricle. No quantitative measurements of hippocampi were performed for this study. The distribution of hippocampal ratings was ‘0’ (7.9%), ‘1’ (10.8%), ‘2’ (30.8%), ‘3’ (16.5%), ‘4’ (20.4%), ‘5’ (8.6%), and ‘6’ (4.2%). For analyses, the 0 to 2 groups were again combined into a single group yielding 5 hippocampal atrophy groups from none/mild (‘0’) to severe (‘4’) for analysis.
We assessed large vessel disease (atherosclerosis) semiquantitatively based on the number and extent of vascular involvement at the circle of Willis. The neuropathologist rated atherosclerosis on a scale of ‘0’ to ‘6’ (‘0’= none; ‘1’ = possible; ‘2’= minimal (one to a few yellow patches), ‘3’ = mild to moderate, ‘4’ = moderate (dispersed patches across several vessels or one vessel at least 50% affected), ‘5’ = moderate to severe, and ‘6’ = severe (almost all visualized vessels involved). The rating did not take into account the narrowing of the lumen. The distribution of large vessel ratings was ‘0’ (4%), ‘1’ (11.9%), ‘2’ (29.5%), ‘3’ (12.7%), ‘4’ (18.9%), ‘5’ (11.9%), and ‘6’ (11.0%). For analyses, the 7 groups were collapsed to 4 groups by combining the ‘0’ and ‘1’ ratings, the ‘3’ and ‘4’ ratings, and the ‘5’ and ‘6’ ratings and retaining the ‘2’ ratings. This yielded 4 groups rated from none/mild (‘0’) to severe (‘3’).
Small vessel disease refers to arteriolosclerosis and is semiquantitatively assessed based on the severity of wall thickening and luminal occlusion of the small arterioles in one 6 micron section of anterior basal ganglia reviewed on hematoxylin and eosin (H&E) stain; and rated on a 7 point scale (‘0’ = none, ‘1’= mild, ‘2’ = mild to moderate, ‘3’= moderate, ‘4’ = moderate to severe, ‘5’ = severe, and ‘6’ = vascular occlusion; and converted to a 4 point scale for analyses with ‘0’ = none, ‘1’ = minimal; ‘2’ = moderate, and ‘3’=severe [9]. The distribution of small vessel ratings was ‘0’ (22.4%), ‘1’ (35.6%), ‘2’ (30.8%), and 3 (10.8%).
Methods of rating microinfarcts and macroinfarcts have been described previously [10]. Macroscopic infarcts are visualized on slabs of tissue and confirmed on six μ-thick sections stained with H&E. To assess microinfarcts, we used 6μ-thick paraffin-embedded sections from at least 9 regions from one hemisphere including 6 cortical regions, anterior basal ganglia, thalamus, and midbrain. Criteria for chronic microinfarcts included cavitated or incomplete infarcts with fibrillary gliosis and few remaining macrophages. Only chronic infarcts were used in these analyses, as acute or subacute infarcts may be related to increased tissue volume secondary to edema and are unlikely to be related to chronic atrophic changes.
The cortical regions used to assess plaques and tangles included measurements in the same regions assessed for atrophy. Molecularly specific indicators of AD pathology, specifically paired helical filament (PHF)-tau immunoreactive NFT density and Aβ load were assessed as previously described [11]. Briefly, 20μ-thick paraffin-embedded sections entorhinal cortex, CA1/subiculum, anterior cingulate cortex, dorsolateral prefrontal cortex, superior frontal cortex, inferior temporal cortex, inferior parietal cortex, primary visual cortex were immunostained with antibodies to PHF-tau antibody clone AT8 (ThermoScientific, Rockford, IL USA; 1:2000) and Amyloid-β was labeled with an N-terminus-directed monoclonal antibody (10D5; Elan, Dublin, Ireland; 1;1,000).
We used computer assisted sampling to estimate density of tau-immunoreactive neurofibrillary in each cortical region An automated, multistage computational image analysis protocol was used to determine Aβ load. Values were averaged across regions to obtain a composite measures as previously described (11). Methods of apoE genotyping and statin-use determination have been described previously [12–13].
Statistics
We first compared atrophy among the vascular groups with ANOVA. Post hoc comparisons comparing different levels were made with the least significant difference test. When comparing continuous variables and 2 groups, 2-sample t-tests were used. We compared distributions of categorical variables with the Chi-Square test. We assessed the relationship between pathological variables with Pearson correlation controlling for age and sex.
We assessed the relationship between small vessel or large vessel disease with atrophy with ordinal logistic regression models. In all models, amyloid, tangles, age at death, sex, apoE genotype, and statin-use were included as cofactors and covariates. We constructed models for large vessel disease without including small vessel disease, for small vessel disease without large vessel disease.
For the ordinal regression analyses, we report the odds ratio that someone with a specific rating of vascular pathology (compared to someone with the least pathology) would have a higher atrophy score. The odds ratios remain the same at every value of the atrophy ratings. For example, in Table 3a, in the 3rd row, right-most column, we report that the odds ratio for someone with worst large vessel pathology compared to someone with the least for predicting cortical atrophy is 2.66. This means that the odds that a participant with the worst large vessel pathology has cortical atrophy of 3 or more is 2.66 times the odds for participants with the least large vessel pathology. Because the odds ratio remains the same at each value of the atrophy scale, someone with the worst large vessel pathology would also be 2.64 times more likely to have a score of 4 than a score of 3 or lower as someone with the least vascular pathology.
Table 3.
Maximum Likelihood Estimates of the association of large vessel disease and other pathological markers with (a) cortical atrophy and (b) hippocampal atrophy (N =444).
| (a) Cortical Atrophy (Nagelkerke pseudo R2 = 0.064) | |||
|---|---|---|---|
| Estimate (SE) | Significance | OR and CI | |
| Tangles | 0.03 (0.01) | 0.010 | 1.03 (1.01 – 1.06) |
| Amyloid | −0.05 (0.02) | 0.013 | 0.95(0.92–0.99) |
| Worst large vessel disease v least | 0.98 (0.29) | 0.001 | 2.66 (1.51–4.71) |
| 2nd worst v least | 0.57 (0.27) | 0.035 | 1.77 (1.04–3.0) |
| 3rd worst v least | 0.42 (0.27) | 0.12 | 1.52 (0.90 – 2.6) |
| Male sex | −0.17 (0.18) | 0.35 | 1.19 (0.83–1.68) |
| apoE4 genotype | 0.032 (0.22) | .88 | 1.03 (0.68–1.58) |
| Statin use | 0.004 (0.18) | 0.98 | 1.00 (0.71–1.43) |
| (b) Hippocampal atrophy (Nalgelkerke pseudo R2 = 0.113) | |||
|---|---|---|---|
| Estimate (SE) | Significance | OR and CI | |
| Tangles | 0.061 (0.01) | < 0.0001 | 1.06 (1.04–1.09) |
| Amyloid | −0.068 (0.02) | 0.002 | 0.93 (0.90 – 0.98) |
| Worst large vessel disease v least | 1.03 (0.32) | .001 | 2.80 (1.51–5.24) |
| 2nd worst v least | 0.63 (0.30) | .038 | 1.87 (1.04–3.39) |
| 3rd worst v least | 0.55 (0.30) | 0.072 | 1.73 (0.95–3.14) |
| Male Sex | 0.34 (0.19) | 0.075 | 1.40 (0.97–2.03) |
| apoE4 genotype | 0.19 (0.23) | 0.41 | 1.21 (0.77–1.9) |
| Statin use | −.26 (0.19) | .18 | 0.77 (0.53–1.13) |
In Table 3a, row 2, the right-most column, the odds ratio of 1.03 for tangles means that for every unit increase in the tangle value, there is a 1.03 times increase in the odds that they will have a score of 3 rather than a score of 2 or lower.
We used the Nagelkerke pseudo R2 as an estimate of the amount of variance explained by each model. Significance was set at p < 0.05.
RESULTS
Demographic and pathological characteristics of study subjects are shown in Table 1. The mean age at death was 1.5 years lower among participants with the apo E4 genotype than among those without any E4 alleles. Amyloid load and NFT levels were significantly higher in participants with an apoE4 allele than among those without an E4 allele.
Table 1.
Demographics and pathological characteristics of study subjects.
| No Apo E4 (N = 338) | Any apo E4 (Includes 5 Homozygotes) (N = 107) | p-Values | |
|---|---|---|---|
| Age at death | 89.2 (6.2) | 87.8 (5.3) | 0.03 |
| Male sex | 118 (34.9%) | 41 (38.3%) | 0.56 |
| Currently taking statin | 126 (37.7%) | 47 (43.9%) | 0.26 |
| Education (years) | 14.4 (2.8) | 14.4 (3.0) | 0.88 |
| Cortical atrophy | 1.67 (1.22) | 1.73 (1.15) | 0.68 |
| Hippocampal atrophy | 1.00 (1.18) | 1.18 (1.27) | 0.13 |
| Amyloid | 4.24 (4.74) | 7.13 (4.62) | < 0.001 |
| NFTs | 4.87 (5.57) | 10.03 (10.84) | 0.001 |
| Large vessel disease | 1.63 (1.01) | 1.60 (1.01) | 0.79 |
| Small vessel disease | 1.27(0.94) | 1.40 (0.95) | 0.20 |
Values represent means and standard deviations (except for the number of men and the number using a statin). NFTs = neurofibrillary tangles.
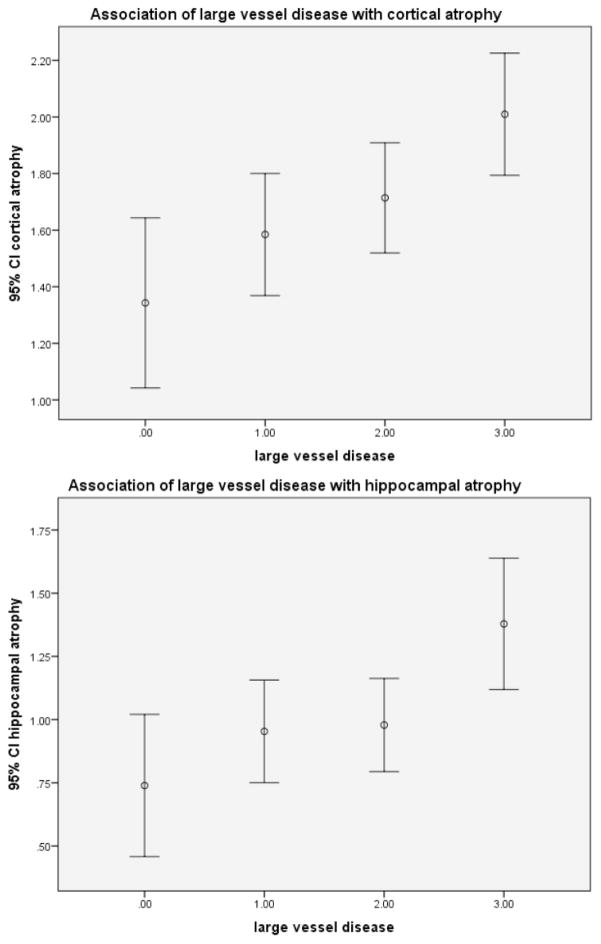
Univariate relationships between large vessel disease and cortical and hippocampal atrophy are shown in (Figs. 1a and b). Large vessel disease was associated with both cortical atrophy (F (3,440) = 4.8, p = 0.003) and hippocampal atrophy (F (3,437)= 4.6, p = 0.003). Post hoc analyses showed that the group with the least severe large vessel disease had less atrophy than the group with the most severe disease (p = 0.001). Similarly, the group with the least severe large vessel disease had less hippocampal atrophy than the group with the most severe disease (p = 0.001).
Fig. 1.
Association of large vessel disease with cortical atrophy (top) or with hippocampal atrophy (bottom). (1a) (top). F(3,440) = 4.8, p = 0.003; post hoc comparisons: 0 v 1, p = .17; 0 v 2, p = 0.034; 0 v 3, p = 0.001. (1b) (bottom). F (3,437) = 4.6, p = 0.003, post hoc comparisons: 0 v 1, 0.23; 0 v 2, 0.17; 0 v 3, 0.001).
Because these results may be influenced by age, sex, apoE genotype, and statin-use, we extended these analyses with ordinal logistic models controlling for these potential confounders. In these analyses, large vessel disease was associated with both cortical and hippocampal atrophy (Table 3). For both cortical and hippocampal atrophy, there was a monotonic significant relationship between the severity of large vessel disease and the degree of atrophy.
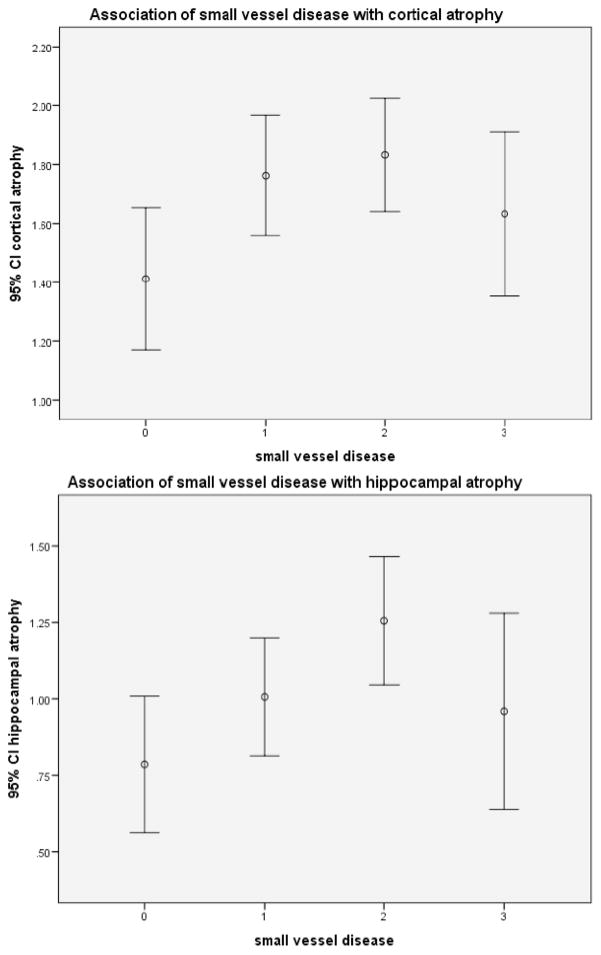
Univariate analyses of the association of small vessel disease with atrophy are shown in (Figs. 2a and 2b). For cortical atrophy, the association was of marginal significance (F(3,440) = 2.7, p = 0.045), The association of small vessel disease with hippocampal atrophy was also significant (F (3,436) = 3.1, p = .03). Post hoc analyses showed that for cortical atrophy, there was no significant difference between the group with the least and the most small vessel disease (p = 0.30). However, significant differences were noted when comparing the other groups. For hippocampal atrophy, the only significant association was between the group with the least SVD and the group with the moderate SVD (i.e. 2nd worst SVD) (p = 0.003). However, there was no significant difference in hippocampal atrophy between the group with the least SVD and the group with the most SVD (p = 0.41).
Fig. 2.
Association of small vessel disease with cortical atrophy (top) or with hippocampal atrophy (bottom). (2a) (top). There was significant association between small vessel disease and cortical atrophy. F (3,440) = 2.7, p = 0.045; post hoc comparisons: 0 v 1, 0.022; 0 v 2, 0.008; 0 v 3, p = 0.290). (2b) (bottom). There was significant association between small vessel disease and hippocampal atrophy. F (3,436) = 3.1, p = 0.028; post hoc comparisons: were 0 v 1, 0.15; 0 v 2, 0.003; 0 v 3, 0.41).
For both cortical and hippocampal atrophy, there appears to be an inverse ‘J-shaped” relationship between small vessel disease and atrophy. For the first 3 small vessel groups, the relationship appears consistent with most severe disease associated with most severe atrophy. However, the group with most severe small vessel disease had less atrophy than the group with moderate small vessel disease.
We also used ordinal logistic models controlling for possible confounders and using the group with the least small vessel disease as the reference. In these analyses, the groups with the 3rd and 2nd worst SVD had more severe cortical atrophy (both 0.02). However, the group with the most severe SVD did not have more atrophy than the group with the least SVD (p= 0.34). A similar pattern was seen for the association of SVD with hippocampal atrophy. The only significant comparison was between the group with the least severe SVD and the group with the 2nd most severe SVD (p= 0.02) (Table 4). In all multivariate models, the amount of variance in cortical or hippocampal atrophy score explained was modest - ranging from 5 to 11%. Vessel disease is related to increased risk of infarcts, therefore we did additional analyses to determine whether infarcts were related to measures of atrophy. Using ordinal regression models adjusted for age, sex, tangles, and amyloid, neither micro or macroscopic infarcts were significantly associated with either cortical or hippocampal atrophy.
Table 4.
Maximum Likelihood Estimates of the association of small vessel vessel disease and other pathological markers with (a) cortical atrophy (top) and (b) hippocampal atrophy (bottom). (N = 444) Age, sex, E4 genotype, and statin-use included in all models.
| (a) Cortical Atrophy (Nagelkerke pseudo R2 = 0.52) | |||
|---|---|---|---|
| Estimate (SE) | Significance | OR and CI | |
| Tangles | 0.03 (0.01) | 0.009 | 1.03 (1.01–1.06) |
| Amyloid | −0.05 (0.02) | 0.007 | 0.95 (0.91 – 0.99) |
| Worst small vessel disease v least | 0.31 (0.32) | 0.34 | 1.34 (0.71–2.53) |
| 2nd worst v least | 0.55 (0.24) | 0.023 | 1.71 (1.07–2.75) |
| 3rd worst v least | 0.54 (0.23) | 0.02 | 1.70 (1.08 – 2.68) |
| Male Sex | −.125 (0.18) | 0.49 | 0.88 (0.62–1.26) |
| apoE4 genotype | 0 | 0.90 | 1 (0.68–1.59) |
| Statin use | −.048 (0.18) | .79 | 0.95 (0.67–1.26) |
| (b) Hippocampal Atrophy (Nagelkerke pseudo R2 = 0.102) | |||
|---|---|---|---|
| Estimate (SE) | Significance | OR and CI | |
| Tangles | 0.06 (0.01) | <0.001 | 1.07 (1.04–1.10) |
| Amyloid | −0.07 (0.02) | 0.001 | 0.93 (0.89–0.97) |
| Worst small vessel disease v least | 0.22 (0.35) | 0.52 | 1.25 (0.63–2.46) |
| 2nd worst v least | 0.62 (0.26) | 0.02 | 1.85 (1.11–3.1) |
| 3rd worst v least | 0.33 (0.25) | 0.19 | 1.39 (0.85–2.28) |
| Male Sex | 0.38 (0.19) | .044 | 1.47 (1.01–2.25) |
| apoE4 genotype | .165 (0.23) | 0.47 | 1.18 (0.76–1.84) |
| Statin use | −.28 (.19) | 0.14 | 0.76 (0.52–1.10) |
As shown in Tables 2, 3, and 4, in both univariate and multivariate analyses, NFTs were significantly positively associated with cortical and with hippocampal atrophy; that is more tangles were associated with worse atrophy. The association was stronger for hippocampal atrophy than for cortical atrophy. On the other hand, the reverse relationship was noted for amyloid – more amyloid was associated with less atrophy. In univariate analyses this association was significant for cortical, but not for hippocampal atrophy. However, in multivariate analyses, the association was significant for both cortical and hippocampal atrophy.
Table 2.
Association of Alzheimer-disease variables with atrophy.
| Cortical Atrophy | Hippocampal Atrophy | Amyloid | Tangles | |
|---|---|---|---|---|
| cortical atrophy | 1 | 0.373, p < 0.001 | −0.112, p = 0.019 | 0.101, p = 0.034 |
| hippocampal atrophy | 0.373, p < .001 | 1 | −0.070, p = 0.141 | 0.205, P < 0.001 |
| Amyloid | −0.112, p = 0.019 | −0.070, p = 0.141 | 1 | 0.305 p < 0.001 |
Values represent partial correlation coefficients (controlled for sex and age at death). (N = 445)
DISCUSSION
In a large cohort of prospectively studied elderly individuals we found that large vessel disease was significantly associated with both cortical and hippocampal atrophy. While small vessel disease was associated with hippocampal atrophy and marginally associated with neocortical atrophy, the results were inconsistent across severity ranges, and did not show the expected dose-response profile. We also found a relationship with AD pathology, but here to the relationship was complex, with tangles showing the expected positive relationship, but amyloid showing an inverse relationship, with higher amyloid loads related to less atrophy.
Although cortical atrophy has been shown in multiple studies to be independently related to cognitive impairment and dementia [1, 14, 15], we are aware of only one previous pathological study that compared the effects of both AD and vascular markers on cortical atrophy [5]. Jagust et al. [5] used postmortem MRI to quantify the extent of atrophy in 93 brains and found that markers of composite measures of brain vascular disease and AD both were significantly associated with cortical atrophy. In contrast to these few pathological studies, there is an extensive literature showing that antemortem measures of cardiovascular disease are associated with gray matter volume as determined using MRI (e.g. [6–7]).
Large vessel disease but not microscopic infarcts nor large vessel infarcts were associated with cortical atrophy. One interpretation of this finding is that some mechanism other than infarcts “drives” the association between vascular disease and atrophy. Possible mechanisms, include intermittent hypoxia and/or transient ischemia with energy failure particularly at times of poor cardiac output and maximal brain metabolism, leading either directly or indirectly (via dendritic pruning or a dying back process) to neuronal loss. Animal and tissue culture models may be able to test this hypothesis. If this hypothesis is supported by empirical models, it will have important implications for the prevention and treatment of dementia.
While the ordinal models suggested a relationship of SVD with both cortical and hippocampal atrophy, the data seemed to follow an inverse ‘J’ shaped pattern. We are not aware of a biological explanation of why the group with the most severe SVD should have less atrophy than the group with moderate SVD. The group with severe small vessel disease was the smallest of the vascular groups consisting of 10.8% of study subjects, raising the possibility that we were underpowered to explore the relationship of atrophy an SVD through those with the most severe SVD. Further studies will need to be done to more fully explore this relationship.
We found a complex relationship between AD pathology and atrophy with severity of amyloid load having an inverse association, and higher tangle density having a positive association with more severe atrophy. In contrast, in a previous study, our group [16] did not find a significant relationship between a global, semi-quantitative measure of AD pathology and cortical atrophy when studying a subset of the patients described in this current report. However, in that study, AD pathology was used a composite and amyloid and tangles were not evaluated separately. Similar reports of no association or a negative association with more amyloid burden associated with less atrophy have been reported previously [17–18]. Using structural MRI and [11 C] compound B (PiB) positron emission tomography, Chetelat et al. [17] found a positive association between PiB standardized uptake value ratio and global gray matter volume among participants with AD. Patients with MCI and controls had no significant association between gray matter volume and PiB uptake. Only among the participants with subjective cognitive complaints and normal cognitive testing was a significant negative correlation found between PiB uptake and gray matter volume. Similarly, La Joie et al. [18] showed that Aβ load was not consistently associated with either hypometabolism or atrophy. In the only other report that looked at the association between both vascular and AD markers, Jagust et al. [5] found that the vascular measure and the AD measure were both significantly associated with atrophy. However, the AD pathology marker in the Jagust et al. paper [5] was a composite measure that combined tangle and amyloid measures. Finally, a surprising finding of the first Aβ immunization trial was that antibody responders with presumably less amyloid in the brain had more atrophy than non-responders [19].
The major strength of our study is the large number brains from community-dwelling older subjects with semiquantitatively assessed atrophy and both small and large vessel disease with a large range of severity. Nonetheless, quantitative measures of both atrophy and vascular disease would strengthen this study. In addition, to the lack of quantification our measure of cortical atrophy is not region specific. Certainly, one might hypothesize if vascular disease is “driving” the atrophic process, that atrophy would be worse in frontal regions than in occipital regions. Bakkour et al. [20] suggested that pathological processes associated with atrophy may be region specific, with AD processes associated with temporal and parietal lobe and ‘normal’ aging associated with frontal atrophy. Quantitative measures of regional gray volume as well as volumes of both normal and abnormal white matter would help address this shortcoming.
Another limitation is that we only assessed intracranial atherosclerosis in the circle of Willis and in the lenticulostriates. However, vascular disease is heterogeneous with some subjects having largely extracranial vascular disease others largely intracranial disease, and thus assessment with the circle of Willis alone may not capture some, more proximal or pial branch large vessel pathology. Similarly, small vessel disease may differ between lenticulostriates (where measurements were made) and long penetrating medullary arteries. The modest amount of variance in atrophy explained by our models may be accounted for in part by the above shortcomings.
Other valuable data that would inform further on the mechanisms by which vessel disease and AD pathology are associated with atrophy would include neuron number, synapse number, fullness of the dendritic arbor, and measures of gliosis in specific atrophic areas.
CONCLUSION
In summary, the data presented in this report demonstrate that large vessel disease and tangles are independently associated with cortical and hippocampal atrophy in older persons. On the other hand, amyloid appears to be associated with preservation of hippocampal and cortical volume. The association of small vessel disease with atrophy is suggestive but requires further study. These observations suggest the need for a reformulation of concepts on hippocampal and cortical atrophy.
Acknowledgments
Supported by grants R21MH09639002, P30AG10161, R01AG15819, R01AG17917, R01HL09644, and R01AG040039.
Footnotes
Send orders for Reprints to reprints@benthamscience.net
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
AUTHOR CONTRIBUTIONS
HAC : Study hypothesis, analysis, drafting and completion of the manuscript
JS : Data collection, review of the drafts
SEL : Data analyses, review of the drafts
DB : Overall responsibility for creating and maintaining study cohort, review of the draft
SL : Review of analysis plans and of the drafts
References
- 1.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360(22):2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 2.Gelber RP, Launer LJ, White LR. The Honolulu-Asia Aging Study: epidemiologic and neuropathologic research on cognitive impairment. Curr Alzheimer Res. 2012;9(6):664–72. doi: 10.2174/156720512801322618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erten-Lyons D, Dodge HH, Woltjer R, Silbert LC, Howieson DB, Kramer P, et al. Neuropathologic Basis of Age-Associated Brain Atrophy. JAMA Neurol. 2013;18:1–7. doi: 10.1001/jamaneurol.2013.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol. 2011;70(5):774–80. doi: 10.1002/ana.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63(1):72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erbay S, Han R, Aftab M, Zou KH, Polak JF, Bhadelia RA. Is intracranial atherosclerosis an independent risk factor for cerebral atrophy? A retrospective evaluation. BMC Neurol. 2008;8:51. doi: 10.1186/1471-2377-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raji CA, Lopez OL, Kuller LH, Carmichael OT, Longstreth WT, Jr, Gach HM, et al. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging. 2012;33(4):834 e7–16. doi: 10.1016/j.neurobiolaging.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–75. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 9.Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA. Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke. 2011;42(11):3183–9. doi: 10.1161/STROKEAHA.111.623462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42(3):722–7. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol. 2012;72(4):599–609. doi: 10.1002/ana.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider JA, Gearing M, Robbins RS, de l’Aune W, Mirra SS. Apolipoprotein E genotype in diverse neurodegenerative disorders. Journal: Annals of neurology. 1995 Jul;38(1):131–5. doi: 10.1002/ana.410380122. [DOI] [PubMed] [Google Scholar]
- 13.Arvanitakis Z, Schneider JA, Wilson RA, Bienias JL, Kelly JF, Evans DA, Bennett DA. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology. 2008;70:1795–1802. doi: 10.1212/01.wnl.0000288181.00826.63. [DOI] [PubMed] [Google Scholar]
- 14.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68(2):231–40. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, O’Brien RJ. Age, Alzheimer’s disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain. 2010;133(Pt 8):2225–31. doi: 10.1093/brain/awq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honer WG, Barr AM, Sawada K, Thornton AE, Morris MC, Leurgans SE, et al. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry. 2012;2:e114. doi: 10.1038/tp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chetelat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67(3):317–24. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- 18.La Joie R, Perrotin A, Barre L, Hommet C, Mezenge F, Ibazizene M, et al. Region-specific hierarchy between atrophy, hypometabolism, and beta-amyloid (Abeta) load in Alzheimer’s disease dementia. J Neurosci. 2012;32(46):16265–73. doi: 10.1523/JNEUROSCI.2170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64(9):1563–72. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 20.Bakkour A, Morris JC, Wolk DA, Dickerson BC. The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: Specificity and differential relationships with cognition. Neuroimage. 2013;76:332–44. doi: 10.1016/j.neuroimage.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]