Abstract
Our previous studies suggested that arsenic is able to induce serine 21 phosphorylation of the EZH2 protein through activation of JNK, STAT3, and Akt signaling pathways in the bronchial epithelial cell line, BEAS-2B. In the present report, we further demonstrated that reactive oxygen species (ROS) were involved in the arsenic-induced protein kinase activation that leads to EZH2 phosphorylation. Several lines of evidence supported this notion. First, the pretreatment of the cells with N-acetyl-l-cysteine (NAC), a potent antioxidant, abolishes arsenic-induced EZH2 phosphorylation along with the inhibition of JNK, STAT3, and Akt. Second, H2O2, the most important form of ROS in the cells in response to extracellular stress signals, can induce phosphorylation of the EZH2 protein and the activation of JNK, STAT3, and Akt. By ectopic expression of the myc-tagged EZH2, we additionally identified direct interaction and phosphorylation of the EZH2 protein by Akt in response to arsenic and H2O2. Furthermore, both arsenic and H2O2 were able to induce the translocation of ectopically expressed or endogenous EZH2 from nucleus to cytoplasm. In summary, the data presented in this report indicate that oxidative stress due to ROS generation plays an important role in the arsenic-induced EZH2 phosphorylation.
Keywords: Arsenic, Oxidative stress, EZH2, Akt, Phosphorylation, Cytoplasmic translocation
Introduction
Arsenic is an environmental and carcinogenic metalloid found in water, earth crust, food, plants, and some other natural depositions (Nordstrom, 2002). Certain industrial settings can also release arsenic into the environment through the industry activities, such as mining, mineral refining, pesticide manufacturing, and wood preservation (Garelick et al., 2008). In general, the organic form of arsenic that is compounded with carbon and found in certain seafood or plant, is considered as less toxic. In contrast, the inorganic form of arsenic that is compounded with sulfur and chloride and, among others, is highly toxic. The International Agency for Research on Cancer (IARC) classifies inorganic arsenic and its compounds as group I carcinogens (IARC, 1987). Well-documented evidence showed that chronic exposure to arsenic increased the risks of cancer development, including cancers in lung, skin, bladder, kidney, and liver (Ren et al., 2011). Currently, more than 137 million people are exposed to arsenic in ground water worldwide.
A number of reports revealed that inorganic arsenic, especially the trivalent arsenic (As3+), may induce excessive generation of the reactive oxygen species (ROS) in mammalian cells (Chou et al., 2004; Hei et al., 1998; Wang et al., 2013; Zhang et al., 2011). ROS were able to cause genetic mutation and cancer through the mechanism of DNA damage, activation of the oncogenic kinases (Wang et al., 2012), or oxidation of the important lipids and proteins to inactivate DNA repairing machineries, such as poly(ADP ribose) polymerase (PARP)-1 (Hubaux et al., 2012; Wang et al., 2013). There are several cellular sources of ROS induced by As3+. First, As3+ can induce the release of redox active iron from ferritin to catalyzes the Haber–Weiss Fenton reaction, leading to the generation of the hydroxyl radical (Ahmad et al., 2000). Second, As3+ has been viewed as a potent activator of the NADPH oxidase that is bound to the cell membrane for the release of superoxide anion (Chou et al., 2004). Third, As3+ may alter the membrane potential of the mitochondria and the subsequent increased generation of ROS from mitochondria (Woo et al., 2002). Lastly, the metabolism of As3+ to pentavalent arsenic involves the reduction of H2O, which generates H2O2, and some metabolic intermediates, such as dimethylarsine peroxyl radical [(CH3)2AsOO•] (Valko et al., 2006; Yamanaka et al., 2001).
Enhancer of Zeste homolog 2 (EZH2) is an enzymatic subunit of the PRC2 complex responsible for the tri-methylation of lysine 27 on histone H3 (H3K27me3) (Hock, 2012). Emerging evidence suggested that many types of cancers exhibited increased expression or activity of EZH2 that represses the expression of tumor suppressors and tumor-suppressive microRNAs, such as, ARF, p57KIP2, FBXO32, p27, BRCA1, and miR-29 (Zhang et al., 2012). In our previous study, we have discovered that As3+ is capable of inducing serine 21 phosphorylation of the EZH2 protein (pEZH2) and provided evidence indicating the importance of the upstream protein kinase pathways in this bioprocess (Chen et al., 2013). The present study is to delineate whether ROS are the key mediators for As3+-induced pEZH2 phosphorylation.
Materials and methods
Cell lines and reagents
The human bronchial epithelial cell line BEAS-2B was obtained from the American Type Culture Collection (ATCC, Manassas, VA). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Grand Island, NY) containing 5% fetal bovine serum (FBS, Invitrogen), 1% penicillin, and 1%l-glutamine (Sigma) at 37 °C humidified incubator with 5% CO2. The human embryonic kidney 293 cell line was purchased from ATCC and maintained in DMEM containing 10% FBS, 1% penicillin, and 1% l-glutamine at 37 °C humidified incubator with 5% CO2. The non-small cell lung cancer cell line A549 was from ATCC and cultured in RPMI-1640 medium supplemented with 10% FBS, 1% penicillin, and 1% l-glutamine (Sigma). The cells were seeded in 6-well plates. When the cells reached 70%–80% confluency, hydrogen peroxide (Sigma), As3+, or NAC (N-acetyl-L-cysteine, Sigma) were added for the indicated concentrations and times.
Plasmid preparation and transfection
pGFP-EZH2-WTand p3myc-EZH2-WT are gifts from Dr. Mien-Chie Hung (Department of Molecular and Cellular Oncology, University of Texas). The plasmid DNA was amplified from DH5α competent cells (Invitrogen) and purified using Plasmid Purification Kit (QIAGEN) according to the manufacturer's protocol. BEAS-2B or HEK-293 cells in 6-well plates were transfected with 2 µg plasmic DNA using Lipofectamine 2000 Transfection Reagent (Invitrogen) or Nucleofector (Lonza) with program T020. The cells were then cultured for 24 h followed by the treatment of the cells with As3+ or H2O2 for the indicated times.
Cellular fractionation and Western blotting
For nuclear and cytoplasmic fractionations, a NE-PER nuclear and cytoplasmic fractionation kit (Thermo Scientific, Rockford IL) was used according to the manufacturer's protocol. Fractions were then analyzed by regular Western blotting using GAPDH and lamin A/C as cytoplamic and nuclear reference, respectively. For regular Western blotting, the cell lysates were prepared by RIPA cell lysis buffer (Cell Signalling) followed by ultrasonication and centrifugation. The supernatants were aspirated and proteins were quantified using a SpectraMax spectrophotometer (MDA Analytical Technologies). LDS sample buffer (Invitrogen) and dithiothreitol were added before denature. The samples were separated on 7.5% or 10% SDS–PAGE running gel and then transferred to methanol wetted PVDF membranes. The membranes were blocked in 5% non-fat milk in TBST and incubated with the indicated primary antibodies at 4 °C overnight and then incubated with second antibodies at room temperature for 1 hour and washed with TBST 3 times for 10 min each. The protein levels were detected using CDP-Star Reagent (New England Biolabs). The primary antibodies used in Western blotting include anti-phopho-EZH2 (Ser21) (Abcam), anti-phospho-JNK (Cell Signaling), anti-phospho-Akt(Ser473) (Cell Signaling), anti-phospho-Stat3(Ser 727) (Cell Signaling), anti-phospho-p38 (Cell Signaling), anti-phopho-ERK (Cell Signaling), anti-EZH2 (Cell Signaling), anti- JNK (Cell Signaling), anti-Stat3 (Cell Signaling), anti-p38 (Cell Signaling), anti-ERK (Cell Signaling), anti-GFP (Santa Cruz), anti-phospho-Akt substrate RXRXXS*/T* (Cell Signaling), anti-GAPDH (Cell Signaling), and anti-β-actin (Sigma).
Immunoprecipitation (IP) and fluorescent microscopy
After the indicated treatments, the cells were collected in IP lysis buffer and fragmented through passing the cells in a syringe equipped with 21.5 needle for 10–15 times and then incubating with the specific antibodies or IgG that were conjugated with agarose beads at 4 °C agitation overnight. For fluorescent microscopy, the cells were fixed by 4% paraformaldehyde at room temperature for 15 min. DNA was stained with 1 µg/ml of DAPI.
Statistics
Microsoft Excel was used for statistical analyses of the quantitative data. The data are expressed as the mean ± standard deviation (SD), and Student's t-tests were used to determine the statistical significance of differences between samples treated under different conditions. Differences were considered statistically significant when *p < 0.05 or **p < 0.01.
Result
As3+-induced EZH2 phosphorylation is ROS dependent in BEAS-2B cells
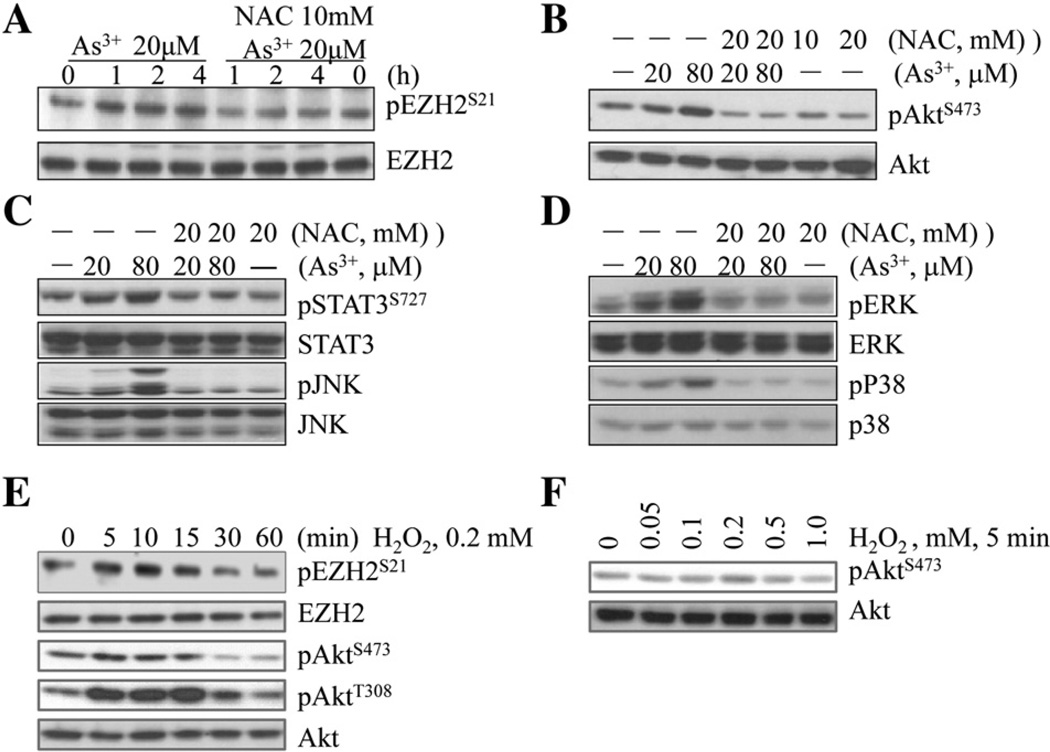
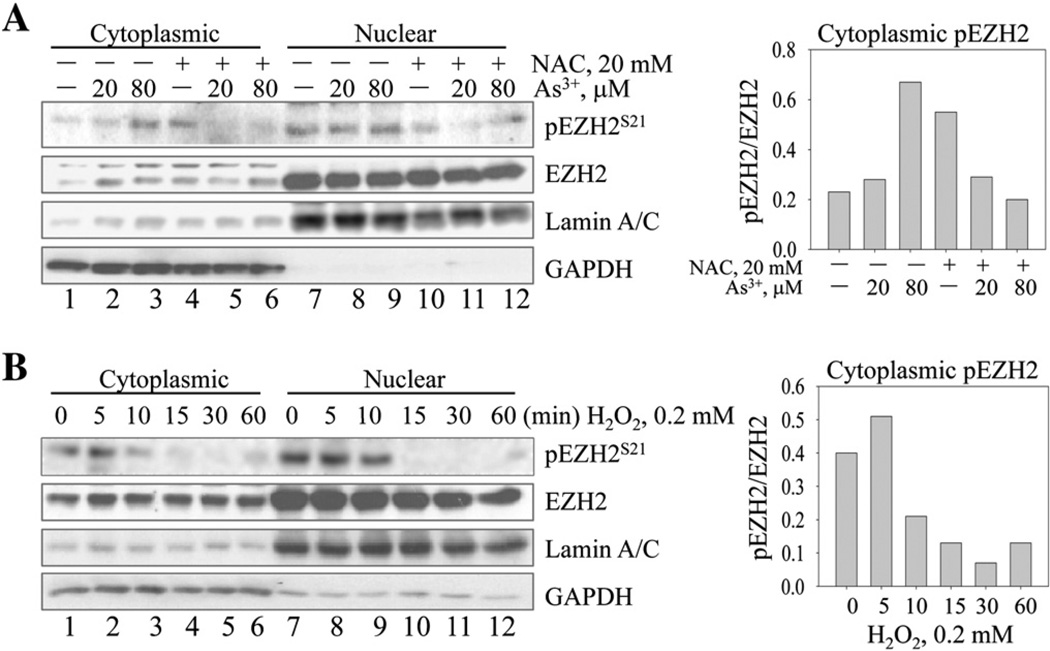
In agreement with our previous report, we observed that As3+ is able to induce serine 21 (S21) phosphorylation of the EZH2 in human bronchial epithelial cell line, BESA-2B cells (Fig. 1A). To determine the involvement of oxidative stress resulted from ROS generation in this As3+-induced EZH2 phosphorylation, we pre-treated the cells with 20 mM N-acetyl-l-cysteine (NAC), a general antioxidant that provides the cells with sufficient amount of glutathione to minimize the oxidation of cellular proteins, lipids, and DNA (Sadowska et al., 2006), for 2 h and then treated the cells with 20 µM As3+ for 1,2, or 4 h. A significant reduction in EZH2 phosphorylation was noted in the cells treated with NAC (Fig. 1A). NAC is also capable of inhibiting As3+-induced activation of Akt, STAT3, and JNK (Fig. 2B and C), the upstream kinases associated with the S21 phosphorylation of the EZH2. In addition, NAC is also potent in diminishing the As3+-induced activation of ERK and p38 (Fig. 1D), two mitogen-activated protein kinases that respond to the oxidative stress. To validate the contribution of ROS in As3+-induced S21 phosphorylation of the EZH2, we also tested the capability of H2O2, one of the most abundant and important ROS, on EZH2 phosphorylation and kinase activation. Indeed, an earlier occurrence of S21 phosphorylation of the EZH2was noted in the cells treated with 0.2 m MH2O2 for 5 to 15 min (Fig. 1E), which correlates with the time-dependent activation of Akt and the dose-dependent Akt activation in the cells treated with different concentrations of H2O2 for 5 min (Figs. 1E and 1F).
Fig. 1.
Involvement of oxidative stress in As3+-induced kinase activation and EZH2 phosphorylation in BEAS-2B cells. (A) BEAS-2B cells were treated with 20 µM As3+ for 0, 1, 2, or 4 h with or without NAC pretreatment for 2 h. S21 phosphorylation of the EZH2 (pEZH2S21) protein was determined by Western blotting. The most right lanes (0 h As3+) are NAC only treatment. (B–D) The activation of Akt (B), STAT3 and JNK (C), and Erk and p38 (D) were determined in the BEAS-2B cells treated with 20 or 80 µM As3+ for 2 h in the presence or absence of NAC pretreatment for 2 h. In each panel, the most right lanes (without As3+, —) are NAC only groups. (E) H2O2 induces pEZH2S21 and Akt activation in a time-dependent manner. (F) Dose-dependent activation of Akt kinase in the BEAS-2B cells treated with the indicated concentrations of H2O2 for 5 min.
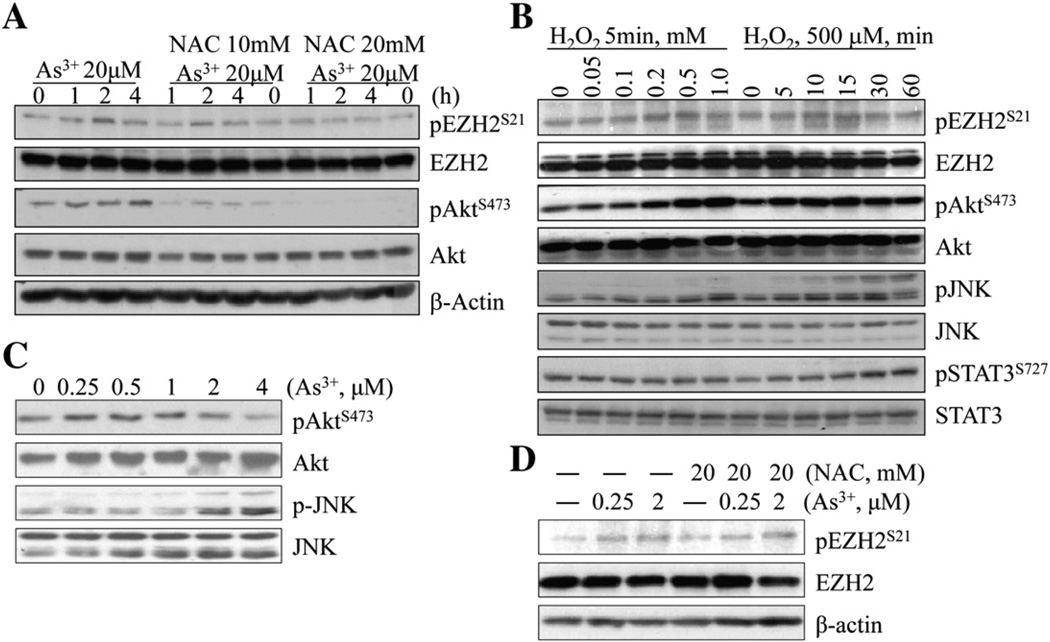
Fig. 2.
Oxidative stress contributes to As3+-induced EZH2 phosphorylation and kinase activation in A549 cells. (A) A549 cells were treated with 20 µM As3+ for the indicated time with or without NAC pretreatment for 2 h. The levels of pEZH2S21 and Akt activation were determined by Western blotting. The NAC only groups were indicated as 0 h As3+ treatment. (B) Dose- and time-dependent EZH2 phosphorylation and kinases activation in A549 cells treated with H2O2. (C) Lower concentrations of As3+ induce JNK but not Akt activation in the A549 cells treated with As3+ for 72 h. (D) NAC was unable to inhibit lower concentration As3+-induced EZH2 phosphorylation in the cells cultured for 72 h.
ROS contribute to As3+-induced EZH2 phosphorylation in A549 cells
To explore whether the above observations are cell type specific or not, we extended this study in other type of cells too. A549 is a cell line derived from the non-small cell lung cancer (NSCLC) with some features of alveolar type II cells. The S21 phosphorylation of the EZH2 could be observed in the A549 cells treated with 20 µM As3+ for 1 to 4 h with a peak phosphorylation at 2 h, which is roughly parallel with the pattern of Akt activation by As3+ (Fig. 2A). A significant decrease of both EZH2 phosphorylation and Akt activation in response to As3+ was noted in the cells pre-treated with 10 or 20 mM NAC (Fig. 1A), indicating that oxidative stress due to ROS induction by As3+ is also involved in the S21 phosphorylation of the EZH2 protein in A549 cells. To address this notion further, the A549 cells were treated with different concentrations of H2O2 for 5 min or 500 µMH2O2 for 5 to 60 min. As depicted in Fig. 2B, H2O2 is able to induce S21 phosphorylation of the EZH2 along with the activation of the upstream kinases, including JNK, STAT3, and Akt.
To extend above observations, we also tested the inducibility of EZH2 phosphorylation by As3+ at much lower concentrations from 0.25 to 4 µM in the cells cultured for a prolonged time, 72 h. We noted that lower concentrations of As3+ was able to induce JNK and p38 activation in a clear dose-dependent manner (Fig. 2C and data not shown). However, a significant Akt activation by lower concentrations of As3+ could not be detected (top two panels, Fig. 2C). The treatment of the cells with 0.25 or 2 µM As3+-induced S21 phosphorylation of EZH2 (Fig. 2D). Unexpectedly, NAC appeared to be unable to inhibit the EZH2 phosphorylation induced by As3+ at lower concentrations. In other experiments, we demonstrated that prolonged incubation of the cells with NAC, e.g., 72 h, enhanced both basal and As3+-induced p38 activation, possibly because of stress responses due to the overwhelmed reduction condition. Accordingly, we speculate that different mechanisms may be involved in the EZH2 phosphorylation induced by low and high concentrations of As3+, respectively.
Both As3+ and H2O2 induce exogenous EZH2 phosphorylation through the direct interaction of Akt and EZH2
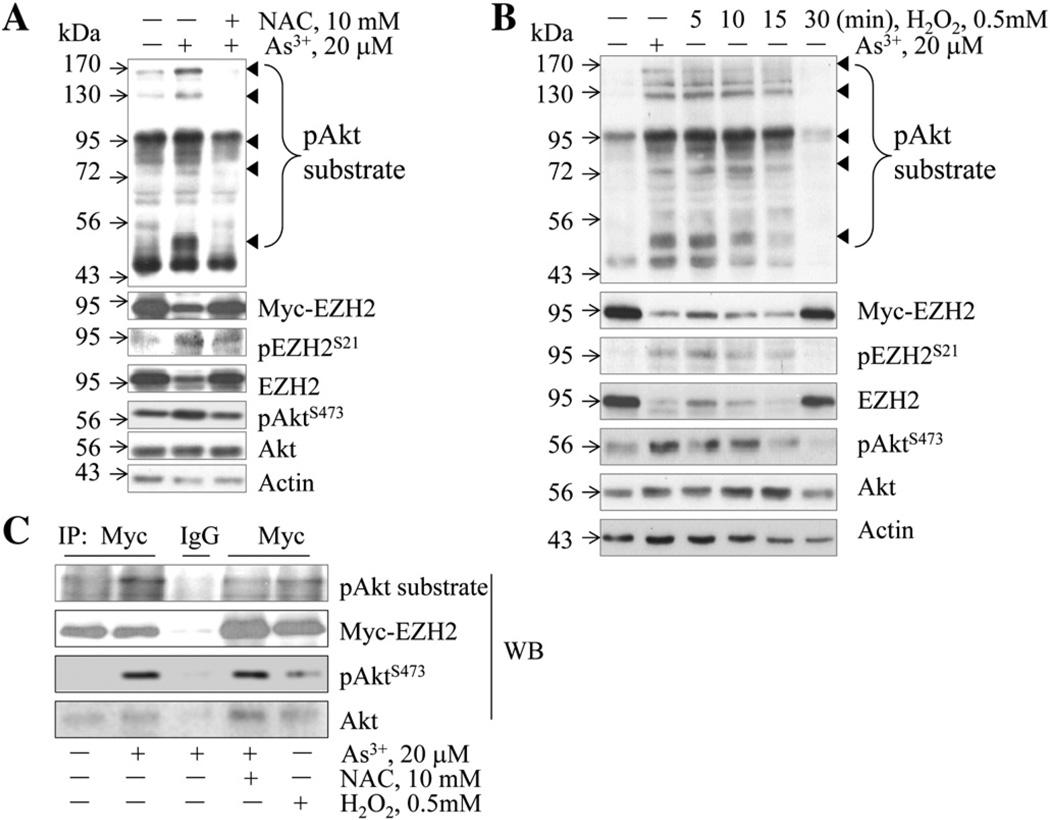
As an arginine (Arg, R)-directed or AGC-family kinase, Akt can directly phosphorylate serine (Ser)/Threonine (Thr) in a conserved motif, RXRXXS/T, characterized by R at positons – 5 and – 3 (Alessi et al., 1996). Accordingly, proteins containing RXRXXS/T motif can be phosphorylated by Akt, which can be recognized by anti-RXRXXS*/T* motif antibody (anti-Akt substrate antibody, “*” indicates phosphorylation). The human EZH2 protein contains RKRVKS21 motif that is in consensus with the conserved Akt phosphorylation site. To determine if As3+ can induce S21 phosphorylation of the exogenous EZH2 by Akt, the HEK-293 cells were transfected with a 3myc-tagged EZH2 followed by As3+ treatment. In the Western blotting results as shown in Fig. 3A, the anti-Akt substrate antibody detected several bands with molecular weights around 170, 130, 95, 72, and 50 kDa (Fig. 3A, pointed by small triangles) in the cells treated with As3+. Re-probing of the membrane with antibodies against myc-tag and EZH2 suggested that the band at position 95 kDa contains both the endogenous and the exogenously transfected EZH2 proteins that were phosphorylated by Akt. It remains to be determined about the nature of other bands at the positions 170, 130, 72, and 50 kDa that are phosphorylated by Akt as indicated by the anti-pAkt substrate antibody. The intensity of these bands was significantly decreased in the cells treated with NAC, suggesting that ROS play important role in As3+-induced phosphorylation of these proteins by Akt. To further validate the involvement of ROS in the phosphorylation of these proteins, the transfected HEK-293 cells were treated with 0.5 mM H2O2 for 5 to 30 min and then the cells were subjected to Western blotting using the anti-pAkt substrate antibody. A similar pattern of band detecting was noted in the cells treated with As3+ and H2O2 (comparing lane 2 with lanes 3–5, Fig. 3B).
Fig. 3.
Both As3+ and H2O2 induce the interaction of Akt and EZH2 and Akt-dependent phosphorylation of the exogenous EZH2 overexpressed in HEK-293 cells. (A) HEK-293 cells were transfected with 3myc-tagged EZH2 expression vector for 24 h followed by the treatment of the cells with 20 µM As3+ for 2 h in the presence or absence of 10 mM NAC, followed by Western blotting using the indicated antibodies. (B) The transfected HEK-293 cells were treated with As3+ or H2O2, followed by Western blotting as in (A). (C) Anti-myc tag or control IgG was used in immunoprecipitation (IP) to pull down the exogenousmyc-tagged EZH2. The IP was then subjected to Western blotting using antibodies recognizing the phosphorylated Akt substrate motif (RXRXXS*/T*), myc tag, pAkt, and Akt.
To determine whether As3+-induced EZH2 S21 phosphorylation is through direct interaction of Akt and EZH2 in the HEK-293 cells transfected with myc-tagged EZH2, an immunoprecipitation (IP) was performed to pull down the myc-tagged exogenous EZH2. As shown in Fig. 3C, the anti-pAkt substrate antibody recognized the phosphorylated form of myc-tagged EZH2 that was precipitated from the As3+-treated cells. The pretreatment of the transfected cells with NAC diminished As3+-induced phosphorylation of the exogenous EZH2 protein. Again, H2O2 was able to induce this Akt-dependent phosphorylation of the exogenous EZH2. Furthermore, when the precipitates from Myc-tag IP were subjected to Western blotting using antibodies against pAkt and Akt, respectively, a predominant pAktS473 signal was detected in the IP from As3+- or H2O2-treated cells (Fig. 3C), indicating the direct interaction of the activated Akt with the myc-tagged EZH2 in the cells in response to As3+ and H2O2. The interaction of the non-activated Akt with the myc-tagged EZH2 was negligible.
Involvement of ROS in As3+-induced cytoplasmic translocation of the EZH2 protein
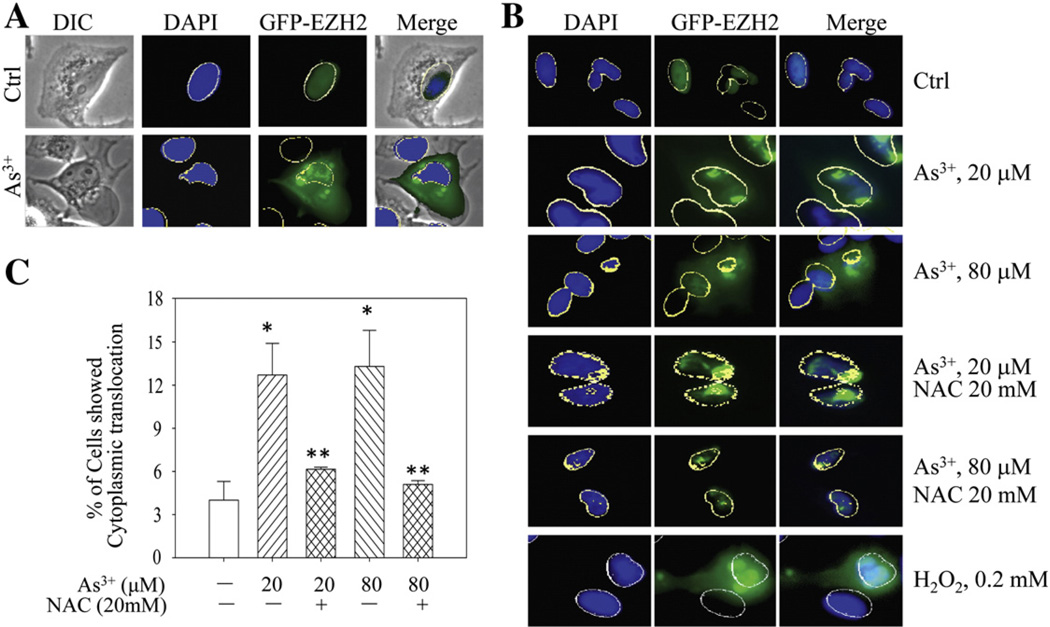
In BEAS-2B cells, we had previously shown the cytoplasmic translocation of the S21-phosphorylated endogenous EZH2 from nuclei in response to As3+ treatment (Chen et al., 2013). To investigate if As3+ is able to induce the same translocation of the exogenous EZH2, the BEAS-2B cells expressing GFP-EZH2 were treated with 20 µM As3+ for 2 h. The GFP-EZH2 is exclusively localized in the nuclei of the cells without As3+ treatment (Fig. 4A, upper panels). Following As3+ treatment, both cytoplasmic and nuclear locations of the GFP-EZH2 were observed (Fig. 4A, bottom panels). Furthermore, As3+ appeared to be able to induce the clustering of GFP-EZH2 proteins. These clusters were randomly distributed in both cytoplasm and nucleus. To explore whether oxidative stress is involved in As3+-induced re-distribution of the GFP-EZH2, NAC was applied prior to As3+ treatment. As shown in Fig. 4B, NAC prevented the cytoplasmic localization of the GFP-EZH2 induced by either 20 µM or 80 µM As3+, indicating that the oxidative stress is involved in As3+-induced cytoplasmic localization of the EZH2 protein. However, NAC was unable to prevent the nuclear clustering of the exogenous GFP-EZH2 proteins induced by As3+. The possible role of oxidative stress on the intracellular distribution of the EZH2 protein was confirmed by the treatment of the cells with 0.2 mM H2O2 that clearly induced cytoplasmic localization of the GFP-EZH2 proteins (Fig. 4B, bottom panels). Semi-quantification suggested a more than 50% inhibition of the As3+-induced cytoplasmic localization of the exogenous EZH2 (GFP-EZH2) by NAC (Fig. 4C).
Fig. 4.
Both As3+ and H2O2 induce cytoplasmic localization of the EZH2 protein in BEAS-2B cells. (A) BEAS-2B cells expressing GFP-EZH2 were treated with 20 µM As3+ for 2 h. The intracellular distribution of EZH2 was determined by immunofluorescent microscopy. (B) NAC prevented cytoplasmic localization of the EZH2 protein induced by As3+ in BEAS-2B cells transfected with GFP-EZH2. H2O2 was used as a control of ROS. (C) Statistical analysis of the cytoplasmic translocation ratio of the EZH2 protein in the cells treated with As3+ in the presence or absence of NAC. Data are expressed as the mean ± SD, n=3, ** p < 0.05.
To additionally confirm the effect of As3+ and/or oxidative stress on the cytoplasmic localization of the EZH2 proteins, we fractionated the cell lysates by isolating cytoplasmic and nuclear fractions, respectively, followed by measuring the levels of S21-phosphorylated and the total endogenous EZH2 proteins in response to As3+ or H2O2 treatment. Although As3+ appeared to be unable to affect the nuclear levels of the phosphorylated EZH2 (pEZH2) and EZH2, As3+ was able to increase both pEZH2 and EZH2 in the cytoplasmic fraction (Fig. 5A). The pretreatment of the cells with 20 mM NAC reduced As3+-induced pEZH2 in the cytoplasmic fraction significantly. NAC was also able to reduce the level of total EZH2 in the cytoplasmic fraction, although with a lesser extent relative to its effect on the phosphorylated EZH2.
Fig. 5.
Increased cytoplasmic localization of the phosphorylated and total EZH2 in BEAS-2B cells treated with As3+ or H2O2. (A) BEAS-2B cells were treated with 20 or 80 µM As3+ for 2 h with or without NAC pretreatment. Cellular fractions were made to extract the cytoplasmic and nuclear proteins. Lamin A/C and GAPDH were used as indications of the nuclear and cytoplasmic fractions. Lanes 4 and 10 are NAC only groups. Right panel shows semi-quantification of the ratio between pEZH and EZH2 in the cytoplasm. (B) BEAS-2B cells were treated with 0.2 mM H2O2 for the indicated times. The levels of phosphorylated EZH2, total EZH2, lamin A/C, and GAPDH were determined in the cytoplasmic and nuclear fractions, respectively. Right panel shows semi-quantification of the ratio between pEZH and EZH2 in the cytoplasm.
H2O2 could transiently induce the increase of both phosphorylated and total EZH2 in the cytoplasm (Fig. 5B). However, this effect of H2O2 was rapidly diminished after 10 min of H2O2 treatment, possibly due to the fast elimination of H2O2 by the medium, the cellular peroxisomes that contain catalase and the cytoplasmic glutathione peroxidase (GPx) and NADPH (Makino et al., 2004).
Discussion
In the present report, we provide evidence showing that the oxidative stress due to ROS generation contributes to As3+-induced kinase activation that leads to S21 phosphorylation of the EZH2 protein. In addition, oxidative stress is also involved in the direct interaction of the activated Akt and EZH2. Furthermore, oxidative stress appears to be able to induce cytoplasmic localization of the phosphorylated and the non-phosphorylated EZH2 protein in the cells treated with As3+. The pretreatment of the cells with NAC prevented EZH2 phosphorylation and cytoplasmic localization induced by As3+.
EZH2 along with EED, SUZ12, and RbAp46/48 proteins forms polycomb repressive complex 2 (PRC2) that catalyzes the trimethylation of lysine 27 of histone H3 (H3K27me3), a mark of transcriptionally silent or poised chromatin (Margueron and Reinberg, 2011). In the past few years, EZH2 had been the focus of a significant number of biochemical and molecular studies in cancer cells and cancer stem cells. Many independent studies revealed that EZH2 is overexpressed in several common cancers and that the degree of EZH2 overexpression is associated with the aggressiveness of these tumors (Hock, 2012). Such an overexpression of EZH2 was viewed as a major causative force, rather than a result, of the cancer development. Some genetic studies suggested that missense mutation in the catalytic domain causes hyperactivation of the EZH2 protein and increased incidents of malignancies, such as neuroblastoma and lymphoma (Crea, 2012). Additional evidence also indicated that the overactivation of EZH2 is responsible for the increased aggressiveness of the breast cancer associated with the loss of BRCA1 tumor suppressor (Wang and Huang, 2013). In human prostate cancer, EZH2 is one of the most important oncogenic factors for the initiation and progression of the tumor, as revealed by the fact that the inactivation of EZH2 by chemical inhibitor DZNep reduced tumor size and invasion significantly (Deb et al., 2013). A well-accepted and simplified explanation for the oncogenic role of EZH2 is that EZH2 inhibits the expression of tumor suppressor genes and DNA repair genes through the PRC2-dependent establishment of the silent chromatin that enriched with H3K27me3. A most recent study, however, suggested a PRC2-independent role of EZH2 in glioblastoma stem cells (GSC) (Kim et al., 2013). Following Akt-mediated S21 phosphorylation, EZH2 can directly bind to and induces methylation of lysine 180 (K180) of the STAT3 protein, leading to a sustained activation of the transcriptional activity of the STAT3 and the maintenance of the stemness of the GSC (Kim et al., 2013).
S21 phosphorylation of EZH2 by Akt kinase has been first demonstrated in breast cancer cells treated with insulin-like growth factor I (IGF-I) or estrogen (Bredfeldt et al., 2010; Cha et al., 2005). In BEAS-2B cells, we had also noticed S21 phosphorylation of the EZH2 protein through the activation of the JNK-STAT3-Akt signaling axis in response to As3+ (Chen et al., 2013). The present report further unraveled the importance of oxidative stress or ROS in this As3+-induced EZH2 phosphorylation, which confirmed our previous findings showing that As3+ is able to induce ROS generation in the non-transformed BEAS-2B cells (Chang et al., 2010). Given the critical roles of ROS and EZH2 in human cancers related to environmental exposure, the data from this report may shed new light on the prevention or treatment of human lung cancer or other cancers resulting from environmental exposure to As3+ or other carcinogens.
Acknowledgment
We thank Dr. Mien-Chie Hung from the Department of Molecular and Cellular Oncology, University of Texas, for providing pGFP-EZH2-WT and p3myc-EZH2-WT expression vectors. This work was supported by NIH grant nos. R01 ES017217 and R01 ES020137 to FC.
References
- Ahmad S, Kitchin KT, Cullen WR. Arsenic species that cause release of iron from ferritin and generation of activated oxygen. Arch. Biochem. Biophys. 2000;382:195–202. doi: 10.1006/abbi.2000.2023. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol. Endocrinol. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, Ping B, Otte AP, Hung MC. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310:306–310. doi: 10.1126/science.1118947. [DOI] [PubMed] [Google Scholar]
- Chang Q, Pan J, Wang X, Zhang Z, Chen F, Shi X. Reduced reactive oxygen species-generating capacity contributes to the enhanced cell growth of arsenic-transformed epithelial cells. Cancer Res. 2010;70:5127–5135. doi: 10.1158/0008-5472.CAN-10-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Liu J, Chang Q, Beezhold K, Lu Y, Chen F. JNK and STAT3 signaling pathways converge on Akt-mediated phosphorylation of EZH2 in bronchial epithelial cells induced by arsenic. Cell Cycle. 2013;12:112–121. doi: 10.4161/cc.23030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, Dang CV. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crea F. Histone code, human growth and cancer. Oncotarget. 2012;3:1–2. doi: 10.18632/oncotarget.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb G, Thakur VS, Gupta S. Multifaceted role of EZH2 in breast and prostate tumorigenesis: epigenetics and beyond. Epigenetics. 2013;8:464–476. doi: 10.4161/epi.24532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelick H, Jones H, Dybowska A, Valsami-Jones E. Arsenic pollution sources. Rev. Environ. Contam. Toxicol. 2008;197:17–60. doi: 10.1007/978-0-387-79284-2_2. [DOI] [PubMed] [Google Scholar]
- Hei TK, Liu SX, Waldren C. Mutagenicity of arsenic in mammalian cells: role of reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8103–8107. doi: 10.1073/pnas.95.14.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock H. A complex polycomb issue: the two faces of EZH2 in cancer. Genes Dev. 2012;26:751–755. doi: 10.1101/gad.191163.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubaux R, Becker-Santos DD, Enfield KS, Lam S, Lam WL, Martinez VD. Arsenic, asbestos and radon: emerging players in lung tumorigenesis. Environ. Health. 2012;11:89. doi: 10.1186/1476-069X-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum. 1987;(Suppl 7):1–440. (PMID: 3482203). [PubMed] [Google Scholar]
- Kim E, Kim M, Woo DH, Shin Y, Shin J, Chang N, Oh YT, Kim H, Rheey J, Nakano I, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell. 2013;23:839–852. doi: 10.1016/j.ccr.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino N, Sasaki K, Hashida K, Sakakura Y. A metabolic model describing the H2O2 elimination by mammalian cells including H2O2 permeation through cytoplasmic and peroxisomal membranes: comparison with experimental data. Biochim. Biophys. Acta. 2004;1673:149–159. doi: 10.1016/j.bbagen.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Margueron R, Reinberg D. The polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom DK. Public health. Worldwide occurrences of arsenic in ground water. Science. 2002;296:2143–2145. doi: 10.1126/science.1072375. [DOI] [PubMed] [Google Scholar]
- Ren X, McHale CM, Skibola CF, Smith AH, Smith MT, Zhang L. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ. Health Perspect. 2011;119:11–19. doi: 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowska AM, Verbraecken J, Darquennes K, De Backer WA. Role of N-acetylcysteine in the management of COPD. Int. J. Chron. Obstruct Pulm. Dis. 2006;1:425–434. doi: 10.2147/copd.2006.1.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Wang L, Huang H. EZH2 takes the stage when BRCA1 loses. Cell Cycle. 2013;12:3575–3576. doi: 10.4161/cc.26785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mandal AK, Saito H, Pulliam JF, Lee EY, Ke ZJ, Lu J, Ding S, Li L, Shelton BJ, et al. Arsenic and chromium in drinking water promote tumorigenesis in a mouse colitis-associated colorectal cancer model and the potential mechanism is ROS-mediated Wnt/beta-catenin signaling pathway. Toxicol. Appl. Pharmacol. 2012;262:11–21. doi: 10.1016/j.taap.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhou X, Liu W, Sun X, Chen C, Hudson LG, Jian Liu K. Arsenite-induced ROS/RNS generation causes zinc loss and inhibits the activity of poly(ADP-ribose) polymerase-1. Free Radic. Biol. Med. 2013;61C:249–256. doi: 10.1016/j.freeradbiomed.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Park IC, Park MJ, Lee HC, Lee SJ, Chun YJ, Lee SH, Hong SI, Rhee CH. Arsenic trioxide induces apoptosis through a reactive oxygen species-dependent pathway and loss of mitochondrial membrane potential in HeLa cells. Int. J. Oncol. 2002;21:57–63. [PubMed] [Google Scholar]
- Yamanaka K, Takabayashi F, Mizoi M, An Y, Hasegawa A, Okada S. Oral exposure of dimethylarsinic acid, a main metabolite of inorganic arsenics, in mice leads to an increase in 8-Oxo-2'-deoxyguanosine level, specifically in the target organs for arsenic carcinogenesis. Biochem. Biophys. Res. Commun. 2001;287:66–70. doi: 10.1006/bbrc.2001.5551. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang X, Cheng S, Sun L, Son YO, Yao H, Li W, Budhraja A, Li L, Shelton BJ, et al. Reactive oxygen species mediate arsenic induced cell transformation and tumorigenesis through Wnt/beta-catenin pathway in human colorectal adenocarcinoma DLD1 cells. Toxicol. Appl. Pharmacol. 2011;256:114–121. doi: 10.1016/j.taap.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhao X, Fiskus W, Lin J, Lwin T, Rao R, Zhang Y, Chan JC, Fu K, Marquez VE, et al. Coordinated silencing of MYC-mediated miR-29 by HDAC3 and EZH2 as a therapeutic target of histone modification in aggressive B-cell lymphomas. Cancer Cell. 2012;22:506–523. doi: 10.1016/j.ccr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]