Abstract
Importance
The incidence of early-stage non-small cell lung cancers among the elderly is expected to rise dramatically due to demographic trends and CT screening. However, no modern trials have compared the most commonly delivered treatments.
Objective
To determine clinical characteristics and survival outcomes associated with the three most commonly utilized definitive therapies for early-stage NSCLC in the elderly population.
Design, Setting, and Participants
The Surveillance, Epidemiology, and End Results–Medicare–linked database was used to determine the baseline characteristics and outcomes of 9,093 patients with early-stage, node-negative NSCLC who underwent definitive treatment with lobectomy, sublobar resection, or stereotactic ablative radiation between 2003 and 2009.
Main Outcomes and Measures
Overall survival and lung-cancer specific survival were compared using Medicare claims through December 2012. Both proportional hazards regression and propensity score matching (PSM) were used to adjust outcomes for key patient, tumor, and practice environment factors.
Results
The median age was 75 years, and treatment distribution was as follows: Lobectomy (79.4%), sublobar resection (16.5%), and SABR (4.2%). Unadjusted 90-day mortality was highest for lobectomy (4.0%) followed by sublobar resection (3.7%, P=0.79) and SABR (1.3%, P=0.008). At three years, unadjusted mortality was lowest for lobectomy (25.0%), followed by sublobar resection (35.3%, P<0.001) and SABR (45.1%, P<0.001). Proportional hazards regression demonstrated that sublobar resection was associated with worse overall survival (Adjusted hazard ratio [HR] 1.32; 95% confidence interval [CI] 1.20–1.44) and lung-cancer specific survival (HR 1.50; 95%CI 1.29–1.75) compared to lobectomy. PSM analysis reiterated these findings. In proportional hazards regression, SABR was associated with better overall survival than lobectomy in the first 6 months after diagnosis (HR 0.45; 95%CI 0.27–0.75), but worse survival thereafter (HR 1.66; 95%CI 1.39–1.99). PSM analysis of well-matched SABR and lobectomy cohorts demonstrated similar overall survival in the two groups (HR 1.01; 95%CI 0.74–1.38).
Conclusions
Lobectomy was associated with better outcomes than sublobar resection in elderly patients with early-stage NSCLC. Propensity-score matching suggests that SABR may be a good option among patients with very advanced age and multiple comorbidities.
Introduction
Two public health developments are expected to significantly impact the incidence of early-stage non-small cell lung cancers (NSCLC) in the United States. First, the US Preventative Services Task Force (USPSTF) has recently released new recommendations in favor of CT lung cancer screening for long-term smokers. This development is in response to the findings of the National Lung Screening Trial (NLST), which demonstrated a reduction in lung cancer mortality among appropriately screened patients.1 Secondly, by 2030 the incidence of NSCLC among adults over 65 is expected to rise 67% to 271,000 annual cases as a result of the aging population.2 This demographic trend is expected to occur independently of whether screening disseminates into routine care.
The dramatic rise in the number of early-stage NSCLC cases among the elderly will place pressure on the health care system to provide effective and cost-conscious care. Regrettably no recent randomized trials have compared contemporary treatment strategies for elderly patients. Moreover, the last major trial to address this question in any population was the Lung Cancer Study Group (LCSG) 821 trial, which accrued patients more than two decades ago. This trial randomized patients with early-stage disease to either lobectomy or limited resection and found that lobectomy resulted in fewer local failures and improved survival.3 However, several issues complicate straightforward application of those findings to modern practice. Contemporary imaging technology has become more sensitive, which has allowed identification of smaller and perhaps more indolent lesions than those observed in the trial. Also, the therapeutic challenge of treating elderly patients with comorbid illnesses was not well-addressed as LCSG 821 sought to enroll medically fit patients, a third of whom were younger than 60. Finally, more recent retrospective studies suggest that sublobar resections using modern surgical techniques result in better outcomes than those observed in the older literature.4–8 Therefore, the question of whether the burgeoning population of elderly patients with early NSCLC might be better served with less aggressive strategies than lobectomy remains open.
Given the urgency of this clinical issue, several trials have been opened to directly compare lobectomy, sublobar resection, and SABR. Unfortunately, these studies have been beset by slow accrual, several have been closed, and results from the still active trials are not expected for years.9–12 When randomized trial data are absent, carefully controlled population-based analysis can provide important evidence. Therefore, we used a large population-based registry to determine outcomes for early-stage lung cancer in contemporary practice in the United States. Specifically, we used the latest iteration of the Surveillance, Epidemiology, and End Results (SEER)-Medicare cohort to determine the association of lobectomy, sublobar resection, and SABR with overall survival (OS) and lung cancer-specific survival (LCSS) among elderly patients with early-stage NSCLC.
Methods
Data Source
The SEER-Medicare database captures clinical, pathological, and insurance claims data for incident cancers diagnosed in Medicare beneficiaries who reside within one of 16 geographic areas that account for 26% of the US population. The case ascertainment rate for the SEER data is approximately 98%.13 In this study, demographic and tumor characteristics for incident malignancies diagnosed from January 1, 2003 to December 31, 2009 were linked to Medicare claims for treatment and outcomes from January 1, 2002 to December 31, 2012.
Study Sample
From 2003–2009,186,349 patients aged ≥ 66 years without prior malignancy were diagnosed with lung cancer and reported in the SEER-Medicare cohort. To facilitate use of Medicare billing claims, patients with inadequate Medicare records were excluded as were those with any second cancer diagnosed within 120 days of the index lung cancer, as billing records could not discriminate between procedures performed for the index cancer versus the second cancer (eTable 1). Other exclusion criteria included histologies other than NSCLC, tumors larger than 5 cm, distant metastases or nodal disease at presentation, absence of pathologic confirmation, and the use of non-standard therapies for early-stage NSCLC (eTable 1). To ensure that treatment was not directed at metastatic targets, we excluded patients with codes for brain, bone, liver or adrenal metastases within 120 days of cancer diagnosis. These criteria yielded a sample of 9,093 patients (eTable 1).
Treatment Strategies
Medicare claims using International Classification of Diseases, 9th Revision (ICD-9) and Clinical Modification and Current Procedural Terminology/ Healthcare Common Procedure Coding System (CPT) codes were utilized to extract claims for treatments. Therapies occurring within 4 months of diagnosis were considered to be part of the initial treatment strategy. Lung surgery was determined from SEER and Medicare claims and classified as lobar or sublobar resection (eTable 2). The definitive surgery was defined as the most extensive procedure reported by SEER or Medicare. SABR use was extracted if Medicare claims confirmed actual delivery of 1–5 fractions of radiosurgery (eTable2).
Other Covariables
Patient characteristics from the SEER data included age at diagnosis, race, sex, and whether the county of residence was urban or rural. Baseline clinical characteristics were determined using Medicare claims from an interval of 12 months before to 1 month after diagnosis.14 A modified Charlson comorbidity index with Klabunde modification was determined from ICD-9 codes using published methods15–17; chronic obstructive pulmonary disease (COPD) was not included in the index and was instead included as a separate covariable. Patients were classified as oxygen users if durable medical equipment claims included oxygen equipment. A performance status covariable was generated using claims for medical assistance devices and home healthcare18.
Tumor characteristics extracted from SEER included T-stage, laterality, and lung subsite. To adjust for stage migration, mediastinal sampling and positron emission tomography (PET) use within a time period extending from 2 weeks prior to 4 months following diagnosis were extracted from the SEER registry and Medicare claims codes, respectively (eTable 2). This period of time was chosen to exclude diagnostic orders triggered at first follow-up.
Practice environment characteristics were also evaluated. The 16 SEER regions were categorized into four geographic areas (East, South, Midwest, and West). County-level density of surgeons and radiation oncologists was determined using the Area Resource File for 1998–2009 in accordance with published methods.19 Year of diagnosis was obtained from the SEER data.
Outcomes
OS was determined from Medicare records with follow-up through December 2012. LCSS was determined using cause of death data abstracted from death certificates and reported by SEER with follow up through December 2009. In the United States, the observed sensitivity and specificity of death certificates for reporting lung cancer as the cause of death have been recently reported as approximately 89 and 99 percent, respectively.20 For survival analyses, censorship was performed at the earliest of the following: loss of Medicare coverage, conversion to HMO coverage, death, or the end of the study period.
Statistical Analysis
Baseline characteristics across treatment strata were compared with Pearson’s X2 test. The association between treatment strategy and survival outcomes was determined with multivariable proportional hazards regression with backwards elimination of variables that did not reduce model fit (P > 0.05). The proportional hazards assumption was assessed analytically using Schoenfeld residuals. Violations were addressed by inclusion of a time-varying covariable to the model.21 For the comparison of lobectomy and sublobar resection, additional models limited to pre-specified sub-groups were fitted (age > 75 years, tumor size < 2 cm, sublobar resections billed as video-assisted surgery, sublobar resections billed as segmentectomy).
Because baseline covariable differences may not have been adequately addressed by proportional hazards regression, we performed a second analysis wherein propensity-score matching was used to compare lobectomy patients to those treated with sublobar resection or SABR. Propensity scores were generated using logistic modeling with treatment as the dependent variable. Independent variables included age, gender, comorbidity score, oxygen use, performance score, tumor size, PET staging, and pathological staging with mediastinal sampling.22 Patients were matched 1:1 using nearest neighbor technique with caliper distance limited to 25% of the standard deviation of the pooled propensity scores. Covariable balance between cohorts was assessed with a standardized difference threshold of 0.15.23 Proportional hazards models, stratified by matched pair and adjusted for unbalanced covariables, were generated to compare the cohorts.24 Two sensitivity analyses were performed using more strict or less strict criteria for matching. In the more strict analysis, all 20 covariables were used for propensity score calculation. In the less strict analysis, nearest neighbor matching was performed without a specified caliper distance.
All statistical analyses were 2-sided with P ≤ 0.05 and conducted using SAS v. 9.3 (Cary, NC). Our institutional review board granted this study exempt status.
Results
Baseline Characteristics and Unadjusted Mortality
Among the 9,093 patients treated definitively for early-stage NSCLC between 2003 and 2009, median age was 75 years and 54% were female. Treatment strategy was as follows: 7,215 lobectomy (79.4%), 1,496 sublobar resection (16.5%), and 389 SABR (4.2%). Pathologic node-negative status was established with mediastinal sampling in 94% of the lobectomy patients, 45% of the sublobar resection patients, and 5% of the SABR patients. Surgical patients were younger and carried fewer comorbidities than SABR patients. Baseline characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics Stratified by Treatment
| Lobectomy N=7215 |
Sublobar Resection N=1496 |
SABR N=382 |
P>X2 | |
|---|---|---|---|---|
|
Patient Factors | ||||
| Age | ||||
| 66–69 | 1,515 (21%) | 235 (16%) | 39 (10%) | <.001 |
| 70–74 | 2,182 (30%) | 415 (28%) | 71 (19%) | |
| 75–79 | 2,069 (29%) | 435 (29%) | 94 (25%) | |
| ≥ 80 | 1,449 (20%) | 411 (27%) | 178 (47%) | |
| Sex | ||||
| Male | 3,365 (47%) | 693 (46%) | 143 (37%) | 0.002 |
| Female | 3,850 (53%) | 803 (54%) | 239 (63%) | |
| Race | ||||
| White | 6,456 (89%) | 1,360 (91%) | 340 (89%) | 0.005 |
| Black | 394 (5%) | 73 (5%) | >31 (>5%) | |
| Other or Unspecified | 365 (5%) | <65 (<5%) | <11 (<5%) | |
| Educational Attainment (% with Less than High School Education in Census Tract) | ||||
| 1st Quartile (0 – 10%) | 2,077 (29%) | 424 (28%) | 109 (29%) | 0.56 |
| 2nd Quartile (10 – 17%) | 1,828 (25%) | 411 (27%) | 91 (24%) | |
| 3rd Quartile (17 – 27%) | 1,755 (24%) | 355 (24%) | 103 (27%) | |
| 4th Quartile (>27%) | 1,555 (22%) | 306 (20%) | 79 (21%) | |
| Median Household Income for Zip Code | ||||
| 1st Quartile (<$32,826) | 1,552 (22%) | 316 (21%) | 87 (23%) | 0.40 |
| 2nd Quartile ($32,827 – $43,536) | 1,762 (24%) | 350 (23%) | 96 (25%) | |
| 3rd Quartile ($43,537 – $58,316) | 1,788 (25%) | 404 (27%) | 103 (27%) | |
| 4th Quartile (>$58,317) | 2,113 (29%) | 426 (28%) | 96 (25%) | |
| Type of Residence | ||||
| Urban | 6,409 (89%) | 1,342 (90%) | 341 (89%) | 0.61 |
| Rural | 806 (11%) | 154 (10%) | 41 (11%) | |
| COPD | ||||
| No | 2,756 (38%) | 360 (24%) | 86 (23%) | <.001 |
| Yes | 4,459 (62%) | 1,136 (76%) | 296 (77%) | |
| Charleson Comorbidity Index Excluding COPD | ||||
| 0 | 4,368 (61%) | 792 (53%) | 170 (45%) | <.001 |
| 1 | 1,700 (24%) | 379 (25%) | 108 (28%) | |
| ≥2 | 1,147 (16%) | 325 (22%) | 104 (27%) | |
| Oxygen Supplementation | ||||
| No | 6,348 (88%) | 1,110 (74%) | 220 (58%) | <.001 |
| Yes | 867 (12%) | 386 (26%) | 162 (42%) | |
| Performance Score (Medical Assistance) | ||||
| 0 | 6,374 (88%) | 1,235 (83%) | 294 (77%) | <.001 |
| ≥1 | 841 (12%) | 261 (17%) | 88 (23%) | |
|
Tumor Factors | ||||
| T-Stage | ||||
| T1a (0.0 – 2.0 cm) | 3,169 (44%) | 964 (64%) | 153 (40%) | <.001 |
| T1b (2.1 – 3.0 cm) | 2,370 (33%) | 355 (24%) | 153 (40%) | |
| T2a (3.1 – 5.0 cm) | 1,676 (23%) | 177 (12%) | 76 (20%) | |
| Histology | ||||
| NSCLC, NOS | 366 (5%) | 90 (6%) | 82 (21%) | <.001 |
| Adenocarcinoma | 4,371 (61%) | 866 (58%) | 178 (47%) | |
| Squamous | 2,236 (31%) | 482 (32%) | >110 (>25%) | |
| Large Cell | 242 (3%) | <60 (<5%) | <11 (<5%) | |
| Laterality | ||||
| Right | 4,248 (59%) | 828 (55%) | 201 (53%) | 0.004 |
| Left | 2,967 (41%) | 668 (45%) | 181 (47%) | |
| Site | ||||
| Bronchus | <11 (<2%) | <11 (<2%) | <11 (<3%) | <.001 |
| Upper Lobe | >4,400 (>60%) | >900 (>60%) | >210 (>60%) | |
| Middle Lobe | 384 (5%) | 44 (3%) | 13 (3%) | |
| Lower Lobe | 2,269 (31%) | 489 (33%) | 128 (34%) | |
| Overlapping/Unknown | 112 (2%) | 37 (2%) | <11 (<3%) | |
|
Diagnostic Studies | ||||
| PET Staging | ||||
| No | 3,329 (46%) | 701 (47%) | 92 (24%) | <.001 |
| Yes | 3,886 (54%) | 795 (53%) | 290 (76%) | |
| Mediastinal Sampling | ||||
| No | 406 (6%) | 820 (55%) | 362 (95%) | <.001 |
| Yes | 6,809 (94%) | 676 (45%) | 20 (5%) | |
|
Practice Environment Factors | ||||
| Density of Surgeons in County | ||||
| Highest Quartile | 1,684 (23%) | 349 (23%) | 82 (21%) | 0.048 |
| Second Quartile | 1,825 (25%) | 351 (23%) | 78 (20%) | |
| Third Quartile | 1,909 (26%) | 391 (26%) | 124 (32%) | |
| Fourth Quartile | 1,797 (25%) | 405 (27%) | 98 (26%) | |
| Density of Radiation Oncologists in County | ||||
| Highest Quartile | 1,652 (23%) | 341 (23%) | 93 (24%) | 0.016 |
| Second Quartile | 1,799 (25%) | 341 (23%) | 104 (27%) | |
| Third Quartile | 1,917 (27%) | 377 (25%) | 79 (21%) | |
| Fourth Quartile | 1,847 (26%) | 437 (29%) | 106 (28%) | |
| Geographic Region | ||||
| West | 2,618 (36%) | 520 (35%) | 97 (25%) | <.001 |
| Midwest | 814 (11%) | 174 (12%) | 84 (22%) | |
| East | 1,671 (23%) | 360 (24%) | 77 (20%) | |
| South | 2,112 (29%) | 442 (30%) | 124 (32%) | |
| Year of Diagnosis | ||||
| 2003 | 1,040 (14%) | <200 (15%) | <11 (<3%) | <.001 |
| 2004 | 1,013 (14%) | <200 (15%) | <11 (<3%) | |
| 2005 | 1,041 (14%) | 217 (15%) | 11 (3%) | |
| 2006 | 1,033 (14%) | 228 (15%) | 34 (9%) | |
| 2007 | 1,076 (15%) | 237 (16%) | 51 (13%) | |
| 2008 | 1,012 (14%) | 220 (15%) | 105 (27%) | |
| 2009 | 1,000 (14%) | 201 (13%) | 168 (44%) | |
Abbrev: SABR, stereotactic ablative radiotherapy; PET, positron emission tomography; NOS, not otherwise specified
Unadjusted 90-day mortality was highest for lobectomy (4.0%) followed by sublobar resection (3.7%, P=0.79) and SABR (1.3%, P=0.008). At three years, unadjusted mortality was lowest for lobectomy (25.0%), followed by sublobar resection (35.3%, P<0.001) and SABR (45.1%, P<0.001). Unadjusted LCSS followed similar long-term trends. Unadjusted survival curves are presented in eFigure 1.
Association of Baseline Characteristics with Outcomes
Multivariable proportional hazards regression demonstrated that advanced age, male gender, higher burden of comorbid illness, use of oxygen, use of medical assistance devices, and larger tumors were associated with worse outcomes (Table 2,Table 3). Lower educational attainment, but not race or income level, was associated with higher mortality. The use of mediastinal sampling for staging was associated with improved outcomes. These results are summarized in Tables 2 and 3.
Table 2.
Final Proportional Hazards Model for Overall Survival
| Variable | HR | 95%CI | P>X2 |
|---|---|---|---|
| Treatment | |||
| Lobectomy (Baseline) | 1.00 | -- | -- |
| Sublobar Resection | 1.32 | 1.20 – 1.44 | <.001 |
| SABR (t ≤ 6 months) | 0.45 | 0.27 – 0.75 | <.001 |
| SABR (t > 6 months) | 1.66 | 1.39 – 1.99 | <.001 |
| Age | |||
| 66–69 (Baseline) | 1.00 | -- | -- |
| 70–74 | 1.28 | 1.16 – 1.41 | <.001 |
| 75–79 | 1.51 | 1.37 – 1.66 | <.001 |
| ≥80 | 1.93 | 1.74 – 2.13 | <.001 |
| Sex | |||
| Male (Baseline) | 1.00 | -- | -- |
| Female | 0.75 | 0.71 – 0.80 | <.001 |
| Educational Attainment | |||
| 1st Quartile (0 – 10%) | 1.00 | -- | -- |
| 2nd Quartile (10 – 17%) | 1.13 | 1.03 – 1.23 | 0.01 |
| 3rd Quartile (17 – 27%) | 1.13 | 1.03 – 1.23 | 0.01 |
| 4th Quartile (>27%) | 1.23 | 1.12 – 1.35 | <.001 |
| COPD | |||
| No (Baseline) | 1.00 | -- | -- |
| Yes | 1.25 | 1.16 – 1.34 | <.001 |
| Charlson Comorbidity Score Ex. COPD | |||
| 0 (Baseline) | 1.00 | -- | -- |
| 1 | 1.20 | 1.12 – 1.30 | <.001 |
| ≥2 | 1.64 | 1.51 – 1.78 | <.001 |
| Oxygen Supplementation | |||
| No (Baseline) | 1.00 | -- | -- |
| Yes | 1.30 | 1.20 – 1.41 | <.001 |
| Performance Score (Medical Assistance) | |||
| 0 (Baseline) | 1.00 | -- | -- |
| ≥1 | 1.20 | 1.09 – 1.31 | <.001 |
| T-Stage | |||
| T1A (0.0 – 2.0 cm) (Baseline) | 1.00 | -- | -- |
| T1B (2.1 – 3.0 cm) | 1.22 | 1.14 – 1.31 | <.001 |
| T2A (3.1 – 5.0 cm) | 1.47 | 1.36 – 1.59 | <.001 |
| Histology | |||
| NSCLC, NOS (Baseline) | 1.00 | -- | -- |
| Adenocarcinoma | 0.83 | 0.73 – 0.94 | <.001 |
| Squamous carcinoma | 1.00 | 0.88 – 1.14 | 0.95 |
| Large cell | 0.98 | 0.81 – 1.18 | 0.83 |
| Mediastinal Sampling | |||
| Not Performed (Baseline) | 1.00 | -- | -- |
| Performed | 0.82 | 0.74 – 0.90 | <.001 |
| Region | |||
| West (Baseline) | 1.00 | -- | -- |
| Midwest | 0.96 | 0.86 – 1.06 | 0.43 |
| East | 0.95 | 0.87 – 1.03 | 0.22 |
| South | 1.11 | 1.03 – 1.20 | 0.01 |
| Year of Diagnosis | |||
| 2003 (Baseline) | 1.00 | -- | -- |
| 2004 | 0.90 | 0.81 – 1.00 | 0.04 |
| 2005 | 0.93 | 0.83 – 1.03 | 0.15 |
| 2006 | 0.91 | 0.82 – 1.02 | 0.11 |
| 2007 | 0.83 | 0.74 – 0.93 | <.001 |
| 2008 | 0.82 | 0.72 – 0.93 | <.001 |
| 2009 | 0.78 | 0.68 – 0.90 | <.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval; SABR, stereotactic ablative radiation; Ex, excluding; NSCLC, NOS, non-small cell lung cancer, not otherwise specified
Table 3.
Final Proportional Hazards Model for Lung Cancer Specific Survival
| Variable | HR | 95%CI | P>X2 |
|---|---|---|---|
| Treatment | |||
| Lobectomy (Baseline) | 1.00 | -- | -- |
| Sublobar Resection | 1.50 | 1.29 – 1.75 | <.001 |
| SABR | 1.44 | 1.03 – 2.02 | 0.03 |
| Age | |||
| 66–69 (Baseline) | 1.00 | -- | -- |
| 70–74 | 1.29 | 1.09 – 1.52 | <.001 |
| 75–79 | 1.37 | 1.16 – 1.62 | <.001 |
| ≥80 | 1.66 | 1.40 – 1.98 | <.001 |
| Sex | |||
| Male (Baseline) | 1.00 | -- | -- |
| Female | 0.75 | 0.67 – 0.83 | <.001 |
| Educational Attainment | |||
| 1st Quartile (0 – 10%) | 1.00 | -- | -- |
| 2nd Quartile (10 – 17%) | 1.15 | 0.99 – 1.33 | 0.07 |
| 3rd Quartile (17 – 27%) | 1.21 | 1.04 – 1.40 | 0.01 |
| 4th Quartile (>27%) | 1.30 | 1.11 – 1.51 | 0.00 |
| COPD | |||
| No (Baseline) | 1.00 | -- | -- |
| Yes | 1.25 | 1.16 – 1.34 | <.001 |
| Charleson Comorbidity Score Ex. COPD | |||
| 0 (Baseline) | 1.00 | -- | -- |
| 1 | 1.03 | 0.90 – 1.17 | 0.69 |
| ≥2 | 1.30 | 1.13 – 1.49 | <.001 |
| Oxygen Supplementation | |||
| No (Baseline) | 1.00 | -- | -- |
| Yes | 1.35 | 1.17 – 1.55 | <.001 |
| T-Stage | |||
| T1A (0.0 – 2.0 cm) (Baseline) | 1.00 | -- | -- |
| T1B (2.1 – 3.0 cm) | 1.30 | 1.15 – 1.47 | <.001 |
| T2A (3.1 – 5.0 cm) | 1.60 | 1.40 – 1.83 | <.001 |
| Histology | |||
| NSCLC, NOS (Baseline) | 1.00 | -- | -- |
| Adenocarcinoma | 0.82 | 0.67 – 1.01 | 0.06 |
| Squamous carcinoma | 0.95 | 0.77 – 1.17 | 0.63 |
| Large cell | 0.99 | 0.72 – 1.35 | 0.94 |
| Mediastinal Sampling | |||
| Not Performed (Baseline) | 1.00 | -- | -- |
| Performed | 0.78 | 0.78 – 0.78 | <.001 |
| Year of Diagnosis | |||
| 2003 (Baseline) | 1.00 | -- | -- |
| 2004 | 0.92 | 0.79 – 1.08 | 0.31 |
| 2005 | 0.96 | 0.81 – 1.13 | 0.59 |
| 2006 | 0.90 | 0.75 – 1.08 | 0.26 |
| 2007 | 0.76 | 0.62 – 0.94 | 0.01 |
| 2008 | 0.76 | 0.59 – 0.97 | 0.03 |
| 2009 | 0.55 | 0.36 – 0.84 | 0.01 |
Abbreviations: HR, hazard ratio; CI, confidence interval; SABR, stereotactic ablative radiation; Ex, excluding; NSCLC, NOS, non-small cell lung cancer, not otherwise specified
Comparison of Lobectomy and Sublobar Resection
Compared to lobectomy, sublobar resection was associated with worse overall survival (Hazard ratio [HR] 1.32; 95% confidence interval [CI] 1.20–1.44; P<0.001) and worse lung-cancer specific survival (HR 1.50; 95%CI 1.29–1.75; P<0.001) in proportional hazards regression. This finding was unchanged if the study cohort was restricted to any of the prespecified sub-groups (age ≥ 75 years, tumor size ≤ 2 cm) (eTable3). Likewise, this finding was preserved even if the sublobar resection cohort was limited to those billed as video-assisted surgery or anatomic segmentectomy (eTable 3).
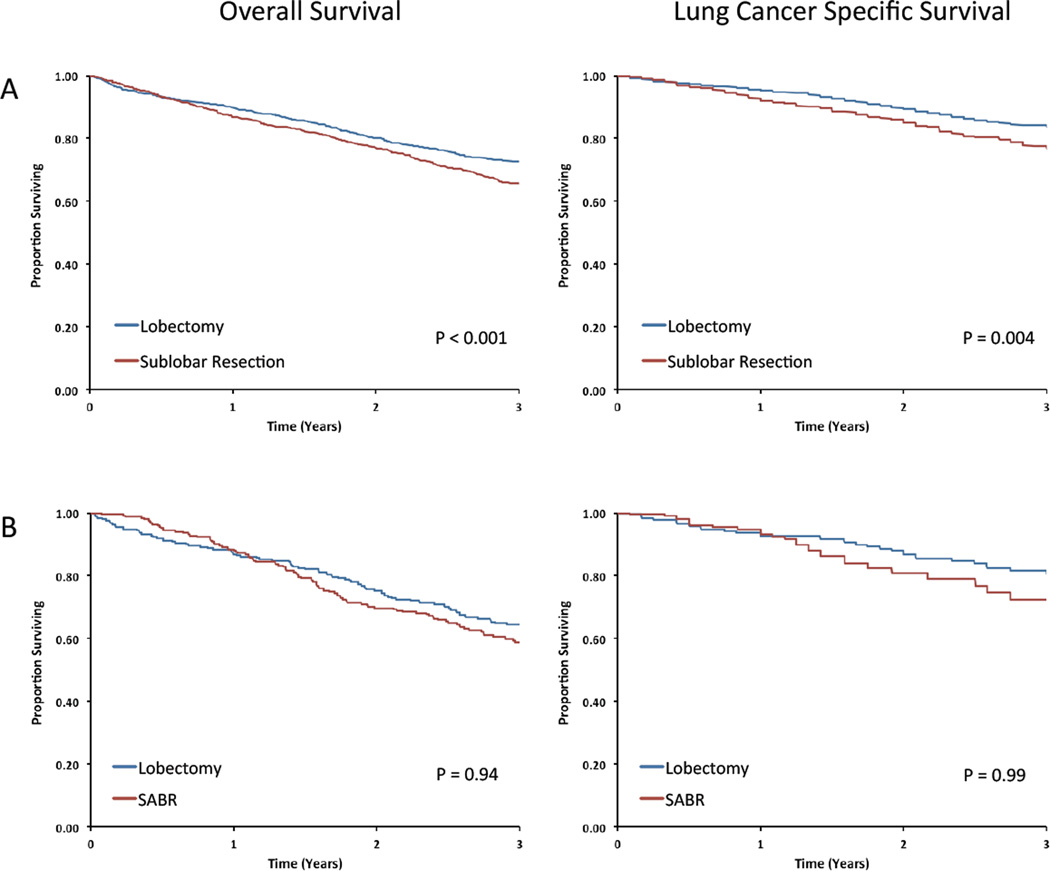
PSM analysis yielded sublobar resection and lobectomy cohorts that were well-balanced (eTable 4). Survival analysis of the cohorts demonstrated significantly worse LCSS and OS among sublobar resection patients (Table 4, Figure 1). Sensitivity analyses yielded qualitatively similar results (Table 4).
Table 4.
Propensity-Score Matching Sensitivity Analysis
| Overall Survival | Lung Cancer Specific Survival |
|||||
|---|---|---|---|---|---|---|
| Comparison | HR1 | 95%CI | P | HR1 | 95%CI | P |
| Sublobar Resection v Lobectomy | ||||||
| Main Analysis2 (1077 Matched Pairs) |
1.36 | (1.17–1.58) | <0.001 | 1.46 | (1.13–1.90) | 0.004 |
| More Strict Match3 (1057 Matched Pairs) |
1.20 | (1.03–1.39) | 0.016 | 1.30 | (1.00–1.69) | 0.05 |
| Less Strict Match4 (1496 Matched Pairs) |
1.25 | (1.08–1.45) | 0.004 | 1.40 | (1.08–1.82) | 0.013 |
| SABR v Lobectomy | ||||||
| Main Analysis2 (251 Matched Pairs) |
1.01 | (0.74–1.38) | 0.94 | 1.00 | (0.52–1.92) | 0.99 |
| More Strict Match3 (149 Matched Pairs) |
1.28 | (0.86–1.91) | 0.23 | 1.30 | (0.57–2.97) | 0.53 |
| Less Strict Match4 (382 Matched Pairs) |
1.16 | (0.87–1.56) | 0.31 | 1.18 | (0.59–2.38) | 0.64 |
Abbreviations: HR, hazard ratio; CI, confidence interval; SABR, stereotactic ablative radiation
Lobectomy is the referent group.
Independent variables for propensity score calculation include age, gender, comorbidity score, oxygen use, performance score, tumor size, use of PET staging, and use of mediastinal sampling. Patients were matched using nearest neighbor technique with caliper distance limited to 25% of the standard deviation of the pooled propensity scores. In all comparisons, independent covariables were balanced between treatments.
Independent variables for propensity score calculation include all available covariables. Patients were matched using nearest neighbor technique with caliper distance limited to 25% of the standard deviation of the pooled propensity scores. In all comparisons, independent covariables were balanced between treatments.
Independent variables for propensity score calculation include age, gender, comorbidity score, oxygen use, performance score, tumor size, use of PET staging, and use of mediastinal sampling. Patients were matched using nearest neighbor technique without specified caliper distance. Hazard ratios were adjusted for covariables whose standardized difference between cohorts was greater than 0.15.
Figure 1.
Outcomes for propensity-score matched cohorts treated with (A) lobectomy or sublobar resection and (B) lobectomy or SABR.
Comparison of Lobectomy and SABR
For overall survival, the proportional hazards assumption between lobectomy and SABR was violated. Therefore, a time-interaction term was introduced for the first 6 months following diagnosis and the period thereafter. In the initial six months, SABR was associated with a lower risk of death (HR 0.45; 95%CI 0.27–0.75; P<0.001) compared to lobectomy (Table 2). After the initial six months, SABR was associated with a higher risk of death (HR 1.66; 95%CI 1.39–1.99; P<0.001). For LCSS, SABR was associated with inferior outcomes (HR 1.44; 95%CI 1.03 – 2.02; P=0.03).
In PSM analysis – which restricted the comparison to well-matched cohorts characterized by very advanced age, more comorbid illness, increased use of oxygen, and low likelihood of mediastinal sampling (eTable 4) – the two modalities were associated with similar OS and LCSS (Table 4, Figure 1). Again, the PSM findings were unchanged in sensitivity analyses (Table 4).
Discussion
The adoption of widespread CT screening for lung cancer is expected to considerably increase the incidence of NSCLC in the United States. On the one hand, this development is to be applauded as well-executed studies confirm that screening is able to identify lung cancer at an earlier stage, and that a mortality benefit accrues from this timely identification of malignant nodules.1 On the other hand, screening, in conjunction with demographic headwinds, will present a challenge to the American health care system as more elderly individuals with comorbid illnesses such as COPD and coronary disease become diagnosed with NSCLC. Because the median age of lung cancer patients is 70 years, evidence is needed to guide clinical decision-making that balances both surgical risk and therapeutic efficacy in this population.
Recently, there has been increased enthusiasm for utilizing sublobar resection instead of the current standard, lobectomy, for elderly patients.25,26 Proponents of sublobar resection argue that the clinical trial upon which current standards of care are based, LCSG 821, was conceived and carried out in an era that is fundamentally different from the current one. To wit, modern imaging is able to identify ever-smaller tumors, and sublobar surgical techniques have improved to provide better local control outcomes than those observed in the limited resection arm of LCSG 821.4–8 Our study of outcomes among patients treated during the last ten years did not reinforce these arguments. In both traditional multivariable and propensity-score matched analysis, we found that sublobar resection was associated with worse lung cancer-specific survival and overall survival. Furthermore, this result was consistent if the analysis was limited to specific subsets of sublobar resection (VATS, segmentectomy) or to sub-populations for whom sublobar resection may be especially appropriate (patients older than 75 years, tumors less than 2 cm). These results reflect overall population outcomes and may underestimate the efficacy of formal anatomic segmentectomy at highly specialized centers of excellence. Still, these findings should give pause to the notion that, in general, sublobar resections are equally efficacious as lobectomy for elderly patients.27 This question will be definitively addressed in stage IA patients by Cancer and Leukemia Group B Trial 140503, but the results of this trial are not expected to be available until after 2020.12
Though our findings are concordant with LCSG 821, they are different than earlier SEER analyses of NSCLC patients treated before 2005, which found that lobectomy did not confer a survival advantage over sublobar resection in various subgroups of elderly patients.28–30 Several possibilities may explain the dissimilar findings. First, our data represents the latest iteration of SEER-Medicare and may reflect improved surgical technology and better perioperative care in the community over the last decade, which in turn may have narrowed perioperative differences between sublobar resections and full lobectomies. Secondly, methodological differences may account for the disparate conclusions. Whereas the earlier studies adjusted for five to ten baseline characteristics from the SEER registry, we incorporated 20 covariables and conducted multiple sensitivity analyses to address statistical uncertainties. We conjecture that this rich set of baseline data helped diminish confounding by indication.
We also examined outcomes associated with a newer technology, stereotactic ablative body radiotherapy (SABR). This technology, which utilizes precise delivery of high-dose radiation in a few sessions, was introduced during the study interval.31 Thus, we identified nearly 400 patients who underwent SABR during the initial adoption phase of the technology. The overall survival curve for these patients was characterized by two phases and was qualitatively different than the curves for surgical patients. In the first phase, these patients had better survival, possibly because they were spared the risk of perioperative mortality. Over the long-term, they had worse survival, perhaps because of their tendency to be octogenarians with multiple comorbidities or because of inferior local control with this modality. With regard to disease-specific survival, this two-phase pattern was not observed, and multivariable regression demonstrated higher risk of cause-specific mortality than lobectomy.
An important drawback to traditional multivariable analysis for comparing treatment effects in this context is that, in addition to their demographic differences, SABR patients were rarely staged pathologically. Therefore these patients may have harbored occult mediastinal disease that was not captured by clinical staging. To better adjust for this possibility, a secondary analysis with propensity-score matching was performed. This analysis compared lobectomy and SABR cohorts with balanced baseline characteristics and similar rates of pathological staging. It found no significant differences in overall survival or lung cancer-specific survival between the two treatment strategies. A caveat to this finding, however, is that its clinical relevance is restricted to patients well represented by the matched cohorts (ie, clinically staged patients with very advanced age and multiple comorbidities). The use of this analysis to rationalize SABR utilization instead of lobectomy in the general population of elderly patients with early stage NSCLC is not justified.
The matched comparison of SABR with lobectomy is similar to single institution studies32,33 and population-based analyses34,35 that retrospectively compared stereotactic radiation with surgery. Single-arm prospective trials of SABR in “operable” patients have also yielded efficacy similar to historical outcomes after surgery.36,37 Though this body of evidence is compelling, a definitive conclusion regarding the comparative effectiveness of SABR and surgery must be derived from randomized clinical trials. However, three major trials addressing this question have been terminated due to slow accrual.9–11 The promising outcomes observed among the SABR patients in this study will hopefully promote speedier recruitment in future comparative trials, especially in the elderly population.
Our study has several limitations. Confounders pertinent to the care of lung cancer patients including pulmonary function and performance status are not available in the SEER-Medicare registry. To address this limitation, proxy covariates including COPD status, supplemental oxygen use, and claims for medical assistance were utilized to approximate the traditional prognostic factors. A second limitation is the small sample size for the SABR cohort compared to the other two treatments, which reflects the fact that SABR was first introduced into practice during the study interval.38 A related issue is that outcomes associated with SABR during the earlier years of the study period may not reflect modern outcomes because specific quality measures, such as the minimum necessary biologically effective dose, had not yet been established. Finally, statistical adjustments are unable to fully account for confounding by indication in population-based analyses.39 Therefore, prospective trials are required to confirm the findings reported here.
In summary, our analysis of patients with early-stage NSCLC lung cancer in the contemporary period supports lobectomy as the optimal surgery for older adults able to undergo either lobectomy or sublobar resection. Our findings are also promising regarding the comparative effectiveness of SABR in frail patients with very advanced age, as this technology appears to offer a lower risk of periprocedural mortality and promising long-term survival.
Supplementary Material
Acknowledgments
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Dr. Benjamin Smith had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr. Benjamin Smith is supported by grants from the Cancer Prevention & Research Institute of Texas [Grant RP101207]. This work was also supported by the Department of Health and Human Services National Cancer Institute [Grants CA16672, T32CA77050]. Dr. Smith also reports research funding from Varian Medical Systems. These entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Dr. Anna Likhacheva reports research funding from Elekta Incorporated. This entity had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Dr. James Welsh reports a compensated, consultory role at Reflexion Medical. This entity had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Dr. Stephen G. Swisher reports a compensated, consultory role at GlaxoSmithKline. This entity had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
There are no other conflicts of interest to report.
Contributor Information
Shervin M Shirvani, Email: smshirvani@mdanderson.org.
Jing Jiang, Email: jjiang@mdanderson.org.
Joe Y. Chang, Email: jychang@mdanderson.org.
James Welsh, Email: jwelsh@mdanderson.org.
Anna Likhacheva, Email: alikhacheva@mdanderson.org.
Thomas A Buchholz, Email: tbuchhol@mdanderson.org.
Stephen G. Swisher, Email: sswisher@mdanderson.org.
References
- 1.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011 Aug 4;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009 Jun 10;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg R, Rubinstein L. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. The Annals of Thoracic Surgery. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Ucar AE, Nakas A, Pilling JE, West KJ, Waller DA. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2005 Apr;27(4):675–679. doi: 10.1016/j.ejcts.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007 Sep;84(3):926–932. doi: 10.1016/j.athoracsur.2007.05.007. discussion 932-923. [DOI] [PubMed] [Google Scholar]
- 6.El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg. 2006 Aug;82(2):408–415. doi: 10.1016/j.athoracsur.2006.02.029. discussion 415-406. [DOI] [PubMed] [Google Scholar]
- 7.Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg. 2009 Jun;87(6):1662–1666. doi: 10.1016/j.athoracsur.2009.02.097. discussion 1667–1668. [DOI] [PubMed] [Google Scholar]
- 8.Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. The Journal of thoracic and cardiovascular surgery. 2006 Oct;132(4):769–775. doi: 10.1016/j.jtcvs.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 9.A Randomized Phase III Study of Sublobar Resection (+/− Brachytherapy) versus Stereotactic Body Radiation Therapy in High Risk Patients with Stage I Non-Small Cell Lung Cancer (NSCLC) [Accessed on January 20, 2014]; http://clinicaltrials.gov/show/NCT01336894.
- 10.International Randomized Study to Compare CyberKnife Stereotactic Radiotherapy With Surgical Resection In Stage I Non-small Cell Lung Cancer (STARS) [Accessed on June 1, 2013]; http://clinicaltrials.gov/ct2/show/NCT00840749.
- 11.Trial of Either Surgery or Stereotactic Radiotherapy for Early Stage (IA) Lung Cancer (ROSEL) [Accessed on June 1, 2013]; http://clinicaltrials.gov/ct2/show/NCT00687986.
- 12.Comparison of Different Types of Surgery in Treating Patients With Stage IA Non-Small Cell Lung Cancer. [Accessed on July 1, 2013]; http://clinicaltrials.gov/show/NCT00499330.
- 13.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl) doi: 10.1097/01.MLR.0000020942.47004.03. IV-3-18. [DOI] [PubMed] [Google Scholar]
- 14.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993 Oct;46(10):1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 15.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000 Dec;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 18.Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010 May 1;28(13):2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 19.Smith BD, Pan IW, Shih YC, et al. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. J Natl Cancer Inst. 2011 May 18;103(10):798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 20.Doria-Rose VP, Marcus PM. Death certificates provide an adequate source of cause of death information when evaluating lung cancer mortality: an example from the Mayo Lung Project. Lung Cancer. 2009 Feb;63(2):295–300. doi: 10.1016/j.lungcan.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein JP, Moeschberger ML. Survival analysis techniques for censored and truncated data. New York: Springer; 2003. [Google Scholar]
- 22.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. American journal of epidemiology. 2006 Jun 15;163(12):1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine. 2009 Nov 10;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in medicine. 1998 Oct 15;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Donington JS. Point: are limited resections appropriate in non-small cell lung cancer? Yes. Chest. 2012 Mar;141(3):588–590. doi: 10.1378/chest.11-3108. discussion 593–584. [DOI] [PubMed] [Google Scholar]
- 26.Chamogeorgakis T, Ieromonachos C, Georgiannakis E, Mallios D. Does lobectomy achieve better survival and recurrence rates than limited pulmonary resection for T1N0M0 non-small cell lung cancer patients? Interactive cardiovascular and thoracic surgery. 2009 Mar;8(3):364–372. doi: 10.1510/icvts.2008.178947. [DOI] [PubMed] [Google Scholar]
- 27.Detterbeck FC. Counterpoint: are limited resections appropriate in non-small cell lung cancer? No: don't overdo it, and don't get confused. Chest. 2012 Mar;141(3):590–592. doi: 10.1378/chest.11-3110. discussion 592–593. [DOI] [PubMed] [Google Scholar]
- 28.Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest. 2005 Jul;128(1):237–245. doi: 10.1378/chest.128.1.237. [DOI] [PubMed] [Google Scholar]
- 29.Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest. 2011 Mar;139(3):491–496. doi: 10.1378/chest.09-2547. [DOI] [PubMed] [Google Scholar]
- 30.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Annals of surgery. 2010 Mar;251(3):550–554. doi: 10.1097/SLA.0b013e3181c0e5f3. [DOI] [PubMed] [Google Scholar]
- 31.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA : the journal of the American Medical Association. 2010 Mar 17;303(11):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2011 Feb 20;28(6):928–935. doi: 10.1200/JCO.2009.25.0928. [DOI] [PubMed] [Google Scholar]
- 33.Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery. 2010 Aug;140(2):377–386. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 34.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman B, Senan S. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2011 Nov;101(2):240–244. doi: 10.1016/j.radonc.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 35.Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. International journal of radiation oncology, biology, physics. 2012 Dec 1;84(5):1060–1070. doi: 10.1016/j.ijrobp.2012.07.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. International journal of radiation oncology, biology, physics. 2012 May 1;83(1):348–353. doi: 10.1016/j.ijrobp.2011.06.2003. [DOI] [PubMed] [Google Scholar]
- 37.Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? International journal of radiation oncology, biology, physics. 2011 Dec 1;81(5):1352–1358. doi: 10.1016/j.ijrobp.2009.07.1751. [DOI] [PubMed] [Google Scholar]
- 38.Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011 Oct 1;117(19):4566–4572. doi: 10.1002/cncr.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosco JL, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010 Jan;63(1):64–74. doi: 10.1016/j.jclinepi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.