Abstract
It is well known that immunoproteasome generates peptides for MHC Class I occupancy and recognition by cytotoxic T lymphocytes (CTL). The present study focused on evidence for alternative roles for immunoproteasome. Retina and brain were analyzed for expression of immunoproteasome subunits using immunohistochemistry and western blotting under normal conditions and after injury/stress induced by CTL attack on glia (brain) or neurons (retina). Normal retina expressed substantial levels of immunoproteasome in glia, neurons, and retinal pigment epithelium. The basal level of immunoproteasome in retina was two-fold higher than in brain; CTL-induced retinal injury further up-regulated immunoproteasome expression. Immunoproteasome up-regulation was also observed in injured brain and corresponded with expression in Purkinje cells, microglia, astrocytes, and oligodendrocytes. These results suggest that the normal environment of the retina is sufficiently challenging to require on-going expression of immunoproteasome. Further, immunoproteasome up-regulation with retinal and brain injury implies a role in neuronal protection and/or repair of damage.
Keywords: brain, immunoproteasome, neuronal injury, proteasome, retina
The proteasome is an intracellular protease complex that regulates processes essential for cell survival, including cell cycle regulation, control of signal transduction and gene expression, and protein quality control (Coux et al. 1996). The 20S proteasome makes up the catalytic core of all proteasome species (i.e., 26S, immunoproteasome) that are defined by both the composition of the 20S catalytic subunits and the association of various regulatory complexes, such as PA700 and PA28. Three subtypes of 20S catalytic cores have been described. The standard proteasome core contains the catalytic subunits β1, β2, and β5 and is the major core in tissues outside the immune system. These standard subunits can be replaced in nascent proteasomes with the inducible subunits, LMP2 (β1i), MECL (β2i), and LMP7 (β5i), which form the core of the immunoproteasome. The third type of core particle, the intermediate-type 20S proteasome, contains a mixture of the standard and immunoproteasome catalytic subunits (Dahlmann et al. 2000; Klare et al. 2007). Analysis of the three types of catalytic cores has shown that they differ substantially in their enzymatic characteristics and cleavage of model substrates (Dahlmann et al. 2000; Klare et al. 2007). Furthermore, while most cells contain a heterogenous population of 20S cores, the relative ratio of different subtypes is cell-specific (Dahlmann et al. 2000; Noda et al. 2000) and can be altered under different cellular conditions. For example, we have previously reported a three-fold up-regulation of the immunoproteasome subunits in aged muscle undergoing significant muscle atrophy (Husom et al. 2004; Ferrington et al. 2005). These findings support the hypotheses that the proteasome population is highly dynamic, and that each proteasome subtype may perform specialized functions that allow the cell to respond to changing conditions. Understanding how proteasome composition changes with specific environmental perturbations will provide valuable insight into the strategic functions of each proteasome subtype.
This study focused on evidence for alternative roles for the immunoproteasome in the brain and retina. While its role in generating immunogenic peptides for antigen presentation has been clearly established (Rock et al. 1994; Goldberg et al. 2002), the expression of immunoproteasome in non-inflamed immune-privileged tissue, such as retina (Louie et al. 2002; Kapphahn et al. 2007), brain (Diaz-Hernandez et al. 2003; Mishto et al. 2006), and lens epithelial cells (Singh et al. 2002) implies other non-immune functions are possible. Notably, the up-regulation of immunoproteasome in diseased retina (Ethen et al. 2007) and brain (Diaz-Hernandez et al. 2003; Mishto et al. 2006) suggests the immunoproteasome responds to challenges that induce stress and injury. In the current study, we show that in normal retina, immunoproteasome expression is approximately two-fold higher than in normal brain. In response to injury, immunoproteasome is significantly up-regulated in both retina and brain. These results suggest additional non-immune functions for the immunoproteasome that provide protection from damage and/or facilitate repair of injured tissue.
Materials and methods
Animals
The two beta-galactosidase (β-gal) transgenic mice (B10.A background) have been described (Gregerson et al. 1999; Gregerson and Xiao 2001; McPherson et al. 2003). β-gal expression in the rod photoreceptor cells of hi-arr-β-gal mice is under control of the arrestin promotor (Kikuchi et al. 1993; Gregerson and Dou 2002). The GFAP-β-gal mice express β-gal in astrocytes of brain, retina, and optic nerve, under the control of the glial fibrillary acidic protein (GFAP) promoter (Johnson et al. 1995). Mice used in this study were 2–3 months old. Mice were handled in accordance with guidelines of the Institutional Animal Care and Use Committee of the University of Minnesota and the National Institutes of Health.
Generation, activation, and transfer of β-gal-specific CD8 T cells
CD8 T cells specific for an immunodominant, H-2Ld-restricted epitope of β-gal (TPHPARIGL) (Dick et al. 1994), were prepared from vaccinia virus VSC 56-vaccinated female B10.A mice. Use of the virus, and generation and maintenance of the T cells were carried out as previously described (McPherson et al. 2003; Ferrington et al. 2006; Gregerson et al. 2006). For i.v. inoculations (1–15 × 106 cells), the cells were resuspended in phosphate-buffered saline at 5 × 107 cells/mL.
Immunohistochemistry
Eyes and brains were removed and either immediately snap-frozen in Tragacanth (Sigma, St. Louis, MO, USA) or tissues were fixed by perfusion with 4% paraformaldehyde and cryoprotected with 30% sucrose. Prior to antibody labeling, tissue sections (12 μm) were fixed in cold acetone for 30 min and washed in phosphate-buffered saline. Non-specific immunoglobulin binding was blocked for 30 min with normal donkey serum. The tissue sections were stained with the primary antibody (Table 1) for 30 min at 23°C, then incubated with biotinylated secondary antibody for 30 min followed by incubation with an avidin–biotin complex (Vector Laboratories, Burlingaine, CA, USA). Antibody binding was visualized using 3′, 3′-diaminobenzidine as the chromogen (DAB kit, Vector Laboratories). To confirm the specificity of the primary antibody, controls included pre-absorption with the corresponding synthetic peptide or omission of the primary antibody. Some sections were lightly counterstained with nuclear fast red (Vector Laboratories).
Table 1.
Antibodies used for immunohistochemistry or western immunoblotting
| Antibodya | Typeb | Assayc | Dilution | Company |
|---|---|---|---|---|
| 20Sα5 | P | I | 1 : 1000 | Affinity BioReagents, Golden, CO, USA |
| 20Sα7 | M | W | 1 : 1000 | Biomol, Plymouth Meeting, PA, USA |
| 20Sβ1i (LMP2) | M | W | 1 : 1000 | Biomol |
| 20Sβ5i (LMP7) | P | I | 1 : 1000 | Biomol |
| 20Sβ5i (LMP7) | P | W | 1 : 1000 | Biomol |
| 20Sβ1 (20SY) | P | W | 1 : 1000 | Affinity BioReagents |
| 20Sβ5 (20SX) | P | W | 1 : 1000 | Affinity BioReagents |
| 20Sβ5 (20SX) | P | I | 1 : 200 | Affinity BioReagents |
| Arrestin (A9C6) | M | I | 1 : 1000 | Dale Gregerson, University of Minnesota, USA |
| GFAP | M | I | 1 : 2000 | Chemicon, Temecula, CA, USA |
| GFAP | M | W | 1 : 1000 | Chemicon |
| MBP | M | I | 1 : 500 | Chemicon |
| CD45 | M | I | 1 : 20 | Biolegend, San Diego, CA, USA |
Glial fibrillary acidic protein (GFAP), myelin basic protein (MBP);
Monoclonal (M), polyclonal (P);
Immunohistochemistry or Immunofluorescence (I), Western blotting (W).
Immunofluorescence
For co-localization experiments, sections were incubated for 1 h with blocking reagent followed by an overnight staining with the primary antibody (Table 1). After washing, the sections were incubated for 2 h in the dark with the corresponding fluorescent secondary antibody (Rhodamin Red, 1 : 200, Jackson ImmunoResearch, West Grove, PA or Alexa Fluor 488, 1 : 200, Molecular Probes, Carlesbad, CA, USA). For double labeling, sections underwent a further cycle of primary and secondary staining. After washing, the slides were cover slipped with Immuno Mount (Thermo Electron Corporation, Waltham, MA, USA). Images were captured using the Bioquant Nova Prime V6 software (Nashville, TN, USA).
Comparison of proteasome content
To compare the relative content of immunoproteasome in the retina and brain, minimal sample processing was used to capture essentially all proteasomes. To minimize day-to-day assay variability and differences between animals, tissues from individual mice were analyzed in parallel, with paired t-tests used to test statistical differences between tissues. Both retinas and the brain from each mouse were homogenized in 5× the tissue volume in DNase buffer (20 mM Tris (pH 7.0), 1 mM CaCl2, 5 mM MgCl2, 150 units/mL DNase) using a glass homogenizer with a Teflon pestle and incubated for 20 min. The sample was then diluted to 25× the original tissue volume in 20 mM Tris (pH 7.0), 6 M urea, 2% sodium dodecyl sulfate and homogenized. Protein concentrations were determined using the Bicinchoninic Acid Protein Assay (Pierce, Rockford, IL, USA) with bovine serum albumin as the standard. Multiple lanes containing increasing amounts of protein from either the retina or brain (20–60 μg) were resolved using a 13% sodium dodecyl sulfate gel, transferred to a polyvinylidene difluoride membrane, and probed with antibodies to α7, LMP2, LMP7, β1, and β5. Immunoblots were imaged using a ChemiDoc (BioRad, Hercules, CA, USA). Densitometry was performed on the immune reactions using Quantity One (BioRad) and density was plotted as a function of protein load. The relative content of individual proteasome subunits was determined by comparing the slope of the immune reactions (density/μg protein) for the retina and brain from each mouse. A paired t-test was performed comparing slopes from the retina and brain, with the level of significance set at p ≤ 0.05.
Sample preparations
Retinas were processed as outlined (Louie et al. 2002; Kapphahn et al. 2006, 2007), using an homogenization buffer containing 20 mM Tris (pH 7.4), 20% sucrose, 2 mM MgCl2, 10 mM glucose, and 2% 3-[3-cholamidopropyl] dimethylammonio-2-hydroxy-1-propanesulfonate (CHAPS). The supernatant containing soluble retinal proteins from the final step of processing was retained. Brains were homogenized in the retina buffer and centrifuged at 4000 g at 4°C for 20 min. Pellets were rehomogenized, centrifuged, and supernatants from the two spins were combined and centrifuged at 11 800 g for 20 min. The supernatant was then centrifuged at 100 000 g for 16 h and the pellet was suspended in 20 mM Tris (pH 7.5), 5 mM MgCl2, and 20 mM KCl. Aliquots of protein from the final processing step for retina and brain were stored at −80°C. Protein concentration was determined using the Bicinchoninic Acid Protein Assay.
Western blotting
Western blotting was performed as described (Kapphahn et al. 2006, 2007). A sample of 20S purified from liver was run along with samples on each blot. Membranes were incubated for 16 h at 4°C with one of the primary antibodies (Table 1). Appropriate secondary antibodies were used in conjunction with chemiluminescence to visualize the immune reactions.
Immunoblots were imaged using a ChemiDoc (BioRad) and quantified using Quantity One (BioRad). Samples were normalized to a reference sample run on each blot. To insure that the β-subunits were incorportated into the mature 20S complex, the migration of the β-subunits in samples were aligned with the β-subunits of 20S proteasome purified from liver that was run on each blot. The relative content of proteasome subunits or GFAP in tissue post-cytotoxic T lymphocytes (CTL) injury is plotted relative to the control tissue. Note that the third immunoproteasome catalytic subunit MECL is not included in our analysis because the specificity of the antibody was not adequate.
Proteasome activity measurements
Proteasome activity was measured using 75 μM LLVY-AMC (EMD Biosciences, San Diego, CA, USA) as the fluorogenic peptide substrate (Kapphahn et al. 2007).
Statistical analysis
Immunoproteasome content in retina and brain (Fig. 1) was compared using a paired t-test. Other analyses for testing statistical significance between treatment groups used a Student’s unpaired t-test analysis or analysis of variance (ANOVA) with a Tukey post hoc test. Statistical software in Origin v. 7.5 was used for the analysis. The level of significance was set at p ≤ 0.05. Data are reported as mean ± SEM for all groups.
Fig. 1.
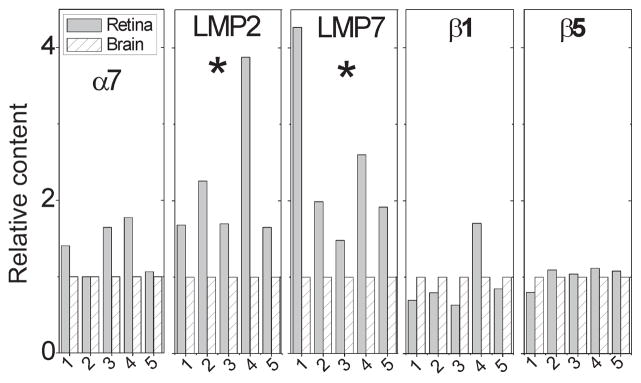
Quantitative analysis of proteasomal subunits in murine retina and brain. Western blotting was used to determine the relative content of the α7 subunit and the catalytic subunits for the immunoproteasome (LMP7, LMP2) and standard proteasome (β1, β5) in retina and brain from five mice (labeled 1–5). Relative content of proteasome subunits is the retinal reaction density normalized to the brain reaction density for each mouse. * Retina significantly higher than brain by paired t-test, p ≤ 0.05.
Results
Immunoproteasome in the retina and brain
There are many similarities between retina and brain, especially with respect to parenchymal composition (i.e., neurons, glia, microglia) and the blood-tissue barriers. However, there are also substantial differences, including the retina’s exposure to light, the shedding and degradation of spent tips of photoreceptors, and the rapid recycling of bleached photoreceptor molecules (Burnside and Bost-Usinger 1998). These conditions place a heavy burden of environmental stress and metabolic demands on the retina; consequently, we predicted that endogenous levels of retinal immunoproteasome would be higher than in brain. To test this hypothesis, proteasomal subunit content in the retina and brain was analyzed by western blotting using subunit-specific antibodies. Since the α-subunits are present in the 20S catalytic core of all proteasome subtypes, the immune reaction of the α7 subunit provided a measure of the total proteasome content. Blots were also probed for two immunoproteasome (LMP2, LMP7) and standard (β1, β5) catalytic subunits.
To evaluate the content of immunoproteasome in retina and brain, minimal tissue processing (i.e., homogenization in buffers containing Dnase and detergents without centrifugation) was used so that essentially all protein in the tissue could be captured. To minimize the effect of variability between animals, subunit content was measured in retina and brain from five individual mice and paired t-tests were performed to compare the relative tissue content of immunoproteasome (Fig. 1). Based on densitometry of the α7 immune reaction, total proteasome content was slightly higher in the retina compared with brain (1.38 ± 0.15), but this increase did not reach statistical significance (p = 0.12). When calculating the relative amount of immunoproteasome, the content of LMP2 and LMP7 was consistently higher in the retina of all five mice. Comparing retina to brain, the relative content of LMP2 and LMP7 was elevated 2.2 ± 0.4 (p = 0.05) and 2.5 ± 0.5 (p = 0.001)-fold, respectively. The content of β1 and β5 were not different from retina and brain. These results show that there was approximately two-fold more immunoproteasome in the retina, consistent with our hypothesis that endogenous levels of retinal immunoproteasome are higher than in brain.
Localization of retinal immunoproteasome
To determine if immunoproteasome was localized to specific regions of the retina, immunohistochemistry was performed on retinal sections. Using antibody specific for the α5 subunit to track all proteasome subtypes, diffuse staining was observed throughout the retina (Fig. 2a), which was expected based on proteasome’s ubiquitous presence in all cells. The notably darker staining in the photoreceptor cell inner segments (IS) suggests higher total proteasome content in this region. Specificity of the reaction was shown in control experiments where the antigenic peptide of the α5 subunit was incubated with the α5 antibody prior to staining led to the loss of staining. Omission of the primary antibody further confirmed the specificity (Fig. 2b and c). Retinal sections stained with anti-β5 antibody showed a pattern of staining identical to the α5 antibody (data not shown).
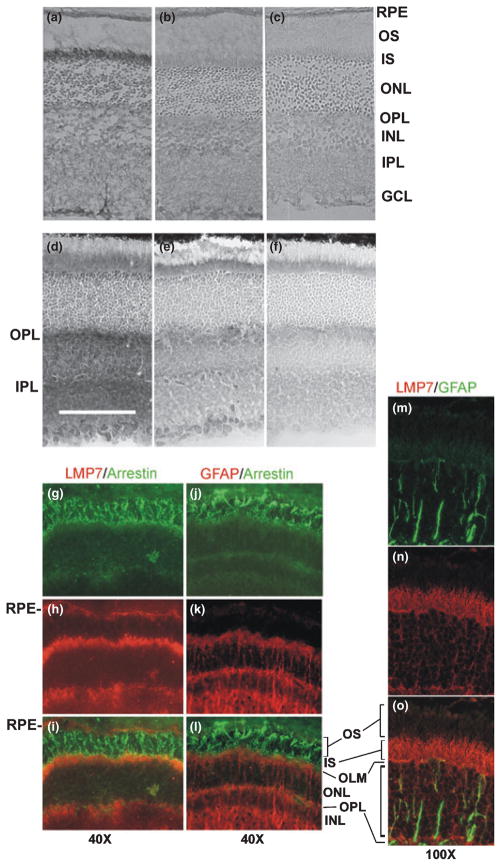
Fig. 2.
Immunolocalization of retinal immunoproteasome. (a) Retinal sections stained with an antibody that recognizes the α5 subunit showed the distribution of all proteasome subtypes. The specificity of the antibody was demonstrated by the inhibition in staining when antibody is pre-incubated with the antigenic peptide (b) or without the primary antibody (c). (d) Retinal sections stained with an antibody specific for the LMP7 subunit showed the distribution of the immunoproteasome. The specificity of the LMP7 antibody is demonstrated by the inhibition in staining when antibody was pre-incubated with 20S immunoproteasome purified from spleen (e) or when the primary antibody was omitted (f). Bar in (d) indicates 100 μm. Micrographs (a–f) were taken at a magnification of 10×. (g–o) Immunofluorescence of retinal sections stained with antibodies specific for the immunoproteasome (LMP7), photoreceptors (arrestin), and Mueller cells (GFAP). (g) Anti-arrestin (green) labeled the photoreceptor cells from the OS to OPL. (h) Anti-LMP7 (red) intensely stained the RPE, OS and IPL. (i) Overlay showing the IS stain yellow/orange, indicated co-localization of arrestin and immunoproteasome. (j) Anti-arrestin (green) shows labeling as in G. (k) Anti-GFAP (red) staining the Mueller cells, including the Mueller end feet in the region of the IS. (l) Overlay showed that separate cell populations of photoreceptors and Mueller cells are distinctly labeled with very little overlap. (m) Anti-GFAP (green) stained for Mueller cells between the IS and OPL. (n) Anti-LMP7 (red) stained immunoproteasome. (o) Immunoproteasome staining was concentrated in the inner segments. The OLM was labeled yellow, which suggests that the Mueller cell end feet contain some immunoproteasome. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; IS, inner segments; OLM, outer limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer;. OS, outer segments; RPE, retinal pigment epithelium.
For immunoproteasome, intense staining with the LMP7 antibody was less concentrated in the IS and more heavily stained in the inner and outer plexiform layers (IPL, OPL) (Fig. 2d). The specificity of the anti-LMP7 reaction was confirmed by the difference in intensity when the antibody was pre-incubated with 20S immunoproteasome purified from spleen, or the primary antibody was omitted (Fig. 2e and f). Since the IS region also contains the Mueller cell end feet, we double-labeled retinal sections with antibodies to GFAP to stain the Mueller cells, or arrestin to stain photoreceptors, to further define the cell type containing the immunoproteasome. The absence of overlapping stains for GFAP and arrestin show that distinct cell populations can be labeled (Fig. 2j–l). Conversely, co-localization of arrestin and LMP7 confirms immunoproteasome is present in the inner segments (Fig. 2g–i). Additionally, robust staining for LMP7 is also observed in the retinal pigment epithelium. In Fig. 2m–o, minimal overlap in staining for LMP7 and GFAP is seen, including in the outer limiting membrane, suggesting immunoproteasome is present, albeit in very low abundance in the Mueller end feet and is concentrated in the photoreceptor cell inner segments.
Up-regulation of retinal immunoproteasome following injury from cytotoxic T lymphocytes
The hypothesis is that immunoproteasome plays a role in protecting from damage and/or repairing injury. Therefore, the prediction is that immunoproteasome will be up-regulated in response to challenges that induce injury. To test this idea, the retina was injured using an experimental model that mimics autoimmune retinitis, where photoreceptor injury is mediated by CTL specific for beta-galactosidase (β-gal). Previously, we showed loss of photoreceptors following CTL injection into transgenic mice expressing β-gal in photoreceptor cells (McPherson et al. 2003, 2006). In the current experiments, the protocol included sacrificing β-gal transgenic mice at 17–21 days after injection of CTLs. At this time, the initial inflammation and photoreceptor cell injury has occurred and the active inflammation has resolved. Staining of retinal sections with anti-LMP7 and nuclear fast red counterstain showed the expected retinal morphology and pattern of staining for immunoproteasome in untreated mice (Fig. 3a). In β-gal transgenic mice treated with CTLs, complete loss of the photoreceptor layer and intense overall staining with the anti-LMP7 antibody was observed (Fig. 3b).
Fig. 3.
Immunoproteasome up-regulation in CTL-injured retina. (a and b) Retinal sections were stained with anti-LMP7 and counterstained with nuclear fast red in control mice (a) and following 21 days after injection of CTLs into transgenic mice expressing β-gal in the photo-receptors (b). Both images were taken with a 20× objective. Scale bar: 100 μm. INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, outer segments. (c) Western immunoblot probed for proteins to evaluate retinal stress (GFAP), and the content of total proteasome (α7), and catalytic subunits of immunoproteasome (LMP7, LMP2) and standard proteasome (β1, β5). Protein load was 35 μg per lane. (d) Summary of western blot densities. Relative density is the average density for each group normalized to the control (untreated) density. Data are mean ± SEM. N = 4 per group. *Significantly different by t-test, p ≤ 0.05.
The content of GFAP was used as a marker of retinal stress or injury. This protein is expressed mainly in glial cells and was previously reported to be up-regulated in the injured retina (Chen and Weber 2002). Western blotting of retinal homogenates showed GFAP content was increased ~13-fold over control levels (Fig. 3c and d). This level of stress response is consistent with the significant damage observed on retinal sections. The reaction for proteasomal subunits showed that both LMP2 and LMP7 subunits were up-regulated approximately four-fold, while content of α7, β1, and β5 remained unchanged. Measurement of total proteasome activity in retinal homogenates was maintained; activity for control and CTL-treated mice was 16 ± 1 pmol/mg/min and 15 ± 1 pmol/mg/min, respectively. Taken together, these results suggest up-regulation of immunoproteasome content as a mechanism for maintaining proteasome-dependent functions following retinal injury.
Immunoproteasome up-regulation in the brain following CTL-induced injury
We extended our investigation of immunoproteasome to the brain to determine if the CTL injury-induced up-regulation was unique to the retina or was a more universal response in other immune-privileged tissue. The CTLs were injected into transgenic mice expressing β-gal in brain astrocytes. Mice were killed during the time of acute onset of CTL-mediated attack (day 6 post-CTL-transfer) and after active inflammation had subsided (day 21 post-CTL-transfer).
To evaluate proteasome changes in response to CTL-induced injury, proteasome activity was measured using a fluorogenic peptide substrate, and western immunoblotting was performed to evaluate the complement of proteasomal subunits. Proteasome activity demonstrated a significant 1.7-fold increase during the acute phase of attack (day 6) but returned to control values by 21 days post-CTL injection (Fig. 4, left panel). Results from western immunoblots showed GFAP content was increased two- to six-fold post-CTL-transfer, indicating a stress response that was elicited by CTL-induced injury continued and was magnified after active inflammation had subsided (Fig. 4, right panel). Quantitative assessment of LMP2 and LMP7 indicates content is increased 2.8- and 1.9-fold, respectively, at day 6 post-injection. Immunoproteasome content returned to near normal values by 21 days post-injection. Total proteasome content, estimated from the α7 reaction, increased 1.8-fold by 21 days. There was no significant change in the β1 and β5 subunits. These results show that, like the retina, immunoproteasome can be up-regulated in the brain in response to injury.
Fig. 4.

Immunoproteasome up-regulation in CTL-injured brain. (left panel) Proteasome activity measured using the LLVY-AMC peptide substrate in controls, and at 6 and 21 days post-CTL injection. *p = 0.005, 6 days different than control and 21 days. (right panel) Summary of densitometry from Western blots probed for proteins to evaluate stress (GFAP), total proteasome content (α7), and catalytic subunits of immunoproteasome (LMP7, LMP2) and standard proteasome (β1, β5). Relative density is the average density for each group normalized to the control density. Symbols indicate significant differences by Tukey’s post hoc analysis; *control different than 21 days, ** control different than 6 days, @ 6 days different than 21 days. Data are mean ± SEM. N=6 (control), 5 (6 days), and 3 (21 days).
Immunohistochemistry was performed to determine if immunoproteasome was localized to specific brain regions and specific cells. In non-injured brain, very minimal anti-LMP7 staining of parenchymal tissue and a few anti-LMP7 positive cells in the meninges were observed (Fig. 5i and j). Positive staining in the meninges is expected because of the presence of many CD11b+ immune cells (Fig. 5a). CD11b+ microglia were present in the parenchyma, but not readily observed in thin sections (Fig. 5b). During the acute phase of attack (day 6), there was significant staining with anti-LMP7 antibody, indicating immunoproteasome was up-regulated in the leptomeninges, in active lesions, and in parenchymal tissue distant from lesions (Fig. 5k–m). Part of this staining was due to activation and injury of microglia, as well as the recruitment of inflammatory cells (Fig. 5c–e). Interestingly, anti-LMP7 staining of the leptomeninges returned to normal levels by 21 days post-injury while remaining highly positive for CD11b+ cells (Fig. 5n and f). However, anti-LMP7 positive cells were often more prominent and widespread in the damaged lesion than were the anti-CD11b+ cells (Fig. 5o and g). This result raised the possibility that non-bone marrow-derived cells were expressing LMP7, as seen in the normal retina.
Fig. 5.
Immunoproteasome up-regulation in specific regions of CTL-injured brain. Brain sections stained with anti-CD11b (a–h), anti-LMP7 (i–p), or secondary antibody only (q–s) showing the leptomeninges, sites of active lesions, and parenchymal tissue far from lesions. Mice were either untreated (Normal) or treated with CTLs and examined at 6 days post-transfer (6 days) when acute inflammation was ongoing or at 21 days post-transfer (21 days) when inflammation had subsided.
To estimate the contribution of the immune cells in the meninges to the overall content of immunoproteasome in the brain, we compared the immune densities of LMP2 and LMP7 in preparations from either the entire brain (pia/leptomeninges plus parenchymal tissue) or from brain tissue from which the pia was removed in normal (untreated) mice. We found the densities for immunoproteasomal subunits were only 25% lower in preparations in which pia was removed (data not shown). These results suggest that while the meninges contained significant amounts of immunoproteasome, non-bone marrow-derived elements of the parenchymal tissue also contained measurable amounts of immunoproteasome.
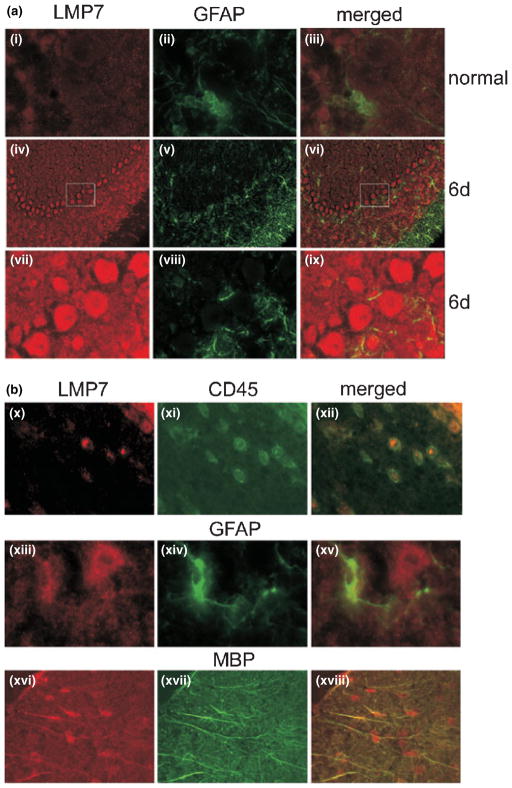
To more clearly define the cell-type contributing to the increased staining in CTL-injured brain, brain sections were double-labeled with anti-LMP7 and antibodies to proteins specific for astrocytes (GFAP), bone marrow-derived cells (CD45), and oligodendrocytes (myelin basic protein, MBP) (Fig. 6). In the cerebellum, where the majority of the CTL-induced damage has been previously observed (McPherson et al. 2006), minor staining with anti-LMP7 is observed in normal mouse brain (Fig. 6a-i–iii). In the injured brain, a robust pattern of staining with anti-LMP7 is observed in the cerebellar loops under low magnification (Fig. 6a-iv–vi). At higher magnification, intensely stained individual cells are obvious (Fig. 6a-vii–ix). Based on their location and appearance, these anti-LMP7 positive/GFAP negative cells in the acutely inflamed brain are Purkinje cells.
Fig. 6.
Localization of immunoproteasome to specific non-immune cells in the brain. (a) Staining with anti-LMP7 and GFAP in the cerebellum of normal mice (i–iii) and mice 6 days post-CTL injection (iv–ix). Panels iv–vi are low magnification images of the cerebellar region showing intense staining of the Purkinje cells with anti-LMP7. High magnification images from the boxed area are shown in panels vii–ix. Magnification was 100× (i–iii, vii–ix) and 20× (iv–vi). (b) Staining of brain sections in a mouse 6 days post-CTL injection. Co-localization of staining for anti-LMP7 and cell specific antibodies show immunoproteasome is found in bone marrow-derived immune cells (CD45 positive) and astrocytes (GFAP positive) in the cerebellum, and oligodendrocytes (MBP positive) in the stria. Magnification was 100× (x–xii), 53× (xiii–xv), and 63× (xvi–xviii). GFAP, glial fibrillar acidic protein; MBP, myelin basic protein.
Other cells examined in the cerebellum include bone marrow-derived immune cells and astrocytes. The expected co-localization of anti-CD45 with anti-LMP7 in immune cells was observed (Fig. 6b-x–xii). Co-localization of anti-GFAP and anti-LMP7 was also observed (Fig. 6b-xiii–xv) indicating that astrocytes also express immunoproteasome in the injured brain. Notably, only one of the two adjacent anti-LMP7-positive cells stained with anti-GFAP, indicating multiple cell types contain immunoproteasome. Micrographs from the stria brain region stained with anti-LMP7 and anti-MBP showed that oligodendrocytes also contained immunoproteasome (Fig. 6b, xvi–xviii). These results show immunoproteasome was up-regulated in multiple non-immune cells in the injured brain.
Discussion
In this study, we investigated the possibility that the immunoproteasome performs functions unrelated to its well-characterized role in generating immunogenic peptides for presentation to CD8 T cells as part of immune surveillance. Our hypothesis is that immunoproteasome’s alternative roles include protecting from injury and repairing damage. In the current study, we used injury protocols that evoked damage in the retina and brain. Our in vivo data provide convincing evidence through both increased staining of tissue sections and immune reactions on western blots that injury induces an up-regulation in immunoproteasome content in both retina and brain that was not limited to immune cells.
A critical distinction is whether the increases in immunoproteasome are solely because of the cellular elements of the innate and adaptive immune systems, or if the increases can be attributed in part to non-myeloid components of retina and brain. The retina contains CD11b+ microglia that resemble, morphologically and phenotypically, the CD11b+ microglia of brain (Gregerson and Yang 2003; Gregerson et al. 2004). Myeloid-derived cells have the well-known ability to up-regulate immunoproteasome in response to numerous stimuli. Cultured CNS microglia were found to express mainly the standard proteasome subunits with very low LMP2 and LMP7, but no MECL (Stohwasser et al. 2000). Addition of interferon gamma (IFNγ) or endotoxin-induced expression of MECL, but LMP7 was unchanged. Microglia play a critical role in maintaining tissue homeostasis, especially after injury. For example, optic nerve crush and light-induced retinal degeneration lead to microglial activation and migration to the ganglion cell layer and outer nuclear layer, respectively, where dying retinal cells are phagocytosed (Moore and Thanos 1996; Panagis et al. 2005; Zhang et al. 2005). While the contribution of cells of the innate immune system to total immunoproteasome complement cannot be ignored, the microglia, even under conditions of significant injury, are drastically limited in number relative to the total cellular content of the retina. We also presented evidence showing immunoproteasome is present in neurons (photoreceptor and Purkinje cells), glia (Mueller cells and astrocytes), and oligodendrocytes and conclude that these non-immune cells produce a substantial portion of the immunoproteasome.
Immunoproteasome in the retina and brain
Although immunoproteasome structure and function have been extensively studied in the immune system, a paucity of information is available for immunoproteasome in the CNS. Our laboratory provided the first reports of readily detectable levels of LMP2 and LMP7 in both rat and human retina (Louie et al. 2002; Ethen et al. 2007; Kapphahn et al. 2007). Studies of immunoproteasome subunits in homogenates of young, non-diseased human, rodent, and bovine brains were consistent with our observation that immunoproteasome is expressed in low amounts in the uninjured brain of young mice (Noda et al. 2000; Diaz-Hernandez et al. 2003; Mishto et al. 2006). In parallel analysis of proteasome subunits, our results show that while the standard proteasome subunits β1 and β5 in the brain and retina are approximately equal, the relative proportion of LMP2 and LMP7 immunoproteasomal subunits were ~two-fold higher in the normal retina compared with normal brain. This is despite the presence of the meninges in the brain, especially the pia, which is rich in cells of the innate immune system that could contribute to brain immunoproteasome content (McMenamin 1999).
It is important to mention that our measures of β-subunit content included only subunits that were integrated into the mature 20S particle. Newly synthesized β-subunits contain a pro-peptide that is cleaved upon incorporation into the 20S core (Chen and Hochstrasser 1996), and therefore, the unprocessed and processed subunits can be distinguished by their difference in migration on high percentage polyacryl-amide gels. Alignment of β-subunits in samples with the β-subunits in 20S purified from liver was done on each western blot to ensure that we were measuring only subunits integrated into the mature 20S particle. In contrast, the α-subunits do not contain a pro-peptide, so our measures of the α7 subunit include both subunits incorporated into the 20S core and those that have not incorporated. The inability to discriminate between the ‘free’ and incorporated α7 subunits could explain why there is not complete correspondence between changes in β-subunits and α7 content.
This study and others have shown that disease and injury can up-regulate immunoproteasome content in the CNS. Retinal injury induced by CTLs resulted in a four-fold increase in both LMP2 and LMP7, which is consistent with the reported increase in retinal immunoproteasome subunit content in diseased human retinas with age-related macular degeneration (Ethen et al. 2007). With brain injury induced by CTLs, a two-fold increase in both LMP2 and LMP7 was observed. Increased expression of the immunoproteasomal subunits has been reported in the hippocampus of human brain with aging and with Alzheimer’s disease (Mishto et al. 2006), in HD94 mice, a model of Huntington’s disease (Diaz-Hernandez et al. 2003), and a four-fold increase in only the LMP7 subunit after traumatic brain injury in rats (Yao et al. 2007).
Proteasome activity was measured to determine how the injuries affected function. In the brain, proteasome activity increased at early injury (6 days), then returned to control levels at late injury (21 days). In retina, activity was measured only at late injury and showed proteasome activity was equal between control and treated retina, which is consistent with our results from late injury in the brain. A limitation of using peptide hydrolysis as a measure of proteasome activity is that it does not distinguish between different proteasome subtypes and therefore, the contribution of immunoproteasome to total proteasome activity is not known. However, our results suggest up-regulation of immunoproteasome content is a mechanism for maintaining proteasome function following injury.
To study the cellular distribution of immunoproteasome, immunohistochemistry was used to localize the cells showing immunoproteasome expression. In control retinas, immunoproteasome staining was heaviest in the retinal pigment epithelium, and specific neuronal regions (i.e., IS, OPL and IPL). The localization of immunoproteasome to specific retinal regions may provide important insight regarding its non-immune alternative roles.
Staining for immunoproteasome in injured brain was mostly in the leptomeninges and in lesions where astrocytes, microglia, oligodendrocytes, and neuronal Purkinje cells stained intensely for immunoproteasome. Other laboratories have reported immunoproteasome staining in neurons, astrocytes, and the vascular endothelium of elderly and diseased brains and in degenerating neurons in the cortex and striatum of Huntington’s disease patients (Diaz-Hernandez et al. 2003; Mishto et al. 2006). The mechanisms responsible for inducing immunoproteasome expression in injured, aged, or diseased tissue has not been defined, but may reflect the occurrence of oxidative stress and inflammation that are common to these conditions. The induction of immunoproteasome subunits in cultured neurons following peroxide treatment is consistent with oxidative stress as a potential regulator of immunoproteasome expression (Ding et al. 2003).
Regulation of gene expression
Induction of immunoproteasome subunit expression by IFNγ has been well established for both cultured immune cells (Akiyama et al. 1994; Nandi et al. 1996) and cultured non-immune cells, such as neurons (Diaz-Hernandez et al. 2004) and epithelial cells of the retina (Gregerson et al. 2006). This cytokine-induced expression results from binding of the Stat transcription factor to multiple interferon gamma consensus/activation sequences in the promoter region of the LMP2, LMP7, and MECL-1 genes (Cruz et al. 1997; Chatterjee-Kishore et al. 1998; Yawata et al. 2001). However, using IFNγ−/− mice, Barton et al. (2002) showed that constitutive expression of immunoproteasome occurred independent of IFNγ under basal conditions. The presence of promoter consensus sequences for multiple transcription factors that are not regulated by IFNγ (James et al. 2006; Zanelli et al. 1993) provides additional evidence for a cytokine-independent regulation.
Potential alternative roles
The induction of immunoproteasome in the CNS under conditions of disease and injury suggest roles in protecting from injury and repairing damage. One possibility is that immunoproteasome is more efficient at degrading specific protein substrates that are present in greater abundance as a result of injury or disease. For injury that leads to increased production of free radicals, immunoproteasome may be involved in protecting the cell by efficiently degrading oxidized proteins. Consistent with this idea, oxidized proteins accumulate in the brain and liver of LMP2 deficient mice (Ding et al. 2006).
Immunoproteasome may perform roles related to normal cell functions, such as cell signaling or synaptic remodeling. Data from cultured lymphocytes of LMP2−/− mice and in splenocytes from non-obese diabetic mice that were devoid of the LMP2 protein suggest an important putative role for LMP2 in activation of NF-κB. Cell lines lacking LMP2 showed defects in proteolytic processing of both the NF-κB precursor and IκB inhibitory protein, linking immunoproteasome with cell signaling (Hayashi and Faustman 1999, 2001).
The localized, intense staining for LMP7 in the retinal IPL and OPL, which are the sites of neuronal synapses, implies that immunoproteasome may participate in neuronal maintenance or synaptic vesicle formation. Although a role for distinct proteasome subtypes has not yet been defined, considerable evidence suggests proteasome modulates synaptic plasticity by translocating to the site of remodeling (Bingol and Schuman 2006) and degrading proteins in the synaptic junction (i.e., NMDA receptor subunits, glutamate transporters, post-synaptic density scaffolding molecules) (Boehmer et al. 2003; Colledge et al. 2003; Ehlers 2003).
In summary, we show that while immunoproteasome is present in both retina and brain, the content is approximately two-fold higher in the normal uninjured retina suggesting a role for immunoproteasome in maintaining retinal homeostasis. Furthermore, immunoproteasome is significantly up-regulated with injury/stress in vivo in both the retina and brain. These data support the idea that an alternative role for immunoproteasome is in protecting from injury and/or repairing damage in the CNS.
Acknowledgments
This work was supported by grants from the National Institutes of Health EY013623 (D.A.F.), EY011542 (D.S.G.), P30-EY11374 (Core Grant for Vision Research), and an unrestricted grant from the Research to Prevent Blindness, and the Minnesota Lions Clubs.
Abbreviations used
- ANOVA
analysis of variance
- CTL
cytotoxic T lymphocytes
- GCL
ganglion cell layer
- GFAP
glial fibrillary acidic protein
- IFNγ
interferon gamma
- INL
inner nuclear layer
- IPL
inner plexiform layer
- IS
inner segments
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- OS
outer segments
- RPE
retinal pigment epithelium
- β-gal
beta-galactosidase
References
- Akiyama K, Yokota K, Kagawa S, Shimbara N, Tamura T, Akioka H, Nothwang HG, Noda C, Tanaka K, Ichihara A. cDNA cloning and interferon gamma down-regulation of proteasomal subunits X and Y. Science. 1994;265:1231–1234. doi: 10.1126/science.8066462. [DOI] [PubMed] [Google Scholar]
- Barton LF, Cruz M, Rangwala R, Deepe GS, Monaco JJ. Regulation of immunoproteasome subunit expression in vivo following pathogenic fungal infection. J Immunol. 2002;169:3046–3052. doi: 10.4049/jimmunol.169.6.3046. [DOI] [PubMed] [Google Scholar]
- Bingol B, Schuman EM. Activity-dependent dynamics and sequestration of proteasomes in dendritic spines. Nature. 2006;441:1144–1148. doi: 10.1038/nature04769. [DOI] [PubMed] [Google Scholar]
- Boehmer C, Henke G, Schniepp R, Palmada M, Rothstein JD, Broer S, Lang F. Regulation of the glutamate transporter EAAT1 by the ubiquitin ligase Nedd4-2 and the serum and glucocorticoid inducible kinase isoforms SGK1/3 and protein kinase B. J Neurochem. 2003;86:1181–1188. doi: 10.1046/j.1471-4159.2003.01937.x. [DOI] [PubMed] [Google Scholar]
- Burnside B, Bost-Usinger L. The retinal pigment epithelial cytoskeleton. In: Marmore MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium. Oxford University Press; New York: 1998. pp. 41–67. [Google Scholar]
- Chatterjee-Kishore M, Kishore R, Hicklin DJ, Marincola FM, Ferrone S. Different requirements for signal transducer and activator of transcription 1 alpha and interferon regulatory factor 1 in the regulation of low molecular mass polypeptide 2 and transporter associated with antigen processing 1 gene expression. J Biol Chem. 1998;273:16177–16183. doi: 10.1074/jbc.273.26.16177. [DOI] [PubMed] [Google Scholar]
- Chen P, Hochstrasser M. Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell. 1996;86:961–972. doi: 10.1016/s0092-8674(00)80171-3. [DOI] [PubMed] [Google Scholar]
- Chen H, Weber AJ. Expression of glial fibrillary acidic protein and glutamine synthetase by Mueller cells after optic nerve damage and intravitreal application of brain-derived neurotrophic factor. Glia. 2002;38:115–125. doi: 10.1002/glia.10061. [DOI] [PubMed] [Google Scholar]
- Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coux O, Tanaka K, Goldberg A. Structure and function of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- Cruz M, Elenich LA, Smolarek TA, Menon AG, Monaco JJ. DNA sequence, chromosomal localization, and tissue expression of the mouse proteasome subunit lmp10 (Psmb10) gene. Genomics. 1997;45:618–622. doi: 10.1006/geno.1997.4977. [DOI] [PubMed] [Google Scholar]
- Dahlmann B, Ruppert T, Kuehn L, Merforth S, Kloetzel PM. Different proteasome subtypes in a single tissue exhibit different enzymatic properties. J Mol Biol. 2000;303:643–653. doi: 10.1006/jmbi.2000.4185. [DOI] [PubMed] [Google Scholar]
- Diaz-Hernandez M, Hernandez F, Martin-Aparicio E, Gomez-Ramos P, Moran MA, Castano JG, Ferrer I, Avila J, Lucas JJ. Neuronal induction of the immunoproteasome in Huntington’s Disease. J Neurosci. 2003;23:1653–1661. doi: 10.1523/JNEUROSCI.23-37-11653.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Hernandez M, Martin-Aparicio E, Avila J, Hernandez F, Lucas JJ. Enhanced induction of the immunoproteasome by interferon gamma in neurons expressing Huntingtin. Neurotox Res. 2004;6:463–468. doi: 10.1007/BF03033282. [DOI] [PubMed] [Google Scholar]
- Dick LR, Aldrich C, Jameson SC. Proteolytic processing of ovalbumin and beta-galactosidase by the proteasome to a yield antigenic peptides. J Immunol. 1994;152:3884–3894. [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Reinacker K, Dimayuga E, Nukala V, Drake J, Butterfield DA, Dunn JC, Martin S, Bruce-Keller AJ, Keller JN. Role of the proteasome in protein oxidation and neural viability following low-level oxidative stress. FEBS Lett. 2003;546:228–232. doi: 10.1016/s0014-5793(03)00582-9. [DOI] [PubMed] [Google Scholar]
- Ding Q, Martin S, Dimayuga E, Bruce-Keller AJ, Keller JN. LMP2 knock-out mice have reduced proteasome activities and increased levels of oxidatively damaged proteins. Antioxid Redox Signal. 2006;8:130–135. doi: 10.1089/ars.2006.8.130. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Ethen CE, Hussong SA, Reilly C, Feng X, Olsen TW, Ferrington DA. Transformation of the proteasome with age-related macular degeneration. FEBS Lett. 2007;581:885–890. doi: 10.1016/j.febslet.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 2005;19:644–646. doi: 10.1096/fj.04-2578fje. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Tran TN, Lew KL, Van Remmen H, Gregerson DS. Different death stimuli evoke apoptosis via multiple pathways in retinal pigment epithelial cells. Exp Eye Res. 2006;83:638–650. doi: 10.1016/j.exer.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol. 2002;39:147–164. doi: 10.1016/s0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, Dou C. Spontaneous induction of immunoregulation by an endogenous retinal protein. Invest Ophthalmol Vis Sci. 2002;43:2984–2991. [PubMed] [Google Scholar]
- Gregerson DS, Xiao J. Failure of memory (CD44 high) CD4 T cells to recognize their target antigen in retina. J Neuroimmunol. 2001;120:34–41. doi: 10.1016/s0165-5728(01)00406-4. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, Yang J. CD45-positive cells of the retina and their responsiveness to in vivo and in vitro treatment with IFN-gamma or anti-CD40. Invest Ophthalmol Vis Sci. 2003;44:3083–3093. doi: 10.1167/iovs.02-1014. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, Torseth JW, McPherson SW, Roberts JP, Shinohara T, Zack DJ. Retinal expression of a neo-self antigen, beta-galactosidase, is not tolerogenic and creates a target for autoimmune uveitis. J Immunol. 1999;163:1073–1080. [PubMed] [Google Scholar]
- Gregerson DS, Sam TN, McPherson SW. The antigen-presenting activity of fresh, adult parenchymal microglia and perivascular cells from retina. J Immunol. 2004;172:6587–6597. doi: 10.4049/jimmunol.172.11.6587. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, Lew KL, McPherson SW, Heuss ND, Ferrington DA. RPE cells resist bystander killing by CTLs, but are highly susceptible to antigen-dependent CTL killing. Invest Ophthalmol Vis Sci. 2006;47:5385–5394. doi: 10.1167/iovs.06-0636. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Faustman D. NOD mice are defective in proteasome production and activation of NF-κB. Mol Cell Biol. 1999;19:8646–8659. doi: 10.1128/mcb.19.12.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Faustman D. Selected contribution: Association of gender-related LMP2 inactivation with autoimmune pathogenesis. J Appl Physiol. 2001;91:2804–2815. doi: 10.1152/jappl.2001.91.6.2804. [DOI] [PubMed] [Google Scholar]
- Husom AD, Peters EA, Kolling EA, Fugere NA, Thompson LV, Ferrington DA. Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys. 2004;421:67–76. doi: 10.1016/j.abb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- James AB, Conway AM, Morris BJ. Regulation of the neuronal proteasome by Zif268 (Egr1) J Neurosci. 2006;26:1624–1634. doi: 10.1523/JNEUROSCI.4199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WB, Ruppe MD, Rockenstein EM. Indicator expression directed by regulatory sequences of the glial fibrillary acidic protein (GFAP) gene: in vivo comparison of distinct GFAP-lacZ transgenes. Glia. 1995;13:174–184. doi: 10.1002/glia.440130304. [DOI] [PubMed] [Google Scholar]
- Kapphahn RJ, Giwa BM, Berg KM, Roehrich H, Feng X, Olsen TW, Ferrington DA. Retinal proteins modified by 4-hydroxynonenal: Identification of molecular targets. Exp Eye Res. 2006;83:165–175. doi: 10.1016/j.exer.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Kapphahn RJ, Bigelow EJ, Ferrington DA. Age-dependent inhibition of proteasome chymotrypsin-like activity in the retina. Exp Eye Res. 2007;84:646–654. doi: 10.1016/j.exer.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Raju K, Breitman ML, Shinohara T. The proximal promoter of the mouse arrestin gene directs gene expression in photoreceptor cells and contains an evolutionarily conserved retinal factor-binding site. Mol Cell Biol. 1993;13:4400–4408. doi: 10.1128/mcb.13.7.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klare N, Seeger M, Janek K, Jungblut PR, Dahlmann B. Intermediate-type 20S proteasome in HeLa Cells: ‘Asymmetric’ subunit composition, diversity and adaptation. J Mol Biol. 2007;373:1–10. doi: 10.1016/j.jmb.2007.07.038. [DOI] [PubMed] [Google Scholar]
- Louie JL, Kapphahn RJ, Ferrington DA. Proteasome function and protein oxidation in the aged retina. Exp Eye Res. 2002;75:271–284. [PubMed] [Google Scholar]
- McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroids plexus of the rat brain as demonstrated in whole-mount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- McPherson SW, Yang J, Chan CC, Dou C, Gregerson DS. Resting CD8 T cells recognize beta-galactosidase expressed in the immune-privileged retina and mediate autoimmune disease when activated. Immunology. 2003;110:386–396. doi: 10.1046/j.1365-2567.2003.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson SW, Heuss ND, Roehrich H, Gregerson DS. Bystander killing of neurons by cytotoxic T cells specific for a glial antigen. Glia. 2006;53:457–466. doi: 10.1002/glia.20298. [DOI] [PubMed] [Google Scholar]
- Mishto M, Bellavista E, Santoro A, et al. Immunoproteasome and LMP2 polymorphism in aged and Alzheimer’s disease brains. Neurobiol Aging. 2006;27:54–66. doi: 10.1016/j.neurobiolaging.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Moore S, Thanos S. The concept of microglia in relation to central nervous system disease and regeneration. Prog Neurobiol. 1996;48:441–460. doi: 10.1016/0301-0082(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Nandi D, Jiang H, Monaco JJ. Identification of MECL-1 (LMP10) as the third IFN-gamma inducible proteasome subunit. J Immunol. 1996;156:2361–2364. [PubMed] [Google Scholar]
- Noda C, Tanahashi N, Shimbara N, Hendil KB, Tanaka K. Tissue distribution of standardproteasomes, immunoproteasomes, and PA28 in rats. Biochem Biophys Res Commun. 2000;277:348–354. doi: 10.1006/bbrc.2000.3676. [DOI] [PubMed] [Google Scholar]
- Panagis L, Thanos S, Fischer D, Dermon CR. Unilateral optic nerve crush induces bilateral retinal glial cell proliferation. Eur J Neurosci. 2005;21:2305–2309. doi: 10.1111/j.1460-9568.2005.04046.x. [DOI] [PubMed] [Google Scholar]
- Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Singh S, Awasthi N, Egwuagu CE, Wagner BJ. Immunoproteasome expression in a nonimmune tissue, the ocular lens. Arch Biochem Biophys. 2002;405:147–153. doi: 10.1016/s0003-9861(02)00341-7. [DOI] [PubMed] [Google Scholar]
- Stohwasser R, Giesebrecht J, Kraft R, Mueller EC, Hausler KG, Kettenmann H, Hanisch UK, Kloetzel PM. Biochemical analysis of proteasomes from mouse microglia: induction of immunoproteasome by interferon-gamma and lipopolysaccharide. Glia. 2000;29:355–365. [PubMed] [Google Scholar]
- Yao X, Liu J, McCabe JT. Alterations of cerebral cortex and hippocampal proteasome subunit expression and function in a traumatic brain injury rat model. J Neurochem. 2007;104:353–363. doi: 10.1111/j.1471-4159.2007.04970.x. [DOI] [PubMed] [Google Scholar]
- Yawata M, Murata S, Tanaka K, Ishigatsubo Y, Kasahara M. Nucleotide Sequence analyis of the approximately 35 kb segment containing interferon-γ-inducible mouse proteasome activator genes. Immunogenetics. 2001;53:119–129. doi: 10.1007/s002510100308. [DOI] [PubMed] [Google Scholar]
- Zanelli E, Zhou P, Cao H, Smart MK, David CS. Genomic organization and tissue expression of the mouse proteasome gene LMP-7. Immunogenetics. 1993;38:400–407. doi: 10.1007/BF00184520. [DOI] [PubMed] [Google Scholar]
- Zhang C, Shen JK, Lam TT, Zeng HY, Chiang SK, Yang F, Tso MO. Activation of microglia and chemokines in light-induced retinal degeneration. Mol Vis. 2005;11:887–895. [PubMed] [Google Scholar]