Abstract
Purpose of the study
Defibrotide (DF), an orally bioavailable polydisperse oligonucleotide has promising activity in hepatic veno-occlusive disease (VOD), a stem cell transplantation-related toxicity, characterized by microangiopathy. The anti-thrombotic properties of DF and its minimal hemorrhagic risk could serve for treatment of cancer-associated thrombotic complications. Given its cytoprotective effect on endothelium, we investigated whether DF protects tumor cells from cytotoxic anti-tumor agents. Further, given its anti-adhesive properties, we evaluated whether DF modulates the protection conferred to multiple myeloma (MM) cells by bone marrow stromal cells (BMSCs).
Methods-Results
DF lacks significant single-agent in vitro cytotoxicity on MM or solid tumor cells and does not attenuate their in vitro response to dexamethasone, bortezomib, immunomodulatory thalidomide derivatives, and conventional chemotherapeutics, including melphalan and cyclophosphamide. Importantly, DF enhances in vivo chemosensitivity of MM and mammary carcinoma xenografts in animal models. In co-cultures of MM cells with BMSCs in vitro, DF enhances the MM cell sensitivity to melphalan and dexamethasone, decreases MM-BMSC adhesion and its sequelae, including NF-κB activation in MM and BMSCs, and associated cytokine production. Moreover, DF inhibits expression and/or function of key mediators of MM interaction with BMSC and endothelium, including heparanase, angiogenic cytokines and adhesion molecules.
Conclusion
Defibrotide’s in vivo chemosensitizing properties and lack of direct in vitro activity against tumor cells suggest that it favorably modulates antitumor interactions between BMSC and endothelia in the tumor microenvironment. These data support clinical studies of DF in combination with conventional and novel therapies to potentially improve patient outcome in MM and other malignancies.
INTRODUCTION
Thrombotic and microangiopathic complications of malignancy are well established, and constitute a major source of morbidity and mortality in cancer patients because of the disease process itself and/or because of complications of treatment (1, 2). Therapeutic strategies targeting endothelial injury may be beneficial in preventing or helping the treatment of cancer-associated thrombotic complications. Defibrotide (DF) is a novel agent which fits such a profile. This polydisperse polydeoxyribonucleotide, derived from mammalian tissue (porcine mucosa) by controlled depolymerization (3), exhibits diverse biological properties, including anti-thrombotic, thrombolytic, and anti-adhesive effects, and can also protect endothelial cells from chemotherapy-mediated cytotoxicity (3, 4). It is orally bioavailable and, importantly, because it confers no significant hemorrhagic risk (5), DF is an attractive therapeutic option for clinical settings where cytoprotective and anti-thrombotic interventions are needed with a small margin for compromise of hemostasis. Hepatic veno-occlusive disease (VOD) is one such clinical setting where DF has been successfully evaluated as an important therapeutic option (6–12). This clinical syndrome of painful hepatomegaly, jaundice, ascites, fluid retention and otherwise unexplained weight gain is a common (present in up to 60% of patients) regimen-related complication of hematopoietic stem cell transplantation (SCT), with severity ranging from mild, reversible disease to a severe syndrome associated with multi-organ failure and death (13–16). Established severe VOD has a mortality rate approaching 100% by 100 days post-SCT (13, 14, 16, 17). VOD is currently considered to result from conditioning regimen-induced injury to the hepatic sinusoidal endothelium (18), leading to hepatocellular injury, stellate cell activation and subendothelial edema, which eventually result in a triad of sinusoidal obstruction, hepatocellular necrosis and venous occlusion, which are the basic pathophysiologic processes contributing to the clinical presentation of VOD (as reviewed in (19)). Many of the pleiotropic biologic effects of DF directly counteract the fundamental pathophysiological sequelae of VOD and potentially confer protection to hepatic microvasculature against chemotherapy-mediated injury (3, 4). These considerations have provided a basis for the use of DF for both the treatment and prevention of VOD (6–12)
The clinical efficacy and safety profile of DF in VOD prompted us to hypothesize that it could be used for the prevention and/or treatment of cancer-associated thromboembolic events, such as those emerging in the field of multiple myeloma (MM) with the use of certain types of novel agents (2). It was however important to first evaluate whether DF administration could interfere with the anti-tumor activity of certain conventional or novel therapeutics for both hematologic malignancies and solid tumors. We specifically studied 2 distinct questions, both of which derive from our current understanding of its properties. First, we sought to determine whether DF could attenuate the anti-tumor activity of various therapeutic agents, including cytotoxic chemotherapy, glucocorticoids, or novel therapies, including the first-in-class proteasome inhibitor bortezomib. Given its cytoprotective properties, specifically its ability to protect endothelial cells from chemotherapy-mediated cytotoxicity (4), it was critical to assess whether DF could similarly protect tumor cells from cytotoxic injury, because DF use might theoretically compromise the benefit derived from the treatment regimen. Second, we wanted to evaluate whether DF could interfere with tumor-stromal interactions and their impact on the response of malignant cells to various therapeutic agents. Extensive data in various disease settings, most importantly multiple myeloma (MM), as well as in other hematologic malignancies (e.g. various forms of leukemia) and bone metastases of various solid tumors (e.g. breast or prostate carcinomas) (as reviewed in (20, 21)), have indicated that adhesion of malignant cells to bone marrow stromal cells (BMSCs) can protect the tumor cells from the effects of conventional therapeutics such as cytotoxic chemotherapeutics or, in the case of MM, dexamethasone. Given the anti-adhesive properties of DF in other model systems (22–26), we hypothesized that DF could play a similar role in abrogating the tumor-stromal adhesive interaction and thus enhance the responsiveness of malignant cells to various anti-tumor agents.
In this current study, we observed that in conventional in vitro assays, DF has no direct anti-MM activity against MM, breast and colon cancer cells, and does not attenuate the anti-tumor activity of a several different classes of anti-neoplastic drug. Interestingly, with in vitro co-cultures of MM cells with BMSCs, DF enhances the anti-MM activity of certain drugs, such as melphalan or dexamethasone. This effect is associated with suppressed adhesive interaction between the 2 cell compartments; abrogation of NF-κB activation triggered by MM-BMSC adhesion; decreased production of proliferative/anti-apoptotic cytokines; as well as perturbed expression and/or function of key mediators of MM-microenvironment interactions, such as heparanase, angiogenic cytokines and adhesion molecules.
Importantly, in in vivo animal models of xenografted tumor cells (from MM or from breast carcinoma), administration of DF increased the anti-tumor activity of cytotoxics, including cyclophosphamide. Furthermore, pharmacokinetic analyses revealed that the levels of DF achieved in vivo in mice treated with DF are consistent with the levels required (based on in vitro studies) for the anti-tumor properties of DF.
These results suggest that DF administration is not only compatible with conventional and many novel anti-tumor treatment strategies, but also indicate that DF exhibits microenvironment-modulatory properties which serve to attenuate key aspects of tumor pathophysiology and drug resistance (e.g. tumor cell adhesion to stroma and ensuing production of anti-apoptotic cytokines). This therefore allows DF to enhance the responsiveness of tumor cells to certain conventional therapies. These results, coupled with the particular role of tumor-stromal interactions in the pathophysiology of MM, provide the basis for further pre-clinical and clinical evaluation of DF as an adjuvant agent for the management of MM and other malignancies, including breast cancer.
MATERIALS AND METHODS
Cell lines and primary cells
Our studies included the human breast adenocarcinoma cell line MCF-7 (American Type Culture Collection, ATCC, Manassas, VA); the human colorectal adenocarcinoma cell line HT-29 (purchased from ATCC); a series of human MM cell lines, including MM-1S, MM-1R (kindly provided by Dr. Steve Rosen, Feinberg School of Medicine, Northwestern University, Chicago, IL), RPMI-8226 and Dox40 (kindly provided by William Dalton, Moffitt Cancer Center, Tampa FL), U266 (purchased from the ATCC) and OPM-1. Human dermal microvascular endothelial cells (HMEC) were kindly provided by the Centers for Disease Control and Prevention (Atlanta, Georgia, USA). Human neo-natal dermal microvascular endothelial cells (HMVEC) and human umbilical vein endothelial cells (HUVEC) were purchased from Cambrex (East Rutherford, NJ) and propagated in EGM2-MV medium (Cambrex, East Rutherford, NJ). Primary MM tumor cells were obtained as previously described (27). BMSCs were cultured as previously described (27). All cultures were performed at 37°C in a humidified atmosphere and in medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S) (Invitrogen, Inc., Carlsbad, CA).
Compounds
Defibrotide (DF) was provided by Gentium (Como, Italy). Carboplatin, melphalan, paclitaxel, vinblastine, vincristine, doxorubicin, dexamethasone, cyclophosphamide, BCNU, and monocrotaline (28) were purchased from SIGMA-Aldrich (St. Louis, MO). Bortezomib (PS-341, Velcade™) was obtained from Millennium Pharmaceuticals (Cambridge, MA). CC4047 (Actimid) and CC5013 (Lenalidomide, Revlimid) were obtained from Celgene (Warren, NJ).
Cell viability assays
MM1-S and MM1-R cells were detached from the flask using a cell scraper and were washed once in RPMI-2%FBS. 2,000 cells were plated per well in a 96-well format in RPMI-2%FBS. The cells were allowed to recover for 24 hours after which they were exposed to the drugs in RPMI-2%FBS for 96 hours. MCF-7 cells and HT-29 cells were detached from the flask using 0.25% trypsin in EDTA (Invitrogen, Inc., Carlsbad, CA) and washed once in RPMI-2%FBS. 2,000 cells were plated per well in a 96-well format in RPMI-2%FBS. The cells were allowed to recover for 24 hours after which they were exposed to the drugs in RPMI-2%FBS for 96 hours. HMVEC and HUVEC were detached from the flask using 0.25% trypsin in EDTA (Invitrogen, Inc., Carlsbad, CA) and washed once in EGM2-MV. 2,000 cells were plated per well in a 96-well format in EGM2-MV. The cells were allowed to recover for 24 hours after which they were exposed to the drugs in EGM2-MV for 96 hours. The final volume of each well during the 96h incubation with the drugs was 100 μl and the incubation took place at 37°C in a humidified atmosphere. Following the 96h drug exposure, 10 μl of the WST-1 reagent (BioVision Research Products, Mountain View, CA) were added to each well and incubated for 30 minutes at 37°C. WST-1 is a tetrazolium salt that is cleaved to formazan by mitochondrial dehydrogenases (29). Increased cell number results in increased mitochondrial dehydrogenase activity and therefore increased formazan dye formation. Measurement of the absorbance at 440 nm allowed quantification of the formazan dye. A reference wavelength of 650 nm was used per recommendations from the manufacturer of the reagent. The 650 nm reference absorbance value was subtracted from the 440 nm absorbance value. Background was measured using an empty well containing medium and no cells.
For in vitro co-culture assays of MM cells with BMSCs, primary MM tumor cells were cultured in the presence or absence of BMSCs, and exposed to DF, Dex or Melphalan, or combination of DF with Dex or Mel. Treatment-induced myeloma cell death was measured by flow cytometry using the Apo2.7 mitochondrial early apoptosis marker. MM cells were distinguished from BMSCs by staining for CD38 (30)
In vivo studies of DF as single agent and in combination with other therapeutics
To evaluate the potential impact of DF on in vivo growth of tumors, we performed studies in the following animal models: a SCID/NOD mouse model of subcutaneous plasmacytoma xenografts of MM-1S cells; a SCID/NOD mouse model of diffuse MM bone lesions generated by MM-1S cells; and a rat 13762 mammary carcinoma model In the SCID/NOD mouse model of subcutaneous plasmacytoma xenografts of MM-1S cells, 60 male SCID/NOD mice (6–8 weeks old) were irradiated (450 rads) and, 24 hours later, injected s.c. with 5×106 MM-1S human MM cells. Upon formation of palpable tumors, mice were randomly assigned to 6 cohorts (10 mice each) receiving a) vehicle; b) DF i.v. 450 mg/kg b.i.d.; c) melphalan (MEL) 2.5 mg/kg i.p. once weekly; d) cyclophosphamide (CTX) 50 mg/kg i.p., on days 8, 10, 12, 20, 22 and 24; e) and f) combinations of DF (300 mg/kg i.v.) with MEL or CTX, respectively. Mice were monitored every 3 days for body weight, potential toxicity, and electronic caliper-based tumor volumes.
In the SCID/NOD mouse model of diffuse MM bone lesions, 20 male SCID/NOD mice (6–8 weeks old) were irradiated (450 rads) and, 24 hours later, injected i.v. with 1×106 MM-1S human MM cells, leading to formation of diffuse MM tumors, as previously described (31). Mice were then randomly assigned to 4 cohorts (5 mice each) receiving a) vehicle; b) DF p.o. 45 mg/kg b.i.d.; c) melphalan (MEL) 2.5 mg/kg i.p. once weekly; and d) combinations of DF (45 mg/kg) with MEL. Mice were monitored for body weight, potential toxicity. If they developed signs of morbidity (e.g. hind limb paralysis due to tumor growth in the spine, infection, major bleeding et.c.), they were sacrificed by CO2 inhalation according to Animal Care Use Committee guidelines. The primary endpoint for this model was the overall survival of mice (defined as time between initiation of treatment and sacrifice).
Rat mammary adenocarcinoma 13762 is a carcinogen-induced tumor syngeneic for the female Fisher 344 rat. For experiments, 13762 mammary carcinoma cells (2 × 106) prepared from a brei of donor tumors were implanted subcutaneously into a hind-leg of female Fisher 344 rats weighing between 140 and 160 grams Taconic Farms, Germantown, PA). Animals were housed and handled in accordance with Animal Care & Use Committee guidelines. The 13762 tumor grew to 100 mm3 in about 8 days (32, 33). Each group consisted of 5 rats. Defibrotide administration was initiated either at the same time as cytotoxic treatment or 2 days after the first dose of cytotoxic treatment. Monocrotaline (350 mg/kg) or BCNU (150 mg/kg) were administered by intraperitoneal injection on days 8 and 18 post tumor cell implant (28). Defibrotide (200 mg/kg) was administered twice per day by intravenous injection on days 8 through 26 or on days 10 through 26. Tumor volumes were calculated using the formula (w2 × l)/2 where ‘w’ is the width of the tumor and ‘l’ is the length of the tumor. Individual rats were weighed and tumor measurements taken by calipers twice weekly. Antitumor activity of the treatments was determined by calculating tumor growth delay (T-C) in days. Tumor growth delay was obtained by determining the difference between treatment and control group mean tumor growth in days at a predetermined tumor volume. The data are presented as means+/- SEM. caliper-based tumor volumes.
Pharmacokinetic Studies
Eight-week old male Fisher 344 rats (Charles River Laboratories) were treated (10 mice per treatment cohort), either orally or i.v., with DF (48 mg/kg). Peripheral blood (PBL) plasma samples were serially collected (0–8hrs) via pre-inserted jugular vein catheter for determination of pharmacokinetic (PK) profile by high-pressure liquid chromatography (HPLC) analyses and validation by agarose gel determination, as previously described. Briefly, blood samples taken before the administration (basal condition) of DF and at 1, 3, 5, 10, 20, 30, 40 min and 1hr, 2hrs, and 4 hrs after administration. The anti-coagulated blood was centrifuged to obtain the plasma samples and 1ml of N perchloric acid was added to each 1 ml of plasma. After stirring, the sample was heated for 15 min in water bath at 70°C degrees. The samples were centrifuged at 3000g. The pellet was discarded and 1 ml of diphenylamine reagent (100ml of glacial acetic acid + 1.5 g diphenylamine + 1.5 ml conc. Sulfuric acid and 0.6 ml of 0.16% (w/v) solution of acetaldehyde in water) was added to 1 ml of supernatant. The samples were incubated for 17–20 hrs at 25–30°C. The absorbance of the samples at 600nm was read in spectrophotometer. A standard curve was also prepared with DF dissolved in plasma taken from control animals. For the HPLC determination, Plasma samples were heated to 70°C for 3 min and were then left to cool to 37°C. After adding 12 mg trypsin, samples were kept in water bath at 37°C for 5.5 hrs and then diluted with 10mM phosphate buffer, pH 7.0-Mobile phase of Chromatography, injected in to liquid phase chromatograph. For agarose gel determination, plasma samples were heated to 70°C for about 3 min, then left cool to 37°C. After addition of trypsin, the samples were kept in water bath at 37°C for 5.5 hrs and then were diluted with Tris-acetate buffer, pH 7.8 to give concentrations of DF from 10 to 50ug/ml. The samples were run in 2mm thick 0.5% agarose gel in 5% sorbitol in acetate buffer, pH 7.8 at +4°C degrees, 90V, 30–35mA for 75 min, in a LKB model 2117 multiphor II electrophoretic chamber (from reference materials). The gels were stained with acridine orange (0.006% w/v), in 0.01 M phosphate buffer, pH 7.0. The gel slabs, after drying were analyzed by a TLC scanner photo densitometer. The measurements are carried at 470 nm.
Molecular profiling studies and functional experiments
mRNA levels of heparanase, VEGF, FGF-2, ICAM-1 and E-selectin were measured through Syber-green Real-Time PCR of cDNA prepared from target cell populations (MM cells, endothelial cells) treated with and without DF using the following primers heparanase: F- TCACCATTGACGCCAACCT and R- CTTTGCAGAACCCAGGAGGAT; VEGF: F- CTACCTCCACCATGCCAAGT and R- GCAGTAGCTGCGCTGATAGA; FGF-2: F- CCACTTCAAGGACCCCAAG and R- ATAGCCAGGTAACGGTTAGC; ICAM-1: F- CTGTTCCCAGGACCTGGCAAT and R- AGGCAGGAGCAACTCCTTTTTA; E-selectin: F- CTCTGACAGAAGAAGCCAAG and R- ACTTGAGTCCACTGAAGCCA); β-actin: F- TCACCCACACTGTGCCCATCTACGA AND R- CAGCGGAACCGCTCATTGCCAATGG (housekeeping gene). Intracellular levels of heparanase were measured by intracellular flow cytometry through FACSCalibur flow cytometer and the CellQuest analysis program (Becton Dickinson). using goat polyclonal anti-human heparanase antibodies (Santa Cruz Biotecnology Inc). Omitting the first antibody served as a negative control to detect unspecific fluorescence. Heparanase enzymatic activity was measured in extracts of DF-treated vs, control cells by a commercial heparan degrading enzymatic kit (Takara-bio Inc.).
Adhesion assays of multiple myeloma cells on bone marrow stromal cells were performed using carboxyl fluorescent succinimidyl ester (CFSE) labeling of multiple myeloma cells with the CellTrace CFSE Cell Proliferation Kit (Molecular Probes) according to the instructions of the manufacturer. After CFSE labeling, MM cells were plated in optical 96-well plates on which BMSCs had been pre-seeded and DF or control medium. At the conclusion of the co-cultures, each well was processed by gentle aspiration of their supernatant and washing with 1x PBS. After repeating the aspiration and addition of 1xPBS twice, the plates were read in a fluorescence plate reader (excitation and emission wavelengths of 485 and 520 nm, respectively) to evaluate the % of fluorescent MM cells that remained adhered to BMSCs in the presence vs. absence of DF treatment.
NF-kB binding ELISAs for evaluation of transcription factor activity were performed with NF-kB transcription factor ELISA kits (Active Motif, Carlsbad, CA) according to the instructions of the manufacturer. IL-6 and VEGF protein levels were measured with corresponding ELISA kits (R&D Diagnostics, Minneapolis, MN) according to the instructions of the manufacturer.
Tumor cell invasion assays were performed to assess in vitro the invasive potential of MM cells. Briefly, MM cells overexpressing heparanase or supplemented with heparanase recombinant enzyme (GenWay Biotech inc.) treated with and without DF were layered on the top chamber of a dual-chamber in vitro culture system (Becton Dickinson). The 2 chambers were separated by a polyethylene terephthalate (PET) membrane and matrigel and the number of MM cells that invaded the matrigel and the PET membrane towards the lower chamber were evaluated by fluorescence microscopy and fluorescence plate readers. The invasive potential of the MM cells was assessed on the basis of the number of fluorescent (calcein-labeled) MM cells penetrating the lower chamber. In order to have MM cells overexpressing heparanase, heparanase cDNA (HPSE1) was subcloned into the pcDNA3.1/CT-GFP (Invitrogen) vector to give an in-frame C-terminal green fluorescent protein (GFP) fusion construct. The pcDNA3.1/CT-GFP/HPSE1 construct was transfected into the RPMI 8226 human MM cells using Lipofectin reagents (Invitrogen) following the manufacturers’s instructions.
Statistical Methods
In the cell viability assays, each experimental point was set up in duplicate wells and each assay was repeated identically and independently at least once, and for each experiment, two repetitions of the assay were combined into a single data set in which each experimental point was represented by four values. The mean and standard deviation of these four values were determined for each experimental point. The final data were expressed as a percentage of the proliferation that took place in control wells where cells were not exposed to any drugs. IC50 values were determined for each experiment. In the in vivo models, the overall survival of mice was evaluated with Kaplan-Meier survival analyses and differences between the various cohorts of each experiments were assessed by log-rank tests.
RESULTS
Assessment of direct effects of DF on in vitro viability of tumor cells and endothelial cells
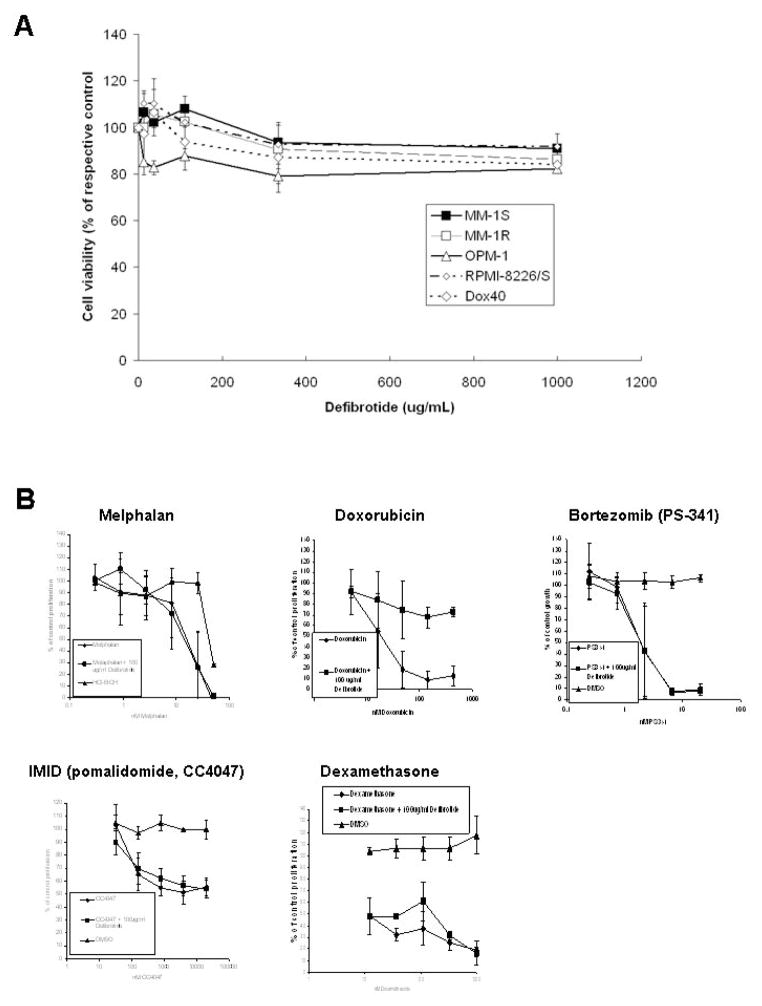
We performed colorimetric survival assays to test the in vitro effects of DF (0–1000 μg/mL for 96 hrs) on human MM cell lines. We observed that exposure to DF did not significantly affect the in vitro viability of these cell lines (except for a modest decline in viability of MM-1R cells treated with the highest concentration of DF tested, i.e. 1000 μg/mL) (Fig. 1A and Suppl. Fig. 1). We extended these observations to other tumor types (e.g. the breast carcinoma cell line MCF-7 and the colon cancer cell line HT-29) (Suppl. Fig. 1C and 1D, respectively); to 2 different endothelial cell models (HMVECs and HUVECs) (Suppl. Fig. 1E and 1F, respectively); as well as to additional well-characterized human MM cell line models (e.g. OPM-1, Dox40 and RPMI-8226/S) (Suppl. Fig. 1G).
Figure 1. In vitro viability of MM cell lines treated with defibrotide (DF) alone or its combination or novel anti-MM agents.
Panel A: The results of in vitro DF treatment of MM cell lines, including MM-1S, its dexamethasone-resistant subline MM-1R, the chemo-sensitive RPMI-8226/S and its Doxorubucin-resistant subline Dox40, as well as the OPM-1 cells. Panel B: WST-1 assays were performed to assess the putative impact of DF on the in vitro response of MM cells to diverse agents, including melphalan; doxorubicin, bortezomib (PS341), the IMID pomalidomide (CC-4047), and dexamethasone. These results represent the combination of two separate independent experiments.
Evaluation of in vitro effects of combinations of DF with diverse anti-tumor agents
Colorimetric survival assays (with WST-1) were performed to evaluate whether DF can modulate the activity of various therapeutics against MM cells, cell lines from other tumor models, or endothelial cells. We specifically evaluated the in vitro effects of combinations of DF with a series of anti-tumor agents, including dexamethasone (Dex), the proteasome inhibitor bortezomib (PS-341), the immunomodulatory derivatives CC-5013 (lenalidomide, Revlimid™) and CC-4047 (pomalidomide, Actmid™), as well as carboplatin, melphalan, paclitaxel, vinblastine, vincristine, and doxorubicin. When used as single agents, carboplatin, melphalan, paclitaxel, vinblastine, vincristine, doxorubicin, bortezomib, CC-4047 and dexamethasone significantly decreased the numbers of viable MM-1S (Fig. 1, Suppl. Fig. 2 and Suppl. Table 1). When these MM1S cells were exposed to a combination of 100 μg/ml defibrotide with increasing concentrations of carboplatin, melphalan, paclitaxel, vinblastine, vincristine, bortezomib, CC-4047, CC-5013 or dexamethasone, the effect of each one of these combinations on the viability of MM-1S was similar to the effect observed with each agent alone in the absence of defibrotide (Fig. 1, Suppl. Fig. 2 and Suppl. Table 1). We then extended these combination studies to other types of tumor cells and to endothelial cells and observed that DF also did not sensitize MCF-7, HT-29, HMVEC or HUVEC to any of these agents or antagonize the activity of any of these agents (Suppl. Table 1). The only exception to that general pattern was observed in combinations of DF with doxorubicin: when DF was used (at a fixed concentration of 100 μg./mL) in combination with doxorubicin, the decrease in MM-1S cell viability was significantly attenuated, with an IC50 of 20 nM in the absence of defibrotide and of >400 nM in the presence of defibrotide (Fig. 1 and Suppl. Table 1). Similarly, DF significantly increased the IC50 value of doxorubicin against MCF-7 cells (IC50 value of 20 nM in the absence of defibrotide vs. 550 nM in the presence of DF); HT-29 cells (IC50 value of 60 vs. 25,000 nM, respectively); HMVECs (200 vs. 2900 nM respectively); and HUVECs (200 vs. 1100 nM, respectively) (Suppl. Table 1).
We also evaluated whether defibrotide could revert the resistance to dexamethasone. To this end, we treated the MM-1R cells (a dexamethasone-resistant subline of the MM-1S cells) with dexamethasone alone vs. a combination of dexamethasone and DF. We observed that dexamethasone alone failed (at concentrations up to 1 μM) to inhibit the survival of MM-1R cells (while it inhibited MM-1S cell viability with an IC50 of 12 nM) and that in the presence of DF, the IC50 for inhibition of MM-1R cell viability by was still > 1μM, suggesting that defibrotide does not overcome the dexamethasone resistance of MM-1R cells (Suppl. Fig. 2F and Suppl. Table 1).
In vivo anti-tumor activity of DF
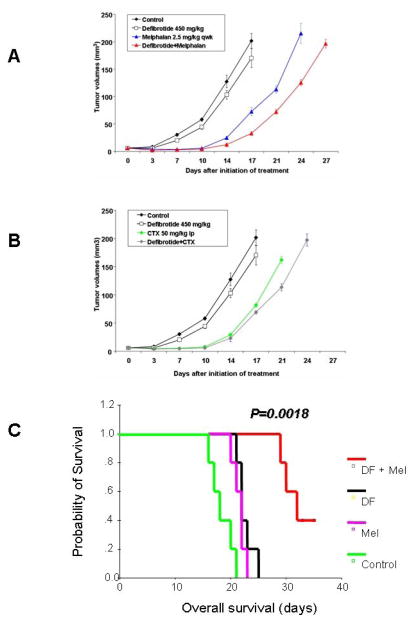
The in vivo anti-tumor activity of DF was evaluated in a series of different animal models of tumor growth, including a SCID/NOD mouse model of subcutaneous plasmacytoma xenografts of MM-1S cells; a SCID/NOD mouse model of diffuse MM bone lesions generated by MM-1S cells; and a rat 13762 mammary carcinoma model. In each one of these models, tumor-bearing mice where treated with DF alone; with various anti-tumor therapeutics (the choice of which depended on the particular tumor type that was studied); with combinations of DF with these anti-tumor therapeutics; or with the respective vehicles. In the first of these models, SCID/NOD mice bearing subcutaneous plasmacytoma xenografts of MM-1S cells were randomly assigned to cohorts treated with vehicle; melphalan alone; cyclophosphamide alone; DF alone; DF plus melphalan; and DF plus cyclophosphamide. DF, either as single agent or in combination with MEL or CTX, was well tolerated without hemorrhagic complications or body weight loss (P>0.05) in all groups. The major endpoints for efficacy were a) tumor volume changes and b) overall survival (time-to-sacrifice, performed when tumor diameters > 2 cm). DF treatment resulted in significantly lower tumor volumes than in control mice (P<0.05 for all comparisons by analysis of variance and post-hoc tests). The combination of DF with MEL or CTX induced significantly lower tumor volumes than the respective single-agent cytotoxic chemotherapy (Fig. 2A and 2B, P<0.05 for all comparisons). Kaplan-Meier survival analyses showed that DF administration, either as single agent or in combination with cytotoxic chemotherapy (MEL or CTX), was associated with statistically significant prolongation of overall survival, in comparison to vehicle-treated control group or MEL- or CTX-treated groups, respectively (P<0.001 for all comparisons, log-rank test).
Figure 2. In vivo studies of DF in combination with other anti-tumor agents.
Changes in tumor volume over time in mice xenografted with human MM-1S cells (subcutaneous xenograft model in panels A and B, and model of diffuse MM lesions in panel C) and treated with DF either alone or in combination with melphalan (panels A and C) or cyclophosphamide (CTX, panel B).
In the second model, SCID/NOD mice injected i.v. with MM cells to generate diffuse MM bone lesions were randomly assigned to cohorts treated with vehicle; melphalan alone; DF alone; and DF plus melphalan. In this model, the combination of DF plus melphalan led to significant prolongation in overall survival of mice compared to each drug alone (Fig. 2C, P=0.0018, log rank test).
In the third tumor model of rat 13762 mammary carcinoma, tumor-bearing animals were treated with monocrotaline alone, BCNU alone, or with combinations thereof with DF. The primary endpoint in this model was the growth delay that was achieved in each treatment cohort, defined as the difference in the number of days necessary for mice in the control cohort to reach tumor volumes of 500 mm3 compared to mice in the respective treatment cohort. (Day 0 was the day of tumor cell implantation and chemotherapy was administered on day 8 and 18) (Suppl. Table 2). In this model, the growth delay achieved in the cohorts of mice treated with the combination of DF with either monocrotaline or BCNU was longer than the growth delay in the cohorts treated with the respective cytotoxic chemotherapeutic alone.
Pre-clinical pharmacokinetic studies of DF
We evaluated the in vivo pharmacokinetic profile of DF in one of our in vivo animal models. Specifically, blood samples were collected at specific intervals (as described in the Materials and Methods) from pre-inserted jugular vein catheters in Fisher male rats treated with DF (48 mg/kg single dose) either through p.o. or i.v. route (Suppl. Fig. 3). Plasma levels of DF were evaluated by HPLC and agarose gel determination and provided highly consistent pharmacokinetic data between the 2 techniques (with mean and median variability of measurement of 8.07% and 7.89%, respectively). With i.v. and p.o administration, peak DF levels (Cmax) were 1253 and 474 μg/mL, and area under the curve (AUC0–240 min) 14.71 and 17.19 μg*min/Lt, respectively. Time to Cmax was ~30 min for p.o. administration and returned to undetectable levels by 240 min post-treatment for both p.o. and i.v treatment (Suppl. Fig. 3). These results suggest that the levels of DF which are required for some of the mechanistic sequelae of DF in our pre-clinical in vitro models are achievable in vivo and could be involved in the in vivo mechanism(s) whereby DF sensitizes MM tumor cells to certain anti-tumor therapies.
Effect of DF on in vitro co-cultures of MM cells with BMSCs
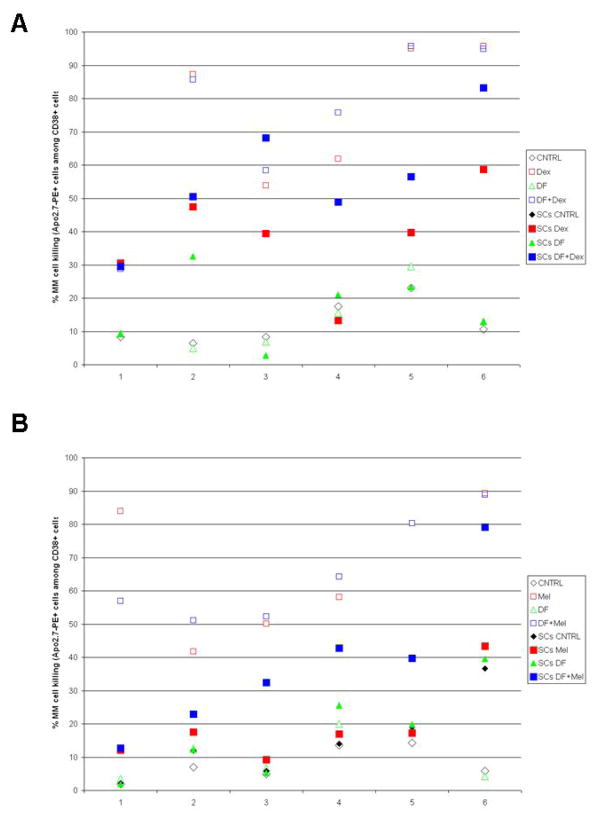
Adhesion to BMSCs attenuates the response of MM cells to conventional anti-neoplastic agents, e.g. Dex and cytotoxic chemotherapeutics (as reviewed in (21)). Because of the proposed anti-adhesive properties of DF in the context of vascular biology (22–26), we hypothesized that DF may have a similar effect in the setting of tumor-stromal interactions in MM. We thus evaluated whether DF can modify the response of primary MM cells to such conventional therapeutics in the context of stromal co-culture. Primary MM tumor cells (CD38+) isolated from MM patients were co-cultured with BMSCs (CD38-) and flow cytometry was used to evaluate the fraction of dead cells (Apo2.7-PE+) within the CD38+ MM cells. Treatment with DF (100 μg/mL) alone did not trigger significant changes in MM cell viability compared to DF-free control cultures, either in the presence or absence of BMSCs (Fig. 3 and Suppl. Fig. 4). Furthermore, in the absence of BMSCs, DF administration did not significantly increase the % killing of MM cells exposed to Dex (Fig. 3A and Suppl. Fig 4A) or melphalan (Fig. 3B and Suppl. Fig 4B), consistent with the colorimetric survival assay results obtained with MM cell lines. When MM cells were treated with Dex or melphalan alone in the presence of BMSCs, the % MM cell killing was in most cases significantly lower compared to single-agent Dex- or melphalan-induced cell killing in the absence of BMSCs. However, in the context of MM cell co-culture with BMSCs, the combination of DF with Dex (Fig. 3A and Suppl. Fig 4A) or melphalan (Fig. 3B and Suppl. Fig 4B) significantly increased in a large proportion of cases the % cell killing of MM cells compared to treatment with Dex or melphalan alone.
Figure 3. Defibrotide (DF) treatment enhances the anti-MM activity of conventional therapeutics in the context of MM-BMSCs interactions.
Illustrative examples of results from treatment of primary MM cells with dexamethasone (Dex, panel A) or melphalan (Mel, panel B), in the presence or absence of bone marrow stromal cells (BMSCs), with or without treatment with DF. Among MM samples which exhibit constitutive responsiveness to these agents in the absence of stroma, but significantly less pronounced response in MM-BMSC co-cultures, DF is able to lead to variable degrees of sensitization to these agents in the presence of stromal cells. In each panel, primary samples 3 to 6 are examples of how DF treatment can increase the presence of stromal cells higher response to treatment with Dex or Mel, respectively.
Cellular and molecular sequelae of DF treatment in the MM-BMSC co-culture
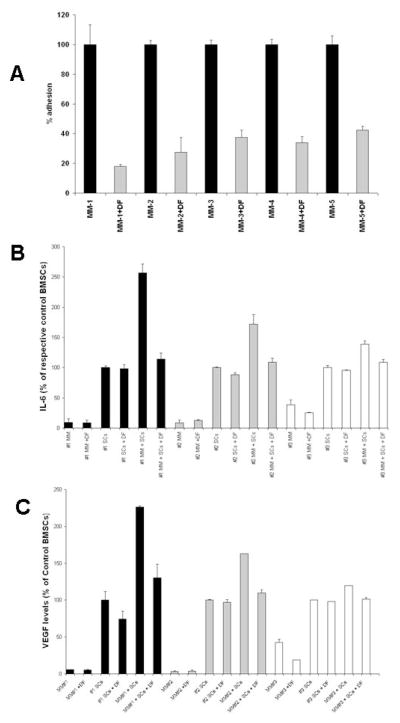
To evaluate the mechanisms whereby DF can attenuate protective effects conferred to MM cells by BMSCs, we performed in vitro cell adhesion assays and observed that DF significantly decreased the adhesion of MM cells to BMSCs (Fig. 4A). The MM cell adhesion to BMSCs has been previously associated with increased transcriptional activity of NF-κB in both cellular compartments (34, 35); leading to increased secretion of anti-apoptotic cytokines (such as IL-6) and pro-angiogenic growth factors (such as VEGF) (27). We therefore hypothesized that the decrease in MM-BMSC adhesion could have an impact on these molecular sequelae. Indeed, separate assays in the MM cell compartment vs. BMSCs of the in vitro co-culture model showed that DF treatment decreased the NF-κB transcriptional activity in both MM cells (Suppl. Fig. 5A) and BMSCs (Suppl. Fig. 5B). This finding was also associated with suppression in secretion of IL-6 (Fig. 4B) and VEGF (Fig. 4C), consistent with the anti-adhesive properties of DF and with the impact of MM-BMSCs adhesion on secretion of these cytokines.
Figure 4. Defibrotide (DF) modulates multiple myeloma (MM) cell adhesion to bone marrow stromal cells (BMSCs or SCs) and its sequelae.
In vitro adhesion assays (with CFSE labeling of MM cells and quantification of adhesion with fluorescence plate reader) show that DF treatment decreases the adhesion of primary MM tumor cells to BMSCs (panel A). Furthermore, DF treatment suppresses the increase, triggered by MM-BMSCs interaction, in secretion of IL-6 (panel B) or VEGF (panel C). Cytokine levels in panels B and C are expressed, in each experiment, as % of levels in cultures of stromal cells alone.
DF modulates the expression and function of heparanase
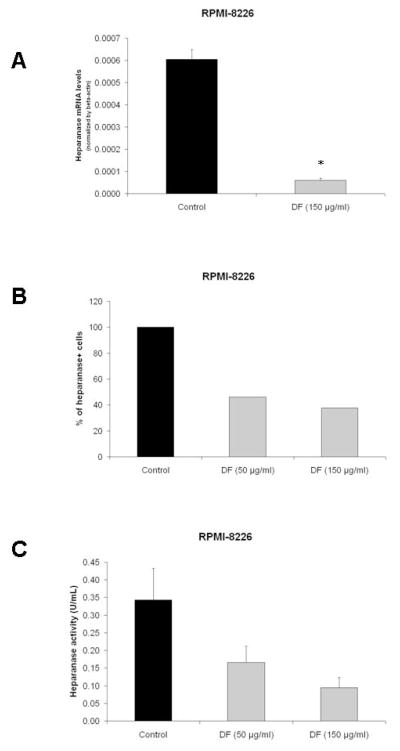
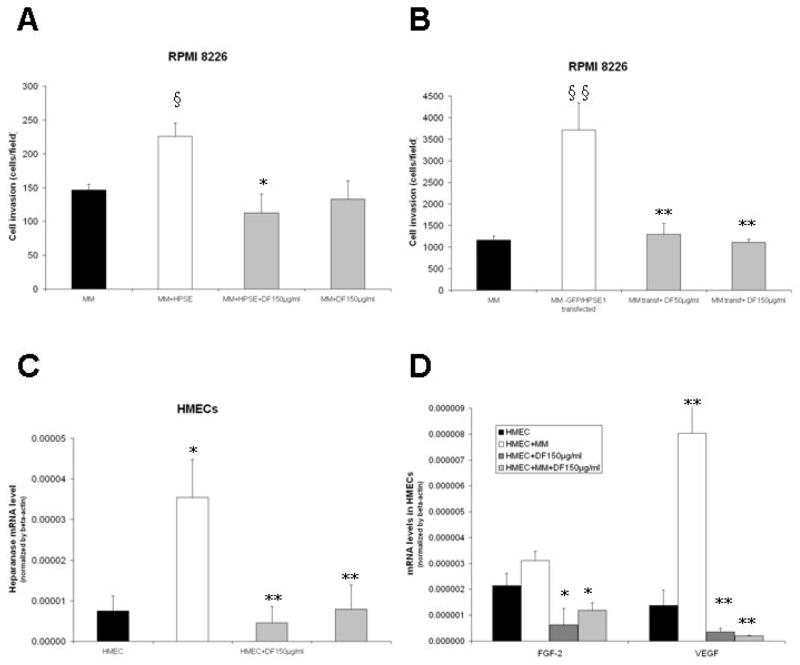
Heparanase is an enzyme that cleaves heparan sulfate chains of proteoglycans, and its expression has been associated with increased growth, metastasis, and angiogenesis of several tumor types, including MM (36–40). Because of the increasing interest in the potential role of heparanase as a mediator of tumor-microenvironment interactions in MM, we evaluated whether DF modulates the biological activity of heparanase. We first confirmed that DF treatment suppresses the expression of heparanase transcripts (assessed by Real-Time RT-PCR) in RPMI-8226 and U266 MM cells (Fig. 5A and Suppl. Fig. 6A); and decreases the intracellular protein expression of heparanase (as evaluated by intracellular flow cytometry) (Fig. 5B). These effects correlated with DF-induced decreased in heparanase activity in cultures of RPMI-8226 cells (Fig. 5C) and U266 cells (Suppl. Fig. 6B). To further probe the functional significance of these observations, we evaluated the impact of DF on an in vitro tumor invasion assay in dual-chamber culture system, where MM cells labeled with calcein were incubated in the upper chamber of the system. Exogeneous addition of recombinant human heparanase in the culture (Fig. 6A) or transfection of RPMI-8226 cells with heparanase construct (Fig. 6B) increased the invasion potential of the MM cells, but in both cases DF treatment abrogated that effect (Fig. 6A–B).
Figure 5. Defibrotide (DF) treatment suppresses the expression and function of heparanase.
RT-PCR was performed as described in “Material and methods”. Results are expressed as mean mRNA heparanase level normalized by β-actin housekeeping gene. DF treatment suppresses the levels of heparanase transcript in RPMI-8226 (panel A) MM cells. Furthermore, DF suppresses the intracellular protein expression of heparanase (measured by flow cytometric analysis) (panel B); as well as the heparanase activity in cellular extracts of RPMI-8226 (panel C) MM cells. Student test: *p< 0.05 and **p<0.01.
Figure 6. Defibrotide (DF) modulates the invasive potential of MM cells and the molecular sequelae of their interactions with endothelial cells.
The invasive potential of MM cells was assessed in vitro on the basis of their ability to invade from the top to the bottom chamber of a dual-chamber in vitro culture system when these 2 chambers are separated by matrigel and a PET membrane. The invasive potential of the MM cells was enhanced by either exogenous addition of heparanase (HSPE) in the culture (panel A) or MM cell transfection with heparanase construct (panel B), but was suppressed, in both cases, by DF treatment. The interaction with MM cells triggers in human microvascular endothelial cells (HMECs) upregulation of transcripts for heparanase (panel C); VEGF and FGF-2 (panel D), but these events are suppressed by DF treatment. Student test: §,*p< 0.05 and §§,**p<0.01.
Defibrotide modulates the molecular events triggered by interaction of endothelial cells with MM cells
We established an in vitro system where MM cells and endothelial cells (HMECs) were cultured in close proximity to each other, with HMECs cultured in the lower chamber of a dual-chamber in vitro system, while the upper chamber contained either medium only (control) or medium with MM cells. In that system, HMECs were cultured (for 48 hours) and after removal of the upper chamber inset, the HMECs and MM cells were separately harvested and processed for mRNA extraction and Real-Time PCR analysis for heparanase transcripts. In this experiment, interaction with MM cells triggered in HMECs significant increase in heparanase expression (Fig. 6C), which was abrogated by DF. In this same system, the interaction of HMECs with MM cells triggered increased gene expression of VEGF, FGF-2 (Fig. 6D), ICAM-1 (Suppl. Fig. 7A), and E-selectin (Suppl. Fig. 7B) in HMECs; as well as increased expression of heparanase, VEGF and FGF-2 in MM cells (Suppl. Fig. 7C). Importantly, treatment with DF was able to suppress these events triggered by HMEC-MM interaction (Fig. 6D and Suppl. Fig. 7).
DISCUSSION
The key focus of this study was to evaluate whether DF can be administered concomitantly with various anti-neoplastic agents currently used for the management of MM and other neoplasias. DF DF has clinical activity for the treatment of severe VOD (6, 7, 10, 41), a clinical setting which historically has >90% mortality rate (42). Among the diverse properties of DF, its anti-thrombotic effects and relative lack of severe hemorrhage or other serious treatment-related toxicity, led us to hypothesize that DF may be an attractive agent for prevention and/or treatment of thromboembolic complications associated with cancer treatment (2). Specifically, we hypothesized that the pleiotropic features of DF which confer protection of endothelial cells from diverse forms of injury could also be useful in treating vascular damage associated with neoplasia-related pro-thrombotic states. In order for extensive clinical evaluation of defibrotide in cancer-related clinical settings to occur, it was essential to address whether its administration is compatible with anti-cancer treatments. In particular, given the known protective effect of DF on the endothelium, it would be potentially detrimental if DF were to extend similar protective effects to neoplastic tissues. Specifically, if neoplastic cells were among those tissues protected by DF, its administration (e.g. as prophylaxis against development of VOD following high-dose chemotherapy and SCT), might theoretically counteract the anti-tumor effects of the cytotoxic conditioning regimen, thus defeating the purpose of the SCT approach.
Our present study indicates that DF does not counteract the anti-tumor activity of several cytotoxic chemotherapy agents commonly used in the management of hematologic malignancies (including MM) and/or solid tumors. This observation was made in in vitro experiments with cell lines from MM, colon and breast carcinoma. We also confirmed that DF does not blunt the anti-MM effect of dexamethasone or the proteasome inhibitor borterzomib (PS-341). Notably, among the diverse classes of anti-cancer drugs that we tested, DF attenuated the anti-tumor effect (in MM or other tumor models) of doxorubicin. Further studies in our group are addressing whether this effect represents a specific feature of doxorubicin or a phenomenon pertinent to the entire class of anthracyclines. It is possible that the 3-dimensional structure of DF retains sufficient similarity to to the “stacked ring” structure of its precursor DNA to allow for anthracyclines to intercalate with defibrotide, similarly to anthracycline intercalation with DNA strands in target cells. For the moment, based on these results, we would suggest caution in terms of concurrent use of DF with doxorubicin or other anthracyclines.
While evaluating the possible impact DF on anti-tumor activities of various classes of agents, we observed no major inhibition of their anti-neoplastic activity. However, we also did not observe a pronounced sensitization of tumor cells to these drug classes (an exception included a modest sensitization of some, but not all, solid tumor cell lines to certain platinum agents). Furthermore, we observed that DF had no significant effect on tumor cell survival as a single agent. These results taken together suggest that, in respect to the tumor models and majority of cancer drug classes that we tested, DF does not exhibit direct anti-tumor effects but also does not function to decrease drug responsiveness in these neoplastic cells.
However, conventional assays for in vitro drug sensitivity of tumor cells cultured in isolation have the key limitation that they do not take into account the interactions of tumor cells with their local microenvironment. It is known that responses of MM and other tumor types to various anti-cancer therapies can be attenuated by cell adhesion-mediated and cytokine/growth factor-driven interactions of tumor cells with their local microenvironment (reviewed in (21)). For instance, MM cell adhesion to BMSCs stimulates paracrine (BMSC-derived) and/or autocrine (MM cell-derived) production of anti-apoptotic cytokines, such as IL-6; as well as triggers direct cell-to-cell contact mediated anti-apoptotic signaling cascades in MM cells (21). The composite effect of these interactions is to attenuate the response of MM cells to various conventional treatments, such as dexamethasone or cytotoxic chemotherapeutics (21). Because this effect is mediated at least in part by adhesion of MM cells on BMSCs, we hypothesized that the anti-adhesive properties of DF (22), could potentially serve to modulate MM-BMSCs interactions and and so sensitize multiple myeloma cells to some of these therapies against which the bone marrow milieu and its stromal cells confer protection.
Our in vitro and in vivo studies indeed support this hypothesis. In ex vivo co-cultures of MM cells with BMSCs, DF had no significant single-agent effect on MM cell viability, either in the presence or absence of BMSCs, and had no sensitizing effect on MM cells exposed to melphalan in the absence of BMSCs. However, the protection conferred by BMSCs to MM cells against Dex or melphalan was significantly suppressed by DF. Further studies at a mechanistic level showed that DF treatment of MM-BMSC co-cultures is associated with decreased adhesion of MM cells to BMSCs and suppression of several key molecular events triggered by MM-BMSC adhesion, including IL-6 and VEGF secretion; NF-κB activation in both the MM and BMSC compartment. IL-6 secretion and NF-κB activation play well-characterized roles in conferring decreased sensitivity of MM cells (or other tumor models) to pro-apoptotic agents, including dexamethasone and/or cytotoxic chemotherapy.
Our in vitro studies support the notion that DF’s effects extend to other aspects of tumor-microenvironment functional interface, such as the interaction of MM cells with HMECs and the invasiveness of MM cells. While interaction of MM cells with human microvascular endothelial cells (HMECs) upregulates the expression of heparanase; VEGF; FGF-2; ICAM-1; and E-selectin in HMECs and of VEGF and FGF-2 in MM cells, these events are suppressed by DF treatment. In addition, DF suppresses in MM cell cultures the transcript and protein expression of heparanase, as well as its enzymatic activity. Recent studies in MM and solid tumors have identified heparanase as an important regulator of tumor cell proliferation; invasiveness and metastatic potential (36, 38, 40). To further evaluate the functional significance of the effects of DF on heparanase, we performed ex vivo tumor cell invasion assays, which showed that DF treatment suppressed the increased in MM cell invasive potential triggered by either exogenous addition of heparanase in the culture or MM cell transfection with heparanase construct.
Importantly, DF exhibits anti-tumor activity in vivo. We observed in three different animal models that DF can sensitize MM or solid tumor cells to various cytotoxic agents, without significant side-effects. In addition, we performed in vivo PK studies of DF (both in p.o. and i.v. administration) which suggest that the DF levels required for in vitro tumor sensitization to Dex and cytotoxic chemotherapy are achievable in vivo.
Our in vitro results indicate that the ability of DF to enhance the anti-tumor activity of Dex or chemotherapeutics in the context of MM cell interactions with their milieu is multi-factorial. Specifically, DF modulates tumor cell interactions with different non-malignant accessory cell compartments of the local milieu (e.g. BMSCs and endothelial cells); influences the protection that these cells can confer to MM cells against conventional anti-tumor agents; interferes with the ability of tumor cells and their microenvironment to trigger cytokine production necessary for recruitment of new blood vessels, without significant direct cytotoxic effect on endothelial cells; and targets heparanase and its role in facilitating the invasiveness potential of tumor cells. These tumor-microenvironment interactions are not exclusive to MM, but are features of a broad spectrum of hematologic malignancies and solid tumors (43–45). Therefore, the activity of DF in both MM and solid tumor models suggests that its anti-tumor properties may have applications in a broad spectrum of neoplasias. With its proven chemo-protective activity towards normal tissues, clinically leveraged to treat VOD (6–9, 11) and its activity in sensitizing tumor cells to anti-cancer therapeutics, DF presents an encouraging profile of biological effects that warrant further evaluation in clinical trials.
Our results do not preclude additional potential mechanism(s) of anti-tumor effects of DF. For instance, the effects of DF on veno-occlusive disease seem to occur via cytoprotective effects on liver sinusoidal endothelium and are related to the antithrombotic and profibrinolytic properties of DF (as reviewed in (3)). Because there is evidence that pro-coagulation mechanisms, and in particular thrombin, may promote tumor progression and neo-angiogenesis (46, 47), the demonstration that DF antagonizes thrombin (48) suggests that thrombin inhibition may also contribute to the anti-cancer effect of DF.
The present study aimed at determining whether DF would interfere with the anti-cancer effect of chemotherapy if administered concurrently with it. In view of the emerging role of DF for the management of VOD and other vascular complications of cancer treatment, this question has direct implication to the future use of this agent in the context of patients with neoplasias. The results of this study show that DF does not adversely interfere with the anti-tumor activity of many important classes of anti-neoplastic agents, with the exception of protection of tumor cells against doxorubicin. Interestingly, our results indicate that DF has intriguing anti-cancer properties in its own right: in multiple in vivo tumor models that were tested, DF confers a modest tumor growth delay as a single agent but also enhances the anti-tumor activity of several chemotherapeutic agents, including melphalan and cyclophosphamide. These results provide a rationale for clinical trials of DF in the treatment of MM and other neoplasias, as well as to the multi-center phase I/II study of melphalan, prednisone, thalidomide and defibrotide in advanced MM patients (49, 50).
Supplementary Material
Suppl. Figure 1. In vitro viability of tumor cell lines and endothelial cells treated with defibrotide. WST-1 assays were performed to assess the putative impact of defibrotide (DF) on the in vitro viability of the human MM cell lines MM-1S and MM1-R (panels A and B, respectively); MCF-7 human breast adenocarcinoma cells (panel C); HT-29 human colorectal adenocarcinoma cells (panel D); human dermal microvascular endothelial cells (HMVEC) (panel E); and human umbilical cord endothelial cells (HUVEC) (panel F). Results for each cell type represent the combination of two separate independent experiments.
Suppl. Figure 2. In vitro viability of MM cells treated with combinations of DF with various anti-tumor drug classes. WST-1 assays were performed to assess the putative impact of DF on the vitro response of MM cells to diverse agents, including (A) carboplatin; (B) paclitaxel; (C) vinblastine; (D) vincristine; and (E) lenalidomide (CC-5013). These results represent the combination of two separate independent experiments. Panel F depicts the results of in vitro treatment of MM-1R cells with dexamethasone in the presence vs. absence of DF
Suppl. Figure 3. Preclinical Pharmacokinetic studies of DF (48 mg/kg) after i.v. (panel A) or p.o. (panel B) administration of DF in the male Fisher rat. Peripheral blood (PBL) plasma samples were serially collected (0–8hrs) via pre-inserted jugular vein catheter for determination of pharmacokinetic (PK) profile by high-pressure liquid chromatography (HPLC) analyses and validation by agarose gel determination.
Suppl. Figure 4. DF treatment enhances the anti-MM activity of conventional therapeutics in the context of MM-BMSCs interactions. Primary MM cells were cultured with Dex (panel A) or Melphalan (panel B), in the presence or absence of BMSCs, with or without treatment with DF. Among MM samples which exhibit constitutive responsiveness to these agents in the absence of stroma, but significantly less pronounced response in MM-BMSC co-cultures, DF is able to lead to variable degrees of sensitization to these agents (e.g. for samples H through N in panel A and I through Q in panel B) in the presence of stromal cells. This supplemental figure includes the results shown in Figure 3: samples, B, E, H, K, M, and N, in panel A are also shown (samples 1–6) in Figure 3 panel A, while samples G, H, J, K, P, and Q are also are also shown (samples 1–6) in Figure 3 panel B.
Suppl. Figure 5: DF modulates sequelae of MM cell adhesion to BMSCs. DF treatment attenuates the increase in NF-κB transcriptional activity triggered in primary MM cells (panel A) and BMSCs (panel B) upon their interaction with each other.
Suppl. Figure 6: DF treatment suppresses the expression and function of heparanase. RT-PCR was performed as described in “material and methods”. Results are expressed as mean mRNA heparanase level normalized by β-actin housekeeping gene. DF treatment suppresses the levels of heparanase transcript in U266 (panel A) MM cells. Furthermore, DF suppresses the heparanase activity in cellular extracts of U266 (panel B) cells. Student test: *p< 0.05 and **p<0.01.
Suppl. Figure 7: DF modulates the molecular sequelae of interactions of MM cells with endothelial cells. The interaction with MM cells triggers in human microvascular endothelial cells (HMECs) upregulation of transcripts for ICAM-1 (panel A) and E-selectin (panel B), but these events are suppressed by DF treatment. Furthermore, interaction with HMECs triggers in MM cells upregulation of transcripts for heparanase, VEGF and FGF-2 (panel C), which is again suppressed by DF. Student test: §,*p< 0.05 and §§,**p<0.01.
STATEMENT OF CLINICAL RELEVANCE.
Thrombotic and microangipathic complications of malignancy are well established, and constitute a major source of morbidity and mortality in cancer patients both from treatment and the disease process itself. Therapeutic strategies targetting endothelial injury may have the dual benefit of reducing toxicity and enhancing efficacy in patients. Defibrotide constitutes an orally bioavailable novel agent with anti-thrombotic properties and minimal toxicity. Our study shows that Defibrotide modulates the interaction of stromal cells with tumor cells in a manner that increases the responsiveness of the latter to existing anti-neoplastic therapies. These data indicate that the Defibrotide may have considerable potential as part of combination treatment in specific settings, including multiple myeloma and solid tumors, such as breast cancer
Acknowledgments
Supported by NIH grants PO-1 78373 and SPORE P50 CA100707 (KCA); NIH Orphan Drug Grant (PGR); the Richard Corman Fund (PGR, CSM) and the Chambers Medical Foundation (PGR and CSM)
Footnotes
Disclosure of Potential Conflicts of Interest
C. Echart, M. Distaso, M. Iacobelli, employees, Gentium. C. Rouleau B. Teicher, employees, Genzyme. M. Iacobelli, ownership interest, Gentium. B. Teicher, ownership interest, Genzyme. P. Richardson, commercial research grant, and advisory board, Gentium; honoraria, Celgene and Millennium. A. Palumbo, honoraria and advisory board, Celgene, and Johnson & Johnson. C. Mitsiades, honoraria, Millennium, Novartis and Pharmion. K. Anderson, board Gentium; advisory board and honoraria, Celgene, Millennium, Novartis, Johnson and Johnson
References
- 1.Lee AY. Management of thrombosis in cancer: primary prevention and secondary prophylaxis. Br J Haematol. 2005;128:291–302. doi: 10.1111/j.1365-2141.2004.05292.x. [DOI] [PubMed] [Google Scholar]
- 2.Knight R, DeLap RJ, Zeldis JB. Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med. 2006;354:2079–80. doi: 10.1056/NEJMc053530. [DOI] [PubMed] [Google Scholar]
- 3.Pescador R, Porta R, Ferro L. An integrated view of the activities of defibrotide. Semin Thromb Hemost. 1996;22 (Suppl 1):71–5. [PubMed] [Google Scholar]
- 4.Eissner G, Multhoff G, Gerbitz A, et al. Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: protective effect of defibrotide. Blood. 2002;100:334–40. doi: 10.1182/blood.v100.1.334. [DOI] [PubMed] [Google Scholar]
- 5.Palmer KJ, Goa KL. Defibrotide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in vascular disorders. Drugs. 1993;45:259–94. doi: 10.2165/00003495-199345020-00007. [DOI] [PubMed] [Google Scholar]
- 6.Richardson PG, Elias AD, Krishnan A, et al. Treatment of severe veno-occlusive disease with defibrotide: compassionate use results in response without significant toxicity in a high-risk population. Blood. 1998;92:737–44. [PubMed] [Google Scholar]
- 7.Richardson PG, Murakami C, Jin Z, et al. Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk population and factors predictive of outcome. Blood. 2002;100:4337–43. doi: 10.1182/blood-2002-04-1216. [DOI] [PubMed] [Google Scholar]
- 8.Abecasis MM, Conceicao Silva JP, Ferreira I, Guimaraes A, Machado A. Defibrotide as salvage therapy for refractory veno-occlusive disease of the liver complicating allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;23:843–6. doi: 10.1038/sj.bmt.1701650. [DOI] [PubMed] [Google Scholar]
- 9.Chopra R, Eaton JD, Grassi A, et al. Defibrotide for the treatment of hepatic veno-occlusive disease: results of the European compassionate-use study. Br J Haematol. 2000;111:1122–9. doi: 10.1046/j.1365-2141.2000.02475.x. [DOI] [PubMed] [Google Scholar]
- 10.Richardson P, Guinan E. Hepatic veno-occlusive disease following hematopoietic stem cell transplantation. Acta Haematol. 2001;106:57–68. doi: 10.1159/000046590. [DOI] [PubMed] [Google Scholar]
- 11.Corbacioglu S, Greil J, Peters C, et al. Defibrotide in the treatment of children with veno-occlusive disease (VOD): a retrospective multicentre study demonstrates therapeutic efficacy upon early intervention. Bone Marrow Transplant. 2004;33:189–95. doi: 10.1038/sj.bmt.1704329. [DOI] [PubMed] [Google Scholar]
- 12.Chalandon Y, Roosnek E, Mermillod B, et al. Prevention of veno-occlusive disease with defibrotide after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:347–54. doi: 10.1016/j.bbmt.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 13.McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:116–22. doi: 10.1002/hep.1840040121. [DOI] [PubMed] [Google Scholar]
- 14.McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–67. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85:3005–20. [PubMed] [Google Scholar]
- 16.Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venoocclusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11:1729–36. doi: 10.1200/JCO.1993.11.9.1729. [DOI] [PubMed] [Google Scholar]
- 17.Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 18.King PD, Perry MC. Hepatotoxicity of chemotherapeutic and oncologic agents. Gastroenterol Clin North Am. 1995;24:969–90. [PubMed] [Google Scholar]
- 19.Richardson P. Hemostatic complications of hematopoietic stem cell transplantation: from hemorrhage to microangiopathies and VOD. Pathophysiol Haemost Thromb. 2003;33 (Suppl 1):50–3. doi: 10.1159/000073293. [DOI] [PubMed] [Google Scholar]
- 20.Bogdanos J, Karamanolakis D, Tenta R, et al. Endocrine/paracrine/autocrine survival factor activity of bone microenvironment participates in the development of androgen ablation and chemotherapy refractoriness of prostate cancer metastasis in skeleton. Endocr Relat Cancer. 2003;10:279–89. doi: 10.1677/erc.0.0100279. [DOI] [PubMed] [Google Scholar]
- 21.Mitsiades CS, Mitsiades NS, Munshi NC, Richardson PG, Anderson KC. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: Interplay of growth factors, their receptors and stromal interactions. Eur J Cancer. 2006 doi: 10.1016/j.ejca.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Carlo-Stella C, Di Nicola M, Magni M, et al. Defibrotide in combination with granulocyte colony-stimulating factor significantly enhances the mobilization of primitive and committed peripheral blood progenitor cells in mice. Cancer Res. 2002;62:6152–7. [PubMed] [Google Scholar]
- 23.Scalia R, Kochilas L, Campbell B, Lefer AM. Effects of defibrotide on leukocyte-endothelial cell interaction in the rat mesenteric vascular bed: role of P-selectin. Methods Find Exp Clin Pharmacol. 1996;18:669–76. [PubMed] [Google Scholar]
- 24.Pellegatta F, Lu Y, Radaelli A, et al. Drug-induced in vitro inhibition of neutrophil-endothelial cell adhesion. Br J Pharmacol. 1996;118:471–6. doi: 10.1111/j.1476-5381.1996.tb15427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellegatta F, Ferrero E, Marni A, Chierchia S, Forti D, Ferrero ME. The anti-ischemic drugs defibrotide and oligotide analogously inhibit leukocyte-endothelial cell adhesion in vitro. Transpl Int. 1996;9 (Suppl 1):S420–4. doi: 10.1007/978-3-662-00818-8_101. [DOI] [PubMed] [Google Scholar]
- 26.Alberico P, Porta R, Pescador R, Ferro L. Is defibrotide’s activity on leukocytes adenosine-receptor mediated? An “in vitro”--”ex vivo” appraisal. Thromb Res. 1995;80:281–9. doi: 10.1016/0049-3848(95)00178-t. [DOI] [PubMed] [Google Scholar]
- 27.Gupta D, Treon SP, Shima Y, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950–61. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 28.Teicher BA, Crawford JM, Holden SA, et al. Glutathione monoethyl ester can selectively protect liver from high dose BCNU or cyclophosphamide. Cancer. 1988;62:1275–81. doi: 10.1002/1097-0142(19881001)62:7<1275::aid-cncr2820620705>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 29.Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 1996;19:1518–20. doi: 10.1248/bpb.19.1518. [DOI] [PubMed] [Google Scholar]
- 30.Koester SK, Roth P, Mikulka WR, Schlossman SF, Zhang C, Bolton WE. Monitoring early cellular responses in apoptosis is aided by the mitochondrial membrane protein-specific monoclonal antibody APO2. 7. Cytometry. 1997;29:306–12. [PubMed] [Google Scholar]
- 31.Mitsiades CS, Mitsiades NS, Bronson RT, et al. Fluorescence imaging of multiple myeloma cells in a clinically relevant SCID/NOD in vivo model: biologic and clinical implications. Cancer Res. 2003;63:6689–96. [PubMed] [Google Scholar]
- 32.Kakeji Y, Maehara Y, Ikebe M, Teicher BA. Dynamics of tumor oxygenation, CD31 staining and transforming growth factor-beta levels after treatment with radiation or cyclophosphamide in the rat 13762 mammary carcinoma. Int J Radiat Oncol Biol Phys. 1997;37:1115–23. doi: 10.1016/s0360-3016(96)00573-1. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez E, Westmore M, Galvin RJ, et al. Properties of bisphosphonates in the 13762 rat mammary carcinoma model of tumor-induced bone resorption. Clin Cancer Res. 2003;9:5705–13. [PubMed] [Google Scholar]
- 34.Chauhan D, Uchiyama H, Akbarali Y, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–12. [PubMed] [Google Scholar]
- 35.Chauhan D, Uchiyama H, Urashima M, Yamamoto K, Anderson KC. Regulation of interleukin 6 in multiple myeloma and bone marrow stromal cells. Stem Cells. 1995;13 (Suppl 2):35–9. [PubMed] [Google Scholar]
- 36.Fontelonga A, Kelly AJ, MacKintosh FR, et al. A novel high-dose chemotherapy protocol with autologous hematopoietic rescue in patients with metastatic breast cancer or recurrent non-Hodgkin’s lymphoma. Bone Marrow Transplant. 1997;19:983–8. doi: 10.1038/sj.bmt.1700783. [DOI] [PubMed] [Google Scholar]
- 37.Mahtouk K, Hose D, Raynaud P, et al. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 2007;109:4914–23. doi: 10.1182/blood-2006-08-043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldshmidt O, Zcharia E, Abramovitch R, et al. Cell surface expression and secretion of heparanase markedly promote tumor angiogenesis and metastasis. Proc Natl Acad Sci U S A. 2002;99:10031–6. doi: 10.1073/pnas.152070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafat I, Vlodavsky I, Ilan N. Characterization of mechanisms involved in secretion of active heparanase. J Biol Chem. 2006;281:23804–11. doi: 10.1074/jbc.M602762200. [DOI] [PubMed] [Google Scholar]
- 40.Yang YL, Lu MY, Jou ST, Lin KH, Lin DT. Hematopoietic stem cell transplantation in Taiwanese children with primary immunodeficiency. J Formos Med Assoc. 2005;104:101–6. [PubMed] [Google Scholar]
- 41.Richardson P, Bearman SI. Prevention and treatment of hepatic venocclusive disease after high-dose cytoreductive therapy. Leuk Lymphoma. 1998;31:267–77. doi: 10.3109/10428199809059219. [DOI] [PubMed] [Google Scholar]
- 42.Wadleigh M, Ho V, Momtaz P, Richardson P. Hepatic veno-occlusive disease: pathogenesis, diagnosis and treatment. Curr Opin Hematol. 2003;10:451–62. doi: 10.1097/00062752-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Murry BP, Greiter-Wilke A, Paulsen DP, Hiatt KM, Beltrami CA, Marchetti D. Selective heparanase localization in malignant melanoma. Int J Oncol. 2005;26:345–52. [PubMed] [Google Scholar]
- 44.Chen JQ, Zhan WH, He YL, et al. Expression of heparanase gene, CD44v6, MMP-7 and nm23 protein and their relationship with the invasion and metastasis of gastric carcinomas. World J Gastroenterol. 2004;10:776–82. doi: 10.3748/wjg.v10.i6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Assal ON, Yamanoi A, Ono T, Kohno H, Nagasue N. The clinicopathological significance of heparanase and basic fibroblast growth factor expressions in hepatocellular carcinoma. Clin Cancer Res. 2001;7:1299–305. [PubMed] [Google Scholar]
- 46.Rickles FR, Patierno S, Fernandez PM. Tissue factor, thrombin, and cancer. Chest. 2003;124:58S–68S. doi: 10.1378/chest.124.3_suppl.58s. [DOI] [PubMed] [Google Scholar]
- 47.Falanga A. Biological and clinical aspects of anticancer effects of antithrombotics. Pathophysiol Haemost Thromb. 2003;33:389–92. doi: 10.1159/000083834. [DOI] [PubMed] [Google Scholar]
- 48.Bracht F, Schror K. Isolation and identification of aptamers from defibrotide that act as thrombin antagonists in vitro. Biochem Biophys Res Commun. 1994;200:933–7. doi: 10.1006/bbrc.1994.1539. [DOI] [PubMed] [Google Scholar]
- 49.Palumbo A, Rus C, Rossi D, et al. A multi-center phase I/II study of melphalan, prednisone, thalidomide and defibrotide in avanced multiple myeloma patients. Blood. 2006;108:1016A–17A. doi: 10.3324/haematol.2009.017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larocca A, Rossi D, Pregno P, et al. A multicenter phase I/II trial on combination of Melphalan, Prednisone, Thalidomide and Defibrotide in advanced stage Multiple Myeloma patients. Haematologica-the Hematology Journal. 2007;92:138–39. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1. In vitro viability of tumor cell lines and endothelial cells treated with defibrotide. WST-1 assays were performed to assess the putative impact of defibrotide (DF) on the in vitro viability of the human MM cell lines MM-1S and MM1-R (panels A and B, respectively); MCF-7 human breast adenocarcinoma cells (panel C); HT-29 human colorectal adenocarcinoma cells (panel D); human dermal microvascular endothelial cells (HMVEC) (panel E); and human umbilical cord endothelial cells (HUVEC) (panel F). Results for each cell type represent the combination of two separate independent experiments.
Suppl. Figure 2. In vitro viability of MM cells treated with combinations of DF with various anti-tumor drug classes. WST-1 assays were performed to assess the putative impact of DF on the vitro response of MM cells to diverse agents, including (A) carboplatin; (B) paclitaxel; (C) vinblastine; (D) vincristine; and (E) lenalidomide (CC-5013). These results represent the combination of two separate independent experiments. Panel F depicts the results of in vitro treatment of MM-1R cells with dexamethasone in the presence vs. absence of DF
Suppl. Figure 3. Preclinical Pharmacokinetic studies of DF (48 mg/kg) after i.v. (panel A) or p.o. (panel B) administration of DF in the male Fisher rat. Peripheral blood (PBL) plasma samples were serially collected (0–8hrs) via pre-inserted jugular vein catheter for determination of pharmacokinetic (PK) profile by high-pressure liquid chromatography (HPLC) analyses and validation by agarose gel determination.
Suppl. Figure 4. DF treatment enhances the anti-MM activity of conventional therapeutics in the context of MM-BMSCs interactions. Primary MM cells were cultured with Dex (panel A) or Melphalan (panel B), in the presence or absence of BMSCs, with or without treatment with DF. Among MM samples which exhibit constitutive responsiveness to these agents in the absence of stroma, but significantly less pronounced response in MM-BMSC co-cultures, DF is able to lead to variable degrees of sensitization to these agents (e.g. for samples H through N in panel A and I through Q in panel B) in the presence of stromal cells. This supplemental figure includes the results shown in Figure 3: samples, B, E, H, K, M, and N, in panel A are also shown (samples 1–6) in Figure 3 panel A, while samples G, H, J, K, P, and Q are also are also shown (samples 1–6) in Figure 3 panel B.
Suppl. Figure 5: DF modulates sequelae of MM cell adhesion to BMSCs. DF treatment attenuates the increase in NF-κB transcriptional activity triggered in primary MM cells (panel A) and BMSCs (panel B) upon their interaction with each other.
Suppl. Figure 6: DF treatment suppresses the expression and function of heparanase. RT-PCR was performed as described in “material and methods”. Results are expressed as mean mRNA heparanase level normalized by β-actin housekeeping gene. DF treatment suppresses the levels of heparanase transcript in U266 (panel A) MM cells. Furthermore, DF suppresses the heparanase activity in cellular extracts of U266 (panel B) cells. Student test: *p< 0.05 and **p<0.01.
Suppl. Figure 7: DF modulates the molecular sequelae of interactions of MM cells with endothelial cells. The interaction with MM cells triggers in human microvascular endothelial cells (HMECs) upregulation of transcripts for ICAM-1 (panel A) and E-selectin (panel B), but these events are suppressed by DF treatment. Furthermore, interaction with HMECs triggers in MM cells upregulation of transcripts for heparanase, VEGF and FGF-2 (panel C), which is again suppressed by DF. Student test: §,*p< 0.05 and §§,**p<0.01.